In Search of Ideal Solutions for Cancer Diagnosis: From Conventional Methods to Protein Biomarkers in Liquid Biopsy
Abstract
1. Introduction
1.1. Challenges of Conventional Cancer Diagnostic
1.2. Evolution of Cancer Diagnostic Technologies
1.3. The Promise of Liquid Biopsy and Future Directions
2. Limitations of Conventional Diagnostic Paradigms
2.1. Serum Biomarkers Limitations
2.2. Histopathology Limitations
2.3. Imaging Methods Limitations
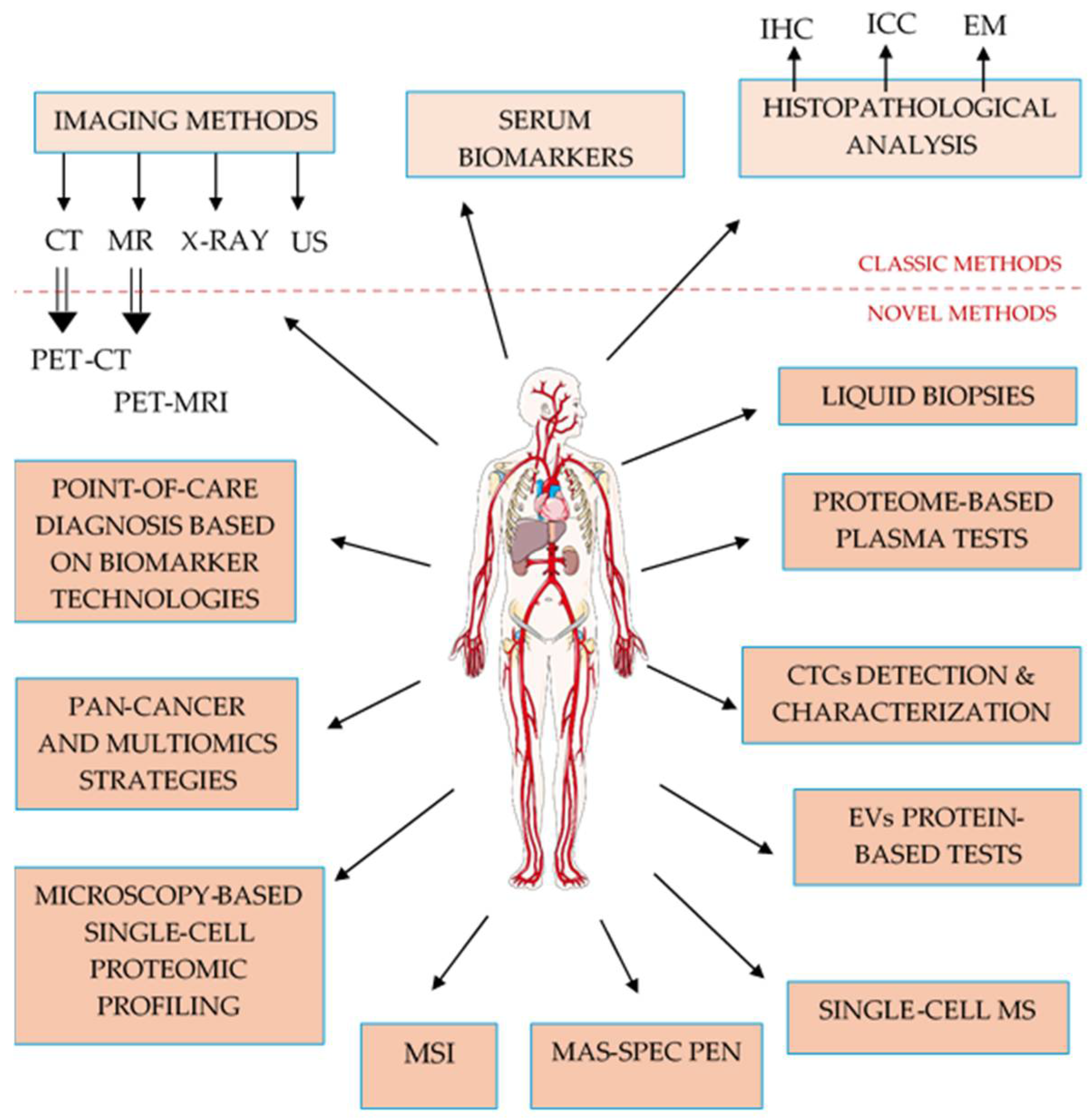
3. What’s Innovative in Cancer Detection and Management?
3.1. Established Clinical Standards as a Foundation for Innovation
- Multimodal imaging
- Genetic and genomic testing
- Liquid biopsy sources for detection of protein biomarkers
- Blood/Serum/Plasma
- Urine
- Saliva or oral fluid
- Human breast milk
- Nipple aspirate fluid (NAF)
- Pleural fluid (PF)
- Tear fluid
- Aqueous humor (AH)
3.2. Near-Clinical or Translational Tools
- Pan-cancer analysis and multi-omics approaches
- Artificial intelligence (AI) and machine learning (ML)
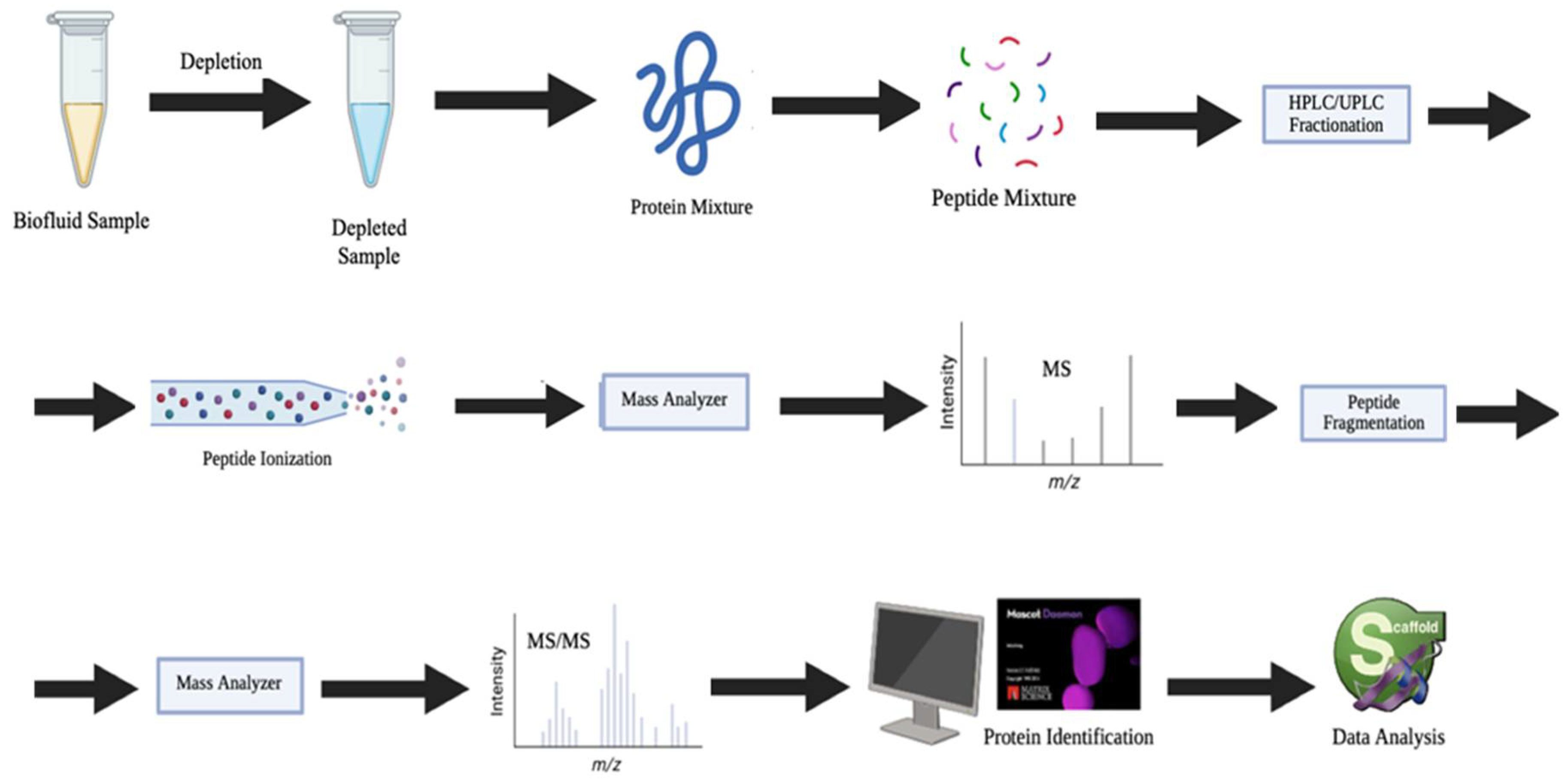
3.3. Advanced Proteomic Methods with Growing Translational Promise
- Protein isoforms as precision biomarkers
- Mass spectrometry imaging (MSI)
- Single-cell proteomic analysis
3.4. Innovative, Emerging Clinical Tools
- MasSpec Pen technology
- Nanotechnology in cancer molecular diagnosis
3.5. Future Point-of-Care (PoC) and Multiplexing Tools
4. Biomarkers: Challenges and Limits
5. Evaluation of Diagnostic Performance in Cancer Detection for Clinical Applications
| Detection Methods | Sensitivity | Specificity | Clinical Applications |
|---|---|---|---|
| BSE | 20% to 30%; 58,3% | 87.4% | BC detection [83,260] |
| iBE | 34.3% to 86%, | 59% to 94% | BC detection [85] |
| CBE | 40% to 69% | - | BC detection [263] |
| CBE (average-risk women) | - | 99.4% (screening); 68.7% (diagnostic) | [264] |
| CBE (increased-risk women) | - | 97.1% (screening) 57.1% (doagnostic) | |
| DRE | 51% | 59% | PCa detection [141] |
| serum PSA | 32% (3.1 ng/mL); 21% (4 ng/mL) | - | PCa detection [142] |
| US | 80.1% (overall pooled sensitivity) | 88.4% | BC diagnosis [87] |
| US | 89.2% (in low and middle-income country) | 99.1% | |
| mammography | 77–95% | - | BC detection [263] |
| modern film mammography alone, without CBE, among asymptomatic Japanese women (40-49 years) | 47.4% | - | BC screening [90] |
| biennial modern film mammography, without CBE, among asymptomatic Japanese women (40-49 years) | 71.7% | 92.6% | |
| digital mammography | 97% | 64.5%, (89.3% accuracy) | BC detection [87,88] |
| FNAB/FNAC | 74%/97% | 96% (95% accuracy) | BC detection [67,68,69] |
| CNB | 95%/97% | 98% (96% accuracy) | BC detection [67,68,69] |
| conventional imaging (CT, US, radiography, skeletal scintigraphy) | 85.9% | 67.3% | detection of BC distant metastasis [99] |
| salivary tests | 71.7% | 72.7% | BC detection [168] |
| salivary chemerin and MMP-9, and serum chemerin | 100% | 100% | differentiating OSCC from OPMLs [176] |
| CEUS (polled sensitivity) | 70% | 74% | PCa detection [265] |
| grey-scale TRUS | 40% to 50% | 40% to 50% | |
| TRUS-guided biopsy | 48% | 96% | PCa detection [142] |
| mpMRI | 93% | 48% | |
| mpMRI and PI-RADS | 89% | 73% | PCa detection [142,266] |
| MRI | 72% | 96% | PCa staging [142] |
| FDG-PET/CT | 97.4% | 91.2% | detection of BC distant metastases [99] |
| latest-generation digital PET/CT | 96.4% | 86.4% | detection of cervical LN metastases in HNSCC [100] |
| HE4 | 76% to 88% (depending on disease stage) | 97.87% | OC detection [43] |
| 4MP (pro-SFPB, CA125, CYFRA21-1, CEA) | 63% | 83% | distinguishing benign from malign indeterminate pulmonary nodules [258] |
| serum CEA | 86.25% | 70% | NSCLC diagnosis [256] |
| serum OPN | 71.61% | 91.25% | |
| serum DKK1 | 92.5% | 65% | |
| serum CEA and OPN | 87.5% | 86.67% | |
| serum CEA and DKK1 | 92.5% | 76.67% | |
| MMP9 and KPYM (uterine aspirate) | 94% | 87% | EC detection [262] |
| CTNB1, XPO2, and CAPG (uterine aspirate) | 95% | 96% | discrimination of endometrioid EC and serous EC [262] |
| blood test based on EVs | 71.2% | 99.5% | detection of OC, PC, bllader cancer (early-stage) [16] |
| calprotectin (CP) (≤6233.2 ng/mL cutoff) | 96% | 60% | benign and malignant pleural effusion differentiation [195] |
| fecal CP (50 µg/g cutoff) | 83% | 61% | CRC detection [254] |
| 76.9% | 88% | esofago-gastric cancer detection [261] | |
| MasSpec Pen technology | 95.5% | 89.7% | differentiation between normal pancreas and PC [226] |
| 92% | 96.2% | differentiation between bile duct and pancreatic cancer [226] | |
| 96.4% | 96.2% | prediction of cancer subtype of lung and thyroid cancer [63] |
6. Future Perspectives
- Ultra-early cancer detection, on even cellular or molecular level, identifying malignant transformation of cells and signaling pathways before they become anatomically detectable;
- Wearable or implantable biosensors continuously sampling and analyzing blood or interstitial fluids in real-time, alerting both patient and physician to molecular changes before symptoms appear;
- Fully personalized protein biomarker panels generated through ML and AI, dynamically adapting to individual genomic and proteomic profiles, lifestyle factors, and environmental exposures;
- PoC diagnostics in everyday life, including smartwatches or home health stations, enabling regular health checks without needing clinical visits;
- Integration with preventive medicine, where LB not only detects cancer but predicts risk, monitors predisposition, and guides proactive interventions years before clinical cancer manifests.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siffels, L.E.; Sharon, T.; Hoffman, A.S. The participatory turn in health and medicine: The rise of the civic and the need to ‘give back’ in data-intensive medical research. Humanit. Soc. Sci. Commun. 2021, 8, 306. [Google Scholar] [CrossRef]
- Loud, J.T.; Murphy, J. Cancer Screening and Early Detection in the 21st Century. Semin. Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef]
- Ginghina, O.; Hudita, A.; Zamfir, M.; Spanu, A.; Mardare, M.; Bondoc, I.; Buburuzan, L.; Georgescu, S.E.; Costache, M.; Negrei, C.; et al. Liquid Biopsy and Artificial Intelligence as Tools to Detect Signatures of Colorectal Malignancies: A Modern Approach in Patient’s Stratification. Front. Oncol. 2022, 12, 856575. [Google Scholar] [CrossRef]
- Mukherjee, N.; Chatterjee, N.; Manna, K.; Das Saha, K. Chapter 2-Types of cancer diagnostics, the current achievements, and challenges. In Biosensor Based Advanced Cancer Diagnostics; Khan, R., Parihar, A., Sanghi, S.K., Eds.; Academic Press: Cambridge, UK, 2022; pp. 27–45. [Google Scholar] [CrossRef]
