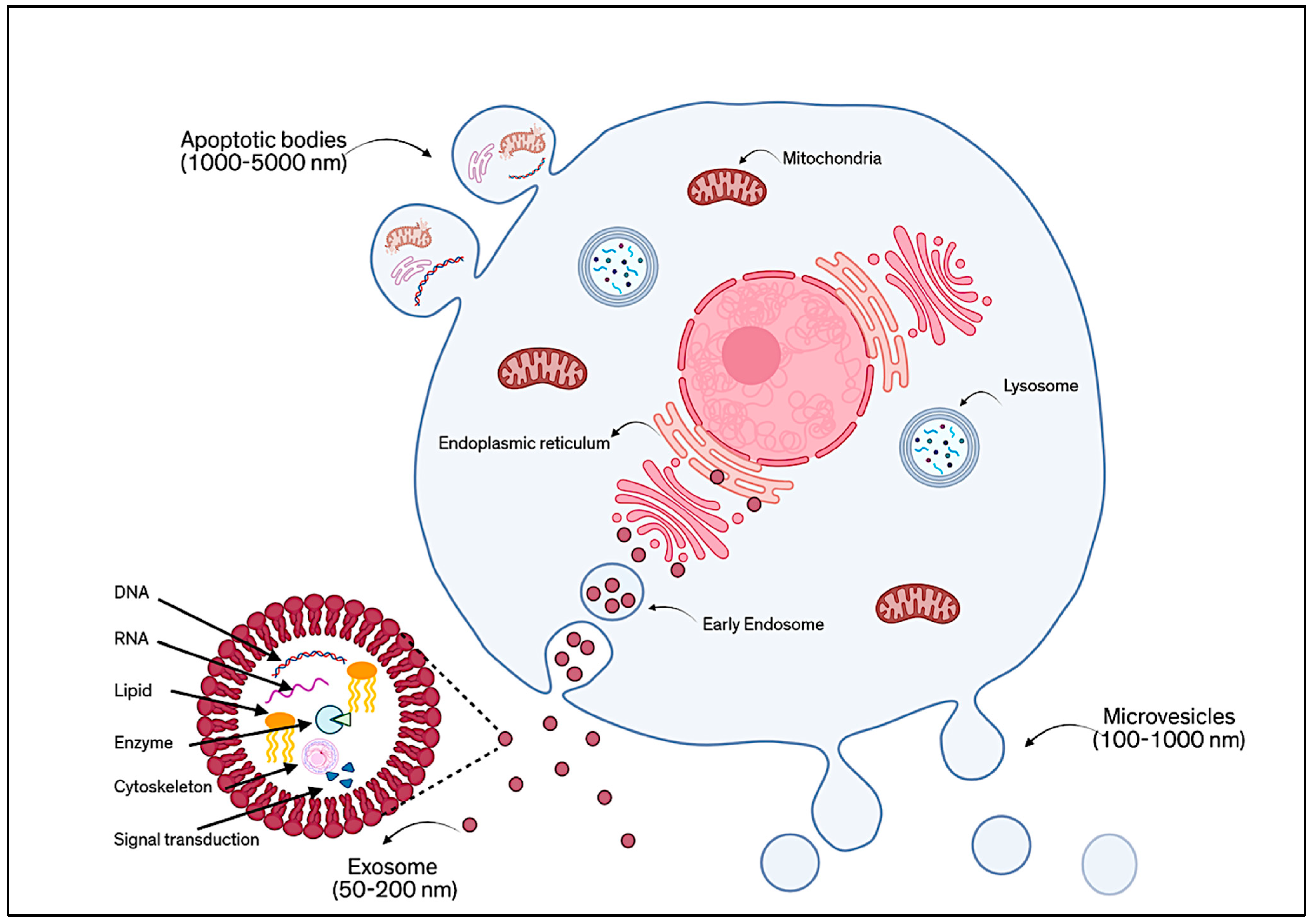
Extracellular Vesicle (EV) Proteomics in Corneal Regenerative Medicine
Abstract
1. Introduction
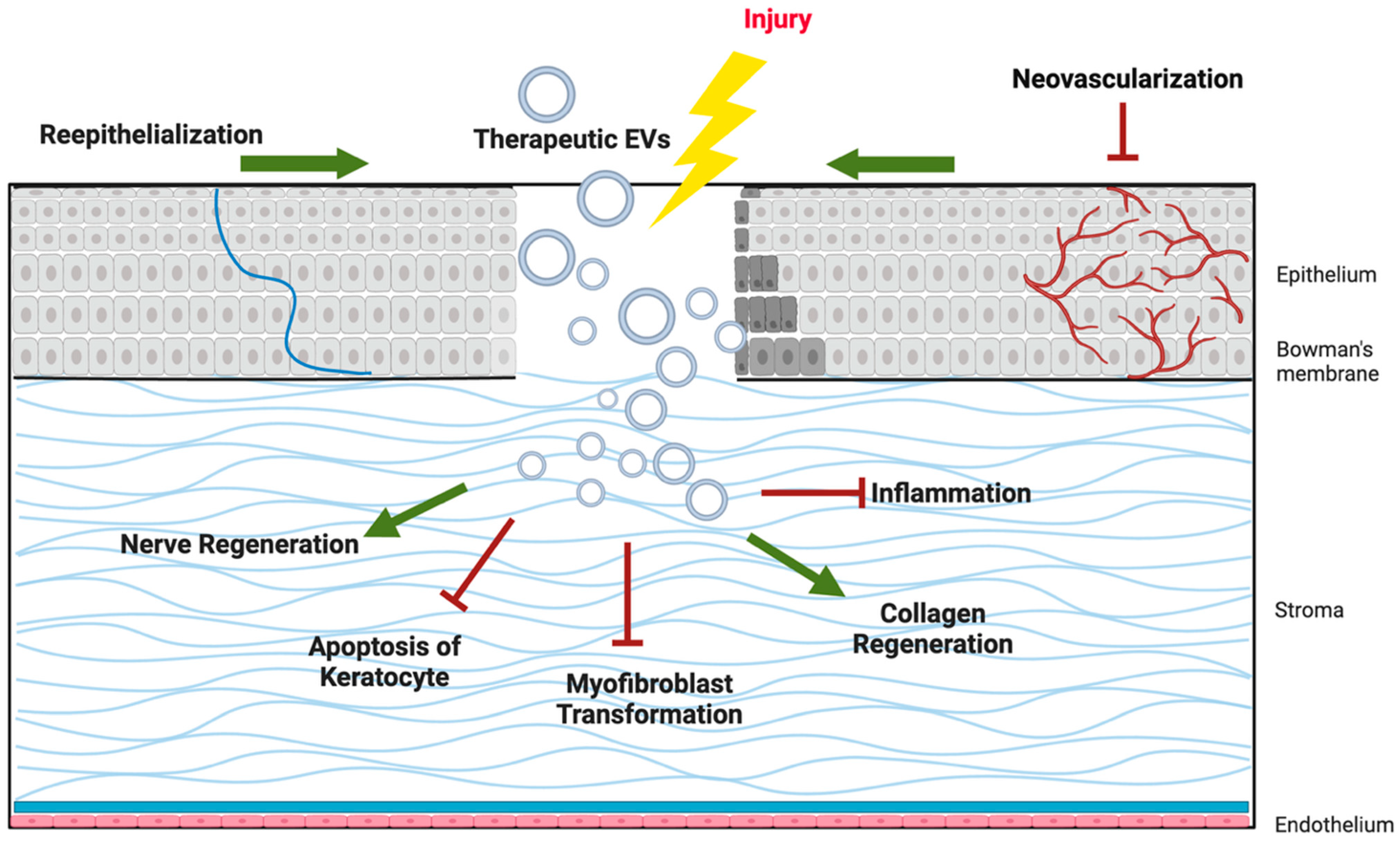
2. EVs in Corneal Regeneration
2.1. Epithelial Regeneration
2.2. Nerve Regeneration
2.3. Stromal Regeneration
2.4. Other EV Sources
3. Proteomic Profiling of EVs
3.1. EV Isolation Methods
3.2. Analytical Platforms for EV Proteome Characterization
4. Proteome Variation
4.1. Priming and Preconditioning
4.2. Source-Dependent
4.3. Protein and microRNA Cargo
5. Functional Roles of Identified Proteins
5.1. Proteins Involved in Angiogenesis and Vascular Modulation
5.2. Anti-Fibrotic and Extracellular Matrix Remodeling Proteins
5.3. Neuroprotective and Nerve Regeneration Factors
5.4. Anti-Inflammatory and Immunomodulatory Proteins
5.5. Cell Survival and Proliferation Factors
6. Challenges and Opportunities
6.1. Clinical Applications
6.2. Challenges in Translation
7. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Yeung, V.; Boychev, N.; Kanu, L.N.; Ng, V.; Ross, A.E.; Hutcheon, A.E.K.; Ciolino, J.B. Proteomic Characterization of Corneal Epithelial and Stromal Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 10338. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpouri, A.; Saberi, M.J.; Yazdansetad, Z.; Arabpour, Z.; Zarei-Behjani, Z. Exosomes from Cancer Cells: Innovative Approach for Targeted Cancer Treatment. Regen. Eng. Transl. Med. 2025, 1–14. [Google Scholar] [CrossRef]
- Nix, C.; Fillet, M. New insights into extracellular vesicles of clinical and therapeutic interest using proteomic mass spectrometry approaches. TrAC Trends Anal. Chem. 2024, 178, 117823. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Rubinstein, E.; Théry, C.; Zimmermann, P. Tetraspanins affect membrane structures and the trafficking of molecular partners: What impact on extracellular vesicles? Biochem. Soc. Trans. 2025, 53, 371–382. [Google Scholar] [CrossRef]
- Zheng, T.; Xu, S.; Xu, J. A review of the roles of specialized extracellular vesicles, migrasomes, and exosomes in normal cell physiology and disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2023, 29, e940118-1. [Google Scholar] [CrossRef]
- Massoumi, H.; Amin, S.; Soleimani, M.; Momenaei, B.; Ashraf, M.J.; Guaiquil, V.H.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R.; Jalilian, E. Extracellular-Vesicle-Based Therapeutics in Neuro-Ophthalmic Disorders. Int. J. Mol. Sci. 2023, 24, 9006. [Google Scholar] [CrossRef]
- Gorgun, C.; Ceresa, D.; Lesage, R.; Villa, F.; Reverberi, D.; Balbi, C.; Santamaria, S.; Cortese, K.; Malatesta, P.; Geris, L.; et al. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials 2021, 269, 120633. [Google Scholar] [CrossRef]
- Yeung, V.; Zhang, T.C.; Yuan, L.; Parekh, M.; Cortinas, J.A.; Delavogia, E.; Hutcheon, A.E.K.; Guo, X.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Myofibroblasts Promote Corneal Epithelial Cell Migration. Int. J. Mol. Sci. 2022, 23, 3136. [Google Scholar] [CrossRef]
- Verma, N.; Khare, D.; Poe, A.J.; Amador, C.; Ghiam, S.; Fealy, A.; Ebrahimi, S.; Shadrokh, O.; Song, X.-Y.; Santiskulvong, C.; et al. MicroRNA and Protein Cargos of Human Limbal Epithelial Cell-Derived Exosomes and Their Regulatory Roles in Limbal Stromal Cells of Diabetic and Non-Diabetic Corneas. Cells 2023, 12, 2524. [Google Scholar] [CrossRef]
- Willmann, D.; Fu, L.; Melanson, S.W. Corneal Injury. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Nuzzi, A.; Giuffrida, F.P.; Luccarelli, S.; Nucci, P. Corneal Epithelial Regeneration: Old and New Perspectives. Int. J. Mol. Sci. 2022, 23, 13114. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Chen, X.; Cao, H.; Zheng, L.; Li, Q.; Zhang, K.; Han, Z.; Han, Z.-C.; Guo, Z.; Li, Z.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Corneal Wound Repair. Stem Cells Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Saccu, G.; Menchise, V.; Gai, C.; Bertolin, M.; Ferrari, S.; Giordano, C.; Manco, M.; Dastrù, W.; Tolosano, E.; Bussolati, B.; et al. Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Corneal Wound Repair by Regulating Inflammation and Angiogenesis. Cells 2022, 11, 3892. [Google Scholar] [CrossRef]
