Abstract
Marine-derived bioactive peptides (MBPs) are emerging as promising natural agents for regulating inflammatory responses. MBPs, typically obtained through enzymatic hydrolysis of proteins from various marine organisms such as fish, mollusks, and algae, exhibit diverse biological activities, including antioxidant, immunomodulatory, and anti-inflammatory effects. The ability of MBPs to modulate key inflammatory mediators such as TNF-α, IL-6, and COX-2, primarily through pathways like NF-κB and MAPK, highlights the therapeutic potential of MBPs in managing chronic inflammatory diseases. However, most existing studies are confined to in vitro assays or animal models, with limited translation to human clinical applications. This review explores the stability, bioavailability, and metabolic rate of MBPs under physiological conditions, which remain poorly understood. In addition, a lack of standardized protocols for peptide extraction, purification, and efficacy evaluation hinders comparative analysis across studies and also different proteomics approaches for separation, purification, identification, and quantification of marine-derived peptides with therapeutic properties. The structure–function relationship of MBPs is also underexplored, limiting rational design and targeted applications in functional foods or therapeutic products. These limitations are largely due to a lack of consolidated information and integrated research efforts. To address these challenges, this review summarizes recent progress in identifying MBPs with anti-inflammatory potentials, outlines key mechanisms, and highlights current limitations. Additionally, this review also emphasizes the need to enhance mechanistic understanding, optimize delivery strategies, and advance clinical validation to fully realize the therapeutic potential of MBPs.
1. Introduction
Inflammation is the body’s first immune response to foreign substances such as toxins, allergens, pathogens, injury, or damaged tissue. It involves complex interactions between blood vessels, immune cells, and molecular and cellular mediators [1,2,3,4]. This response aims to restore normal body function by eliminating the initial cause of injury and removing damaged cells by producing pro-inflammatory enzymes and cytokines such as inducible nitric oxide synthase (iNOS), cyclooxygenase (COX-2), tumor necrosis factor (TNF-α), and interleukins (IL-1β) (IL-6), primarily through several pathways, including nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3), and NOD-like receptor family pyrin domain containing 3 (NLRP3) [5,6]. This inflammatory process plays a significant role in wound healing and microbial resistance [7]. However, when inflammation becomes dysregulated and prolonged, it can lead to different chronic diseases and significant threats to individual health and longevity [8]. In the past decade, inflammation has been identified as a silent global issue due to long-term inflammation underlying several human diseases, such as cancer, diabetes, arthritis, neurodegeneration, cardiovascular disease, and inflammatory bowel disease (IBD) [9]. To alleviate or prevent inflammatory diseases, several therapeutic strategies, including corticosteroid medications, nonsteroidal anti-inflammatory drugs (NSAIDs), biological agents, and lifestyle modifications, are implemented [10]. Despite that, several chronic inflammatory disorders have no fully effective cure and are primarily managed through symptomatic therapies, while common existing drugs show considerable side effects with long-term use [11]. For instance, currently available drugs, aspirin and indomethacin, which are NSAIDs, often help reduce pain caused by inflammation. However, despite their therapeutic effectiveness, they are also associated with potential adverse reactions, mostly stomach ulcers, and rarely, stroke and myocardial infarction [12]. Therefore, recent studies have focused on searching for novel anti-inflammatory drugs from natural bioresources to overcome limitations present in synthetic medications.
The marine ecosystem provides a rich habitat for immense biodiversity, encompassing a diverse range of habitats from highly productive coastal regions to deep-sea environments. This wide range of distribution supports a varied series of life on the planet, allowing for extraordinary interactions among marine plants, animals, and microorganisms, forming complex food webs and contributing to global biogeographical cycles, such as carbon, nitrogen, and oxygen [11]. Consequently, the ocean maintains marine ecosystems and the ecological balance by stabilizing the sustainable biosphere. To date, humans are the most advantageous users of marine resources that extend beyond food and medicines. Therefore, further comprehensive explorations of marine biodiversity are essential for developing new scientific uses [13,14]. Extreme maritime conditions, such as salinity, temperature, pressure, illumination, and variable oxygen levels, produce unique and diverse compounds when compared to the terrestrial environment [3]. The adaptation of these conditions has been directed to the production of by-products, including proteins and bioactive peptides, lipids, polysaccharides, phenolic compounds, and pigments with distinct structural features, diversity, and functional properties [15]. This variety of natural bioactivities has become a more sustainable solution for various industries, especially in medicine, cosmetics, and food, which is due to their abundance, low toxicity, and high specificity. Among them, the medical industry has become more exploratory in research on natural compounds because synthetic compounds have become less effective and are often highly toxic [16]. Marine bioactive compounds exhibit diverse biological activities, including antioxidant, anti-diabetic, anticancer, antimicrobial, immunomodulatory, angiotensin-converting enzyme (ACE) inhibitory activity, and anti-inflammatory effects, and many other biomarkers which have recently been implicated in human health [17]. To date, marine bioactive compounds are directly or indirectly responsible for approximately 60% of pharmaceutical bioactivities as alternative and or complementary therapies [18]. According to the recently reported data, about 68% of cancer-treated drugs are derived from marine organisms, while the remaining are utilized for inflammation, pain, and other medical conditions [19]. Proteomics has been identified as an essential approach for identifying anti-inflammatory targets and assessing therapeutic responses associated with inflammatory diseases. Rather than proteomics, peptidomics has become an emerging and novel field that improves efforts in peptide drug discovery [20]. However, most current research remains confined to in vitro assays or animal models, with minimal translation to human clinical applications. Aquatic proteomics is one of the advancing analytical technologies, useful for studying the dynamic changes within the proteome [21]. It enables detailed insights into protein-related processes, such as protein synthesis, post-translational modifications (PTMs), and degradation, in response to diverse marine environmental factors and developmental (ontogenetic) stages in marine organisms [22].
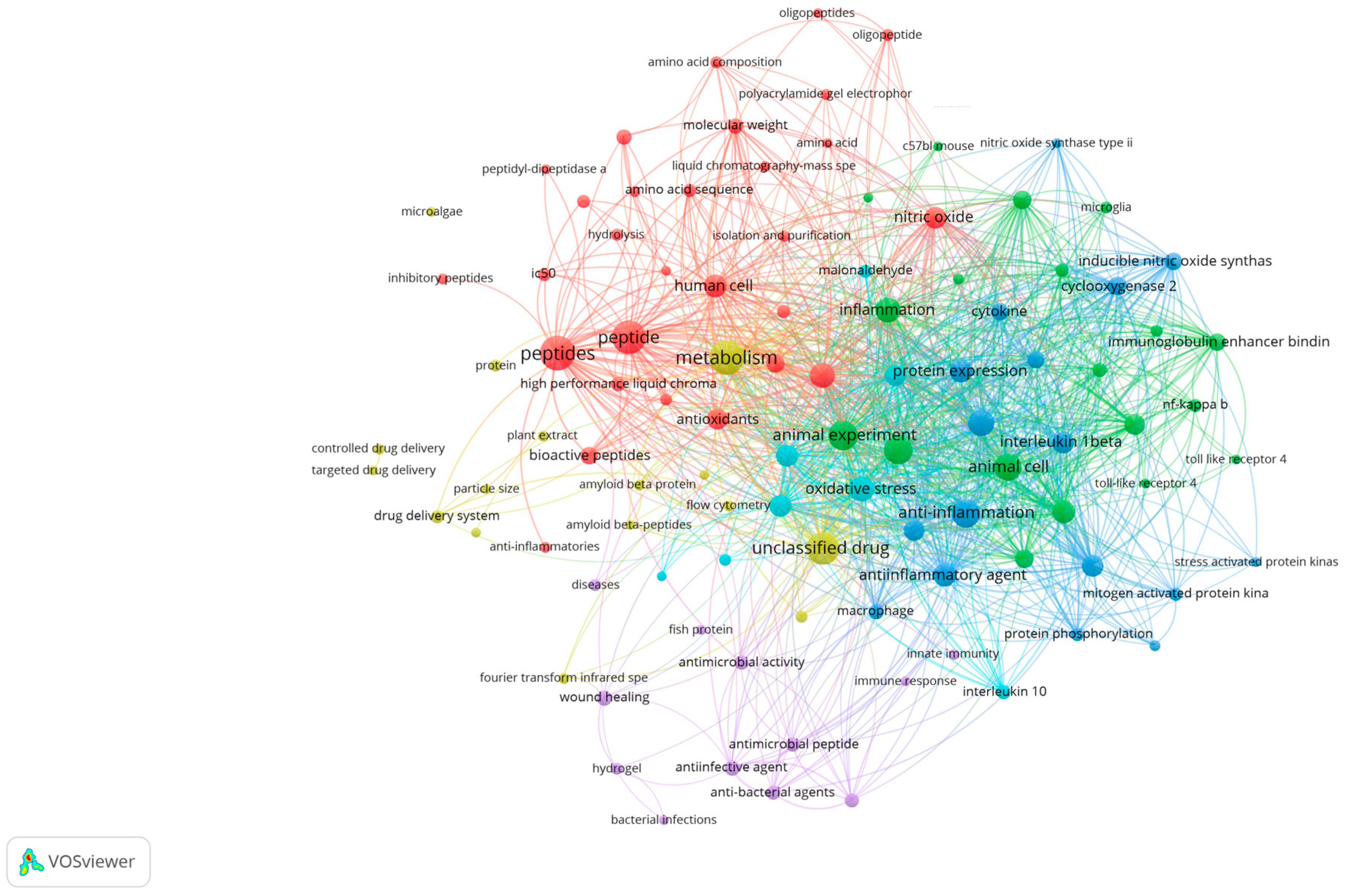
This review aims to present an overview of research trends on MBPs, mainly focusing on their anti-inflammatory potential and the molecular mechanisms reported over the years 2020–2025. Additionally, the advantages and drawbacks associated with marine peptide separation, purification, identification, and quantification techniques are discussed, with an emphasis on current scientific knowledge and future perspectives in the pharmaceutical industry. The Scopus database and Google Scholar were searched to screen relevant articles in this review article. Articles reviewed in the results and discussion sections were based on novelty, time of publication, and the number of citations. For this study, a bibliographic analysis was conducted covering the studies published during 2020–2025, scanning the keywords MBPs, inflammation, marine bioresources, and pro-inflammatory cytokines to locate anti-inflammatory properties of marine bioactive compounds (Figure 1).
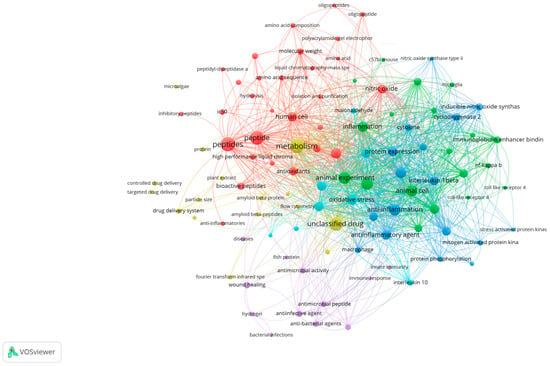
Figure 1.
Bibliometric network analysis of recent publications (2020–2025) using VOSviewer (version 1.6.20), based on Scopus data. Clusters reveal thematic areas most relevant to marine bioactive peptides and inflammation.