- Chubak, J.; Burnett-Hartman, A.N.; Barlow, W.E.; Corley, D.A.; Croswell, J.M.; Neslund-Dudas, C.; Vachani, A.; Silver, M.I.; Tiro, J.A.; Kamineni, A. Estimating Cancer Screening Sensitivity and Specificity Using Healthcare Utilization Data: Defining the Accuracy Assessment Interval. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Santini, A.; Man, A.; Voidăzan, S. Accuracy of Diagnostic Tests. J. Crit. Care Med. 2021, 7, 241–248. [Google Scholar] [CrossRef] [PubMed]
- McKay, K.M.; Lim, L.L.; Van Gelder, R.N. Rational laboratory testing in uveitis: A Bayesian analysis. Surv. Ophthalmol. 2021, 66, 802–825. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, J.; Horton, S.; Kuo, A.; Tomasetti, C. Evaluating the impact of multicancer early detection testing on health and economic outcomes: Toward a decision modeling strategy. Cancer 2022, 128, 892–908. [Google Scholar] [CrossRef]
- Englisz, A.; Smycz-Kubańska, M.; Mielczarek-Palacz, A. Sensitivity and Specificity of Selected Biomarkers and Their Combinations in the Diagnosis of Ovarian Cancer. Diagnostics 2024, 14, 949. [Google Scholar] [CrossRef]
- Combes, G.F.; Vučković, A.-M.; Bakulić, M.P.; Antoine, R.; Bonačić-Koutecky, V.; Trajković, K. Nanotechnology in Tumor Biomarker Detection: The Potential of Liganded Nanoclusters as Nonlinear Optical Contrast Agents for Molecular Diagnostics of Cancer. Cancers 2021, 13, 4206. [Google Scholar] [CrossRef]
- Forgrave, L.M.; Wang, M.; Yang, D.; DeMarco, M.L. Proteoforms and their expanding role in laboratory medicine. Pract. Lab. Med. 2022, 28, e00260. [Google Scholar] [CrossRef]
- Michela, B. Liquid Biopsy: A Family of Possible Diagnostic Tools. Diagnostics 2021, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer: Current status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBioMedicine 2022, 83, 104237. [Google Scholar] [CrossRef]
- Hinestrosa, J.P.; Kurzrock, R.; Lewis, J.M.; Schork, N.J.; Schroeder, G.; Kamat, A.M.; Lowy, A.M.; Eskander, R.N.; Perrera, O.; Searson, D.; et al. Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun. Med. 2022, 2, 29. [Google Scholar] [CrossRef]
- Giunta, E.F.; Malapelle, U.; Russo, A.; De Giorgi, U. Blood-based liquid biopsy in advanced prostate cancer. Crit. Rev. Oncol. Hematol. 2024, 194, 104241. [Google Scholar] [CrossRef]
- Kong, T.; Qu, Y.; Zhao, T.; Niu, Z.; Lv, X.; Wang, Y.; Ding, Q.; Wei, P.; Fu, J.; Wang, L.; et al. Identification of novel protein biomarkers from the blood and urine for the early diagnosis of bladder cancer via proximity extension analysis. J. Transl. Med. 2024, 22, 314. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calle, A.; Garranzo-Asensio, M.; Torrente-Rodríguez, R.M.; Montiel, V.R.-V.; Poves, C.; Dziaková, J.; Sanz, R.; del Arco, C.D.; Pingarrón, J.M.; Fernández-Aceñero, M.J.; et al. p53 and p63 Proteoforms Derived from Alternative Splicing Possess Differential Seroreactivity in Colorectal Cancer with Distinct Diagnostic Ability from the Canonical Proteins. Cancers 2023, 15, 2102. [Google Scholar] [CrossRef]
- Montero-Calle, A.; Garranzo-Asensio, M.; Moreno-Casbas, M.T.; Campuzano, S.; Barderas, R. Autoantibodies in cancer: A systematic review of their clinical role in the most prevalent cancers. Front. Immunol. 2024, 15, 1455602. [Google Scholar] [CrossRef]
- Preethi, K.; Jeyarajan Selvakumaran, S.C.; Ross, K.; Jayaraman, S.; Deusdedit, T.; Sekar, D. Liquid biopsy: Exosomal microRNAs as novel diagnostic and prognostic biomarkers in cancer. Mol. Cancer 2022, 21, 54. [Google Scholar] [CrossRef]
- van der Poort, E.K.J.; van Ravesteyn, N.T.; van den Broek, J.J.; de Koning, H.J. The Early Detection of Breast Cancer Using Liquid Biopsies: Model Estimates of the Benefits, Harms, and Costs. Cancers 2022, 14, 2951. [Google Scholar] [CrossRef] [PubMed]
- Manea, I.; Iacob, R.; Iacob, S.; Cerban, R.; Dima, S.; Oniscu, G.; Popescu, I.; Gheorghe, L. Liquid biopsy for early detection of hepatocellular carcinoma. Front. Med. 2023, 10, 1218705. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, H.; Chen, N.; Hao, J.; Jin, H.; Ma, X. Diagnostic value of various liquid biopsy methods for pancreatic cancer: A systematic review and meta-analysis. Medicine 2020, 99, e18581. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Kong, S.-N.; Liu, Y.-P.; Yang, Y.; Zhang, H.-M. Can Liquid Biopsy Based on ctDNA/cfDNA Replace Tissue Biopsy for the Precision Treatment of EGFR-Mutated NSCLC? J. Clin. Med. 2023, 12, 1438. [Google Scholar] [CrossRef]
- Bogdan, B.; Hossein, A.; Mohammad, H.F.; Ashkan, A. Novel proteomics-based plasma test for early detection of multiple cancers in the general population. BMJ Oncol. 2024, 3, e000073. [Google Scholar] [CrossRef]
- Tessitore, A.; Gaggiano, A.; Cicciarelli, G.; Verzella, D.; Capece, D.; Fischietti, M.; Zazzeroni, F.; Alesse, E. Serum Biomarkers Identification by Mass Spectrometry in High-Mortality Tumors. Int. J. Proteom. 2013, 2013, 125858. [Google Scholar] [CrossRef]
- Merriel, S.W.D.; Pocock, L.; Gilbert, E.; Creavin, S.; Walter, F.M.; Spencer, A.; Hamilton, W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med. 2022, 20, 54. [Google Scholar] [CrossRef]
- De Souza Dutra, C.; Da Cruz Schafhauser, D.; Hentz, M.; Mayer, N.R.; Pinheiro, R.M.; Baierle, G.; Kist, D.R.; Bullé, D.J.; Donaduzzi, R.C.; Bohmgahren, M.F.; et al. Urinary endogenous peptides as biomarkers for prostate cancer. Oncol. Lett. 2023, 25, 173. [Google Scholar] [CrossRef] [PubMed]
- Shaheed, S.-u.; Tait, C.; Kyriacou, K.; Linforth, R.; Salhab, M.; Sutton, C. Evaluation of nipple aspirate fluid as a diagnostic tool for early detection of breast cancer. Clin. Proteom. 2018, 15, 3. [Google Scholar] [CrossRef]
- Saxby, H.; Mikropoulos, C.; Boussios, S. An Update on the Prognostic and Predictive Serum Biomarkers in Metastatic Prostate Cancer. Diagnostics 2020, 10, 549. [Google Scholar] [CrossRef]
- Nepal, A.; Sharma, P.; Bhattarai, S.; Mahajan, Z.; Sharma, A.; Sapkota, A.; Sharma, A. Extremely Elevated Prostate-Specific Antigen in Acute Prostatitis: A Case Report. Cureus 2023, 15, e43730. [Google Scholar] [CrossRef]
- Khoo, A.; Liu, L.Y.; Nyalwidhe, J.O.; Semmes, O.J.; Vesprini, D.; Downes, M.R.; Boutros, P.C.; Liu, S.K.; Kislinger, T. Proteomic discovery of non-invasive biomarkers of localized prostate cancer using mass spectrometry. Nat. Rev. Urol. 2021, 18, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Catalona, W.J. The Prostate Health Index: A new test for the detection of prostate cancer. Ther. Adv. Urol. 2014, 6, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.K.-F.; Liu, A.Q.; Lau, S.-Y.; Teoh, J.Y.-C.; Ho, C.-C.; Yee, C.-H.; Hou, S.-M.; Chan, C.-K.; Tang, W.-L.; Bangma, C.H.; et al. A 2-year prospective evaluation of the Prostate Health Index in guiding biopsy decisions in a large cohort. BJU Int. 2025, 135, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Punnen, S.; Pavan, N.; Parekh, D. Finding the Wolf in Sheep’s Clothing: The 4Kscore Is a Novel Blood Test That Can Accurately Identify the Risk of Aggressive Prostate Cancer. Rev. Urol. 2015, 17, 3–13. [Google Scholar]
- Cui, Y.; Cao, W.; Li, Q.; Shen, H.; Liu, C.; Deng, J.; Xu, J.; Shao, Q. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 25776. [Google Scholar] [CrossRef]
- He, Y.; Lu, H.; Zhang, L. Chapter Ten-Serum AFP levels in patients suffering from 47 different types of cancers and noncancer diseases. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Academic Press: Cambridge, UK, 2019; Volume 162, pp. 199–212. [Google Scholar]
- Miyoshi, A.; Takashi, M.; Komiya, S.; Mimura, M.; Nagamatsu, M.; Yokoi, T. Highly Elevated Level of Serum CA125 Produced by a Large Uterine Leiomyoma in a 20-Year-Old Woman. J. Clin. Gynecol. Obstet. 2015, 4, 275–278. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Dehshiri-Zadeh, N.; Sekhavat, L.; Nosouhi, F. Correlation of CA-125 serum level and clinico-pathological characteristic of patients with endometriosis. Int. J. Reprod. Biomed. 2016, 14, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Khadoor, S.; Ibrahim, R.; Redwan, F. The Significance of Serum CA-125 in patients with Polycystic Ovarian Syndrome and its Association with their Hormonal Status. Res. J. Pharm. Technol. 2024, 17, 5445–5451. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, B.R.; Yang, W.J. Dilution effect of serum CA125 and CA19-9 over a cutoff value, according to obesity. Int. J. Biol. Markers 2015, 30, 122–126. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.-C.; Luo, L.; Mu, C.-Y.; Xu, J.; Feng, Q.; Li, S.-B.; Gu, B.; Ma, P.; Lan, T. The clinical value of the combined detection of sEGFR, CA125 and HE4 for epithelial ovarian cancer diagnosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 604–610. [Google Scholar] [PubMed]
- Sun, S.; Wei, L.; Zou, L.; Wang, T.; Liu, Z.; He, J.; Sun, X.; Zhong, W.; Zhao, F.; Li, X.; et al. Preoperative serum CA125 level and age at diagnosis: An effective prognosis prediction tool for patients with early-stage endometrial cancer. Asia-Pac. J. Clin. Oncol. 2023, 19, e258–e266. [Google Scholar] [CrossRef]
- Hao, C.; Zhang, G.; Zhang, L. Chapter Eleven-Serum CEA levels in 49 different types of cancer and noncancer diseases. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Academic Press: Cambridge, UK, 2019; Volume 162, pp. 213–227. [Google Scholar]
- Blatt, S.; Kämmerer, P.W.; Krüger, M.; Surabattula, R.; Thiem, D.G.E.; Dillon, S.T.; Al-Nawas, B.; Libermann, T.A.; Schuppan, D. High-Multiplex Aptamer-Based Serum Proteomics to Identify Candidate Serum Biomarkers of Oral Squamous Cell Carcinoma. Cancers 2023, 15, 2071. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, N.; Ji, N.; Chen, Z.-S. Proteomics technologies for cancer liquid biopsies. Mol. Cancer 2022, 21, 53. [Google Scholar] [CrossRef]
- Roy, D.; Pascher, A.; Juratli, M.A.; Sporn, J.C. The Potential of Aptamer-Mediated Liquid Biopsy for Early Detection of Cancer. Int. J. Mol. Sci. 2021, 22, 5601. [Google Scholar] [CrossRef]
- Wu, L.; Niu, Q.; Yang, C. Aptamer Molecular Evolution for Liquid Biopsy. In Handbook of Chemical Biology of Nucleic Acids; Sugimoto, N., Ed.; Springer Nature Singapore: Singapore, 2023; pp. 1453–1496. [Google Scholar] [CrossRef]
- Lin, B.; Jiang, J.; Zhou, X. Recent advances in design strategies of aptamer-based liquid biopsy. J. Polym. Sci. 2024, 62, 2848–2870. [Google Scholar] [CrossRef]
- de Araújo, N.S.; Moreira, A.d.S.; Abreu, R.d.S.; Junior, V.V.; Antunes, D.; Mendonça, J.B.; Sassaro, T.F.; Jurberg, A.D.; Ferreira-Reis, R.; Bastos, N.C.; et al. Aptamer-Based Recognition of Breast Tumor Cells: A New Era for Breast Cancer Diagnosis. Int. J. Mol. Sci. 2024, 25, 840. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ma, Y.; Wu, M.; Chen, Z.; Zhang, L.; Lu, J. Recent progress in aptamer-based microfluidics for the detection of circulating tumor cells and extracellular vesicles. J. Pharm. Anal. 2023, 13, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Hanžek, A.; Ducongé, F.; Siatka, C.; Duc, A.-C.E. Identification and Characterization of Aptamers Targeting Ovarian Cancer Biomarker Human Epididymis Protein 4 for the Application in Urine. Cancers 2023, 15, 452. [Google Scholar] [CrossRef]
- Yoon, S.; Rossi, J.J. Emerging cancer-specific therapeutic aptamers. Curr. Opin. Oncol. 2017, 29, 366–374. [Google Scholar] [CrossRef]
- Mahmoudian, F.; Ahmari, A.; Shabani, S.; Sadeghi, B.; Fahimirad, S.; Fattahi, F. Aptamers as an approach to targeted cancer therapy. Cancer Cell Int. 2024, 24, 108. [Google Scholar] [CrossRef] [PubMed]
- Coorssen, J.R.; Padula, M.P. Proteomics—The State of the Field: The Definition and Analysis of Proteomes Should Be Based in Reality, Not Convenience. Proteomes 2024, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Dupree, E.J.; Jayathirtha, M.; Yorkey, H.; Mihasan, M.; Petre, B.A.; Darie, C.C. A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of this Field. Proteomes 2020, 8, 14. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Q.; Wang, Q.; Sun, L. Mass spectrometry-intensive top-down proteomics: An update on technology advancements and biomedical applications. Anal. Methods 2024, 16, 4664–4682. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, K.; Andonovski, M.; Coorssen, J.R. Proteomes Are of Proteoforms: Embracing the Complexity. Proteomes 2021, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Hillman, H. Limitations of clinical and biological histology. Med. Hypotheses 2000, 54, 553–564. [Google Scholar] [CrossRef]
- Galant, C.; Malghem, J.; Sibille, C.; Docquier, P.-L.; Delloye, C. Current limitations to the histopathological diagnosis of some frequently encountered bone tumours. Acta Orthop. Belg. 2008, 74, 1–6. [Google Scholar]
- Soare, I.; Muntean, P.; Mirica, R. Brain Tumors Presumed Malignant without Biopsy-Evaluation and Treatment Problems. Austin J. Clin. Neurol. 2021, 8, 1148. [Google Scholar] [CrossRef]
- Zhang, J.; Rector, J.; Lin, J.Q.; Young, J.H.; Sans, M.; Katta, N.; Giese, N.; Yu, W.; Nagi, C.; Suliburk, J.; et al. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci. Transl. Med. 2017, 9, eaan3968. [Google Scholar] [CrossRef]
- Kramer, C.; Vreeswijk, M.; Thijssen, B.; Bosse, T.; Wesseling, J. Beyond the snapshot: Optimizing prognostication and prediction by moving from fixed to functional multidimensional cancer pathology. J. Pathol. 2022, 257, 403–412. [Google Scholar] [CrossRef]
- Deng, X.; Yan, Y.; Zhan, Z.; Xie, J.; Tang, H.; Zou, Y.; Tu, J.; Liu, P. A glimpse into the future: Integrating artificial intelligence for precision HER2-positive breast cancer management. iMetaOmics 2024, 1, e19. [Google Scholar] [CrossRef]
- Tseng, L.-J.; Matsuyama, A.; MacDonald-Dickinson, V. Histology: The gold standard for diagnosis? Can. Vet. J. 2023, 64, 389–391. [Google Scholar]
- Masood, S.; Rosa, M.; Kraemer, D.F.; Smotherman, C.; Mohammadi, A. Comparative cost-effectiveness of fine needle aspiration biopsy versus image-guided biopsy, and open surgical biopsy in the evaluation of breast cancer in the era of affordable care act: A changing landscape. Diagn. Cytopathol. 2015, 43, 605–612. [Google Scholar] [CrossRef]
- Silva, E.; Meschter, S.; Tan, M.P. Breast biopsy techniques in a global setting—Clinical practice review. Transl. Breast Cancer Res. 2023, 4, 14. [Google Scholar] [CrossRef]
- Wang, M.; He, X.; Chang, Y.; Sun, G.; Thabane, L. A sensitivity and specificity comparison of fine needle aspiration cytology and core needle biopsy in evaluation of suspicious breast lesions: A systematic review and meta-analysis. Breast 2017, 31, 157–166. [Google Scholar] [CrossRef]
- Park, H.E.; Han, D.; Lee, J.S.; Nikas, I.P.; Kim, H.; Yang, S.; Lee, H.; Ryu, H.S. Comparison of Breast Fine-Needle Aspiration Cytology and Tissue Sampling for High-Throughput Proteomic Analysis and Cancer Biomarker Detection. Pathobiology 2024, 91, 359–369. [Google Scholar] [CrossRef]
- Hwang, W.; Raymond, T.; McPartland, T.; Jeong, S.; Evans, C.L. Fluorescence Lifetime Multiplexing (FLEX) for simultaneous high dimensional spatial biology in 3D. Commun. Biol. 2024, 7, 1012. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, M.; Couto, S.; Hansel, D.; Miller, K.; Lopez-Girona, A.; Bjorklund, C.; Gandhi, A.; Thakurta, A.; Chopra, R.; et al. A Dual Color Immunohistochemistry Assay for Measurement of Cereblon in Multiple Myeloma Patient Samples. Appl. Immunohistochem. Mol. Morphol. 2015, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Mito, J.K.; Conner, J.R.; Hornick, J.L.; Cibas, E.S.; Qian, X. SOX10/keratin dual-color immunohistochemistry: An effective first-line test for the workup of epithelioid malignant neoplasms in FNA and small biopsy specimens. Cancer Cytopathol. 2018, 126, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Rizk, E.M.; Gartrell, R.D.; Barker, L.W.; Esancy, C.L.; Finkel, G.G.; Bordbar, D.D.; Saenger, Y.M. Prognostic and Predictive Immunohistochemistry-Based Biomarkers in Cancer and Immunotherapy. Hematol. Oncol. Clin. N. Am. 2019, 33, 291–299. [Google Scholar] [CrossRef]
- Yamashita-Kashima, Y.; Shu, S.; Yorozu, K.; Hashizume, K.; Moriya, Y.; Fujimoto-Ouchi, K.; Harada, N. Importance of formalin fixing conditions for HER2 testing in gastric cancer: Immunohistochemical staining and fluorescence in situ hybridization. Gastric Cancer 2014, 17, 638–647. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yang, C.-F.; Hsu, C.-Y. The impact of modified staining method on HER2 immunohistochemical staining for HER2-low breast cancer. Pathology 2023, 56, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Zafrani, B.; Aubriot, M.-H.; Mouret, E.; De Crémoux, P.; De Rycke, Y.; Nicolas, A.; Boudou, E.; Vincent-Salomon, A.; Magdelénat, H.; Sastre-Garau, X. High sensitivity and specificity of immunohistochemistry for the detection of hormone receptors in breast carcinoma: Comparison with biochemical determination in a prospective study of 793 cases. Histopathology 2000, 37, 536–545. [Google Scholar] [CrossRef]
- Thanasan, S.; Sukhakul, K.; Chitpakdee, S.; Kitkumthorn, N. Diagnostic Accuracy of Immunohistochemistry for HER2-Positive Breast Cancer. Asian Pac. J. Cancer Prev. 2023, 24, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Shamshirian, A.; Aref, A.R.; Yip, G.W.; Warkiani, M.E.; Heydari, K.; Bazaz, S.R.; Hamzehgardeshi, Z.; Shamshirian, D.; Moosazadeh, M.; Alizadeh-Navaei, R. Diagnostic value of serum HER2 levels in breast cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 1049. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.P.L.; Bollwein, C.; Noske, A.; Jacob, A.; Jank, P.; Loibl, S.; Nekljudova, V.; Fasching, P.A.; Karn, T.; Marmé, F.; et al. Characterization of Hormone Receptor and HER2 Status in Breast Cancer Using Mass Spectrometry Imaging. Int. J. Mol. Sci. 2023, 24, 2860. [Google Scholar] [CrossRef]
- Kurreck, A.; Vandergrift, L.A.; Fuss, T.L.; Habbel, P.; Agar, N.Y.R.; Cheng, L.L. Prostate cancer diagnosis and characterization with mass spectrometry imaging. Prostate Cancer Prostatic Dis. 2018, 21, 297–305. [Google Scholar] [CrossRef]
- Huang, N.; Chen, L.; He, J.; Nguyen, Q.D. The Efficacy of Clinical Breast Exams and Breast Self-Exams in Detecting Malignancy or Positive Ultrasound Findings. Cureus 2022, 14, e22464. [Google Scholar] [CrossRef]
- O’Malley, M.S.; Fletcher, S.W. Screening for Breast Cancer With Breast Self-examination: A Critical Review. JAMA 1987, 257, 2196–2203. [Google Scholar] [CrossRef]
- Mango, V.L.; Olasehinde, O.; Omisore, A.D.; Wuraola, F.O.; Famurewa, O.C.; Sevilimedu, V.; Knapp, G.C.; Steinberg, E.; Akinmaye, P.R.; Adewoyin, B.D.; et al. The iBreastExam versus clinical breast examination for breast evaluation in high risk and symptomatic Nigerian women: A prospective study. Lancet Glob. Health 2022, 10, e555–e563. [Google Scholar] [CrossRef]
- Bhimani, F.; Zhang, J.; Shah, L.; McEvoy, M.; Gupta, A.; Pastoriza, J.; Shihabi, A.; Feldman, S. Can the Clinical Utility of iBreastExam, a Novel Device, Aid in Optimizing Breast Cancer Diagnosis? A Systematic Review. JCO Glob. Oncol. 2023, 9, e2300149. [Google Scholar] [CrossRef]
- Gerami, R.; Joni, S.S.; Akhondi, N.; Etemadi, A.; Fosouli, M.; Eghbal, A.; Souri, Z. A literature review on the imaging methods for breast cancer. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 171–176. [Google Scholar]
- Sood, R.; Rositch, A.F.; Shakoor, D.; Ambinder, E.; Pool, K.-L.; Pollack, E.; Mollura, D.J.; Mullen, L.A.; Harvey, S.C. Ultrasound for Breast Cancer Detection Globally: A Systematic Review and Meta-Analysis. J. Glob. Oncol. 2019, 5, 1–17. [Google Scholar] [CrossRef]
- Zeeshan, M.; Salam, B.; Khalid, Q.S.; Alam, S.; Sayani, R. Diagnostic Accuracy of Digital Mammography in the Detection of Breast Cancer. Cureus 2018, 10, e2448. [Google Scholar] [CrossRef]
- Merriel, S.W.; Akter, N.; Zakkak, N.; Swann, R.; McPhail, S.; Rubin, G.; Lyratzopoulos, G.; Abel, G. Factors affecting prostate cancer detection through asymptomatic prostate-specific antigen testing in primary care in England: Evidence from the 2018 National Cancer Diagnosis Audit. Br. J. Gen. Pract. 2024, 75, e300–e305. [Google Scholar] [CrossRef]
- Uematsu, T. Sensitivity and specificity of screening mammography without clinical breast examination among Japanese women aged 40–49 years: Analysis of data from the J-START results. Breast Cancer 2022, 29, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Gøtzsche, P.C. Mammography screening is harmful and should be abandoned. J. R. Soc. Med. 2015, 108, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Karami, V.; Moazen, J.; Kwee, T.; Bhalla, A.S.; Shahbazi-Gahrouei, D.; Shao, Y.-H.J. Radiation Exposure and Lifetime Attributable Risk of Cancer Incidence and Mortality from Low- and Standard-Dose CT Chest: Implications for COVID-19 Pneumonia Subjects. Diagnostics 2022, 12, 3043. [Google Scholar] [CrossRef]
- Cao, C.-F.; Ma, K.-L.; Shan, H.; Liu, T.-F.; Zhao, S.-Q.; Wan, Y.; Jun, Z.; Wang, H.-Q. CT Scans and Cancer Risks: A Systematic Review and Dose-response Meta-analysis. BMC Cancer 2022, 22, 1238. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.H.; Fairley, R.; Murphy, L.S.-L.; Doss, M. The Risk of Cancer from CT Scans and Other Sources of Low-Dose Radiation: A Critical Appraisal of Methodologic Quality. Prehospital Disaster Med. 2020, 35, 3–16. [Google Scholar] [CrossRef]
- Younis, S.; Emayof, M. Role CT imaging for Cancer Diagnosis by Determining Sensitivity and Specificity: 144 Cancer Cases in Tobruk, Libya. AlQalam J. Med. Appl. Sci. 2024, 7, 1458–1463. [Google Scholar] [CrossRef]
- Toyoda, Y.; Nakayama, T.; Kusunoki, Y.; Iso, H.; Suzuki, T. Sensitivity and specificity of lung cancer screening using chest low-dose computed tomography. Br. J. Cancer 2008, 98, 1602–1607. [Google Scholar] [CrossRef]
- Cochran, T.; Patel, S.; Kruse, T.; Lyden, E.; Comer, S.; Ford, J. Sensitivity and Specificity of Whole-body MRI for the Detection of Pediatric Malignancy. J. Pediatr. Hematol. Oncol. 2023, 45, e26–e30. [Google Scholar] [CrossRef]
- Aziz, S.; Mohamad, M.; Zin, R. Histopathological Correlation of Breast Carcinoma with Breast Imaging-Reporting and Data System. Malays. J. Med. Sci. 2022, 29, 65–74. [Google Scholar] [CrossRef]
- Niikura, N.; Costelloe, C.M.; Madewell, J.E.; Hayashi, N.; Yu, T.K.; Liu, J.; Palla, S.L.; Tokuda, Y.; Theriault, R.L.; Hortobagyi, G.N.; et al. FDG-PET/CT Compared with Conventional Imaging in the Detection of Distant Metastases of Primary Breast Cancer. Oncologist 2011, 16, 1111–1119. [Google Scholar] [CrossRef]
- Butt, F.; Dominguez-Konicki, L.; Tocci, N.; Paydarfar, J.; Seltzer, M.; Pastel, D. Diagnostic accuracy of the latest-generation digital PET/CT scanner for detection of metastatic lymph nodes in head and neck cancer. Front. Nucl. Med. 2023, 3, 1184448. [Google Scholar] [CrossRef] [PubMed]
- Vetrone, L.; Mei, R.; Bianchi, L.; Giunchi, F.; Farolfi, A.; Castellucci, P.; Droghetti, M.; Presutti, M.; Degiovanni, A.; Schiavina, R.; et al. Histology and PSMA Expression on Immunohistochemistry in High-Risk Prostate Cancer Patients: Comparison with 68Ga-PSMA PET/CT Features in Primary Staging. Cancers 2023, 15, 1716. [Google Scholar] [CrossRef]
- Houshmand, S.; Lawhn-Heath, C.; Behr, S. PSMA PET imaging in the diagnosis and management of prostate cancer. Abdom. Radiol. 2023, 48, 3610–3623. [Google Scholar] [CrossRef]
- von Stauffenberg, F.; Poyet, C.; Beintner-Skawran, S.; Maurer, A.; Schmid, F.A. Current Clinical Applications of PSMA-PET for Prostate Cancer Diagnosis, Staging, and Treatment. Cancers 2024, 16, 4263. [Google Scholar] [CrossRef] [PubMed]
- Belal, S.L.; Frantz, S.; Minarik, D.; Enqvist, O.; Wikström, E.; Edenbrandt, L.; Trägårdh, E. Applications of Artificial Intelligence in PSMA PET/CT for Prostate Cancer Imaging. Semin. Nucl. Med. 2024, 54, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, F.; Zhang, J.; Wang, L. Clinical Applications of Liquid Biopsy in Prostate Cancer: From Screening to Predictive Biomarker. Cancers 2022, 14, 1728. [Google Scholar] [CrossRef]
- Frantzi, M.; Culig, Z.; Heidegger, I.; Mokou, M.; Latosinska, A.; Roesch, M.C.; Merseburger, A.S.; Makridakis, M.; Vlahou, A.; Blanca-Pedregosa, A.; et al. Mass Spectrometry-Based Biomarkers to Detect Prostate Cancer: A Multicentric Study Based on Non-Invasive Urine Collection without Prior Digital Rectal Examination. Cancers 2023, 15, 1166. [Google Scholar] [CrossRef]
- Höti, N.; Lih, T.-S.; Dong, M.; Zhang, Z.; Mangold, L.; Partin, A.W.; Sokoll, L.J.; Li, Q.K.; Zhang, H. Urinary PSA and Serum PSA for Aggressive Prostate Cancer Detection. Cancers 2023, 15, 960. [Google Scholar] [CrossRef]
- Tanase, C.P.; Codrici, E.; Popescu, I.D.; Mihai, S.; Enciu, A.-M.; Necula, L.G.; Preda, A.; Ismail, G.; Albulescu, R. Prostate cancer proteomics: Current trends and future perspectives for biomarker discovery. Oncotarget 2017, 8, 18497. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-H.; Lu, P.-X.; Qi, W.-L.; Jin, A.-F.; Liu, Q.; Zeng, Q.-Y.; Lu, P. The sensitivity and specificity of 18F-FDG PET/CT in spinal leptomeningeal metastases: The synergistic effect of the 18F-FDG PET-CT to gadolinium-enhanced MRI. Quant. Imaging Med. Surg. 2023, 13, 6863–6875. [Google Scholar] [CrossRef] [PubMed]
- Bashir, U.; Mallia, A.; Stirling, J.; Joemon, J.; MacKewn, J.; Charles-Edwards, G.; Goh, V.; Cook, G.J. PET/MRI in Oncological Imaging: State of the Art. Diagnostics 2015, 5, 333–357. [Google Scholar] [CrossRef]
- Kitajima, K.; Nakamoto, Y.; Okizuka, H.; Onishi, Y.; Senda, M.; Suganuma, N.; Sugimura, K. Accuracy of whole-body FDG-PET/CT for detecting brain metastases from non-central nervous system tumors. Ann. Nucl. Med. 2008, 22, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, W.; Huang, T.; Zhou, J. Genetic Testing Enhances the Precision Diagnosis and Treatment of Breast Cancer. Int. J. Mol. Sci. 2023, 24, 16607. [Google Scholar] [CrossRef]
- Lynce, F.; Isaacs, C. How Far Do We Go With Genetic Evaluation? Gene, Panel, and Tumor Testing. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e72–e78. [Google Scholar] [CrossRef]
- Venetis, K.; Pescia, C.; Cursano, G.; Frascarelli, C.; Mane, E.; De Camilli, E.; Munzone, E.; Dellapasqua, S.; Criscitiello, C.; Curigliano, G.; et al. The Evolving Role of Genomic Testing in Early Breast Cancer: Implications for Diagnosis, Prognosis, and Therapy. Int. J. Mol. Sci. 2024, 25, 5717. [Google Scholar] [CrossRef]
- Yamamoto, S.; Chishima, T.; Shibata, Y.; Harada, F.; Takeuchi, H.; Yamada, A.; Narui, K.; Misumi, T.; Ishikawa, T.; Endo, I. Clinical Impact of a Novel Model Predictive of Oncotype DX Recurrence Score in Breast Cancer. In Vivo 2021, 35, 2439. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Hwang, Y.S.; Kim, J.; Ahn, S.-H.; Son, B.H.; Kim, H.J.; Ko, B.S.; Kim, J.; Chung, I.Y.; Lee, J.W.; et al. A nomogram for predicting probability of low risk of MammaPrint results in women with clinically high-risk breast cancer. Sci. Rep. 2021, 11, 23509. [Google Scholar] [CrossRef]
- Wallden, B.; Storhoff, J.; Nielsen, T.; Dowidar, N.; Schaper, C.; Ferree, S.; Liu, S.; Leung, S.; Geiss, G.; Snider, J.; et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med. Genom. 2015, 8, 54. [Google Scholar] [CrossRef]
- Dubsky, P.C.; Singer, C.F.; Egle, D.; Wette, V.; Petru, E.; Balic, M.; Pichler, A.; Greil, R.; Petzer, A.L.; Bago-Horvath, Z.; et al. The EndoPredict score predicts response to neoadjuvant chemotherapy and neoendocrine therapy in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer patients from the ABCSG-34 trial. Eur. J. Cancer 2020, 134, 99–106. [Google Scholar] [CrossRef]
- Bartlett, J.M.S.; Sgroi, D.C.; Treuner, K.; Zhang, Y.; Piper, T.; Salunga, R.C.; Ahmed, I.; Doos, L.; Thornber, S.; Taylor, K.J.; et al. Breast Cancer Index Is a Predictive Biomarker of Treatment Benefit and Outcome from Extended Tamoxifen Therapy: Final Analysis of the Trans-aTTom Study. Clin. Cancer Res. 2022, 28, 1871–1880. [Google Scholar] [CrossRef]
- Leapman, M.S.; Sutherland, R.; Gross, C.P.; Ma, X.; Seibert, T.M.; Cooperberg, M.R.; Catalona, W.J.; Loeb, S.; Schulman-Green, D. Patient experiences with tissue-based genomic testing during active surveillance for prostate cancer. BJUI Compass 2024, 5, 142–149. [Google Scholar] [CrossRef]
- McHugh, J.K.; Bancroft, E.K.; Saunders, E.; Brook, M.N.; McGrowder, E.; Wakerell, S.; James, D.; Rageevakumar, R.; Benton, B.; Taylor, N.; et al. Assessment of a Polygenic Risk Score in Screening for Prostate Cancer. N. Engl. J. Med. 2025, 392, 1406–1417. [Google Scholar] [CrossRef]
- Katsuya, Y. Current and future trends in whole genome sequencing in cancer. Cancer Biol. Med. 2024, 21, 16. [Google Scholar] [CrossRef]
- Thierry, A.R. Circulating DNA fragmentomics and cancer screening. Cell Genom. 2023, 3, 100242. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.; Goodman, C.; Nadauld, L.D. Clinical Utility of Genomic Testing in Cancer Care. JCO Precis. Oncol. 2022, 6, e2100349. [Google Scholar] [CrossRef]
- Akhoundova, D.; Rubin, M. The grand challenge of moving cancer whole-genome sequencing into the clinic. Nat. Med. 2024, 30, 39–40. [Google Scholar] [CrossRef]
- Rebello, R.; Posner, A.; Dong, R.; Prall, O.; Sivakumaran, T.; Mitchell, C.; Flynn, A.; Caneborg, A.; Mitchell, C.; Kanwal, S.; et al. Whole genome sequencing improves tissue-of-origin diagnosis and treatment options for cancer of unknown primary. Nat. Commun. 2025, 16, 4422. [Google Scholar] [CrossRef] [PubMed]
- Zalis, M.; Veloso, G.G.V.; Aguiar, P.N., Jr.; Gimenes, N.; Reis, M.X.; Matsas, S.; Ferreira, C.G. Next-generation sequencing impact on cancer care: Applications, challenges, and future directions. Front. Genet. 2024, 15, 1420190. [Google Scholar] [CrossRef]
- Donohue, K.E.; Gooch, C.; Katz, A.; Wakelee, J.; Slavotinek, A.; Korf, B.R. Pitfalls and challenges in genetic test interpretation: An exploration of genetic professionals experience with interpretation of results. Clin. Genet. 2021, 99, 638–649. [Google Scholar] [CrossRef]
- Casolino, R.; Beer, P.A.; Chakravarty, D.; Davis, M.B.; Malapelle, U.; Mazzarella, L.; Normanno, N.; Pauli, C.; Subbiah, V.; Turnbull, C.; et al. Interpreting and integrating genomic tests results in clinical cancer care: Overview and practical guidance. CA Cancer J. Clin. 2024, 74, 264–285. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Wuethrich, A.; Constantin, N.; Shanmugasundaram, K.B.; Mainwaring, P.; Kulasinghe, A.; O’Leary, C.; O’Byrne, K.; Sina, A.A.I.; Carrascosa, L.G.; et al. Liquid Biopsy Snapshots of Key Phosphoproteomic Pathways in Lung Cancer Patients for Diagnosis and Therapy Monitoring. Anal. Chem. 2023, 95, 8522–8532. [Google Scholar] [CrossRef]
- Neagu, A.-N.; Jayathirtha, M.; Whitham, D.; Mutsengi, P.; Sullivan, I.; Petre, B.A.; Darie, C.C. Proteomics-Based Identification of Dysregulated Proteins in Breast Cancer. Proteomes 2022, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Shi, Y.; Del Rosario, A.M.; Jian, W. Method Development Workflow for Quantifying Protein Biomarkers by Hybrid LC–MS/MS. Bioanalysis 2022, 14, 985–1004. [Google Scholar] [CrossRef]
- Macklin, A.; Khan, S.; Kislinger, T. Recent advances in mass spectrometry based clinical proteomics: Applications to cancer research. Clin. Proteom. 2020, 17, 17. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, W.; Krishnamoorthy, G.; Hu, P.; Chen, M.; Tiedje, V.; Acuña-Ruiz, A.; Wang, H.; Wang, Z.; Wang, J.; et al. Comprehensive Mass Spectral Libraries of Human Thyroid Tissues and Cells. Sci. Data 2024, 11, 1448. [Google Scholar] [CrossRef]
- Montero, A.; Garranzo-Asensio, M.; Rejas-González, R.; Feliu, J.; Mendiola, M.; García, A.; Barderas, R. Benefits of FAIMS to Improve the Proteome Coverage of Deteriorated and/or Cross-Linked TMT 10-Plex FFPE Tissue and Plasma-Derived Exosomes Samples. Proteomes 2023, 11, 35. [Google Scholar] [CrossRef]
- Lu, P.; Yang, L.; Chen, W.; Li, K.; Chen, X.; Qu, S. Four-dimensional trapped ion mobility spectrometry proteomics reveals circulating extracellular vesicles encapsulated drivers of nasopharyngeal carcinoma distant dissemination. Talanta 2025, 282, 126907. [Google Scholar] [CrossRef] [PubMed]
- Brzhozovskiy, A.; Kononikhin, A.; Bugrova, A.E.; Kovalev, G.I.; Schmit, P.-O.; Kruppa, G.; Nikolaev, E.N.; Borchers, C.H. The Parallel Reaction Monitoring-Parallel Accumulation–Serial Fragmentation (prm-PASEF) Approach for Multiplexed Absolute Quantitation of Proteins in Human Plasma. Anal. Chem. 2022, 94, 2016–2022. [Google Scholar] [CrossRef]
- Bradley, S.; Watson, J. Testing and cancer diagnosis in general practice. BMJ Qual. Amp. Saf. 2025, 34, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154. [Google Scholar] [CrossRef]
- Streicher, J.; Meyerson, B.L.; Karivedu, V.; Sidana, A. A review of optimal prostate biopsy: Indications and techniques. Ther. Adv. Urol. 2019, 11, 1756287219870074. [Google Scholar] [CrossRef]
- Reza, H.S.; Ali, Z.; Tara, H.; Ali, B. Age-specific reference ranges of prostate-specific antigen in the elderly of Amirkola: A population-based study. Asian J. Urol. 2021, 8, 183–188. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Cai, X.; Xie, L.; He, D.; Zhou, L.; Xu, C.; Gao, X.; Ren, S.; Wang, F.; et al. Age-Specific Cutoff Value for the Application of Percent Free Prostate-Specific Antigen (PSA) in Chinese Men with Serum PSA Levels of 4.0–10.0 ng/mL. PLoS ONE 2015, 10, e0130308. [Google Scholar] [CrossRef]
- Aref, A.T.; Vincent, A.D.; O’Callaghan, M.E.; Martin, S.A.; Sutherland, P.D.; Hoy, A.J.; Butler, L.M.; Wittert, G.A. The inverse relationship between prostate specific antigen (PSA) and obesity. Endocr. Relat. Cancer 2018, 25, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Koc, G.; Akgul, K.; Yilmaz, Y.; Dirik, A. The effects of cigarette smoking on prostate-specific antigen in two different age groups. Can. Urol. Assoc. J. 2013, 7, e704–e707. [Google Scholar] [CrossRef] [PubMed]
- Press, D.J.; Pierce, B.; Lauderdale, D.S.; Aschebrook-Kilfoy, B.; Gomez, S.L.; Hedeker, D.; Wright, N.E.; Fantus, R.J.; Bettencourt, L.; Ahsan, H.; et al. Tobacco and marijuana use and their association with serum prostate-specific antigen levels among African American men in Chicago. Prev. Med. Rep. 2020, 20, 101174. [Google Scholar] [CrossRef]
- Barlow, M.; Down, L.; Mounce, L.T.A.; Merriel, S.W.D.; Watson, J.; Martins, T.; Bailey, S.E.R. Ethnic differences in prostate-specific antigen levels in men without prostate cancer: A systematic review. Prostate Cancer Prostatic Dis. 2023, 26, 249–256. [Google Scholar] [CrossRef]
- Down, L.; Barlow, M.; Bailey, S.E.R.; Mounce, L.T.A.; Merriel, S.W.D.; Watson, J.; Martins, T. Association between patient ethnicity and prostate cancer diagnosis following a prostate-specific antigen test: A cohort study of 730,000 men in primary care in the UK. BMC Med. 2024, 22, 82. [Google Scholar] [CrossRef]
- Urabe, F.; Sumiyoshi, T.; Tashiro, K.; Goto, T.; Kimura, T.; Kobayashi, T. Prostate cancer and liquid biopsies: Clinical applications and challenges. Int. J. Urol. 2024, 31, 617–626. [Google Scholar] [CrossRef]
- Joshi, N.; Garapati, K.; Ghose, V.; Kandasamy, R.K.; Pandey, A. Recent progress in mass spectrometry-based urinary proteomics. Clin. Proteom. 2024, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jin, W.; Xu, Z.; Ju, L.; Shan, D.; Li, S.; Yu, M.; Cao, X.; Liu, N.; Qian, K.; et al. Urine-based liquid biopsy in bladder cancer: Opportunities and challenges. Clin. Transl. Discov. 2023, 3, e176. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, F.; Li, T.; Xue, J.; Yue, S.; Zhao, S.; Lu, H.; He, C. Exploration of potential biomarkers for early bladder cancer based on urine proteomics. Front. Oncol. 2024, 14, 1309842. [Google Scholar] [CrossRef]
- Ha, A.; Khoo, A.; Ignatchenko, V.; Khan, S.; Waas, M.; Vesprini, D.; Liu, S.K.; Nyalwidhe, J.O.; Semmes, O.J.; Boutros, P.C.; et al. Comprehensive Prostate Fluid-Based Spectral Libraries for Enhanced Protein Detection in Urine. J. Proteome Res. 2024, 23, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; McKinney, K.; Pavlopoulos, A.; Niu, M.; Kang, J.; Oh, J.; Kim, K.P.; Hwang, S. Altered Proteome of Extracellular Vesicles Derived from Bladder Cancer Patients Urine. Mol. Cells 2018, 41, 179–187. [Google Scholar] [CrossRef]
- Beasley-Green, A. Urine Proteomics in the Era of Mass Spectrometry. Int. Neurourol. J. 2016, 20, S70–S75. [Google Scholar] [CrossRef]
- Wood, S.L.; Knowles, M.A.; Thompson, D.; Selby, P.J.; Banks, R.E. Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers. Nat. Rev. Urol. 2013, 10, 206–218. [Google Scholar] [CrossRef]
- Jordaens, S.; Zwaenepoel, K.; Tjalma, W.; Deben, C.; Beyers, K.; Vankerckhoven, V.; Pauwels, P.; Vorsters, A. Urine biomarkers in cancer detection: A systematic review of preanalytical parameters and applied methods. Int. J. Cancer 2023, 152, 2186–2205. [Google Scholar] [CrossRef] [PubMed]
- Albakova, Z.; Norinho, D.D.; Mangasarova, Y.; Sapozhnikov, A. Heat Shock Proteins in Urine as Cancer Biomarkers. Front. Med. 2021, 8, 743476. [Google Scholar] [CrossRef]
- Ramirez-Garrastacho, M.; Bajo-Santos, C.; Line, A.; Martens-Uzunova, E.S.; de la Fuente, J.M.; Moros, M.; Soekmadji, C.; Tasken, K.A.; Llorente, A. Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: A decade of research. Br. J. Cancer 2022, 126, 331–350. [Google Scholar] [CrossRef]
- Hamid, Y.; Rabbani, R.D.; Afsara, R.; Nowrin, S.; Ghose, A.; Papadopoulos, V.; Sirlantzis, K.; Ovsepian, S.V.; Boussios, S. Exosomal Liquid Biopsy in Prostate Cancer: A Systematic Review of Biomarkers for Diagnosis, Prognosis, and Treatment Response. Int. J. Mol. Sci. 2025, 26, 802. [Google Scholar] [CrossRef]
- Li, S.; Yi, M.; Dong, B.; Tan, X.; Luo, S.; Wu, K. The role of exosomes in liquid biopsy for cancer diagnosis and prognosis prediction. Int. J. Cancer 2021, 148, 2640–2651. [Google Scholar] [CrossRef] [PubMed]
- Cristina Barbosa da Silva, A.C.B.; Albuquerque, A.; Romario-Silva, D.; Ferreira, S.; Rodrigues, A.; Marinho, S. Salivary Diagnostics, Current Reality and Future Prospects. In Emerging Trends in Oral Health Sciences and Dentistry; Virdi, M.S., Ed.; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Limeres, J.; Dios, P.; Scully, C. Infection Transmission by Saliva and the Paradoxical Protective Role of Saliva. Saliva Prot. Transm. Dis. 2017, 1, 1–18. [Google Scholar] [CrossRef]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Subbarao, K.C.; Nattuthurai, G.S.; Sundararajan, S.K.; Sujith, I.; Joseph, J.; Syedshah, Y.P. Gingival Crevicular Fluid: An Overview. J. Pharm. Bioallied Sci. 2019, 11, S135–S139. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharjee, S. Saliva as a sample for liquid biopsy: A review. J. Oral Med. Oral Surg. Oral Pathol. Oral Radiol. 2024, 10, 261–264. [Google Scholar] [CrossRef]
- Koopaie, M.; Kolahdooz, S.; Fatahzadeh, M.; Manifar, S. Salivary biomarkers in breast cancer diagnosis: A systematic review and diagnostic meta-analysis. Cancer Med. 2022, 11, 2644–2661. [Google Scholar] [CrossRef]
- Rashid, S.; Puttagunta, P.; Pamulapati, S.; Yang, J.; Pocha, S.; Saba, N.F.; Teng, Y. Leveraging Saliva for Insights into Head and Neck Cancer. Int. J. Mol. Sci. 2024, 25, 13514. [Google Scholar] [CrossRef] [PubMed]
- Krapfenbauer, K.; Drucker, E.; Thurnher, D. Identification of tumour-related proteins as potential screening markers by proteome analysis—Protein profiles of human saliva as a predictive and prognostic tool. EPMA J. 2014, 5, 20. [Google Scholar] [CrossRef]
- Archana, N.; Atharva, D. Salivary Biomarkers for Oral Cancer Detection: Insights from Human DNA and RNA Analysis. Cardiovasc. Hematol. Agents Med. Chem. 2024, 22, 249–257. [Google Scholar] [CrossRef]
- Suragimath, G.; Patil, S.; Suragimath, D.G.; Sr, A. Salivaomics: A Revolutionary Non-invasive Approach for Oral Cancer Detection. Cureus 2024, 16, e74381. [Google Scholar] [CrossRef]
- Gupta, S.; Brijesh, S.; Rajul, A.; Sameer, G.; Sachan, M. The emerging role of liquid biopsy in oral squamous cell carcinoma detection: Advantages and challenges. Expert Rev. Mol. Diagn. 2024, 24, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Honda, K. Liquid Biopsy for Oral Cancer Diagnosis: Recent Advances and Challenges. J. Pers. Med. 2023, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Bastías, D.; Maturana, A.; Marín, C.; Martínez, R.; Niklander, S.E. Salivary Biomarkers for Oral Cancer Detection: An Exploratory Systematic Review. Int. J. Mol. Sci. 2024, 25, 2634. [Google Scholar] [CrossRef]
- Ghallab, N.A.; Shaker, O.G. Serum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesions. Clin. Oral Investig. 2017, 21, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Aslebagh, R.; Channaveerappa, D.; Arcaro, K.F.; Darie, C.C. Proteomics analysis of human breast milk to assess breast cancer risk. Electrophoresis 2018, 39, 653–665. [Google Scholar] [CrossRef]
- Aslebagh, R.; Whitham, D.; Channaveerappa, D.; Mutsengi, P.; Pentecost, B.T.; Arcaro, K.F.; Darie, C.C. Mass Spectrometry-Based Proteomics of Human Milk to Identify Differentially Expressed Proteins in Women with Breast Cancer versus Controls. Proteomes 2022, 10, 36. [Google Scholar] [CrossRef]
- Aslebagh, R.; Whitham, D.; Channaveerappa, D.; Lowe, J.; Pentecost, B.T.; Arcaro, K.F.; Darie, C.C. Proteomics analysis of human breast milk by two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) coupled with mass spectrometry to assess breast cancer risk. Electrophoresis 2023, 44, 1097–1113. [Google Scholar] [CrossRef]
- Aslebagh, R.; Channaveerappa, D.; Arcaro, K.F.; Darie, C.C. Comparative two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) of human milk to identify dysregulated proteins in breast cancer. Electrophoresis 2018, 39, 1723–1734. [Google Scholar] [CrossRef]
- Whitham, D.; Aslebagh, R.; Channaveerappa, D.; Pentecost, B.; Arcaro, K.F.; Darie, C.C. Proteomic Analysis of Human Breast Milk to Reveal Potential Protein Biomarkers for Breast Cancer. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Durán-Jara, E.; Vera-Tobar, T.; Lobos-González, L.D.L. Lactadherin: From a Well-Known Breast Tumor Marker to a Possible Player in Extracellular Vesicle-Mediated Cancer Progression. Int. J. Mol. Sci. 2022, 23, 3855. [Google Scholar] [CrossRef] [PubMed]
- Karav, S. Chapter 22-Application of a Novel Endo-β-N-Acetylglucosaminidase to Isolate an Entirely New Class of Bioactive Compounds: N-Glycans. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, UK, 2019; pp. 389–404. [Google Scholar] [CrossRef]
- Gopal, S.H.; Das, S. Role of Lactoferrin in the Carcinogenesis of Triple-Negative Breast Cancer. J. Cancer Clin. Trials 2016, 1, e105. [Google Scholar] [PubMed]
- Aslebagh, R.; Channaveerappa, D.; Pentecost, B.T.; Arcaro, K.F.; Darie, C.C. Combinatorial Electrophoresis and Mass Spectrometry-Based Proteomics in Breast Milk for Breast Cancer Biomarker Discovery. In Advancements of Mass Spectrometry in Biomedical Research; Woods, A.G., Darie, C.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 451–467. [Google Scholar] [CrossRef]
- Keskin, M.; Kompuinen, J.; Harmankaya, İ.; Karaçetin, D.; Nissilä, V.; Gürsoy, M.; Sorsa, T.; Gürsoy, U.K. Oral Cavity Calprotectin and Lactoferrin Levels in Relation to Radiotherapy. Curr. Issues Mol. Biol. 2022, 44, 4439–4446. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, X.; Luo, Z.; Wang, H.; Ismtula, D.; Guo, C. Gelsolin: A comprehensive pan-cancer analysis of potential prognosis, diagnostic, and immune biomarkers. Front. Genet. 2023, 14, 1093163. [Google Scholar] [CrossRef]
- Akkinepally, H.; Tejasvi, M.A.; Kavitha, N.L.; Pokala, A.; Reddy, P.; Keerthi, V.R. Estimation of Alpha-amylase in Smokers with and without Leukoplakia and Oral Cancer—A Comparative Study. J. Indian Acad. Oral Med. Radiol. 2023, 35, 331–334. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Thomasson, S.; Oredsson, S. Alpha-Amylase Inhibits Cell Proliferation and Glucose Uptake in Human Neuroblastoma Cell Lines. BioMed Res. Int. 2022, 2022, 4271358. [Google Scholar] [CrossRef]
- Burgos-Panadero, R.; Noguera, I.; Cañete, A.; Navarro, S.; Noguera, R. Vitronectin as a molecular player of the tumor microenvironment in neuroblastoma. BMC Cancer 2019, 19, 479. [Google Scholar] [CrossRef]
- Jiwa, N.; Ezzat, A.; Holt, J.; Wijayatilake, D.S.; Takats, Z.; Leff, D.R. Nipple aspirate fluid and its use for the early detection of breast cancer. Ann. Med. Surg. 2022, 77, 103625. [Google Scholar] [CrossRef]
- Paweletz, C.; Trock, B.; Pennanen, M.; Tsangaris, T.; Magnant, C.; Liotta, L.; Petricoin, E. Proteomic Patterns of Nipple Aspirate Fluids Obtained by SELDI-TOF: Potential for New Biomarkers to Aid in the Diagnosis of Breast Cancer. Dis. Markers 2001, 17, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Patuleia, S.I.S.; Suijkerbuijk, K.P.M.; van der Wall, E.; van Diest, P.J.; Moelans, C.B. Nipple Aspirate Fluid at a Glance. Cancers 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Sorolla, M.A.; Sorolla, A.; Parisi, E.; Salud, A.; Porcel, J.M. Diving into the Pleural Fluid: Liquid Biopsy for Metastatic Malignant Pleural Effusions. Cancers 2021, 13, 2798. [Google Scholar] [CrossRef]
- Botana-Rial, M.; Vázquez-Iglesias, L.; Casado-Rey, P.; Cadena, M.P.d.l.; Andrade-Olivié, M.A.; Abal-Arca, J.; García-Nimo, L.; Ferreiro-Fernández, L.; Valdés-Cuadrado, L.; San-José, M.E.; et al. Validation of Calprotectin As a Novel Biomarker For The Diagnosis of Pleural Effusion: A Multicentre Trial. Sci. Rep. 2020, 10, 5679. [Google Scholar] [CrossRef]
- Nandi, S.K.; Singh, D.; Upadhay, J.; Gupta, N.; Dhiman, N.; Mittal, S.K.; Mahindroo, N. Identification of tear-based protein and non-protein biomarkers: Its application in diagnosis of human diseases using biosensors. Int. J. Biol. Macromol. 2021, 193, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Daily, A.; Ravishankar, P.; Harms, S.; Klimberg, V. Using tears as a non-invasive source for early detection of breast cancer. PLoS ONE 2022, 17, e0267676. [Google Scholar] [CrossRef]
- Ravishankar, P.; Daily, A. Tears as the Next Diagnostic Biofluid: A Comparative Study between Ocular Fluid and Blood. Appl. Sci. 2022, 12, 2884. [Google Scholar] [CrossRef]
- Daily, A.; Ravishankar, P.; Wang, W.; Krone, R.; Harms, S.; Klimberg, V.S. Development and validation of a short-term breast health measure as a supplement to screening mammography. Biomark. Res. 2022, 10, 76. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Byrum, S.D.; Acott, A.A.; Siegel, E.R.; Washam, C.L.; Klimberg, V.S.; Mancino, A.T. Proteomic profiling of tear fluid as a promising non-invasive screening test for colon cancer. Am. J. Surg. 2022, 224, 19–24. [Google Scholar] [CrossRef]
- Schulz, B.; Oxley, D.; Packer, N.; Karlsson, N. Identification of two highly sialylated human tear-fluid DMBT1 isoforms: The major high-molecular-mass glycoproteins in human tears. Biochem. J. 2002, 366, 511–520. [Google Scholar] [CrossRef]
- Midena, E.; Parrozzani, R.; Midena, G.; Trainiti, S.; Marchione, G.; Cosmo, E.; Londei, D.; Frizziero, L. In vivo intraocular biomarkers: Changes of aqueous humor cytokines and chemokines in patients affected by uveal melanoma. Medicine 2020, 99, e22091. [Google Scholar] [CrossRef]
- Ghiam, B.K.; Xu, L.; Berry, J.L. Aqueous Humor Markers in Retinoblastoma, a Review. Transl. Vis. Sci. Technol. 2019, 8, 13. [Google Scholar] [CrossRef]
- