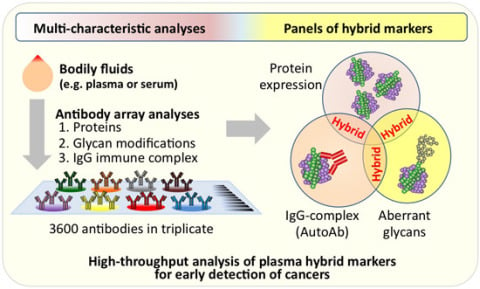
High-Throughput Analysis of Plasma Hybrid Markers for Early Detection of Cancers
Abstract
:1. Introduction
| Protein, glycoprotein and glycan markers | Cancer type | Source | Clinical use | Known glycosylations |
|---|---|---|---|---|
| Alpha-fetoprotein (AFP) | Liver | Blood | Staging | Sialydated [10] |
| Beta-human chorionic gonadotropin (Beta-hCG) | Choriocarcinoma | Urine/blood | Staging, prognosis, treatment response | N- and O-glycans [11] |
| CA125 (MUC16) | Ovarian | Blood | Monitoring | High mannose, complex bisecting N-glycans.Type 1 and 2 O-glycans [12] |
| CA15-3 (MUC1) | Breast | Blood | Monitoring | TF antigen (core 1) and sialyated [13] |
| CA19-9 | Pancreas | Blood | Monitoring | Sialyl Lewis A [14] |
| CEA (carcinoembryonic antigen) | Colon | Blood | Monitoring | Lewis X and Y, high mannose N-glycan [13] |
| Chromogranin A (CgA) | Neuroendocrine tumors | Tumor | Diagnosis, prognosis | O-glycan [15] |
| EGFR | Non-small cell lung cancer | Tumor | Treatment selection | N- and O-glycans [16], sialylated and fucosylated [17] |
| Epididymis protein 4 (HE4) | Ovarian | Blood | Monitoring | N-glycan [18] |
| Fibrin/Fibrinogen (gamma chain) | Bladder | Urine | Monitoring | N-glycan and fucosylated [19] |
| HER2/neu | Breast, gastric, esophageal | Tumor | Monitoring, prognosis and treatment selection | N-glycan [20] |
| KIT | GI stromal tumor and mucosal melanomas | Tumor | Diagnosis, treatment selection | N-glycan [21] |
| Prostate-specific antigen (PSA) | Prostate | Blood | Screening and monitoring | Single N-glycan [22], sialylated [23] |
| Thyroglobulin | Thyroid | Tumor | Monitoring | N-glycan [24] |
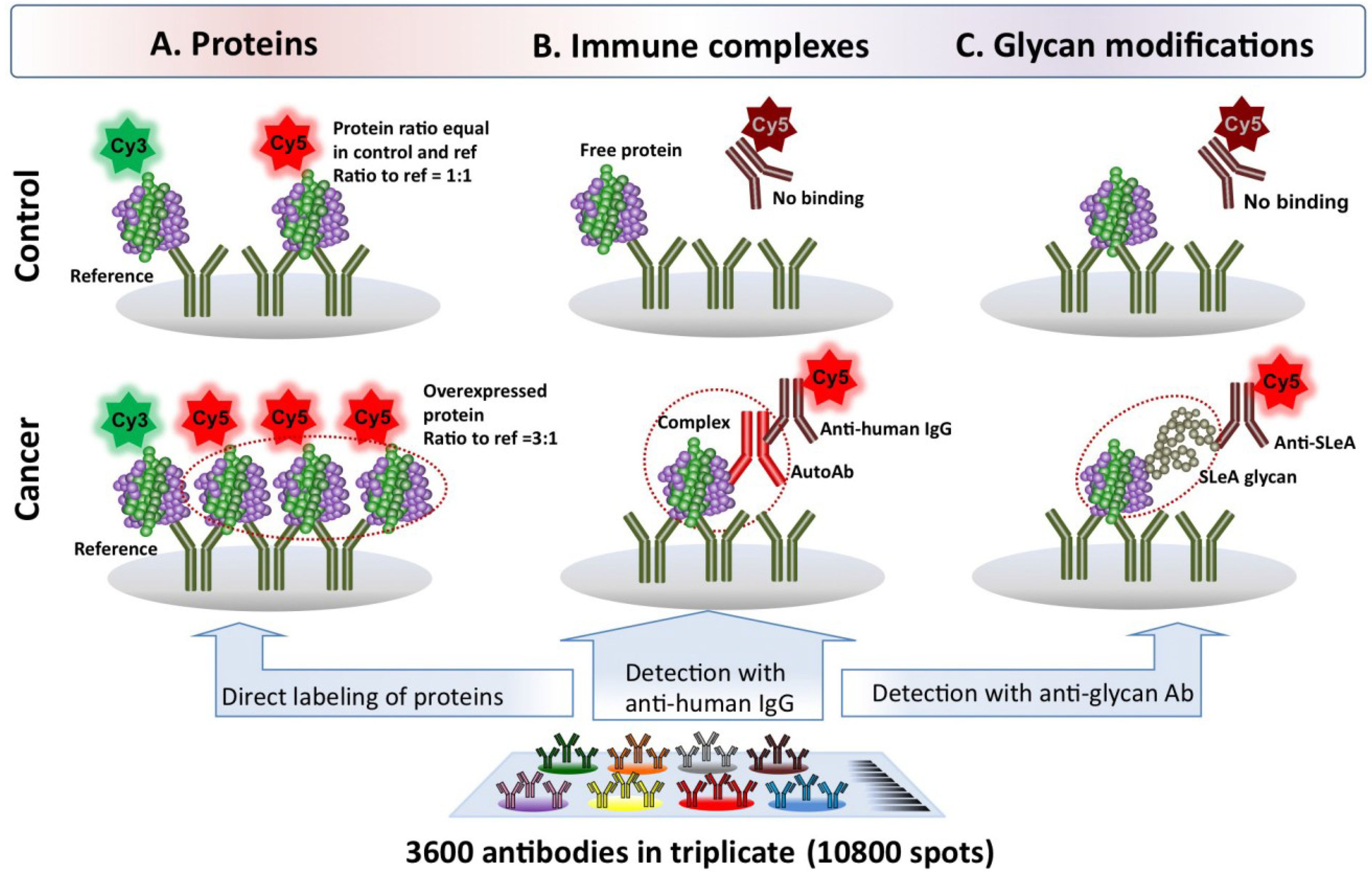
2. A New Paradigm for Novel Blood-Based Cancer Markers: Panels of Hybrid Markers
3. Serum and Plasma as a Source of Biomarkers
3.1. Plasma and Serum Proteomes
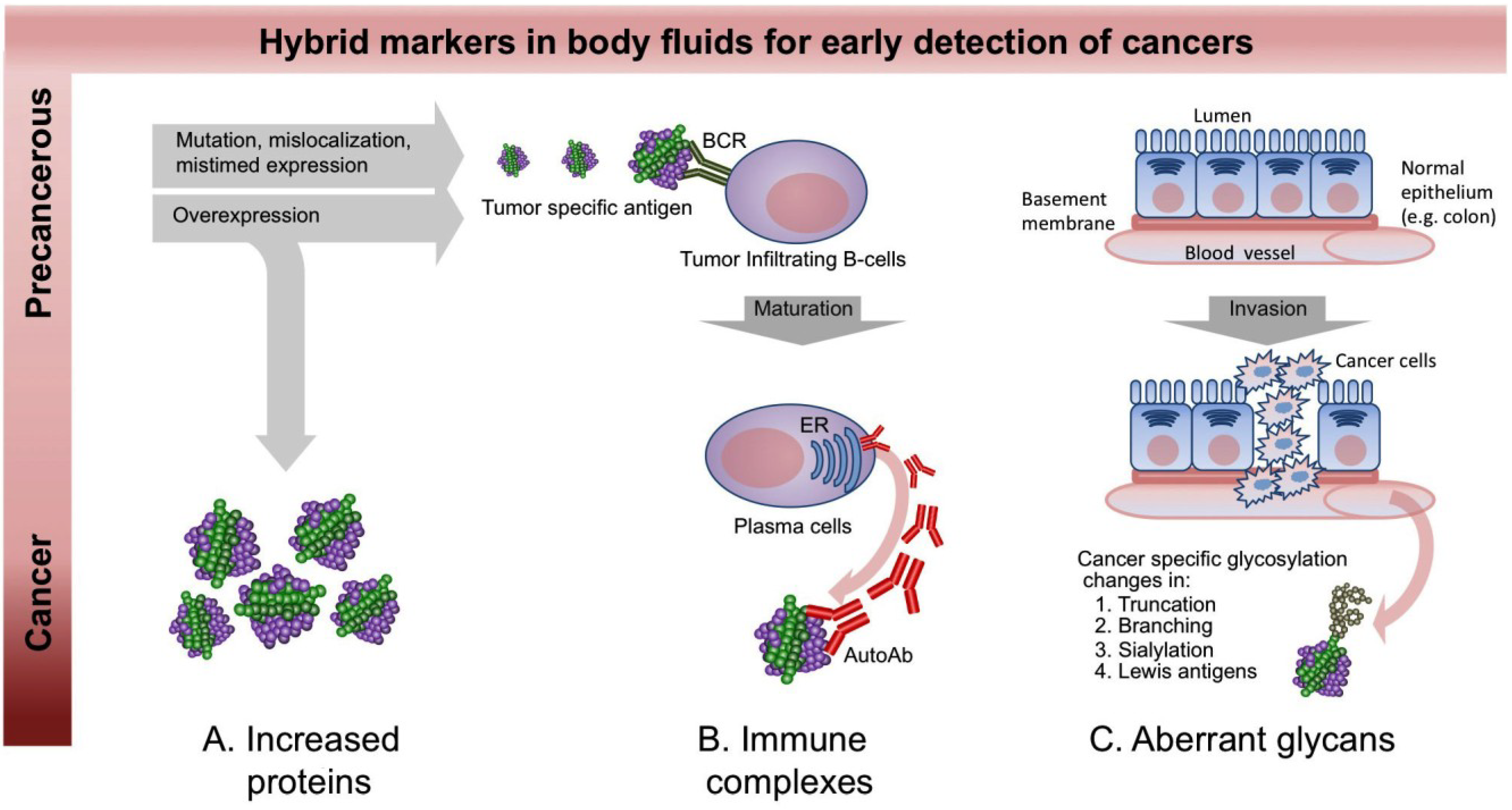
3.2. Autoantibody Markers
3.3. Carbohydrate Markers
4. Enhanced Performance of Hybrid Markers: A Potential Future Direction of Early Detection Biomarker Discovery Research
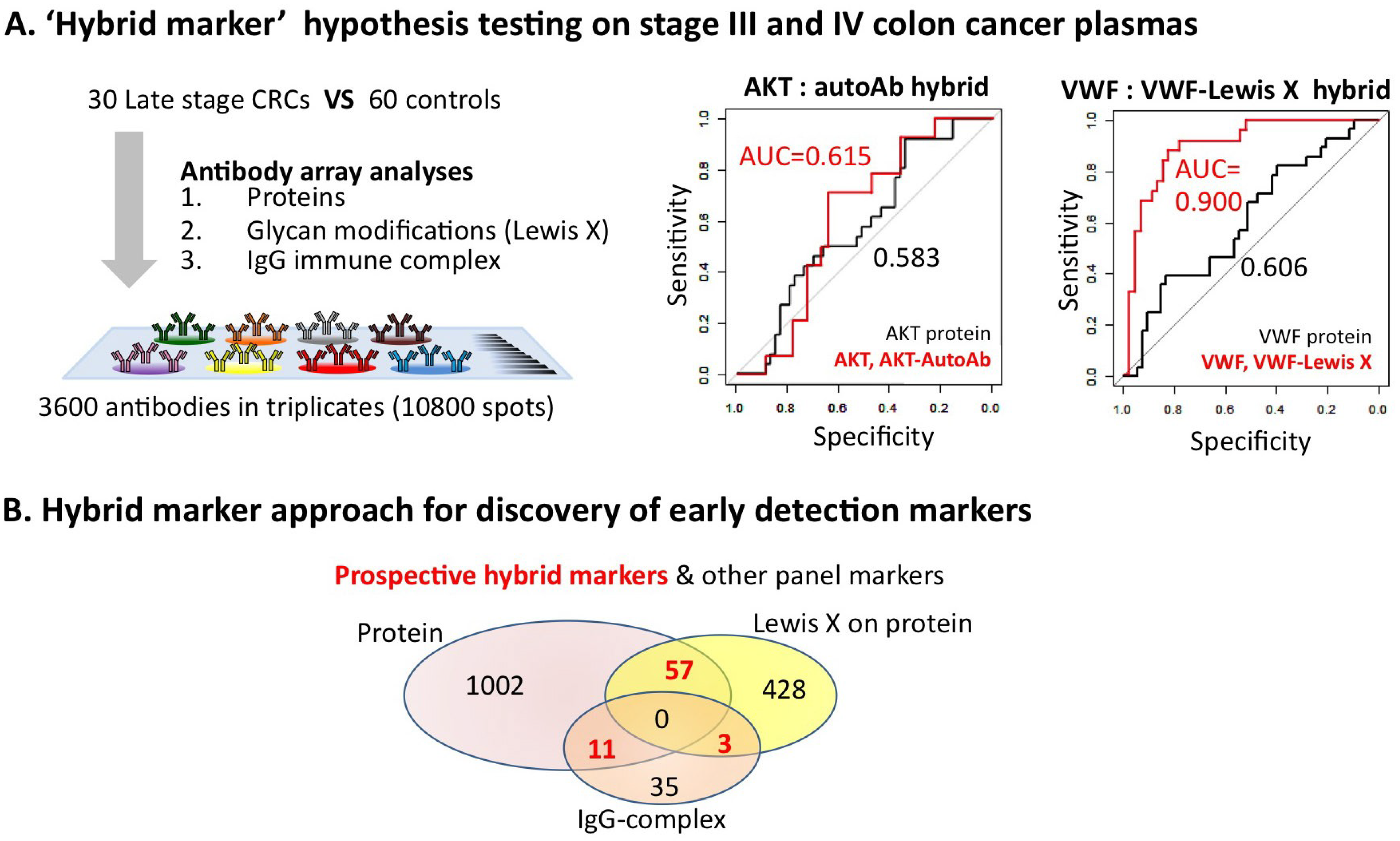
5. Conclusions
Acknowledgments
Authors’ Contribution
Conflicts of Interest
References and Notes
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef]
- Church, T.R.; Black, W.C.; Aberle, D.R.; Berg, C.D.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; Gierada, D.S.; Jones, G.C.; et al. Results of initial low-dose computed tomographic screening for lung cancer. N. Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar] [CrossRef]
- Brodersen, J.; Siersma, V.D. Long-term psychosocial consequences of false-positive screening mammography. Ann. Fam. Med. 2013, 11, 106–115. [Google Scholar] [CrossRef]
- Croswell, J.M.; Kramer, B.S.; Kreimer, A.R.; Prorok, P.C.; Xu, J.L.; Baker, S.G.; Fagerstrom, R.; Riley, T.L.; Clapp, J.D.; Berg, C.D.; et al. Cumulative incidence of false-positive results in repeated, multimodal cancer screening. Ann. Fam. Med. 2009, 7, 212–222. [Google Scholar] [CrossRef]
- Weller, D.; Coleman, D.; Robertson, R.; Butler, P.; Melia, J.; Campbell, C.; Parker, R.; Patnick, J.; Moss, S. The UK colorectal cancer screening pilot: Results of the second round of screening in England. Br. J. Cancer 2007, 97, 1601–1605. [Google Scholar] [CrossRef]
- Tumor markers. Available online: http://www.Cancer.Gov/cancertopics/factsheet/detection/tumor-markers (accessed on 5 November 2013).
- Drucker, E.; Krapfenbauer, K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013, 4, e7. [Google Scholar] [CrossRef]
- Issaq, H.J.; Waybright, T.J.; Veenstra, T.D. Cancer biomarker discovery: Opportunities and pitfalls in analytical methods. Electrophoresis 2011, 32, 967–975. [Google Scholar] [CrossRef]
- Hori, S.S.; Gambhir, S.S. Mathematical model identifies blood biomarker-based early cancer detection strategies and limitations. Sci. Transl. Med. 2011, 3, 109ra116. [Google Scholar]
- Poon, T.C.; Mok, T.S.; Chan, A.T.; Chan, C.M.; Leong, V.; Tsui, S.H.; Leung, T.W.; Wong, H.T.; Ho, S.K.; Johnson, P.J. Quantification and utility of monosialylated alpha-fetoprotein in the diagnosis of hepatocellular carcinoma with nondiagnostic serum total alpha-fetoprotein. Clin. Chem. 2002, 48, 1021–1027. [Google Scholar]
- Lapthorn, A.J.; Harris, D.C.; Littlejohn, A.; Lustbader, J.W.; Canfield, R.E.; Machin, K.J.; Morgan, F.J.; Isaacs, N.W. Crystal structure of human chorionic gonadotropin. Nature 1994, 369, 455–461. [Google Scholar] [CrossRef]
- Kui Wong, N.; Easton, R.L.; Panico, M.; Sutton-Smith, M.; Morrison, J.C.; Lattanzio, F.A.; Morris, H.R.; Clark, G.F.; Dell, A.; Patankar, M.S. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J. Biol. Chem. 2003, 278, 28619–28634. [Google Scholar] [CrossRef]
- Saeland, E.; Belo, A.I.; Mongera, S.; van Die, I.; Meijer, G.A.; van Kooyk, Y. Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. Int. J. Cancer 2012, 131, 117–128. [Google Scholar] [CrossRef]
- Hanisch, F.G.; Uhlenbruck, G.; Dienst, C. Structure of tumor-associated carbohydrate antigen CA 19-9 on human seminal-plasma glycoproteins from healthy donors. Eur. J. Biochem. 1984, 144, 467–473. [Google Scholar] [CrossRef]
- Gadroy, P.; Stridsberg, M.; Capon, C.; Michalski, J.C.; Strub, J.M.; van Dorsselaer, A.; Aunis, D.; Metz-Boutigue, M.H. Phosphorylation and O-glycosylation sites of human chromogranin a (CGA79-439) from urine of patients with carcinoid tumors. J. Biol. Chem. 1998, 273, 34087–34097. [Google Scholar] [CrossRef]
- Wu, Y.M.; Liu, C.H.; Hu, R.H.; Huang, M.J.; Lee, J.J.; Chen, C.H.; Huang, J.; Lai, H.S.; Lee, P.H.; Hsu, W.M.; et al. Mucin glycosylating enzyme GALNT2 regulates the malignant character of hepatocellular carcinoma by modifying the EGF receptor. Cancer Res. 2011, 71, 7270–7279. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yen, H.Y.; Chen, C.Y.; Chen, C.H.; Cheng, P.F.; Juan, Y.H.; Khoo, K.H.; Yu, C.J.; Yang, P.C.; Hsu, T.L.; et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 11332–11337. [Google Scholar] [CrossRef]
- Drapkin, R.; von Horsten, H.H.; Lin, Y.; Mok, S.C.; Crum, C.P.; Welch, W.R.; Hecht, J.L. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005, 65, 2162–2169. [Google Scholar] [CrossRef]
- Pabst, M.; Bondili, J.S.; Stadlmann, J.; Mach, L.; Altmann, F. Mass + retention time = structure: A strategy for the analysis of N-glycans by carbon LC-ESI-MS and its application to fibrin N-glycans. Anal. Chem. 2007, 79, 5051–5057. [Google Scholar] [CrossRef]
- Doherty, J.K.; Bond, C.; Jardim, A.; Adelman, J.P.; Clinton, G.M. The HER-2/neu receptor tyrosine kinase gene encodes a secreted autoinhibitor. Proc. Natl. Acad. Sci. USA 1999, 96, 10869–10874. [Google Scholar] [CrossRef]
- Majumder, S.; Brown, K.; Qiu, F.H.; Besmer, P. C-kit protein, a transmembrane kinase: Identification in tissues and characterization. Mol. Cell. Biol. 1988, 8, 4896–4903. [Google Scholar]
- Meany, D.L.; Zhang, Z.; Sokoll, L.J.; Zhang, H.; Chan, D.W. Glycoproteomics for prostate cancer detection: Changes in serum PSA glycosylation patterns. J. Proteome Res. 2009, 8, 613–619. [Google Scholar] [CrossRef]
- Hakomori, S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989, 52, 257–331. [Google Scholar]
- Yang, S.X.; Pollock, H.G.; Rawitch, A.B. Glycosylation in human thyroglobulin: Location of the n-linked oligosaccharide units and comparison with bovine thyroglobulin. Arch. Biochem. Biophys. 1996, 327, 61–70. [Google Scholar] [CrossRef]
- Wang, V.; Li, C.; Lin, M.; Welch, W.; Bell, D.; Wong, Y.F.; Berkowitz, R.; Mok, S.C.; Bandera, C.A. Ovarian cancer is a heterogeneous disease. Cancer Genet. Cytogenet. 2005, 161, 170–173. [Google Scholar] [CrossRef]
- Zhao, H.; Langerod, A.; Ji, Y.; Nowels, K.W.; Nesland, J.M.; Tibshirani, R.; Bukholm, I.K.; Karesen, R.; Botstein, D.; Borresen-Dale, A.L.; et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol. Biol. Cell. 2004, 15, 2523–2536. [Google Scholar] [CrossRef]
- Warnberg, F.; Nordgren, H.; Bergkvist, L.; Holmberg, L. Tumour markers in breast carcinoma correlate with grade rather than with invasiveness. Br. J. Cancer 2001, 85, 869–874. [Google Scholar] [CrossRef]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar]
- Kravchenko, J.; Akushevich, I.; Seewaldt, V.L.; Abernethy, A.P.; Lyerly, H.K. Breast cancer as heterogeneous disease: Contributing factors and carcinogenesis mechanisms. Breast Cancer Res. Treat. 2011, 128, 483–493. [Google Scholar] [CrossRef]
- West, L.; Vidwans, S.J.; Campbell, N.P.; Shrager, J.; Simon, G.R.; Bueno, R.; Dennis, P.A.; Otterson, G.A.; Salgia, R. A novel classification of lung cancer into molecular subtypes. PLoS One 2012, 7, e31906. [Google Scholar] [CrossRef]
- Berek, J.S.; Bast, R.C., Jr. Ovarian cancer screening. The use of serial complementary tumor markers to improve sensitivity and specificity for early detection. Cancer 1995, 76, 2092–2096. [Google Scholar] [CrossRef]
- Yedema, C.A.; Kenemans, P.; Wobbes, T.; Thomas, C.M.; Bon, G.G.; Mulder, C.; Voorhorst, F.J.; Verstraeten, A.A.; van Kamp, G.J.; Hilgers, J. Use of serum tumor markers in the differential diagnosis between ovarian and colorectal adenocarcinomas. Tumor Biol. 1992, 13, 18–26. [Google Scholar] [CrossRef]
- Firpo, M.A.; Gay, D.Z.; Granger, S.R.; Scaife, C.L.; DiSario, J.A.; Boucher, K.M.; Mulvihill, S.J. Improved diagnosis of pancreatic adenocarcinoma using haptoglobin and serum amyloid a in a panel screen. World J. Surg. 2009, 33, 716–722. [Google Scholar] [CrossRef]
- Wild, N.; Andres, H.; Rollinger, W.; Krause, F.; Dilba, P.; Tacke, M.; Karl, J. A combination of serum markers for the early detection of colorectal cancer. Clin. Cancer Res. 2010, 16, 6111–6121. [Google Scholar] [CrossRef]
- Gormally, E.; Caboux, E.; Vineis, P.; Hainaut, P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: Practical aspects and biological significance. Mutat. Res. 2007, 635, 105–117. [Google Scholar] [CrossRef]
- deVos, T.; Tetzner, R.; Model, F.; Weiss, G.; Schuster, M.; Distler, J.; Steiger, K.V.; Grutzmann, R.; Pilarsky, C.; Habermann, J.K.; et al. Circulating methylated sept9 DNA in plasma is a biomarker for colorectal cancer. Clin. Chem. 2009, 55, 1337–1346. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Li, T.; Kung, H.J.; Mack, P.C.; Gandara, D.R. Genotyping and genomic profiling of non-small-cell lung cancer: Implications for current and future therapies. J. Clin. Oncol. 2013, 31, 1039–1049. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteomics 2002, 1, 845–867. [Google Scholar] [CrossRef]
- Catalona, W.J.; Richie, J.P.; deKernion, J.B.; Ahmann, F.R.; Ratliff, T.L.; Dalkin, B.L.; Kavoussi, L.R.; MacFarlane, M.T.; Southwick, P.C. Comparison of prostate specific antigen concentration versus prostate specific antigen density in the early detection of prostate cancer: Receiver operating characteristic curves. J. Urol. 1994, 152, 2031–2036. [Google Scholar]
- Schroder, F.H.; van der Cruijsen-Koeter, I.; de Koning, H.J.; Vis, A.N.; Hoedemaeker, R.F.; Kranse, R. Prostate cancer detection at low prostate specific antigen. J. Urol. 2000, 163, 806–812. [Google Scholar] [CrossRef]
- Negishi, Y.; Furukawa, T.; Oka, T.; Sakamoto, M.; Hirata, T.; Okabe, K.; Matayoshi, K.; Akiya, K.; Soma, H. Clinical use of CA 125 and its combination assay with other tumor marker in patients with ovarian carcinoma. Gynecol. Obstet. Invest. 1987, 23, 200–207. [Google Scholar] [CrossRef]
- Coghlin, C.; Murray, G.I. Progress in the identification of plasma biomarkers of colorectal cancer. Proteomics 2013, 13, 2227–2228. [Google Scholar] [CrossRef]
- Rho, J.H.; Lampe, P.D. High-throughput screening for native autoantigen-autoantibody complexes using antibody microarrays. J. Proteome Res. 2013, 12, 2311–2320. [Google Scholar] [CrossRef]
- Soussi, T. P53 antibodies in the sera of patients with various types of cancer: A review. Cancer Res. 2000, 60, 1777–1788. [Google Scholar]
- Von Mensdorff-Pouilly, S.; Petrakou, E.; Kenemans, P.; van Uffelen, K.; Verstraeten, A.A.; Snijdewint, F.G.; van Kamp, G.J.; Schol, D.J.; Reis, C.A.; Price, M.R.; et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and n-acetylgalactosamine (GalNAc) peptides. Int. J. Cancer 2000, 86, 702–712. [Google Scholar] [CrossRef]
- Ulanet, D.B.; Torbenson, M.; Dang, C.V.; Casciola-Rosen, L.; Rosen, A. Unique conformation of cancer autoantigen b23 in hepatoma: A mechanism for specificity in the autoimmune response. Proc. Natl. Acad. Sci. USA 2003, 100, 12361–12366. [Google Scholar] [CrossRef]
- Fourneau, J.M.; Bach, J.M.; van Endert, P.M.; Bach, J.F. The elusive case for a role of mimicry in autoimmune diseases. Mol. Immunol. 2004, 40, 1095–1102. [Google Scholar] [CrossRef]
- Casciola-Rosen, L.; Andrade, F.; Ulanet, D.; Wong, W.B.; Rosen, A. Cleavage by granzyme b is strongly predictive of autoantigen status: Implications for initiation of autoimmunity. J. Exp. Med. 1999, 190, 815–826. [Google Scholar] [CrossRef]
- Nozawa, K.; Fritzler, M.J.; Chan, E.K. Unique and shared features of Golgi complex autoantigens. Autoimmun. Rev. 2005, 4, 35–41. [Google Scholar] [CrossRef]
- Rho, J.H.; Zhang, W.; Murali, M.; Roehrl, M.H.; Wang, J.Y. Human proteins with affinity for dermatan sulfate have the propensity to become autoantigens. Am. J. Pathol. 2011, 178, 2177–2190. [Google Scholar] [CrossRef]
- Russo, N.; Wang, X.; Liu, M.; Banerjee, R.; Goto, M.; Scanlon, C.; Metwally, T.; Inglehart, R.C.; Tsodikov, A.; Duffy, S.; et al. A novel approach to biomarker discovery in head and neck cancer using an autoantibody signature. Oncogene 2011, 32, 5026–5037. [Google Scholar]
- Imahayashi, S.; Ichiyoshi, Y.; Yoshino, I.; Eifuku, R.; Takenoyama, M.; Yasumoto, K. Tumor-infiltrating B-cell-derived IgG recognizes tumor components in human lung cancer. Cancer Invest. 2000, 18, 530–536. [Google Scholar] [CrossRef]
- Lim, K.H.; Telisinghe, P.U.; Abdullah, M.S.; Ramasamy, R. Possible significance of differences in proportions of cytotoxic T cells and B-lineage cells in the tumour-infiltrating lymphocytes of typical and atypical medullary carcinomas of the breast. Cancer Immun. 2010, 10, e3. [Google Scholar]
- Milne, K.; Kobel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009, 4, e6412. [Google Scholar]
- Nedergaard, B.S.; Ladekarl, M.; Nyengaard, J.R.; Nielsen, K. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol. Oncol. 2008, 108, 106–111. [Google Scholar] [CrossRef]
- Deschoolmeester, V.; Baay, M.; Van Marck, E.; Weyler, J.; Vermeulen, P.; Lardon, F.; Vermorken, J.B. Tumor infiltrating lymphocytes: An intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010, 11, e19. [Google Scholar] [CrossRef]
- Al-Shibli, K.I.; Donnem, T.; Al-Saad, S.; Persson, M.; Bremnes, R.M.; Busund, L.T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 5220–5227. [Google Scholar] [CrossRef]
- Riemann, D.; Wenzel, K.; Schulz, T.; Hofmann, S.; Neef, H.; Lautenschlager, C.; Langner, J. Phenotypic analysis of T lymphocytes isolated from non-small-cell lung cancer. Int. Arch. Allergy Immunol. 1997, 114, 38–45. [Google Scholar] [CrossRef]
- Nelson, B.H. CD20+ B cells: The other tumor-infiltrating lymphocytes. J. Immunol. 2010, 185, 4977–4982. [Google Scholar] [CrossRef]
- Lubin, R.; Zalcman, G.; Bouchet, L.; Tredanel, J.; Legros, Y.; Cazals, D.; Hirsch, A.; Soussi, T. Serum p53 antibodies as early markers of lung cancer. Nat. Med. 1995, 1, 701–702. [Google Scholar] [CrossRef]
- Pedersen, J.W.; Gentry-Maharaj, A.; Nostdal, A.; Fourkala, E.O.; Dawnay, A.; Burnell, M.; Zaikin, A.; Burchell, J.; Papadimitriou, J.T.; Clausen, H.; et al. Cancer associated auto-antibodies to MUC1 and MUC4—A blinded case control study of colorectal cancer in UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Int. J. Cancer 2013. [Google Scholar] [CrossRef]
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the swiss-prot database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar]
- Varki, A.; Kannagi, R.; Toole, B.P. Glycosylation changes in cancer. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Brockhausen, I. Mucin-type o-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep. 2006, 7, 599–604. [Google Scholar] [CrossRef]
- Adamczyk, B.; Tharmalingam, T.; Rudd, P.M. Glycans as cancer biomarkers. Biochim. Biophys. Acta 2012, 1820, 1347–1353. [Google Scholar] [CrossRef]
- Reis, C.A.; Osorio, H.; Silva, L.; Gomes, C.; David, L. Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 2010, 63, 322–329. [Google Scholar]
- Drake, P.M.; Cho, W.; Li, B.; Prakobphol, A.; Johansen, E.; Anderson, N.L.; Regnier, F.E.; Gibson, B.W.; Fisher, S.J. Sweetening the pot: Adding glycosylation to the biomarker discovery equation. Clin. Chem. 2009, 56, 223–236. [Google Scholar]
- Wada, Y.; Azadi, P.; Costello, C.E.; Dell, A.; Dwek, R.A.; Geyer, H.; Geyer, R.; Kakehi, K.; Karlsson, N.G.; Kato, K.; et al. Comparison of the methods for profiling glycoprotein glycans—HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study. Glycobiology 2007, 17, 411–422. [Google Scholar] [CrossRef]
- Yue, T.; Partyka, K.; Maupin, K.A.; Hurley, M.; Andrews, P.; Kaul, K.; Moser, A.J.; Zeh, H.; Brand, R.E.; Haab, B.B. Identification of blood-protein carriers of the CA 19-9 antigen and characterization of prevalence in pancreatic diseases. Proteomics 2011, 11, 3665–3674. [Google Scholar] [CrossRef]
- Hashii, N.; Kawasaki, N.; Itoh, S.; Nakajima, Y.; Harazono, A.; Kawanishi, T.; Yamaguchi, T. Identification of glycoproteins carrying a target glycan-motif by liquid chromatography/multiple-stage mass spectrometry: Identification of Lewis x-conjugated glycoproteins in mouse kidney. J. Proteome Res. 2009, 8, 3415–3429. [Google Scholar] [CrossRef]
- Haab, B.B.; Yue, T. High-throughput studies of protein glycoforms using antibody-lectin sandwich arrays. Methods Mol. Biol. 2011, 785, 223–236. [Google Scholar] [CrossRef]
- Yue, T.; Goldstein, I.J.; Hollingsworth, M.A.; Kaul, K.; Brand, R.E.; Haab, B.B. The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Mol. Cell. Proteomics 2009, 8, 1697–1707. [Google Scholar] [CrossRef]
- Wu, Y.M.; Nowack, D.D.; Omenn, G.S.; Haab, B.B. Mucin glycosylation is altered by pro-inflammatory signaling in pancreatic-cancer cells. J. Proteome Res. 2009, 8, 1876–1886. [Google Scholar] [CrossRef]
- Yue, T.; Maupin, K.A.; Fallon, B.; Li, L.; Partyka, K.; Anderson, M.A.; Brenner, D.E.; Kaul, K.; Zeh, H.; Moser, A.J.; et al. Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19-9 antigen on specific protein carriers. PLoS One 2011, 6, e29180. [Google Scholar] [CrossRef] [Green Version]
- Rho, J.H.; Mead, J.R.; Wright, W.S.; Brenner, D.E.; Stave, J.W.; Gildersleeve, J.C.; Lampe, P.D. Discovery of sialyl Lewis A and Lewis X modified protein cancer biomarkers using high density antibody arrays. J. Proteomics 2014, 96, 291–299. [Google Scholar] [CrossRef]
- Loch, C.M.; Ramirez, A.B.; Liu, Y.; Sather, C.L.; Delrow, J.J.; Scholler, N.; Garvik, B.M.; Urban, N.D.; McIntosh, M.W.; Lampe, P.D. Use of high density antibody arrays to validate and discover cancer serum biomarkers. Mol. Oncol. 2007, 1, 313–320. [Google Scholar]
- Rangiah, K.; Tippornwong, M.; Sangar, V.; Austin, D.; Tetreault, M.P.; Rustgi, A.K.; Blair, I.A.; Yu, K.H. Differential secreted proteome approach in murine model for candidate biomarker discovery in colon cancer. J. Proteome Res. 2009, 8, 5153–5164. [Google Scholar] [CrossRef]
- Stenman, U.H.; Leinonen, J.; Alfthan, H.; Rannikko, S.; Tuhkanen, K.; Alfthan, O. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: Assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991, 51, 222–226. [Google Scholar]
- Beneduce, L.; Prayer-Galetti, T.; Giustinian, A.M.; Gallotta, A.; Betto, G.; Pagano, F.; Fassina, G. Detection of prostate-specific antigen coupled to immunoglobulin m in prostate cancer patients. Cancer Detect. Prev. 2007, 31, 402–407. [Google Scholar] [CrossRef]
- Weiss, L.; Haydock, K.; Pickren, J.W.; Lane, W.W. Organ vascularity and metastatic frequency. Am. J. Pathol. 1980, 101, 101–113. [Google Scholar]
- Hittelet, A.; Camby, I.; Nagy, N.; Legendre, H.; Bronckart, Y.; Decaestecker, C.; Kaltner, H.; Nifant’ev, N.E.; Bovin, N.V.; Pector, J.C.; et al. Binding sites for Lewis antigens are expressed by human colon cancer cells and negatively affect their migration. Lab. Invest. 2003, 83, 777–787. [Google Scholar] [CrossRef]
- Koh, Y.W.; Lee, H.J.; Ahn, J.H.; Lee, J.W.; Gong, G. Expression of Lewis X is associated with poor prognosis in triple-negative breast cancer. Am. J. Clin. Pathol. 2013, 139, 746–753. [Google Scholar] [CrossRef]
- Remmers, N.; Anderson, J.M.; Linde, E.M.; DiMaio, D.J.; Lazenby, A.J.; Wandall, H.H.; Mandel, U.; Clausen, H.; Yu, F.; Hollingsworth, M.A. Aberrant expression of mucin core proteins and o-linked glycans associated with progression of pancreatic cancer. Clin. Cancer Res. 2013, 19, 1981–1993. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rho, J.-h.; Lampe, P.D. High-Throughput Analysis of Plasma Hybrid Markers for Early Detection of Cancers. Proteomes 2014, 2, 1-17. https://doi.org/10.3390/proteomes2010001
Rho J-h, Lampe PD. High-Throughput Analysis of Plasma Hybrid Markers for Early Detection of Cancers. Proteomes. 2014; 2(1):1-17. https://doi.org/10.3390/proteomes2010001
Chicago/Turabian StyleRho, Jung-hyun, and Paul D. Lampe. 2014. "High-Throughput Analysis of Plasma Hybrid Markers for Early Detection of Cancers" Proteomes 2, no. 1: 1-17. https://doi.org/10.3390/proteomes2010001
APA StyleRho, J.-h., & Lampe, P. D. (2014). High-Throughput Analysis of Plasma Hybrid Markers for Early Detection of Cancers. Proteomes, 2(1), 1-17. https://doi.org/10.3390/proteomes2010001