Marine Bioactive Peptides (MBPs)
Peptides are an intermediate product of protein synthesis, formed by amino acids connected by peptide bonds [23]. Bioactive peptides (BPs) are a type of natural compound consisting of specific protein fragments made up of 3 to 30 amino acid residues. BPs are inactive when integrated within proteins and are only biologically active when disrupted during fermentation, processing, or gastrointestinal digestion [24]. The structure can be linear or cyclic and may contain secondary structures like turns and loops, and exhibit various functional activities, mainly dependent on the sequence and composition of amino acids [25]. Normally, peptides exist as low-molecular-weight compounds of less than 2500 Da, thereby providing a greater possibility of passing the intestinal barriers free of restriction [26]. Peptides can be sourced from natural, synthetic, or recombinant origins [27]. Natural protein sources are diverse, including terrestrial plants, animals, and microorganisms, and each has advantages as well as serious limitations. The reasons for conformational instability, a short half-life, and narrow biological activities have shifted the research attention towards marine-derived resources [28]. Recently, extensive research on marine bioresources has been attributed to their diverse biological properties, as well as their functional properties, such as solubility, emulsification, and gel formation properties, which can be beneficial for industrial applications [24]. At present, exploration of marine bioactive products (MBPs) has reached more than 40,000 compounds, and each year, over 1000 secondary metabolites are discovered, the majority of which exhibit anticancer and cytotoxic effects [29,30].
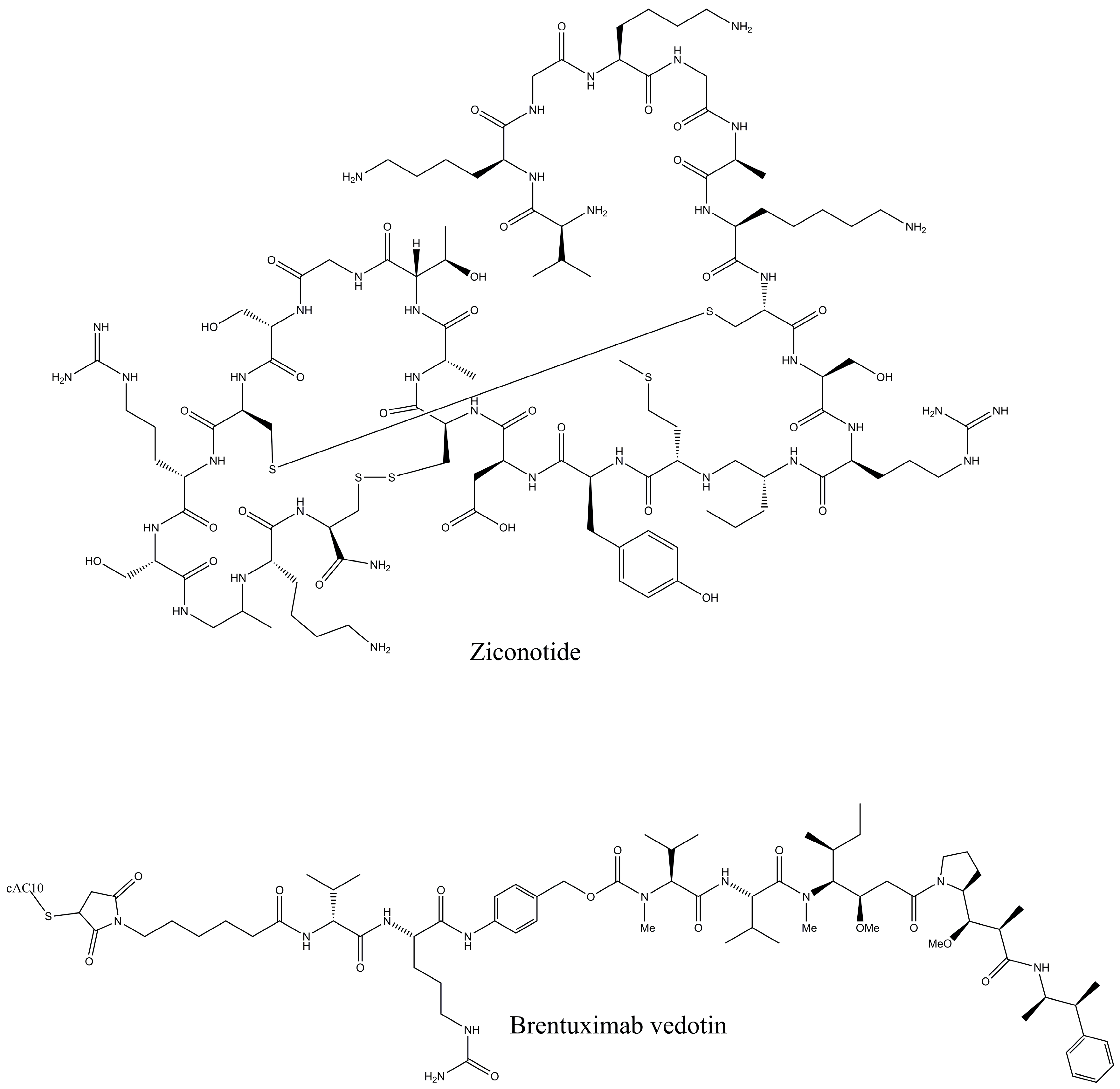
Peptides have been obtained from vast marine sources, including algae (macro and micro), ascidians, sponges, fish, bivalves, mollusks, crustaceans, and some marine by-products, such as shellfish, fish skins, viscera, and muscles. Peptides are highly enriched with multiple pharmacological activities, including anticancer, antimicrobial, antioxidant, anti-diabetic, anticoagulant, anti-inflammatory, hepatoprotective, cardioprotective, immunomodulatory, neurogenerative, and appetite-suppressing effects, and many more [1,24]. Among them, extracted bioactive peptides from seaweeds, sponges, mollusks, and ascidians have shown greater application in pharmaceutical properties (Figure 2) [31]. Moreover, 71.74% of collagen hydrolysate peptides, which contain glycine, proline, hydroxylysine, or hydroxyproline, primarily derived from marine fish skin, scales, and bones, have demonstrated significant potential in preventing and managing various health conditions [32]. Considering the safety aspects of MBPs is essential for pharmaceutical products. In comparison to numerous other bioactive compounds, MBPs are comparatively less allergic and exhibit minimal or no toxicity. However, risk factors such as heavy metals, high iodine levels, anti-nutrients, pesticide residues, ammonia, and radioactive substances may coexist with the final product if the production process fails to implement appropriate safety measures [33]. A number of MBP-based pharmaceutical products have reached the market, while most of the drugs are in preclinical and clinical stages. Nevertheless, some of these products may later be rejected or withdrawn due to emerging safety concerns and limited efficacy. Peptides are the fundamental components of proteins and are essential for both the structure and function of the proteome, providing valuable insights into the dynamic nature and complexity of the cellular proteome. Proteomics approaches have involved unveiling how marine life adapts to its environment and identifying biologically active proteins or peptides that could have mainly therapeutic applications, particularly those that use peptide analysis. Primarily, three techniques, solvent extraction, chemical and enzymatic hydrolysis, and microbial fermentation, are used for releasing peptides from different marine sources. The enzymatic hydrolysis technique is the most common for peptide isolation, but some studies for specific peptides have shown that chemical synthesis and recombinant DNA technologies are more effective for large-scale production. Further, the study performed by Sridhar, Inbaraj et al., 2021 has summarized some more advanced technologies for isolation, purification, and identification, which have been used more recently by suggesting that a combination of different processing technologies can be used for enhancing the yield and purity of the MBPs [34]. To date, Ziconotide (Prialt®) has been recognized as the first marine-derived peptide approved by the Food and Drug Administration (FDA) in 2004 [35]. This drug is isolated from the marine cone snail, Conus magus, which is used to relieve chronic pain by blocking specific voltage-activated calcium channels in neurons [36]. Furthermore, Brentuximab Vedotin (Adcetris®), isolated from the marine mollusk, Dolabella auricularia, was approved in 2011 [36]. This is an antibody–drug conjugate designed to target CD30-positive cells, making it effective in the treatment of classical Hodgkin lymphoma and systemic anaplastic large cell lymphoma [Figure 3].
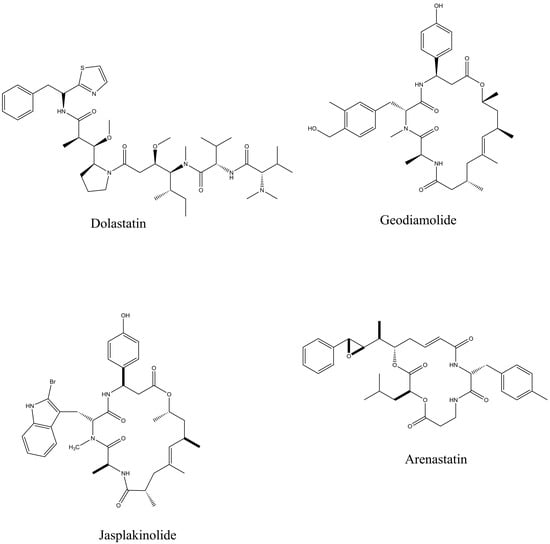
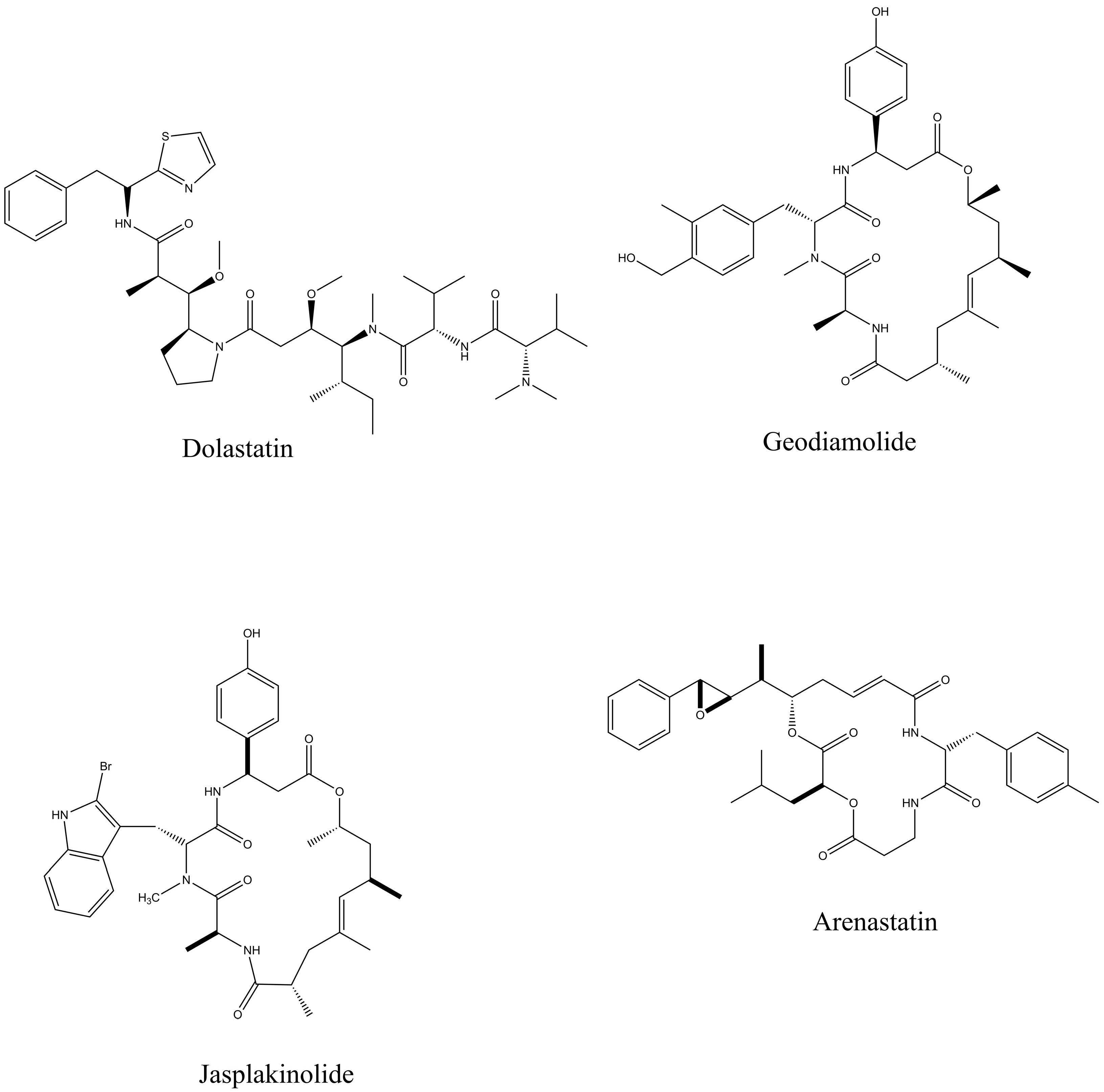
Figure 2.
Bioactive peptides from seaweeds, sponges, mollusks, and ascidians that exhibit therapeutic effects.
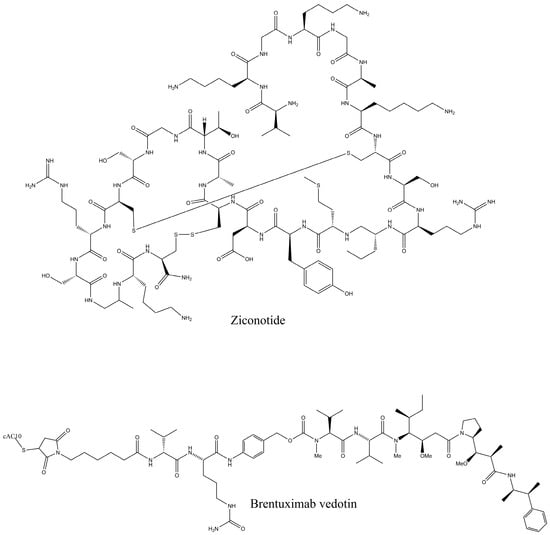
Figure 3.
FDA-approved marine peptide-based compounds.
Further, Jin, Peng et al., 2022 highlighted biological activities, mainly anticancer, antimicrobial, and antioxidant ones, and their relationships with various marine organisms. According to the study, Dolastatin 10, a low-molecular-weight cyclic anticancer peptide, shows the greatest potential for medical use. The development of new Dolastatin 10 analogs has even led to their incorporation into modern conjugated drugs. The peptides Geodiamalide and theopederin A are the marine sponge-derived peptides that show strong cytotoxicity, and theopederin A has indicated the greatest antitumor activity [37].
Nevertheless, a considerable amount of research has been conducted on peptide-based drugs; the majority are in the preclinical stage, and only a small number have reached phase I, II, and/or III of clinical trials (Table 1). Surprisingly, various food and nutraceutical products are being made with ingredients derived from MBPs or peptide-rich hydrolysates of fish gelatin, collagen, and fish proteins. These products have the ability to treat or alleviate chronic diseases, especially for bone and intestinal health, reducing anxiety, controlling postprandial blood glucose levels, and providing overall nutritional supplements.

Table 1.
The status of marine-derived peptide pharmaceutical products undergoing clinical trials.
2. The Structure–Function Relationship of Marine Bioactive Peptides
The structure of MBPs determines their biological activities, and it is essential to analyze the relationship between their structures and the bioactivity of MBPs to develop and optimize therapeutic uses [23]. According to the isolated marine source, the structures of the peptides are complex and highly variable, especially due to the high taxonomic diversity [45]. The molecular weight, amino acid composition and sequence, spatial conformation, and other factors, such as hydrophobicity, balance between charge density, and polymer chain length of the peptides, are crucially impacted on the biological activities [46]. Most commonly, MBPs are small in size and require support to be absorbed more efficiently by the body. Most antihypertensive and antioxidant peptides have low molecular weights (MWs), typically around 1 kDa; likewise, low-MW peptides contribute to the ACE inhibitory effects by increasing the binding ability to the active site of the ACE (Table 2) [23]. Moreover, the amino acid composition and sequence of the MBPs significantly affect several biological activities, such as antioxidant, anticancer, antimicrobial, and ACE inhibition. The anti-inflammatory activity of short sequences (2–3 amino acids) of peptides is generally absorbed more easily and effectively. Surprisingly, HCRG1 and HCRG2, which are long peptides (up to 50 amino acids) derived from the sea anemone (Heteractis crispa), have been reported to have intact absorption ability through the intestine at the tissue level. However, when increasing the chain length of the peptide sequence, the potency of administration tends to be decreased [47,48]. Further, the cyclic structure of peptides is often associated with better passive cell membrane permeability in comparison to the linear structures. Stylissatin A is a cyclic heptapeptide isolated from the marine sponge Stylissa massa that has shown anti-inflammatory effects, facilitating the reduction of nitrogen oxide (NO) production in lipopolysaccharide (LPS)-stimulated murine RAW264.7 macrophage cells (EC50 = 87 and 73 µM) [49]. A number of studies have confirmed that the variation in the type of the sequence and position of the amino acid and chemical properties, such as hydrophobicity, flexibility, and charged residues of the linear structure, along with their secondary and tertiary structure properties for the structure, enhances the functions of MBPs (Table 2). The positively charged amino acids in peptides act as chemokines and interact with the immune system, helping to suppress inflammation. It is reported that peptides derived from tuna juice have two positively charged amino acids, lysine (K) and arginine (R), most abundant at the N-terminal, and have anti-inflammatory activities [47].

Table 2.
The structure of MBPs affects their different functions.
Further, Rivera-Jiménez et al. (2022) reported that, after analyzing 31 peptide sequences, 19 contained between 25% and 100% hydrophobic amino acids, most commonly alanine and isoleucine. The tripeptide PAY, which is derived from salmon, shows 100% hydrophobicity and displays inhibitory activity against NO production [47]. The special conformation (3D structure) of MBPs largely determines their overall efficacy by enhancing their target-binding affinity through changing conformational structure. As a result, the activity will last longer by resisting degradation and preventing the loss of molecular stability.
3. Protein Hydrolysis Mechanisms (Protein Hydrolysis, Purification, Separation, and Identification)
3.1. Protein Extraction Process
Marine protein extraction involves isolating proteins from different marine sources. To date, enzymatic extraction, ultrasound-assisted extraction, deep eutectic solvent extraction, physical aided extraction, supercritical fluid extraction, and acidic extraction are commonly used. Depending on the species, amino acid composition, and sequence, extraction methods can vary [57]. For instance, microalgae are microscopic organisms and develop in various ecological environments. Spirulina (Arthrospira platensis) is a multicellular blue-green alga that belongs to the cyanobacteria, which contain a 55–70% protein content. Chlorella (Chlorella vulgaris) belongs to the phylum Chlorophyta and contains 42–58% protein. Using one-pot ultrasound-assisted extraction (UAE) gives a high yield of protein content simply, effectively, and sustainably [58]. Chlorella vulgaris, Nanochloropsis oceanica, and Tetraselmis chuli are other microalgae that contain high protein content. Researchers have gained high extraction yield using the freeze–thawing method and high-pressure homogenization from those microalgal species [59]. Collagen is one of the most abundant proteins found in animal bone and skin (pig, cow, and fish), and gelatin is the high-protein product derived from collagen [60,61]. Normally, enzymatic extraction is used to obtain high gelatin content from the marine organisms [60]. Based on the extraction types, each method has both advantages and disadvantages (Table 3).

Table 3.
Advantages and disadvantages of protein extraction methods.
3.2. Protein Hydrolysis
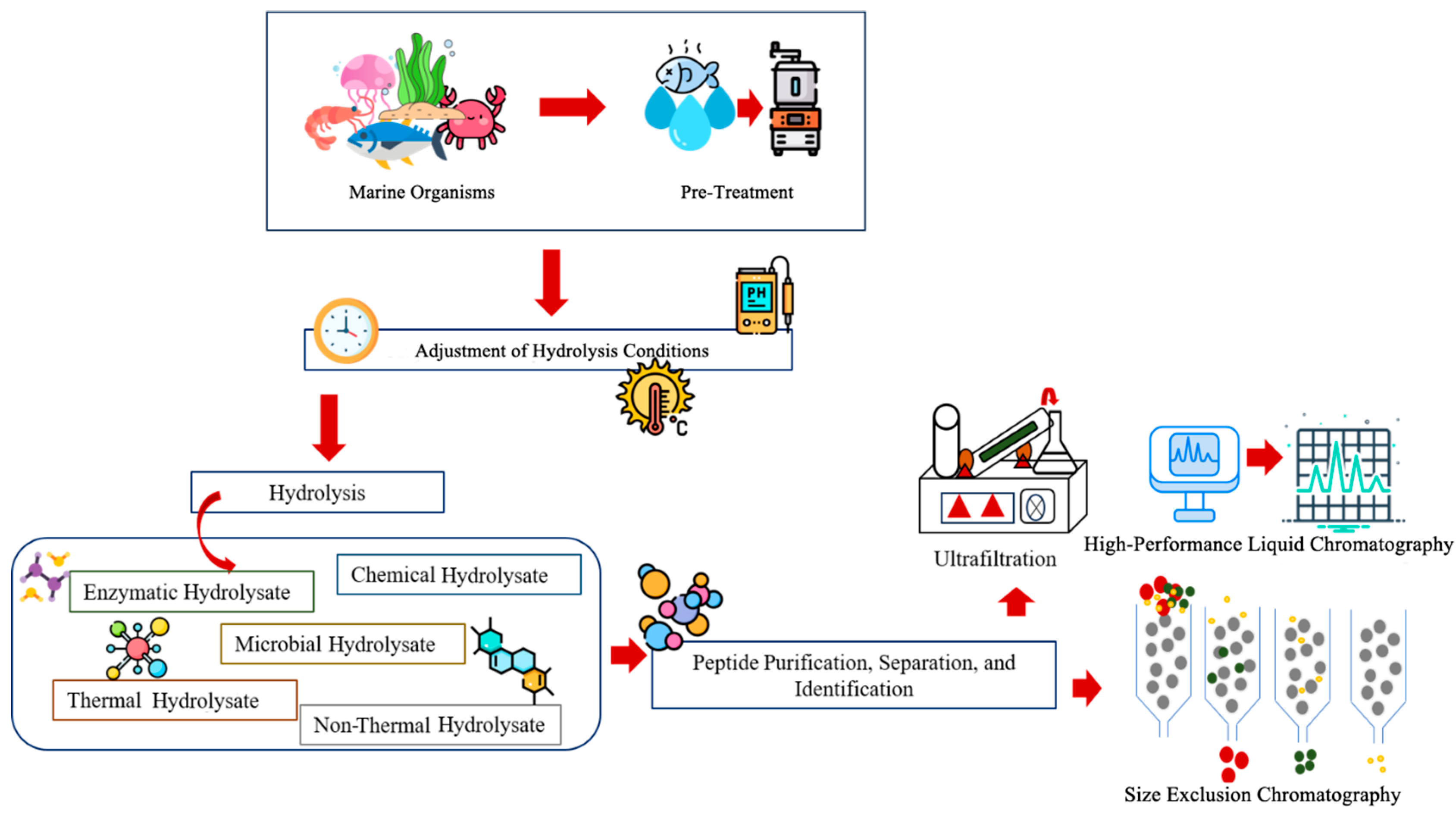
Protein hydrolysis is the process of cleaving large protein molecules into low-molecular-weight peptides or individual amino acids, involving a molecule of water for each broken bond [67]. Nowadays, marine organisms and their by-products, especially those derived from fish, are widely recognized as a significant source of proteins, minerals, and fatty acids. On average, the value of fish by-products reaches a content of 49.22–57.92% proteins. Several methods, such as enzymatic, chemical, microbial, thermal, and non-thermal hydrolysic, are involved in the formation of protein hydrolysate [68]. Following protein hydrolysis, products are subjected to purification, separation, and peptide or amino acid identification (Figure 4). Enzyme hydrolysation is a highly efficient method in comparison to other methods, and uses several enzymes such as pepsin, trypsin, neutrase, and protease while maintaining concentration, temperature, and pH [34,69].
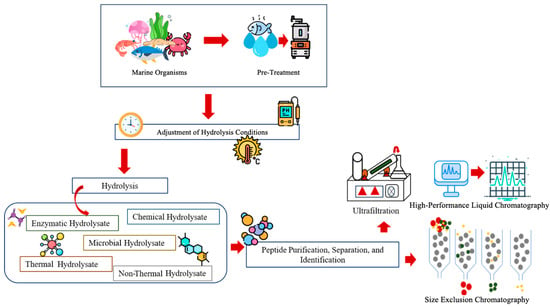
Figure 4.
Preparation and identification of marine protein hydrolysates.
Previous research has reported on enzymatic hydrolysis-based bioactivities and functional properties from fish and their byproducts. Still, few studies have focused on the cod backbone as a substrate for continuing this enzymatic hydrolysis. The defrosted cod fish backbones were hydrolyzed using alcalase, neutrase, and protamex at an enzyme-to-substrate ratio of 1% for 24 h under optimal pH conditions with values of 8.0, 8.0, and 6.5, respectively, while the optimal temperatures were 60 °C, 60 °C, and 50 °C. According to the results, the alcalase enzyme-treated cod backbone sample has shown the highest results compared with other enzymes [69]. In another study with olive flounder (Paralichthys olivaceus), the researchers aimed to optimize the hydrolysis conditions using their byproducts, resulting in a high-quality fish protein hydrolysate using the alcalase, pepsin, trypsin, protamex, and neutrase enzymes [70]. The amino acid profiles of this research showed high concentrations of Glycine, L-glutamic acid, and L-aspartic acid. Further, researchers are focused on evaluating whether sea cucumber intestinal hydrolysates (SCIHs) can enhance the proliferation and migration of bone marrow mesenchymal stem cells. The research used alkaline protease enzyme to prepare an SCIH [71]. The dried gut and skin of the sea cucumber, Holothuria scabra, when mixed with papain enzyme, undergo hydrolysis and contain highly bioactive properties that can have an effective antiproliferative effect on cancer cells. Hydrolysate represents potential as a functional ingredient with natural antioxidant and anticancer properties [72]. In recent years, research has focused on new strategies that can enhance peptide bioactivity through advanced enzymatic hydrolysis techniques, including the use of multiple proteases, membrane–bioreactor systems, and various pretreatment strategies. The results have demonstrated that combining physical pretreatments such as pulsed electric field or high hydrostatic pressure (HHP) with enzymatic hydrolysis can significantly improve protein extraction efficiency, increase the degree of hydrolysis, and generate peptides with stronger bioactive properties, such as antioxidant capacity, which ultimately suppresses inflammation [34].
Chemical hydrolysis is another method that involves the hydrolysis using an acid or base solution to cleave proteins into peptides and individual amino acids. Most commonly, hydrochloric acid (HCl) or sulfuric acid (H2SO4) for acid hydrolysis, and sodium hydroxide (NaOH) or potassium hydroxide (KOH), are utilized. Compared to other hydrolysis methods, chemical hydrolysis comes with several limitations, such as incompatibility with food applications, lack of control over the hydrolysis process, degradation or destruction of certain amino acids, degradation of tryptophan, need for neutralization, and reduced functional properties due to salt formation [73]. Fucus vesiculosus and Saccharina latissima are the brown seaweeds that contain glucose and mannitol. Using acidic hydrolysis (0.2 M H2SO4) and enzymatic hydrolysis (Cellic CTec2 enzyme), one could compare these two types of final results to gain a high yield of glucose and mannitol based on the enzymatic reaction process [74].
Palmaria palmata (Dulse) is a red seaweed that contains a limited variety of dietary uses due to ineffective protein extraction methods. Microwave digestion, acid hydrolysis, and Viscozyme hydrolysis are employed for extracting protein from dulse. Ultimately, microwave digestion and acid hydrolysis show high protein amounts and lower-molecular-weight proteins compared to the enzyme hydrolysis method [75]. Microwave hydrolysis offers a sustainable approach to processing seaweeds for protein and nanocellulose management. Using microwave-assisted hydrolysis for Ascophyllum nodosum and Aegagropila linnaei results in a high yield of proteins, polysaccharides (including alginate and fucoidan), and nanocellulose [76].
3.3. Peptide Separation, Purification, Identification, and Quantification Techniques
3.3.1. Ultrafiltration (UF)
Fractionate peptides are based on molecular weight. The membrane separation process is used to purify peptides by selectively retaining large molecules while allowing smaller ones and water to pass through [77]. This ultrafiltration method belongs to the separation and purification of both steps. Membrane fouling is a significant obstacle in industrial UF to the recovery of bioactive peptides, increased energy, and potentially irreversible membrane clogging [78].
3.3.2. Solid Phase Extraction (SPE)
Solid-phase extraction (SPE) is widely used for isolating, purifying, and concentrating peptides from liquid samples using a solid sorbent [77]. This process is also very useful for desalting proteins and sugar samples. SPE resulted in the development of new extraction techniques, such as solid-phase dynamic extraction (SPDE), matrix solid-phase dispersion (MSPD), microextraction by packed sorbent (MEPS), stir-bar sorptive extraction (SBSE), and solid-phase microextraction (SPME) [78,79].
3.3.3. Gel Filtration Chromatography (Size Exclusion)
These separation techniques distinguish molecules based on their size and shape. Gel filtration chromatography is a simple method in which an inert gel medium made up of spherical beads with stable properties is used. When analytes of various sizes are applied, larger molecules pass through the interstitial space and elute first, while smaller molecules diffuse into the pores [77]. Sephadex G-10 is a gel filtration resin used for the desalting and buffer exchange of peptides and small biomolecules with molecular weights over 700. Different types of Sephadex are available, depending on their degree of cross-linking, swelling point, and molecular fractionation range. Sephadex G-10 for small molecules, G-75 for larger molecules, and G-50 are available in the four main categories of particle size: coarse, resin, fine, and superfine. Coarse and resin are preferred for the large scale. Superfine has the smallest bead size for higher efficiency fractionation with a shorter diffusion distance [80]. The collected Sephadex fraction was then analyzed using a UV/VIS spectrophotometer (220 nm), and after that, peptide identification was performed by using HPLC [81]. The molecular mass of the purified peptide and amino acid sequencing is mostly determined by using a Q-TOF mass spectrometer coupled with an electrospray ionization (ESI) [82].
3.3.4. Ion Exchange
Peptides are separated based on their charge. Cation exchange (CM-Sepharose) attaches positively charged peptides. Anion exchange (Diethylaminomethyl (DEAE)-Sepharose) attaches negatively charged peptides. This method is a high-resolution, acid-resistant technique used for separating peptides [83]. It involves a phase with fixed ionic groups and exchangeable ions that can undergo reversible exchange. Purified immunoglobulin G from rabbit serum is obtained using a DEAE-Sepharose fast flow ion exchange column with a Tris-HCl buffer at pH 7.0 and 8.5 as the mobile phase. The separation of proteolytic enzymes with the same net charge and one basic residue is influenced by specific activities. Based on the high resolution, the serum is resistant to acids and alkalis, and can be easily separated into peptides. This purification method can be introduced as a cost-effective marine peptide purification method compared with the RP-HPLC method [37]. Further, using the UV/Vis spectrophotometer in the 200–280 nm range and coupling liquid chromatography–mass spectrometry (LC-MS/MS) provides optimization for both the separation and identification of peptides [81,84].
3.3.5. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
High-resolution purification is based on hydrophobicity. Reversed-phase liquid chromatography (RPLC) purifies proteins based on their polarity. This method introduced a good standard method for the separation, identification, and purification of peptide molecules. This method’s stationary phase functionalizes with an anionic group that can bind positively charged purified peptides [77,85]. Reverse-phase high-performance liquid chromatography (RP-HPLC) is a specific type of RPLC, and this method refers to the same liquid chromatography method. Polar mobile phase and non-polar stationary phase separate compounds based on their hydrophobicity into C18 and C8 columns [86]. Protein identification is based on which proteins are available in the extracted sample. Proteins after the enzymatic digestion were analyzed by mass spectrometry (MS), and the fragment pattern matched the parental proteins. The combination of HPLC and MS can be used to separate and identify peptides [81].
3.3.6. Capillary Electrophoresis (CE)
Bio-capillary electrophoresis is an analytical separation technique that is used to separate peptide samples based on their electrophoretic mobility. In this technique, only a small sample volume and substrates are used. This CE method is more automated and has high resolution compared with other peptide purification methods [87]. Ahn et al. (2015) discussed the involvement of the CE in marine peptide separation in high-throughput and rapid screening of marine protein hydrolysate from Acetes chinensis, shark meat, Polysiphonia urceolata, Spirulina platensis, and mackerel bone, which are enriched in marine peptides with angiotensin I converting enzyme inhibiting activity, checked by using CE [88].
3.3.7. Matrix-Assisted Laser Desorption/Ionization (MALDI) Time-of-Flight (TOF) Mass Spectrometry (MS)
MALDI-TOF MS, or MALDI TOF, is a peptide identification technique. MALDI is a system in which the sample is mixed with a matrix spotted on a stainless-steel plate, where it is evaporated until dry and eventually ionized with a laser. MALDI is often combined with a TOF Mass analyzer, and these methods are effective for analysis with applications in proteomics and food safety [89]. Generally, MALDI-TOF MS determines the molecular weight as 1570 Da, and MALDI-TOF/TOF MS analyzes structures as cyclic peptides [90,91]. The study performed by Panteleev et al. (2020) discussed the structure elucidation and functional studies of a novel hairpin antimicrobial peptide from the marine Polychaeta Capitella teleta. The purified peptides were subjected to RP-HPLC for fractionation and finally analyzed by MALDI-TOF MS [90]. Further, identification of bioactive peptides (QTDDNHSNVLWAG-FSR) derived from Pyropia haitanensis and converting enzyme inhibitory peptides YRD, AG-GEY, VYRT, VDHY, IKGHY, LKNPG, LDY, LRY, and FEQDWAS from Palmaria palmata were investigated by combination with MALDI-TOF and Edman degradation [89,92].
4. Anti-Inflammatory Mediators and Their Therapeutic Potentials
4.1. Anti-Inflammatory Potential of Marine Bioactive Peptides
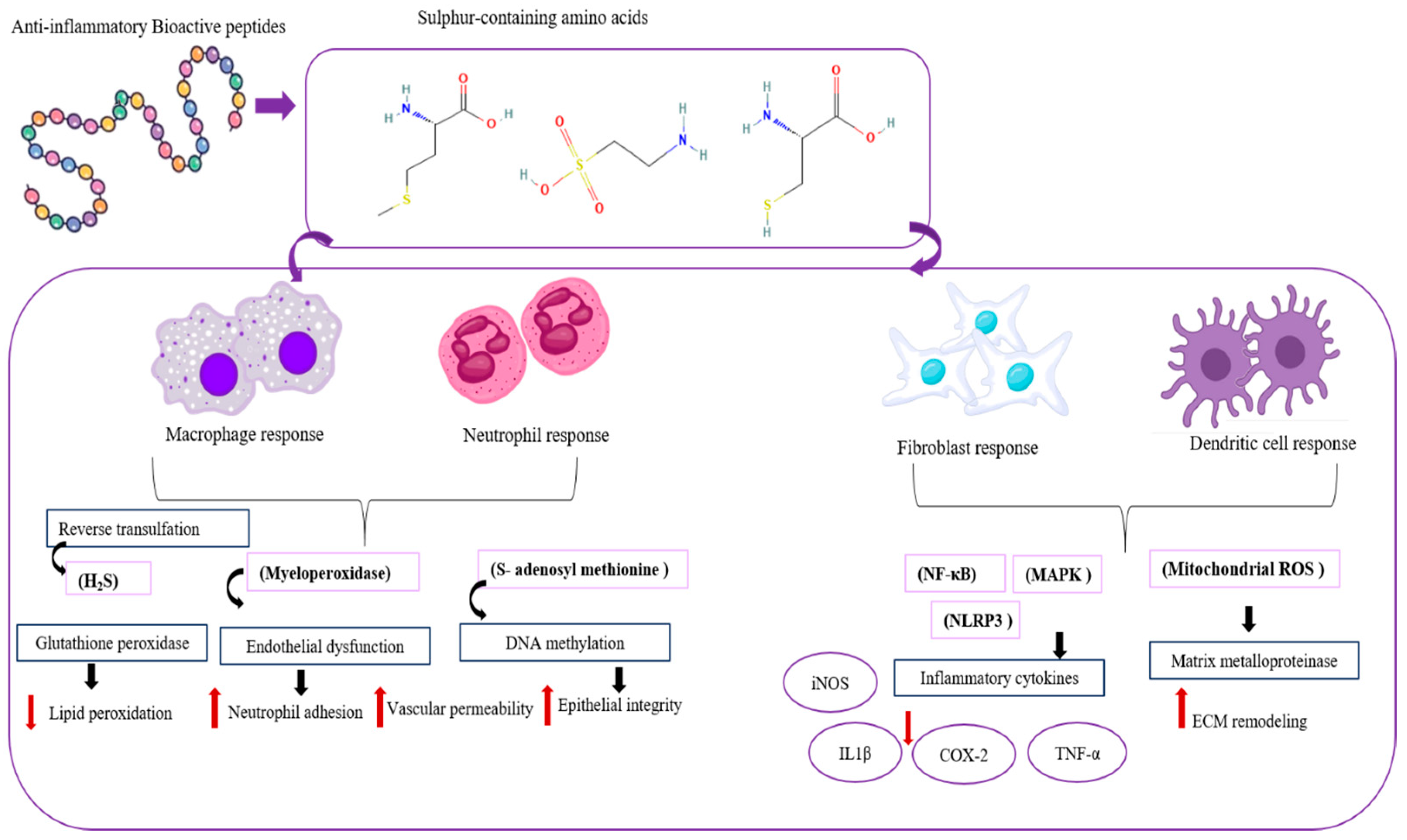
Inflammation is a physiological process that provides an essential defense mechanism for the human body in natural [93]. Often, body tissues and cells are attacked by physical, chemical, and pathogenic hazardous substances; however, acute and chronic diseases are mostly underlined by inflammatory mechanisms of pathogenesis [94]. Primarily, the inflammatory responses can be identified as redness, swelling, heat, pain, and loss of tissue function. Acute (excessive inflammation) and chronic inflammation are the major types of inflammation that can be found in the body. Acute inflammation lasts for short periods and responds by minimizing the approach injury or infection, which helps the reimplantation of tissues. Chronic inflammation lasts for long periods and is mostly not under control, and can develop into chronic inflammation. Recently, excessive inflammation has been discovered as a factor that exacerbates non-communicable diseases (NCDs) such as obesity, diabetes, cancer, respiratory disorders, and cardiovascular disease [95,96]. Marine organisms are a valuable source of proteins and bioactive peptides with medicinal properties. Lectins and phycobiliproteins, like compounds from seaweeds, show biological activity in animal models. These compounds are involved in processes like cell communication, host–pathogen interactions, cancer metastasis, and apoptosis. ESA-2 lectin from Eucheuma serra has been shown to induce colonic carcinogenesis when administered orally. The anti-inflammatory mechanisms of MBPs, iNOS, COX2, NFκB, chemokines, cytokines, and MAPK inhibition effects can be studied (Figure 5).
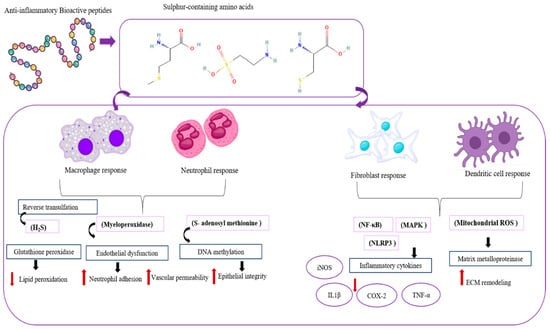
Figure 5.
Mechanisms of anti-inflammatory peptides in regulating the inflammatory signaling pathway.
Macrophages, neutrophils, fibroblasts, and dendritic cells all contribute to the anti-inflammatory mechanisms. Macrophages have different phenotypes: M1 and M2. Type M1 promotes inflammation. M2 type secretes cytokines IL-10 and TGF-β, clears apoptotic cells, promotes tissue repair, and prevents chronic inflammation (R1). Neutrophils mostly cause inflammation to resist threats. After being free from harm, apoptosis releases lipoxins and resolvins that balance the immune system and stop inflammation. Fibroblasts assist in restoring tissue integrity by producing extracellular matrix compounds and releasing TGF-β and PGE2, modulating immune cell activity. Dendritic cells regulate immune responses by IL-10 and are induced by regulatory T cells, and suppress excessive inflammation [97]. Inflammation is associated with iNOS proteins. Mainly, free radicals are generated with this iNOS activity. This iNOS produces nitric oxide (NO) from the conversion of L-arginine to L-citrulline. Reactive nitrogen species (RNS) that cause oxidative stress in cells are produced by NO2, and NO can produce various types of inflammatory responses [96,98].
4.2. Key Mechanisms of the Anti-Inflammatory Mediators
4.2.1. MAPK Inhibition of Marine Organisms
Mitogen-activated protein kinase modules, also known as MAPK or MAP kinase, play a critical role in regulating mammalian cell proliferation, differentiation, and apoptosis [99]. Its primary function is to convert extracellular signals into intracellular responses, facilitating the transformation of stimulus energy into neural activity. The MAPK module consists of three major protein kinases: p38 kinase, c-Jun N-terminal kinase (JNK), and extracellular regulated protein kinase ½ (ERK1/2) [100]. These proteins are activated by various extracellular and intracellular factors, including oxidative stress, osmotic pressure, and pro-inflammatory cytokines. Novel antioxidant peptides (LSPGEL, VYFDR, and PGPTY) derived from the red alga Gracilariopsis lemaneiformis were shown to reduce oxidative stress and modulate the inflammatory pathways. According to the results, the isolated peptides were found to suppress excessive NO production and regulate iNOS expression in macrophages [101]. NO is highly involved in monocyte function differentiation and modulates the bioavailability of cells that respond to pro-inflammatory stimuli. The immunomodulatory effects of proper iNOS inhibition in monocytes suggest potential therapeutic strategies for pathological conditions with imbalanced immune responses [102].
Tripeptides from salmon byproducts hydrolysate have shown notable anti-inflammatory properties. These peptides were identified as Pro-Ala-Tyr (PAY) and inhibit NO/iNOS, PGE2/COX-2 pathways, and can also inhibit pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β). Apostichopus japonicus- and Acaudina leucoprocta-isolated peptides GPSGRP, GPAGPR, PQGETGA, and GFDGPEGPR inactivate MAPK pathways in LPS-induced mouse liver injury [103]. To further understand the anti-inflammatory effects of sea cucumbers, studies have examined mRNA expression in lung tissue related to inflammation signaling. The results indicate that sea cucumber extracts suppressed NF-κB and MAPK transcript levels. This suggests that sea cucumber extracts act as anti-inflammatory agents by inhibiting key mediators of inflammation [100]. Additionally, protein hydrolysates from Ulva spp. (green macroalgae) are shown to modulate MAPK pathways, including ERK1/2, JNK, and P38 MAPK. Lymphocytes and macrophages have the potential to regulate the immune response towards their bioactive peptide [104].
4.2.2. NF-κB Inhibition of Marine Organisms
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a transcription factor crucial for both innate and adaptive immune responses, cell growth, and cell development, mainly for inflammation [105]. Further, it regulates more than 500 cancer-related genes [106]. NF-κB activation causes different autoimmune, inflammatory, and malignant disorders. Therefore, inhibition of NF-κB signaling can be used in therapeutic applications [107]. Further, the above innate and adaptive immune responses are activated using the ligation of T or B cell receptors that are involved in various inflammatory diseases. The inhibition of the activation of the NF-κB helps the pathogenic process of various inflammatory diseases. The NF-κB is a central mediator of pro-inflammatory gene induction. Isostichopus bandionotus and Cucumaria frondosa have strong anti-inflammatory properties [108,109]. The IKK kinase is a central regulator of the NF-κB pathway, composed of two catalytic subunits (IKK α and IKK β) and a regulatory subunit, IKK γ. Phosphorylation of IKK β protein activation, leading to degradation, allows NF-κB dimers to translocate into the nucleus and regulate the transcription of pro-inflammatory and immune-related genes [110]. Chlorella pyrenoidosa is a unicellular photosynthetic green alga rich in protein and fats. Chlorella-11 peptide, which contains C. pyrenoidosa, inhibited the MCP-1 formation in LPS-stimulated Raw 264.7 macrophages, blocked intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 formation in endothelial cells, and lowered the NF-κB abundance [111]. LDAVNR and MMLDF are two peptides purified from Spirulina maxima hydrolysate, found to inhibit cytokine production in endothelial cells, indicating their potential to reduce inflammation [104].
Chemokines are small proteins that contain structurally important cysteine residues, which play a crucial role in organ development, normal physiology, and immune responses. Marine-derived peptides produce anti-inflammatory effects by downregulating, such as COX-2 and iNOS, and modulating cytokine and chemokine signaling. Inhibiting pro-inflammatory molecules such as TNF-α, IL-6, and MCP-1 leads to reduced immune cell infiltration and helps restore immune balance, highlighting their role as immunomodulatory agents [112]. Pacific oysters (Crassostrea gigas) extract oyster β-thymosin water-soluble polypeptide. Peptides were tested for anti-inflammatory activity. These peptides significantly inhibited NO production, suppressed PGE2, iNOS, and reduced the COX-2, TNF-α, IL-1β, and IL-6and blocked NF-κB pathways and activation by preventing κB (IκB) degradation in LPS-induced RAW264.7 cells. The studies have exhibited a strong positive correlation of strong in vivo osteogenic activity with COX-2 inhibition, showing the potential for novel drugs for treating bone disorders related to inflammatory processes using sea cucumber extractions. The authors discussed the involvement of the isolated clam worm (Marphysa sanguinea) peptide NCWPFQGVPLGFQAPP, which suppresses the NF-κB signaling pathway and lowers the NO and iNOS. According to the authors, the anti-inflammatory activity was assessed based on the relative COX-2 expression compared to the control. C- phy-cocyanin is a dominant pigment protein in Spirulina platensis and Synechococcus marine bacteria peptides (AILQSYSAGKTK, ALNKTHLIQTK, LLVHAPVK, IPDAHPVK, and VVVLRDGAVQQLGTPR), effectively inhibiting inflammation by reducing the abundance of NO synthase and COX-2, promoting the production of pro-inflammatory cytokines [104].
5. Current Trends and Future Directions
The diverse bioactivity properties of MBPs have led to significant scientific and industrial interest in various applications. In the pharmaceutical industry, adequate evidence has been provided on production, purification, optimization, and identification in previous studies. Instead of advantages, considerable drawbacks in the production yield, low purity, and high cost on large scales remain. Further, the short half-life of existing peptides and the loss of stability during processing are the other critical aspects that should be addressed immediately [113,114]. Moreover, MBPs often have a strong, unpleasant marine odor and a bitter taste. Flavor modification of MBPs is essential for oral pharmaceutical products to ensure better adherence to treatment [115]. So far, the potential health benefits have mostly been observed in in vitro and in vivo models, and these technologies must be validated through human clinical trials [37].
Therefore, novel technological approaches and regulatory support systems have led to improvements in the process of peptide isolation, purification, identification, and quantification from various marine biological sources. Sustainable and rapid development of each step allows for novel peptides that could be a solution for health issues. Currently, enzymatic hydrolysis is the main and common method for producing bioactive MBPs rather than conventional methods such as ultrasound, high hydrostatic pressure, microwave, pulsed electric field, and subcritical water. Most of these methods are particularly suitable only for laboratory-scale implementations, and large-scale production is often limited [23]. However, the low yield and high cost of the enzymatic method remain, motivating researchers to seek alternative technologies. Application of bioinformatics, computational approaches such as molecular docking, in silico screening, and artificial intelligence (AI) will lead to a better understanding of metabolic pathways, molecular mechanisms, and target interaction of each bioactivity [116]. AI, particularly deep learning (DI), has emerged as a transformative peptide-based drug-designing tool capable of analyzing complex structural data more precisely. Recent advanced tools such as AF3/AFM and RFAA/RoseTTAFold can predict high-resolution 3D structures of protein and peptide complexes. Furthermore, these models capture complex molecular structures and increase target binding potential more accurately and quickly, making it easier to develop new peptide-based therapeutics [117].
Further, the development of omics technologies such as genomics, transcriptomics, and proteomics has greatly advanced the discovery of novel MBPs with potential health benefits. The rapid development of these technologies has facilitated the discovery of novel peptides, their sequences, and their functionality by increasing yield and minimizing the cost. The molecular hybrid approach can be proposed to enhance the therapeutic effects of the doses by reducing or removing toxicity compared to currently available single-molecule-based treatments [28]. Because of their unique and complex biological mechanisms, these hybrids require careful evaluation, including studies on their toxicity and pharmacokinetics. Modification of the flavor and stability of the peptides is important for developing MBPs. Nanotechnology can significantly enhance the performance of marine peptide-based drugs by overcoming the drawbacks of poor stability, short half-life in the body, and difficulty reaching the target. Nano-encapsulation of marine peptide-based drugs into various structures leads to protecting their functional activity and stability. Nanoliposomes, nanoemulsions, and polymeric or lipid nanoparticles are the most common nanocarriers that are essential to overcome these limitations [118].
6. Conclusions
In conclusion, bioactive peptides derived from marine sources are promising biomolecules for treating several diseases due to their diverse bioactivities. For inflammation, several approaches have been explored to combat it so far; however, MBPs allow additional benefits that are still unable to be achieved using conventional treatments, even to treat chronic diseases. Even though MBPs serve as sustainable and highly effective bioactive compounds, it is necessary to accurately identify the relationship between specific amino acid sequences and anti-inflammatory features for various effects, including more reliable targets and mechanisms, controllability, and reproducibility abilities, in order to enhance their powerful anti-inflammatory activities. For this purpose, modifications of many peptide parameters use novel technologies, particularly multi-omics strategies and high-throughput screening methods in biotechnology. Specifically, future studies should be focused on the detailed characterization of proteoforms to better understand the mechanisms underlying the bioactivity of MBPs, as the specific modifications or cleavage patterns in source proteoforms can influence peptide activity. Moreover, clinical studies that are more focused on humans, extending beyond in vitro experiments, are essential. These efforts aim to achieve safe and effective marine peptide-based therapeutics that not only address inflammation but also other life-threatening diseases in the near future.
Author Contributions
Conceptualization, review and editing, supervision, software, K.K.A.S.; review and editing, supervision, software, H.-S.K. and L.W.; data curation, writing—original draft preparation, H.D.T.U.W. and D.M.N.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council, University of Sri Jayewardenepura, Sri Lanka, grant number RC/URG/FOT/2024/59.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by a grant from the Research Council, University of Sri Jayewardenepura, Sri Lanka (Grant number RC/URG/FOT/2024/59).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jegani, K.T.; Balde, A.; Nazeer, R.A. A review on anti-inflammatory and antioxidant peptides derived from marine organisms: Mechanism of action and therapeutic applications. Food Biosci. 2025, 63, 105745. [Google Scholar] [CrossRef]
- Kim, J.-A.; Kim, S.-K. Bioactive Peptides from Marine Sources as Potential Anti-Inflammatory Therapeutics. Curr. Protein Pept. Sci. 2013, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Saeid, A. Bioactivity of Marine-Derived Peptides and Proteins: A Review. Mar. Drugs 2025, 23, 157. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Shi, Y.; Luo, S.; Weng, J.; Wu, Y.; Zheng, X. The role of inflammation in immune system of diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Front. Immunol. 2022, 13, 1055087. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as Anti-Inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef]
- Dadar, M.; Shahali, Y.; Chakraborty, S.; Prasad, M.; Tahoori, F.; Tiwari, R.; Dhama, K. Antiinflammatory peptides: Current knowledge and promising prospects. Inflamm. Res. 2019, 68, 125–145. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Herath, K.; Yang, H.W.; Choi, C.S.; Jeon, Y.J. Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Mar. Drugs 2021, 19, 678. [Google Scholar] [CrossRef] [PubMed]
- Jogpal, V.; Sanduja, M.; Dutt, R.; Garg, V.; Tinku. Advancement of nanomedicines in chronic inflammatory disorders. Inflammopharmacology 2022, 30, 355–368. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Chronic Inflammatory Diseases, Anti-Inflammatory Agents and Their Delivery Nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef]
- Plichta, J.; Kuna, P.; Panek, M. Biologic drugs in the treatment of chronic inflammatory pulmonary diseases: Recent developments and future perspectives. Front. Immunol. 2023, 14, 1207641. [Google Scholar] [CrossRef]
- Dash, S.; Singh, P.A.; Bajwa, N.; Choudhury, A.; Bisht, P.; Sharma, R. Why Pharmacovigilance of Non-steroidal Anti-inflammatory Drugs is Important in India? Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Khan, I. Biotechnological Utilization of the Marine Environment for Food, Drugs, and Energy. In Marine Biotechnology: Applications in Food, Drugs and Energy; Shah, M.D., Ransangan, J., Venmathi Maran, B.A., Eds.; Springer Nature: Singapore, 2023; pp. 23–46. [Google Scholar]
- Sanjeewa, K. Exploring the Blue Bioeconomy: Marine Bioresources and Sustainable Applications; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Senadheera, T.R.L.; Hossain, A.; Shahidi, F. Marine Bioactives and Their Application in the Food Industry: A Review. Appl. Sci. 2023, 13, 12088. [Google Scholar] [CrossRef]
- Tan, L.T. Impact of Marine Chemical Ecology Research on the Discovery and Dev. of New Pharmaceuticals. Mar. Drugs 2023, 21, 174. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Famurewa, A.C.; Oprea, E. Natural Bioactive Compounds and Human Health. Molecules 2024, 29, 3372. [Google Scholar] [CrossRef]
- Moutinho Cabral, I.; Gonçalves, C.; Grosso, A.R.; Costa, P.M. Bioprospecting and marine ‘omics’: Surfing the deep blue sea for novel bioactive proteins and peptides. Front. Mar. Sci. 2024, 11, 1362697. [Google Scholar] [CrossRef]
- Srinivasan, N.; Dhanalakshmi, S.; Pandian, P. Encouraging Leads from Marine Sources for Cancer Therapy A Review Approach. Pharmacogn. J. 2020, 12, 1475–1481. [Google Scholar] [CrossRef]
- Purohit, K.; Reddy, N.; Sunna, A. Exploring the Potential of Bioactive Peptides: From Natural Sources to Therapeutics. Int. J. Mol. Sci. 2024, 25, 1391. [Google Scholar] [CrossRef]
- Gajahin Gamage, N.T.; Miyashita, R.; Takahashi, K.; Asakawa, S.; Senevirathna, J.D.M. Proteomic Applications in Aquatic Environment Studies. Proteomes 2022, 10, 32. [Google Scholar] [CrossRef]
- Forgrave, L.M.; Wang, M.; Yang, D.; DeMarco, M.L. Proteoforms and their expanding role in laboratory medicine. Pract. Lab. Med. 2022, 28, e00260. [Google Scholar] [CrossRef]
- Tang, C.D.; Cheng, J.H.; Sun, D.W. Structure-activity relationships and activity enhancement techniques of marine bioactive peptides (MBPs). Crit. Rev. Food Sci. Nutr. 2024, 65, 4941–4963. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y. Review and perspective on bioactive peptides: A roadmap for research, development, and future opportunities. J. Agric. Food Res. 2022, 9, 100353. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Wu, J. Marine proteins and peptides: Production, biological activities, and potential applications. Food Innov. Adv. 2023, 2, 69–84. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Glowacka, M.; Kamysz, W.; Kleczkowska, P. Marine Peptides: Potential Basic Structures for the Development of Hybrid Compounds as Multitarget Therapeutics for the Treatment of Multifactorial Diseases. Int. J. Mol. Sci. 2024, 25, 12601. [Google Scholar] [CrossRef]
- Izzati, F.; Warsito, M.F.; Bayu, A.; Prasetyoputri, A.; Atikana, A.; Sukmarini, L.; Rahmawati, S.I.; Putra, M.Y. Chemical Diversity and Biological Activity of Secondary Metabolites Isolated from Indonesian Marine Invertebrates. Molecules 2021, 26, 1898. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, B.; Zhao, D.-L.; Newman, D.J. Editorial: The discovery, identification and application of marine microorganisms derived natural products. Front. Mar. Sci. 2024, 11, 1366379. [Google Scholar] [CrossRef]
- Dehghani, M.; Taherizadeh, M.R.; Homaei, A. Marine Origin Bioactive Peptides: Novel Advances in the Therapeutic Potential. In Marine Biomaterials; Jana, S., Jana, S., Eds.; Springer Nature: Singapore, 2022; pp. 351–392. [Google Scholar]
- Xu, N.; Peng, X.L.; Li, H.R.; Liu, J.X.; Cheng, J.S.; Qi, X.Y.; Ye, S.J.; Gong, H.L.; Zhao, X.H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Pham, B.P.; Molnár, Z.; Varga, L.; Greff, B. The structure–activity relationship of marine peptides: A review. Int. J. Food Sci. Technol. 2024, 59, 4437–4445. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef] [PubMed]
- El Nashar, H.A.; Kocaeli, S.; Abdallah, M.; El-Shazly, M. Drug from Marine Sampling to Factory. In Marine Ecosystems: A Unique Source of Valuable Bioactive Compounds; Bentham Science: Sharjah, United Arab Emirates, 2023; Volume 3, pp. 355–393. [Google Scholar]
- Sukmarini, L. Drug Development from Peptide-derived Marine Natural Products. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012063. [Google Scholar] [CrossRef]
- Jin, Q.-H.; Peng, D.-X.; Zheng, Z.-J. Advances in extracting and understanding the bioactivities of marine organism peptides: A review. J. Food Process. Preserv. 2022, 46, e15602. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Y.; Hua, H.; Li, D.; Tang, C. Marine Antitumor Peptide Dolastatin 10: Biological Activity, Structural Modification and Synthetic Chemistry. Mar. Drugs 2021, 19, 363. [Google Scholar] [CrossRef]
- Goel, B.; Jain, S.K. Semisynthesis: Bridging natural products and novel anticancer therapies. Eur. J. Med. Chem. Rep. 2024, 12, 100218. [Google Scholar] [CrossRef]
- Singh, S.B. Discovery and Development of Dolastatin 10-Derived Antibody Drug Conjugate Anticancer Drugs. J. Nat. Prod. 2022, 85, 666–687. [Google Scholar] [CrossRef]
- Trinidad-Calderon, P.A.; Varela-Chinchilla, C.D.; Garcia-Lara, S. Depsipeptides Targeting Tumor Cells: Milestones from In Vitro to Clinical Trials. Molecules 2023, 28, 670. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Sorolla, M.A.; Krishnan, P.D.G.; Sorolla, A. From Seabed to Bedside: A Review on Promising Marine Anticancer Compounds. Biomolecules 2020, 10, 248. [Google Scholar] [CrossRef]
- Bojarska, J.; Breza, M.; Remko, M.; Czyz, M.; Gajos-Michniewicz, A.; Zimecki, M.; Kaczmarek, K.; Madura, I.D.; Wojciechowski, J.M.; Wolf, W.M. Structural and Biofunctional Insights into the Cyclo(Pro-Pro-Phe-Phe-) Scaffold from Experimental and In Silico Studies: Melanoma and Beyond. Int. J. Mol. Sci. 2022, 23, 7173. [Google Scholar] [CrossRef] [PubMed]
- Cappello, E.; Nieri, P. From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial. Life 2021, 11, 1390. [Google Scholar] [CrossRef]
- Pavlicevic, M.; Maestri, E.; Marmiroli, M. Marine Bioactive Peptides-An Overview of Generation, Structure and Application with a Focus on Food Sources. Mar. Drugs 2020, 18, 424. [Google Scholar] [CrossRef]
- Gagat, P.; Ostrowka, M.; Duda-Madej, A.; Mackiewicz, P. Enhancing Antimicrobial Peptide Activity through Modifications of Charge, Hydrophobicity, and Structure. Int. J. Mol. Sci. 2024, 25, 10821. [Google Scholar] [CrossRef]
- Rivera-Jimenez, J.; Berraquero-Garcia, C.; Perez-Galvez, R.; Garcia-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Peptides and protein hydrolysates exhibiting anti-inflammatory activity: Sources, structural features and modulation mechanisms. Food Funct. 2022, 13, 12510–12540. [Google Scholar] [CrossRef]
- Linker, S.M.; Schellhaas, C.; Kamenik, A.S.; Veldhuizen, M.M.; Waibl, F.; Roth, H.-J.; Fouché, M.; Rodde, S.; Riniker, S. Lessons for Oral Bioavailability: How Conformationally Flexible Cyclic Peptides Enter and Cross Lipid Membranes. J. Med. Chem. 2023, 66, 2773–2788. [Google Scholar] [CrossRef]
- Zhang, M.; Sunaba, T.; Sun, Y.; Sasaki, K.; Isoda, H.; Kigoshi, H.; Kita, M. Anti-inflammatory marine cyclic peptide stylissatin A and its derivatives inhibit differentiation of murine preadipocytes. Chem. Commun. 2019, 55, 5471–5474. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, M.; Chen, Y.; Qu, Y.; Shi, W.; Shi, L.; Qiao, Y.; Li, X.; Guo, X.; Wang, L.; et al. Preparation of polysaccharide-based nanoparticles by chitosan and flaxseed gum polyelectrolyte complexation as carriers for bighead carp (Aristichthys nobilis) peptide delivery. Int. J. Biol. Macromol. 2023, 249, 126121. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; He, H.; Zhou, W.; Li, M.; Lu, A.; Che, T.; Shen, S. Purification identification and function analysis of ACE inhibitory peptide from Ulva prolifera protein. Food Chem. 2023, 401, 134127. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-G.; Kim, H.-S.; Lee, H.-G.; Oh, J.-Y.; Lu, Y.A.; Wang, L.; Rho, S.; Jeon, Y.-J. Low-molecular weight peptides isolated from seahorse (Hippocampus abdominalis) improve vasodilation via inhibition of angiotensin-converting enzyme in vivo and in vitro. Process Biochem. 2020, 95, 30–35. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Rui, X.; Simpson, B.K. Interactions of C. frondosa-derived inhibitory peptides against angiotensin I-converting enzyme (ACE), alpha-amylase and lipase. Food Chem. 2022, 367, 130695. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; San, S. Efficacy of a Novel ACE-Inhibitory Peptide from Sargassum maclurei in Hypertension and Reduction of Intracellular Endothelin-1. Nutrients 2020, 12, 653. [Google Scholar] [CrossRef]
- Hu, Y.D.; Xi, Q.H.; Kong, J.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from the Collagens of Monkfish (Lophius litulon) Swim Bladders: Isolation, Characterization, Molecular Docking Analysis and Activity Evaluation. Mar. Drugs 2023, 21, 516. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Tian, C.; Jiang, J.; Zhang, G.L.; Hao, H.; Hou, H.M. Antibacterial Activity and Potential Application in Food Packaging of Peptides Derived from Turbot Viscera Hydrolysate. J. Agric. Food Chem. 2020, 68, 9968–9977. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, Preparation, and Purification of Marine Bioactive Peptides. BioMed Res. Int. 2017, 2017, 9746720. [Google Scholar] [CrossRef] [PubMed]
- Guadalupi, L.S.; Bianco, M.; Cataldi, T.R.I.; Ravnsborg, T.; Jensen, O.N.; Calvano, C.D. Ultrasound-assisted protein extraction for deep proteome analysis of Spirulina and Chlorella microalgae. LWT 2025, 222, 117647. [Google Scholar] [CrossRef]
- Moreira, C.; Ferreira-Santos, P.; Nunes, R.; Carvalho, B.; Pereira, H.; Teixeira, J.A.; Rocha, C.M.R. Influence of different processing techniques on microalgal protein extraction. Algal Res. 2025, 86, 103958. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Li, Y.; Pan, J.; Liu, F.; Dai, H.; Fu, Y.; Huang, T.; Farooq, S.; Zhang, H. Collagen and gelatin: Structure, properties, and applications in food industry. Int. J. Biol. Macromol. 2024, 254, 128037. [Google Scholar] [CrossRef]
- Gallego, C.; Rodil, E.; Rodríguez, H.; Soto, A. Extraction and characterisation of gelatine from yellowfin tuna skin pretreated with a eutectic solvent. Food Hydrocoll. 2025, 159, 110652. [Google Scholar] [CrossRef]
- Zhang, W.; Boateng, I.D.; Xu, J. Novel marine proteins as a global protein supply and human nutrition: Extraction, bioactivities, potential applications, safety assessment, and deodorization technologies. Trends Food Sci. Technol. 2024, 143, 104283. [Google Scholar] [CrossRef]
- Trigo, J.P.; Steinhagen, S.; Stedt, K.; Krona, A.; Verhagen, S.; Pavia, H.; Abdollahi, M.; Undeland, I. A new method for protein extraction from sea lettuce (Ulva fenestrata) via surfactants and alkaline aqueous solutions. Food Chem. 2025, 464, 141839. [Google Scholar] [CrossRef]
- Farooq, S.; Ahmad, M.I.; Zheng, S.; Ali, U.; Li, Y.; Shixiu, C.; Zhang, H. A review on marine collagen: Sources, extraction methods, colloids properties, and food applications. Collagen Leather 2024, 6, 11. [Google Scholar] [CrossRef]
- Gonzaga, L.J.; Pérez Roa, M.E.; Lavecchia, R.; Zuorro, A. Unlocking marine potential: Microwave-assisted extraction of bioactive compounds from marine macroalgae. J. Environ. Chem. Eng. 2025, 13, 116858. [Google Scholar] [CrossRef]
- Gutierrez-Canul, C.D.; Can-Herrera, L.A.; Ramirez-Rivera, E.J.; Prinyawiwatkul, W.; Sauri-Duch, E.; Moo-Huchin, V.M.; Hernandez-Nunez, E. A Review of Classical and Rising Approaches the Extraction and Utilization of Marine Collagen. BioTech 2025, 14, 26. [Google Scholar] [CrossRef]
- Pasarin, D.; Lavric, V.; Enascuta, C.E.; Ghizdareanu, A.-I.; Matei, C.B. Optimal Enzymatic Hydrolysis of Sweet Lupine Protein towards Food Ingredients. Fermentation 2023, 9, 203. [Google Scholar] [CrossRef]
- Vaishnav, A.; Lal, J.; Mehta, N.K.; Mohanty, S.; Yadav, K.K.; Priyadarshini, M.B.; Debbarma, P.; Singh, N.S.; Pati, B.K.; Singh, S.K. Unlocking the potential of fishery waste: Exploring diverse applications of fish protein hydrolysates in food and nonfood sectors. Environ. Sci. Pollut. Res. Int. 2025, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Marinou, D.; Jacobsen, C.; Odelli, D.; Sarigiannidou, K.; Sorensen, A.M. Production of Protein Hydrolysates from Cod Backbone Using Selected Enzymes: Evaluation of Antioxidative and Antimicrobial Activities of Hydrolysates. Mar. Drugs 2025, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Jayawardhana, H.; Liyanage, N.M.; Nagahawatta, D.P.; Lee, H.G.; Jeon, Y.J.; Kang, S.I. Pepsin Hydrolysate from Surimi Industry-Related Olive Flounder Head Byproducts Attenuates LPS-Induced Inflammation and Oxidative Stress in RAW 264.7 Macrophages and In Vivo Zebrafish Model. Mar. Drugs 2023, 22, 24. [Google Scholar] [CrossRef]
- Chai, H.; Bo, Y.; Zhou, L.; Talab, A.S.; Zhang, L.; Wang, Y.; Wang, C.; Zhang, T. Protein enzymatic hydrolysates of sea cucumber intestine promotes proliferation and migration of bone marrow mesenchymal stem cells through up-regulating the PI3K/AKT and JAK/STAT pathways. Food Biosci. 2025, 63, 105690. [Google Scholar] [CrossRef]
- Saiwong, S.; Autsavapromporn, N.; Siriwoharn, T.; Techapun, C.; Wangtueai, S. Enzymatic Hydrolysis Optimization for Preparation of Sea Cucumber (Holothuria scabra) Hydrolysate with an Antiproliferative Effect on the HepG2 Liver Cancer Cell Line and Antioxidant Properties. Int. J. Mol. Sci. 2023, 24, 9491. [Google Scholar] [CrossRef]
- Nemati, M.; Shahosseini, S.R.; Ariaii, P. Review of fish protein hydrolysates: Production methods, antioxidant and antimicrobial activity and nanoencapsulation. Food Sci. Biotechnol. 2024, 33, 1789–1803. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Nawijn, W.; Mironczuk, A.M. Brown seaweed hydrolysate as a promising growth substrate for biomass and lipid synthesis of the yeast yarrowia lipolytica. Front. Bioeng. Biotechnol. 2022, 10, 944228. [Google Scholar] [CrossRef] [PubMed]
- Wijethunga, A.M.; He, Q.S.; Prithiviraj, B.; Sun, X. Acid hydrolysis and microwave digestion enhanced protein extraction from red seaweed Palmaria palmata. Food Chem. X 2025, 25, 102222. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Lê, H.Q.; Leppiniemi, J.; Koso, T.; Välisalmi, T.; Linder, M.B.; Pisano, I.; Dou, J.; Leahy, J.J.; Kontturi, E. Microwave hydrolysis, as a sustainable approach in the processing of seaweed for protein and nanocellulose management. Algal Res. 2024, 78, 103406. [Google Scholar] [CrossRef]
- Peng, Z.; Gao, J.; Su, W.; Cao, W.; Zhu, G.; Qin, X.; Zhang, C.; Qi, Y. Purification and Identification of Peptides from Oyster (Crassostrea hongkongensis) Protein Enzymatic Hydrolysates and Their Anti-Skin Photoaging Effects on UVB-Irradiated HaCaT Cells. Mar. Drugs 2022, 20, 749. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Purification and fractionation of bioactive peptides through membrane filtration: A critical and application review. Trends Food Sci. Technol. 2023, 131, 118–128. [Google Scholar] [CrossRef]
- Bugyi, F.; Toth, G.; Kovacs, K.B.; Drahos, L.; Turiak, L. Comparison of solid-phase extraction methods for efficient purification of phosphopeptides with low sample amounts. J. Chromatogr. A 2022, 1685, 463597. [Google Scholar] [CrossRef]
- Kummari, R.; Bose, K. Gel Filtration Chromatography. In Textbook on Cloning, Expression and Purification of Recombinant Proteins; Bose, K., Ed.; Springer Nature: Singapore, 2022; pp. 199–219. [Google Scholar]
- Saraiva, M.A. Interpretation of α-synuclein UV absorption spectra in the peptide bond and the aromatic regions. J. Photochem. Photobiol. B Biol. 2020, 212, 112022. [Google Scholar] [CrossRef]
- Pandeswari, P.B.; Sabareesh, V. An ESI Q-TOF study to understand the impact of arginine on CID MS/MS characteristics of polypeptides. Int. J. Mass. Spectrom. 2021, 459, 116453. [Google Scholar] [CrossRef]
- Duan, H.; Liu, G.; Liu, J.; Chang, X.; Bao, S.; Song, W.; Yan, W. Research progress on active peptides in marine fish. Food Sci. Anim. Prod. 2024, 2, 9240063. [Google Scholar] [CrossRef]
- Kwon, J.; Shin, D.; Park, G.W.; Lee, G.; Lee, E.; Kang, H.-S. A comprehensive quantitative LC-MS/MS method for rapid gelatin source identification in food products: Comparison with PCR. Food Res. Int. 2025, 201, 115611. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Jaradat, D.s.M.M. Advances in Therapeutic Peptides Separation and Purification. Separations 2024, 11, 233. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural products in cosmetics. Nat. Prod. Bioprospecting 2022, 12, 40. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.-j.; Jo, M.J.; Seo, Y.B.; Park, N.G.; Kim, G.-D. Anti-Inflammatory Activity of β-thymosin Peptide Derived from Pacific Oyster (Crassostrea gigas) on NO and PGE2 Production by Down-Regulating NF-κB in LPS-Induced RAW264.7 Macrophage Cells. Mar. Drugs 2019, 17, 129. [Google Scholar] [CrossRef]
- Ahn, C.B.; Cho, Y.S.; Je, J.Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yi, Y.; Liu, J.; Lin, X.; Yang, K.; Lv, M.; Zhou, X.; Hao, J.; Liu, J.; Zheng, Y.; et al. Isolation and characterization of marine Brevibacillus sp. S-1 collected from South China Sea and a novel antitumor peptide produced by the strain. PLoS ONE 2014, 9, e111270. [Google Scholar]
- Do, T.; Guran, R.; Adam, V.; Zitka, O. Use of MALDI-TOF mass spectrometry for virus identification: A review. Analyst 2022, 147, 3131–3154. [Google Scholar] [CrossRef]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef]
- Wang, S.; Lei, B.; Zhang, E.; Gong, P.; Gu, J.; He, L.; Han, L.; Yuan, Z. Targeted Therapy for Inflammatory Diseases with Mesenchymal Stem Cells and Their Derived Exosomes: From Basic to Clinics. Int. J. Nanomed. 2022, 17, 1757–1781. [Google Scholar] [CrossRef]
- Zotova, N.; Zhuravleva, Y.; Chereshnev, V.; Gusev, E. Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation. Int. J. Mol. Sci. 2023, 24, 1144. [Google Scholar] [CrossRef]
- Sugihara, K.; Nishizawa-Higashi, M.; Joe, G.-H.; Onishi, Y.; Shimizu, Y.; Saeki, H. Improvement of anti-inflammatory activity of salmon muscle peptides by conjugation with alginate oligosaccharide and recovery of the active fraction using ampholyte-free isoelectric focusing. Fish. Sci. 2021, 87, 569–577. [Google Scholar] [CrossRef]
- Michel, P.; Wajs-Bonikowska, A.; Magiera, A.; Wosiak, A.; Balcerczak, E.; Czerwinska, M.E.; Olszewska, M.A. Anti-Inflammatory and Antioxidant Effects of (6S,9R)-Vomifoliol from Gaultheria procumbens L.: In Vitro and Ex Vivo Study in Human Immune Cell Models. Int. J. Mol. Sci. 2025, 26, 1571. [Google Scholar] [CrossRef]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2017, 33, 44–61. [Google Scholar] [CrossRef]
- Farahani, A.; Farahani, A.; Kashfi, K.; Ghasemi, A. Inducible nitric oxide synthase (iNOS): More than an inducible enzyme? Rethinking the classification of NOS isoforms. Pharmacol. Res. 2025, 216, 107781. [Google Scholar] [CrossRef]
- Jin, J.; Wang, W.; Fan, D.; Hao, Q.; Jia, W. Emerging Roles of Mitogen-Activated Protein Kinase Signaling Pathways in the Regulation of Fruit Ripening and Postharvest Quality. Int. J. Mol. Sci. 2024, 25, 2831. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Baek, K.H. E3 ligases and deubiquitinating enzymes regulating the MAPK signaling pathway in cancers. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188736. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Li, J.; Song, Y.; Chen, S.; Zhou, S.; Yang, X. Preparation, purification, and identification of novel antioxidant peptides derived from Gracilariopsis lemaneiformis protein hydrolysates. Front. Nutr. 2022, 9, 971419. [Google Scholar] [CrossRef]
- Zhu, J.; Fan, J.; Xia, Y.; Wang, H.; Li, Y.; Feng, Z.; Fu, C. Potential therapeutic targets of macrophages in inhibiting immune damage and fibrotic processes in musculoskeletal diseases. Front. Immunol. 2023, 14, 1219487. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wang, Y.; Li, K.; Song, J.; Chang, Y. A novel p38 MAPK gene in the sea cucumber Apostichopus japonicus (Ajp38) is associated with the immune response to pathogenic challenge. Fish. Shellfish. Immunol. 2018, 81, 250–259. [Google Scholar] [CrossRef]
- Ghallab, D.S.; Ibrahim, R.S.; Mohyeldin, M.M.; Shawky, E. Marine algae: A treasure trove of bioactive anti-inflammatory compounds. Mar. Pollut. Bull. 2024, 199, 116023. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Nagahawatta, D.P.; Wang, L.; Sanjeewa, K.K.A. The Role of Phlorotannins to Treat Inflammatory Diseases. Chemistry 2025, 7, 77. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Ghiasi, M. Investigating the NF-kappaB signaling pathway in heart failure: Exploring potential therapeutic approaches. Heliyon 2024, 10, e40812. [Google Scholar] [CrossRef]
- Feng, J.; Wang, H.; Luo, X.; Zhang, L.; Zhou, P. Identification and molecular mechanism of the anti-inflammatory effect of sea cucumber peptides: Network pharmacology, molecular docking and animal experiments. Int. J. Biol. Macromol. 2024, 279, 134958. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gallardo, S.; López-Rocha, J.A.; Rosas, C.; Solís-Marín, F.A.; Olvera-Novoa, M.A. Movement and effectiveness of shelters for restocking of the sea cucumber Isostichopus badionotus. Aquac. Rep. 2024, 37, 102191. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Li, W.; Ma, X.C.; Qiu, F.; Sun, C.P. IkappaB kinase beta (IKKbeta): Structure, transduction mechanism, biological function, and discovery of its inhibitors. Int. J. Biol. Sci. 2023, 19, 4181–4203. [Google Scholar] [CrossRef]
- Kaminska, P.; Tempes, A.; Scholz, E.; Malik, A.R. Cytokines on the way to secretion. Cytokine Growth Factor. Rev. 2024, 79, 52–65. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kwon, H.H.; Leem, J.; Jang, Y.Y. Kahweol Inhibits Pro-Inflammatory Cytokines and Chemokines in Tumor Necrosis Factor-alpha/Interferon-gamma-Stimulated Human Keratinocyte HaCaT Cells. Curr. Issues Mol. Biol. 2024, 46, 3470–3483. [Google Scholar] [CrossRef]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldivar, R.; Bilal, M.; Iqbal, H.M.N. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Baraiya, R.; Prakash, S.; Biswas, A.; Chowdhury, B.; Sharma, A. Anti-Inflammatory Potential of Fish-Derived Bioactive Peptides: Molecular Mechanisms, Delivery Strategies, and Clinical Perspectives. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70234. [Google Scholar] [CrossRef] [PubMed]
- Abdo, A.A.A.; Al-Dalali, S.; Hou, Y.; Aleryani, H.; Shehzad, Q.; Asawmahi, O.; Al-Farga, A.; Mohammed, B.; Liu, X.; Sang, Y. Modification of Marine Bioactive Peptides: Strategy to Improve the Biological Activity, Stability, and Taste Properties. Food Bioprocess. Technol. 2024, 17, 1412–1433. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, M.; Wu, P.; Wu, S.; Lee, T.Y.; Bai, C. Application of Computational Biology and Artificial Intelligence in Drug Design. Int. J. Mol. Sci. 2022, 23, 13568. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Liu, T.; Lin, S.; Li, D.; Liu, H.; Yao, X.; Hou, T. Artificial intelligence in peptide-based drug design. Drug Discov. Today 2025, 30, 104300. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Liu, K.; Liu, X.; Rashid, M.T.; Zhang, H.; Wang, M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods 2023, 12, 2999. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).