- Tati, V.; V, S.M.; Shukla, S. Mesenchymal vs. epithelial extracellular vesicles in corneal epithelial repair, apoptosis, and immunomodulation: An in vitro study. Exp. Eye Res. 2024, 247, 110027. [Google Scholar] [CrossRef]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wu, G.; Qi, P.; Zhang, Y.; Liu, Z.; Li, X.; Yu, Y.; Ye, X.; Li, Y.; et al. Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Deliver miR-21 to Promote Corneal Epithelial Wound Healing through PTEN/PI3K/Akt Pathway. Stem Cells Int. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, L.; Gong, C.; Gong, X.; Zhu, T.; Zhu, Y. Extracellular vesicles derived from mouse adipose-derived mesenchymal stem cells promote diabetic corneal epithelial wound healing through NGF/TrkA pathway activation involving dendritic cells. Exp. Eye Res. 2023, 231, 109484. [Google Scholar] [CrossRef]
- Yi, S.; Kim, J.; Kim, M.J.; Yae, C.G.; Kim, K.H.; Kim, H.K. Development of human amniotic epithelial cell-derived extracellular vesicles as cell-free therapy for dry eye disease. Ocul. Surf. 2024, 34, 370–380. [Google Scholar] [CrossRef]
- Braunsperger, M.V.; Martin, G.; Herzig, T.; Kußberger, I.; Gießl, A.; Steimle, S.; Schlötzer-Schrehardt, U.; Schlunck, G.; Reinhard, T.; Polisetti, N. Proteomic Insights into Human Limbal Epithelial Progenitor-Derived Small Extracellular Vesicles. Stem Cell Rev. Rep. 2025, 21, 1578–1593. [Google Scholar] [CrossRef]
- An, S.; Anwar, K.; Ashraf, M.; Lee, H.; Jung, R.; Koganti, R.; Ghassemi, M.; Djalilian, A.R. Wound-Healing Effects of Mesenchymal Stromal Cell Secretome in the Cornea and the Role of Exosomes. Pharmaceutics 2023, 15, 1486. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hayashi, R.; Imaizumi, T.; Harrington, J.; Kudo, Y.; Takayanagi, H.; Baba, K.; Nishida, K. Extracellular vesicles from adipose-derived mesenchymal stem cells promote colony formation ability and EMT of corneal limbal epithelial cells. PLoS ONE 2025, 20, e0321579. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, Z.; Jin, C.; Chen, Q.; Fang, Y.; Jin, J.; Chen, J.; Lu, L.; Tian, H.; Xu, J.; et al. Human amniotic epithelial cell-derived extracellular vesicles provide an extracellular matrix-based microenvironment for corneal injury repair. J. Tissue Eng. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef]
- Soleimani, M.; Baharnoori, S.M.; Massoumi, H.; Cheraqpour, K.; Asadigandomani, H.; Mirzaei, A.; Ashraf, M.J.; Koganti, R.; Chaudhuri, M.; Ghassemi, M.; et al. A deep dive into radiation keratopathy; Going beyond the current frontierss. Exp. Eye Res. 2025, 251, 110234. [Google Scholar] [CrossRef]
- Jalilian, E.; Massoumi, H.; Bigit, B.; Amin, S.; Katz, E.A.; Guaiquil, V.H.; Anwar, K.N.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res. Ther. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Amin, S.; Massoumi, H.; Tewari, D.; Roy, A.; Chaudhuri, M.; Jazayerli, C.; Krishan, A.; Singh, M.; Soleimani, M.; Karaca, E.E.; et al. Cell Type-Specific Extracellular Vesicles and Their Impact on Health and Disease. Int. J. Mol. Sci. 2024, 25, 2730. [Google Scholar] [CrossRef]
- Massoumi, H.; Niktinat, H.; Alviar, M.; Guaiquil, V.H.; Rosenblatt, M.; Djalilian, A.R.; Jalilian, E. Comparative Analysis of miRNA Profiles in Corneal and Bone Marrow Mesenchymal Stem Cells (MSC)-Derived Extracellular Vesicles (EVs): Implications for Nerve Regeneration. Invest. Ophthalmol. Vis. Sci. 2025, 66, 1032. [Google Scholar]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. BioMed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Pieragostino, D.; Lanzini, M.; Cicalini, I.; Cufaro, M.C.; Damiani, V.; Mastropasqua, L.; De Laurenzi, V.; Nubile, M.; Lanuti, P.; Bologna, G.; et al. Tear proteomics reveals the molecular basis of the efficacy of human recombinant nerve growth factor treatment for Neurotrophic Keratopathy. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Yu, M.; Gu, G.; Cong, M.; Du, M.; Wang, W.; Shen, M.; Zhang, Q.; Shi, H.; Gu, X.; Ding, F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021, 134, 190–203. [Google Scholar] [CrossRef]
- Mohan, R.R.; Kempuraj, D.; D’SOuza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef]
- Deng, S.X.; Dos Santos, A.; Gee, S. Therapeutic Potential of Extracellular Vesicles for the Treatment of Corneal Injuries and Scars. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef]
- Lagali, N. Corneal Stromal Regeneration: Current Status and Future Therapeutic Potential. Curr. Eye Res. 2019, 45, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. STEM CELLS Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.-F.; Williams, G.P.; Setiawan, M.; Yusoff, N.Z.B.M.; Lee, X.-W.; Htoon, H.M.; Zhou, L.; Fuest, M.; Mehta, J.S. Nerve regeneration by human corneal stromal keratocytes and stromal fibroblasts. Sci. Rep. 2017, 7, 45396. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Zheng, Q.-Q.; Shen, J.; Li, Q.-S.; Song, X.-H.; Luo, H.-B.; Hong, C.-Y.; Yao, K. Effects of Adipose-derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin. Med J. 2018, 131, 704–712. [Google Scholar] [CrossRef]
- Yam, G.H.-F.; Yang, T.; Geary, M.L.; Santra, M.; Funderburgh, M.; Rubin, E.; Du, Y.; A Sahel, J.; Jhanji, V.; Funderburgh, J.L. Human corneal stromal stem cells express anti-fibrotic microRNA-29a and 381-5p—A robust cell selection tool for stem cell therapy of corneal scarring. J. Adv. Res. 2022, 45, 141–155. [Google Scholar] [CrossRef]
- Ong, H.S.; Riau, A.K.; Yam, G.H.-F.; Yusoff, N.Z.B.M.; Han, E.J.Y.; Goh, T.-W.; Lai, R.C.; Lim, S.K.; Mehta, J.S. Mesenchymal Stem Cell Exosomes as Immunomodulatory Therapy for Corneal Scarring. Int. J. Mol. Sci. 2023, 24, 7456. [Google Scholar] [CrossRef]
- Kumar, N.R.; Khamar, P.; Shetty, R.; Sharma, A.; Shetty, N.; Pahuja, N.; Abilash, V.G.; Jhanji, V.; Ghosh, A.; Mohan, R.R.; et al. Identification of novel predictive factors for post surgical corneal haze. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seth, G.; Singh, S.; Sharma, G.; Suvedi, D.; Kumar, D.; Nagraik, R.; Sharma, A. Harnessing the power of stem cell-derived exosomes: A rejuvenating therapeutic for skin and regenerative medicine. 3 Biotech 2025, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Falcon-Pérez, J.M.; López-Sarrio, S.; González, E.; Alkhraisat, M.H. Advances in Platelet Rich Plasma-Derived Extracellular Vesicles for Regenerative Medicine: A Systematic-Narrative Review. Int. J. Mol. Sci. 2023, 24, 13043. [Google Scholar] [CrossRef] [PubMed]
- Terriaca, S.; Fiorelli, E.; Scioli, M.G.; Fabbri, G.; Storti, G.; Cervelli, V.; Orlandi, A. Endothelial Progenitor Cell-Derived Extracellular Vesicles: Potential Therapeutic Application in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 6375. [Google Scholar] [CrossRef]
- Zhao, K.; Kong, C.; Shi, N.; Jiang, J.; Li, P. Potential angiogenic, immunomodulatory, and antifibrotic effects of mesenchymal stem cell-derived extracellular vesicles in systemic sclerosis. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, S.; Xia, F.; Liu, Y.; Zhang, D.; Wang, F.; Wang, Y.; Li, Q.; Niu, J.; Cao, C.; et al. ExoSD chips for high-purity immunomagnetic separation and high-sensitivity detection of gastric cancer cell-derived exosomes. Biosens. Bioelectron. 2021, 194, 113594. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Duong, P.; Chung, A.; Bouchareychas, L.; Raffai, R.L. Cushioned-Density Gradient Ultracentrifugation (C-DGUC) improves the isolation efficiency of extracellular vesicles. PLoS ONE 2019, 14, e0215324. [Google Scholar] [CrossRef]
- Wang, J.-M.; Li, Y.-J.; Wu, J.-Y.; Cai, J.-X.; Wen, J.; Xiang, D.-X.; Hu, X.-B.; Li, W.-Q. Comparative evaluation of methods for isolating small extracellular vesicles derived from pancreatic cancer cells. Cell Biosci. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-speed centrifugation induces aggregation of extracellular vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Lötvall, J.; Lässer, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23111. [Google Scholar] [CrossRef] [PubMed]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ramos, I.; Bancu, I.; Oliveira-Tercero, A.; Armengol, M.P.; Menezes-Neto, A.; Del Portillo, H.A.; Lauzurica-Valdemoros, R.; Borràs, F.E. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J. Extracell. Vesicles 2015, 4, 27369. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Sitar, S.; Kejžar, A.; Pahovnik, D.; Kogej, K.; Tušek-Žnidarič, M.; Lenassi, M.; Žagar, E. Size Characterization and Quantification of Exosomes by Asymmetrical-Flow Field-Flow Fractionation. Anal. Chem. 2015, 87, 9225–9233. [Google Scholar] [CrossRef]
- Akagi, T.; Kato, K.; Hanamura, N.; Kobayashi, M.; Ichiki, T. Evaluation of desialylation effect on zeta potential of extracellular vesicles secreted from human prostate cancer cells by on-chip microcapillary electrophoresis. Jpn. J. Appl. Phys. 2014, 53, 06JL01. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Z.; Tian, K.; Li, X. Magnetic bead-based adsorption strategy for exosome isolation. Front. Bioeng. Biotechnol. 2022, 10, 942077. [Google Scholar] [CrossRef]
- Kim, H.; Shin, S. ExoCAS-2: Rapid and Pure Isolation of Exosomes by Anionic Exchange Using Magnetic Beads. Biomedicines 2021, 9, 28. [Google Scholar] [CrossRef]
- Kozhevnikova, D.; Chernyshev, V.; Yashchenok, A. Progress in Isolation and Molecular Profiling of Small Extracellular Vesicles via Bead-Assisted Platforms. Biosensors 2023, 13, 688. [Google Scholar] [CrossRef]
- Ma, C.; Xu, Z.; Hao, K.; Fan, L.; Du, W.; Gao, Z.; Wang, C.; Zhang, Z.; Li, N.; Li, Q.; et al. Rapid isolation method for extracellular vesicles based on Fe3O4@ZrO2. Front. Bioeng. Biotechnol. 2024, 12, 1399689. [Google Scholar] [CrossRef]
- Ghosh, A.; Davey, M.; Chute, I.C.; Griffiths, S.G.; Lewis, S.; Chacko, S.; Barnett, D.; Crapoulet, N.; Fournier, S.; Joy, A.; et al. Rapid Isolation of Extracellular Vesicles from Cell Culture and Biological Fluids Using a Synthetic Peptide with Specific Affinity for Heat Shock Proteins. PLoS ONE 2014, 9, e110443. [Google Scholar] [CrossRef]
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 2016, 6, srep33935. [Google Scholar] [CrossRef]
- Fernandes, R.P.; Ruiz, A.B.; Bezemer, S.; Detmers, F.; Hermans, P.; Peixoto, C. Targeted isolation of extracellular vesicles from cell culture supernatant using immuno-affinity chromatography. Sep. Purif. Technol. 2024, 358, 130312. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Draz, M.S.; Zarghooni, M.; Sanati-Nezhad, A.; Ghavami, S.; Shafiee, H.; Akbari, M. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosens. Bioelectron. 2017, 91, 588–605. [Google Scholar] [CrossRef]
- Guo, S.-C.; Tao, S.-C.; Dawn, H. Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1508271. [Google Scholar] [CrossRef]
- Bandu, R.; Oh, J.W.; Kim, K.P. Extracellular vesicle proteins as breast cancer biomarkers: Mass spectrometry-based analysis. Proteomics 2024, 24, e2300062. [Google Scholar] [CrossRef]
- Young, J.W.; Wason, I.S.; Zhao, Z.; Rattray, D.G.; Foster, L.J.; Van Hoa, F.D. His-Tagged Peptidiscs Enable Affinity Purification of the Membrane Proteome for Downstream Mass Spectrometry Analysis. J. Proteome Res. 2020, 19, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.J.; Hisada, Y.; Bae-Jump, V.L.; Mackman, N. Evaluation of a new bead-based assay to measure levels of human tissue factor antigen in extracellular vesicles in plasma. Res. Pr. Thromb. Haemost. 2022, 6, e12677. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tian, Y.; Xue, C.; Niu, Q.; Chen, C.; Yan, X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J. Extracell. Vesicles 2022, 11, e12206. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Hsu, W.; Wang, M.; Daiguji, H. Electroosmotic flow: From microfluidics to nanofluidics. Electrophoresis 2020, 42, 834–868. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Y.; Cheng, S.; Zeng, Y. Advances in Analytical Technologies for Extracellular Vesicles. Anal. Chem. 2021, 93, 4739–4774. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, F.; Qian, H.; Xu, W.; Jiang, J. Preconditioning and Engineering Strategies for Improving the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cell-Free Therapy. Stem Cells Int. 2022, 2022, 1779346. [Google Scholar] [CrossRef]
- Wu, K.Y.; Ahmad, H.; Lin, G.; Carbonneau, M.; Tran, S.D. Mesenchymal Stem Cell-Derived Exosomes in Ophthalmology: A Comprehensive Review. Pharmaceutics 2023, 15, 1167. [Google Scholar] [CrossRef]
- Liu, S.; Liu, F.; Zhou, Y.; Jin, B.; Sun, Q.; Guo, S. Immunosuppressive Property of MSCs Mediated by Cell Surface Receptors. Front. Immunol. 2020, 11, 1076. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Current Strategies and Therapeutic Applications of Mesenchymal Stem Cell-Based Drug Delivery. Pharmaceuticals 2024, 17, 707. [Google Scholar] [CrossRef]
- Liang, Y.C.; Wu, Y.P.; Li, X.D.; Chen, S.H.; Ye, X.J.; Xue, X.Y.; Xu, N. TNF-α-induced exosomal miR-146a mediates mesenchymal stem cell-dependent suppression of urethral stricture. J. Cell. Physiol. 2019, 234, 23243–23255. [Google Scholar] [CrossRef]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Furman, N.E.T.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S., Jr.; Olson, S.D. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells 2018, 36, 79–90. [Google Scholar] [CrossRef]
- Klinker, M.W.; Marklein, R.A.; Surdo, J.L.L.; Wei, C.-H.; Bauer, S.R. Morphological features of IFN-γ–stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E2598–E2607. [Google Scholar] [CrossRef]
- Polchert, D.; Sobinsky, J.; Douglas, G.; Kidd, M.; Moadsiri, A.; Reina, E.; Genrich, K.; Mehrotra, S.; Setty, S.; Smith, B.; et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008, 38, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, P.; Khan, U.Z.; Zhou, Z.; Sui, X.; Li, C.; Dong, K.; Liu, Y.; Qing, L.; Tang, J. Exosomes derived from LPS-preconditioned bone marrow-derived MSC modulate macrophage plasticity to promote allograft survival via the NF-κB/NLRP3 signaling pathway. J. Nanobiotechnol. 2023, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lohan, P.; Murphy, N.; Treacy, O.; Lynch, K.; Morcos, M.; Chen, B.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Third-Party Allogeneic Mesenchymal Stromal Cells Prevent Rejection in a Pre-sensitized High-Risk Model of Corneal Transplantation. Front. Immunol. 2018, 9, 2666. [Google Scholar] [CrossRef] [PubMed]
- Potapova, I.A.; Brink, P.R.; Cohen, I.S.; Doronin, S.V. Culturing of Human Mesenchymal Stem Cells as Three-dimensional Aggregates Induces Functional Expression of CXCR4 That Regulates Adhesion to Endothelial Cells. J. Biol. Chem. 2008, 283, 13100–13107. [Google Scholar] [CrossRef]
- Potapova, I.A.; Gaudette, G.R.; Brink, P.R.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; Doronin, S.V. Mesenchymal Stem Cells Support Migration, Extracellular Matrix Invasion, Proliferation, and Survival of Endothelial Cells In Vitro. STEM CELLS 2007, 25, 1761–1768. [Google Scholar] [CrossRef]
- Carter, K.; Lee, H.J.; Na, K.-S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef]
- Giannasi, C.; Niada, S.; Della Morte, E.; Casati, S.R.; De Palma, C.; Brini, A.T. Serum starvation affects mitochondrial metabolism of adipose-derived stem/stromal cells. Cytotherapy 2023, 25, 704–711. [Google Scholar] [CrossRef]
- Nie, W.; Huang, X.; Zhao, L.; Wang, T.; Zhang, D.; Xu, T.; Du, L.; Li, Y.; Zhang, W.; Xiao, F.; et al. Exosomal miR-17–92 derived from human mesenchymal stem cells promotes wound healing by enhancing angiogenesis and inhibiting endothelial cell ferroptosis. Tissue Cell 2023, 83, 102124. [Google Scholar] [CrossRef]
- Xu, C.M.; Karbasiafshar, C.; Teixeira, R.B.; Ahsan, N.; Corssac, G.B.; Sellke, F.W.; Abid, M.R. Proteomic Assessment of Hypoxia-Pre-Conditioned Human Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Demonstrates Promise in the Treatment of Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1674. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom.–Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Merimi, M.; Buyl, K.; Daassi, D.; Rodrigues, R.M.; Melki, R.; Lewalle, P.; Vanhaecke, T.; Fahmi, H.; Rogiers, V.; Lagneaux, L.; et al. Transcriptional Profile of Cytokines, Regulatory Mediators and TLR in Mesenchymal Stromal Cells after Inflammatory Signaling and Cell-Passaging. Int. J. Mol. Sci. 2021, 22, 7309. [Google Scholar] [CrossRef] [PubMed]
- Elahi, K.C.; Klein, G.; Avci-Adali, M.; Sievert, K.D.; MacNeil, S.; Aicher, W.K. Human Mesenchymal Stromal Cells from Different Sources Diverge in Their Expression of Cell Surface Proteins and Display Distinct Differentiation Patterns. Stem Cells Int. 2015, 2016, 5646384. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, A.; Thomé, C.H.; Ferreira, G.A.; Lanfredi, G.P.; Covas, D.T.; Pitteri, S.J.; Swiech, K.; Faça, V.M. Proteomic Identification and Time-Course Monitoring of Secreted Proteins During Expansion of Human Mesenchymal Stem/Stromal in Stirred-Tank Bioreactor. Front. Bioeng. Biotechnol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Skalnikova, H.K.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- McBride, J.D.; Rodriguez-Menocal, L.; Guzman, W.; Khan, A.; Myer, C.; Liu, X.; Bhattacharya, S.K.; Badiavas, E.V. Proteomic analysis of bone marrow-derived mesenchymal stem cell extracellular vesicles from healthy donors: Implications for proliferation, angiogenesis, Wnt signaling, and the basement membrane. Stem Cell Res. Ther. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, Y.; Zhang, Y.; Zheng, D.; Yan, L.; Guo, M.; Mao, Y.; Yang, L. Exosomes from bone marrow-derived mesenchymal stem cells facilitate corneal wound healing via regulating the p44/42 MAPK pathway. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 261, 723–734. [Google Scholar] [CrossRef]
- Han, Y.; Ren, J.; Bai, Y.; Pei, X.; Han, Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019, 109, 59–68. [Google Scholar] [CrossRef]
- Ma, S.; Yin, J.; Hao, L.; Liu, X.; Shi, Q.; Diao, Y.; Yu, G.; Liu, L.; Chen, J.; Zhong, J. Exosomes From Human Umbilical Cord Mesenchymal Stem Cells Treat Corneal Injury via Autophagy Activation. Front. Bioeng. Biotechnol. 2022, 10, 879192. [Google Scholar] [CrossRef]
- Chu, W.; Zhang, F.; Zeng, X.; He, F.; Shang, G.; Guo, T.; Wang, Q.; Wu, J.; Li, T.; Zhong, Z.Z.; et al. A GMP-compliant manufacturing method for Wharton’s jelly-derived mesenchymal stromal cells. Stem Cell Res. Ther. 2024, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef] [PubMed]
- Galindo, S.; Herreras, J.M.; López-Paniagua, M.; Rey, E.; de la Mata, A.; Plata-Cordero, M.; Calonge, M.; Nieto-Miguel, T. Therapeutic Effect of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Experimental Corneal Failure Due to Limbal Stem Cell Niche Damage. STEM CELLS 2017, 35, 2160–2174. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Masoumi, A.; Momenaei, B.; Cheraqpour, K.; Koganti, R.; Chang, A.Y.; Ghassemi, M.; Djalilian, A.R. Applications of mesenchymal stem cells in ocular surface diseases: Sources and routes of delivery. Expert Opin. Biol. Ther. 2023, 23, 509–525. [Google Scholar] [CrossRef]
- Amable, P.R.; Teixeira, M.V.T.; Carias, R.B.V.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef]
- Shibata, S.; Hayashi, R.; Okubo, T.; Kudo, Y.; Baba, K.; Honma, Y.; Nishida, K. The secretome of adipose-derived mesenchymal stem cells attenuates epithelial–mesenchymal transition in human corneal epithelium. Regen. Ther. 2019, 11, 114–122. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.; Kwon, Y.; Park, K.-S.; Jeong, J.-H.; Choi, S.-J.; Bang, S.I.; Chang, J.W.; Lee, C. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 845. [Google Scholar] [CrossRef]
- Eslani, M.; Putra, I.; Shen, X.; Hamouie, J.; Tadepalli, A.; Anwar, K.N.; Kink, J.A.; Ghassemi, S.; Agnihotri, G.; Reshetylo, S.; et al. Cornea-Derived Mesenchymal Stromal Cells Therapeutically Modulate Macrophage Immunophenotype and Angiogenic Function. STEM CELLS 2018, 36, 775–784. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef]
- Eslani, M.; Putra, I.; Shen, X.; Hamouie, J.; Afsharkhamseh, N.; Besharat, S.; Rosenblatt, M.I.; Dana, R.; Hematti, P.; Djalilian, A.R. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investig. Opthalmology Vis. Sci. 2017, 58, 5507–5517. [Google Scholar] [CrossRef]
- Zoehler, B.; Fracaro, L.; Boldrini-Leite, L.M.; da Silva, J.S.; Travers, P.J.; Brofman, P.R.S.; Bicalho, M.d.G.; Senegaglia, A.C. HLA-G and CD152 Expression Levels Encourage the Use of Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells as an Alternative for Immunosuppressive Therapy. Cells 2022, 11, 1339. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Liu, C.-Y.; Wang, I.-J.; Sieber, M.; Chang, J.; Jester, J.V.; Kao, W.W.Y. Cell Therapy of Congenital Corneal Diseases with Umbilical Mesenchymal Stem Cells: Lumican Null Mice. PLoS ONE 2010, 5, e10707. [Google Scholar] [CrossRef]
- Kacham, S.; Bhure, T.S.; Eswaramoorthy, S.D.; Naik, G.; Rath, S.N.; Parcha, S.R.; Basu, S.; Sangwan, V.S.; Shukla, S. Human Umbilical Cord-Derived Mesenchymal Stem Cells Promote Corneal Epithelial Repair In Vitro. Cells 2021, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.-C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. npj Regen. Med. 2019, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Yu, L.; Wang, Q.; Jin, L.; Yin, X.; Cao, X.; Gao, H. Patterned collagen films loaded with miR-133b@MBG-NH2 for potential applications in corneal stromal injury repair. Biomed. Mater. 2024, 19, 035009. [Google Scholar] [CrossRef] [PubMed]
- Poe, A.J.; Shah, R.; Khare, D.; Kulkarni, M.; Phan, H.; Ghiam, S.; Punj, V.; Ljubimov, A.V.; Saghizadeh, M. Regulatory role of miR-146a in corneal epithelial wound healing via its inflammatory targets in human diabetic cornea. Ocul. Surf. 2022, 25, 92–100. [Google Scholar] [CrossRef]
- Li, D.; Ji, J.; Li, X.; Xie, Y.; Huang, Y.; Qin, J.; Ding, X.; Wang, L.; Fan, Y. LNP-encapsulated miRNA29b for corneal repair: A novel approach to combat fibrosis. Mater. Today Bio 2025, 32, 101695. [Google Scholar] [CrossRef]
- Pedersen, C.; Chen, V.T.; Herbst, P.; Zhang, R.; Elfert, A.; Krishan, A.; Azar, D.T.; Chang, J.-H.; Hu, W.-Y.; Kremsmayer, T.P.; et al. Target specification and therapeutic potential of extracellular vesicles for regulating corneal angiogenesis, lymphangiogenesis, and nerve repair. Ocul. Surf. 2024, 34, 459–476. [Google Scholar] [CrossRef]
- Merino-González, C.; Zuñiga, F.A.; Escudero, C.; Ormazabal, V.; Reyes, C.; Nova-Lamperti, E.; Salomón, C.; Aguayo, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front. Physiol. 2016, 7, 24. [Google Scholar] [CrossRef]
- Ye, Z.; Yu, Z.; Chen, G.; Jia, J. Extracellular vesicles in tumor angiogenesis and resistance to anti-angiogenic therapy. Cancer Sci. 2023, 114, 2739–2749. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Liang, W.; Lu, X.; Piri, N.; Wang, W.; Kaplan, H.J.; Dean, D.C.; Zhang, L.; Liu, Y. Zeb1 promotes corneal neovascularization by regulation of vascular endothelial cell proliferation. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Liu, G.-S.; Chen, H.-A.; Chang, C.-Y.; Chen, Y.-J.; Wu, Y.-Y.; Widhibrata, A.; Yang, Y.-H.; Hsieh, E.-H.; Delila, L.; Lin, I.-C.; et al. Platelet-derived extracellular vesicle drug delivery system loaded with kaempferol for treating corneal neovascularization. Biomaterials 2025, 319, 123205. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Chen, X.; Li, J.; Li, L.; Bian, Z.; Sun, F.; Lu, J.; Yin, Y.; Cai, X.; et al. Secreted Monocytic miR-150 Enhances Targeted Endothelial Cell Migration. Mol. Cell 2010, 39, 133–144. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Lambiase, A.; Aloe, L.; Centofanti, M.; Parisi, V.; Bao, S.N.; Mantelli, F.; Colafrancesco, V.; Manni, G.L.; Bucci, M.G.; Bonini, S.; et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. USA 2009, 106, 13469–13474. [Google Scholar] [CrossRef]
- Rao, J.; Xie, H.; Liang, Z.; Yang, Z.; Chen, P.; Zhou, M.; Xu, X.; Lin, Y.; Lin, F.; Wang, R.; et al. Hypoxic-preconditioned mesenchymal stem cell-derived small extracellular vesicles inhibit neuronal death after spinal cord injury by regulating the SIRT1/Nrf2/HO-1 pathway. Front. Pharmacol. 2024, 15, 1419390. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Bergink, S. Heat shock proteins as potential targets for protective strategies in neurodegeneration. Lancet Neurol. 2016, 15, 748–759. [Google Scholar] [CrossRef]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.-K.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017, 8, 1214–1225. [Google Scholar] [CrossRef]
- Chan, S.-M.; Tsai, C.; Lee, T.-P.; Huang, Z.-R.; Huang, W.-H.; Lin, C.-T. Therapeutic Potential of Umbilical Cord MSC-Derived Exosomes in a Severe Dry Eye Rat Model: Enhancing Corneal Protection and Modulating Inflammation. Biomedicines 2025, 13, 1174. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Antonijevic, A.; Volarevic, V. NLRP3 Inflammasome as a Potentially New Therapeutic Target of Mesenchymal Stem Cells and Their Exosomes in the Treatment of Inflammatory Eye Diseases. Cells 2023, 12, 2327. [Google Scholar] [CrossRef]
- Desjardins, P.; Berthiaume, R.; Couture, C.; Le-Bel, G.; Roy, V.; Gros-Louis, F.; Moulin, V.J.; Proulx, S.; Chemtob, S.; Germain, L.; et al. Impact of Exosomes Released by Different Corneal Cell Types on the Wound Healing Properties of Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 12201. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Nieuwland, R.; Siljander, P.R.-M.; Falcón-Pérez, J.M.; Witwer, K.W. Reproducibility of extracellular vesicle research. Eur. J. Cell Biol. 2022, 101, 151226. [Google Scholar] [CrossRef]
- Bejandi, Z.B.; An, S.; Arabpour, Z.; Araujo, I.; Khandaker, A.N.; Moghtader, A.; Abedi, F.; Mahmud, N.; Ghassemi, M.; Joslin, C.E. Production of GMP-Grade Bone Marrow-Derived Mesenchymal Stromal Cell (BM-MSC) Secretome for Ocular Diseases. Investig. Ophthalmol. Vis. Sci. 2025, 66, 2309. [Google Scholar]
- Verma, N.; Arora, S.; Singh, A.K.; Ahmed, J. Unlocking the potential of exosomes ‘extracellular vesicles’: Drug delivery advancements and therapeutics in ocular diseases. RSC Pharm. 2025. [Google Scholar] [CrossRef]
- Salmond, N.; Williams, K.C. Isolation and characterization of extracellular vesicles for clinical applications in cancer–time for standardization? Nanoscale Adv. 2021, 3, 1830–1852. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Ma, Y.; Hu, Y.; Li, Z. Current Technology for Production, Isolation, and Quality Control of Extracellular Vesicles. In Biomedical Applications of Extracellular Vesicles; Li, Z., Liang, X.J., Cheng, K., Eds.; Wiley: Hoboken, NJ, USA, 2025; pp. 117–146. [Google Scholar]
- Verma, N.; Arora, S. Navigating the Global Regulatory Landscape for Exosome-Based Therapeutics: Challenges, Strategies, and Future Directions. Pharmaceutics 2025, 17, 990. [Google Scholar] [CrossRef]
- Takakura, Y.; Hanayama, R.; Akiyoshi, K.; Futaki, S.; Hida, K.; Ichiki, T.; Ishii-Watabe, A.; Kuroda, M.; Maki, K.; Miura, Y.; et al. Quality and Safety Considerations for Therapeutic Products Based on Extracellular Vesicles. Pharm. Res. 2024, 41, 1573–1594. [Google Scholar] [CrossRef]
| MSC Source | Key Proteomic Features of EVs | Functional Implications | Ref. |
|---|---|---|---|
| Corneal Stromal MSCs (cMSCs) |
|
| [73] |
| Bone Marrow (BM-MSCs) | Proteins involved in apoptosis modulation (BAX, BCL2)
|
| [98,99] |
| Adipose Tissue (AD-MSCs) |
|
| [100] |
| Umbilical Cord (UC-MSCs) | Enriched in growth factors (VEGF, HGF, IGF)
|
| [101] |
| Wharton’s Jelly (WJ-MSCs) |
|
| [102] |
| MSC Source | Key EV Proteins/miRNAs | Functional Role | Demonstrated Effect on Corneal Models | Reference |
|---|---|---|---|---|
| Umbilical Cord MSC (UC-MSC) | TSG-6, PD-L1, IL-10, miR-126 | Anti-inflammatory, immunomodulatory, angiogenesis promotion | Reduced corneal inflammation, promoted endothelial proliferation, enhanced epithelial migration | [77] |
| Bone Marrow MSC (BM-MSC) | MMPs, TIMPs, FN1, IL-13, IL-1RL2 | Anti-fibrotic, ECM remodeling, immunomodulatory | Reduced α-SMA expression, decreased neovascularization, enhanced epithelial proliferation | [16,88,103] |
| Adipose-derived MSC (AD-MSC) | VEGF, miR-146a, MMP-2, ECM proteins | Pro-angiogenic, anti-fibrotic, immunomodulatory | Inhibited epithelial–mesenchymal transition, restored limbal/corneal phenotypes, reduced ocular surface inflammation | [104,105,106,107,108] |
| Corneal Stromal MSC (Cornea-MSC) | sFLT-1, PEDF, bFGF, TGFβ3 | Anti-angiogenic, stromal regeneration | Prevented corneal neovascularization, promoted stromal matrix deposition, keratocyte differentiation | [109,110,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabpour, Z.; Niktinat, H.; Hatami, F.; Yaghmour, A.; Yucel, Z.J.; Ghalibafan, S.; Massoumi, H.; Bibak Bejandi, Z.; Salehi, M.; Jalilian, E.; et al. Extracellular Vesicle (EV) Proteomics in Corneal Regenerative Medicine. Proteomes 2025, 13, 49. https://doi.org/10.3390/proteomes13040049
Arabpour Z, Niktinat H, Hatami F, Yaghmour A, Yucel ZJ, Ghalibafan S, Massoumi H, Bibak Bejandi Z, Salehi M, Jalilian E, et al. Extracellular Vesicle (EV) Proteomics in Corneal Regenerative Medicine. Proteomes. 2025; 13(4):49. https://doi.org/10.3390/proteomes13040049
Chicago/Turabian StyleArabpour, Zohreh, Hanieh Niktinat, Firouze Hatami, Amal Yaghmour, Zarife Jale Yucel, Seyyedehfatemeh Ghalibafan, Hamed Massoumi, Zahra Bibak Bejandi, Majid Salehi, Elmira Jalilian, and et al. 2025. "Extracellular Vesicle (EV) Proteomics in Corneal Regenerative Medicine" Proteomes 13, no. 4: 49. https://doi.org/10.3390/proteomes13040049
APA StyleArabpour, Z., Niktinat, H., Hatami, F., Yaghmour, A., Yucel, Z. J., Ghalibafan, S., Massoumi, H., Bibak Bejandi, Z., Salehi, M., Jalilian, E., Ghassemi, M., Guaiquil, V. H., Rosenblatt, M., & Djalilian, A. R. (2025). Extracellular Vesicle (EV) Proteomics in Corneal Regenerative Medicine. Proteomes, 13(4), 49. https://doi.org/10.3390/proteomes13040049






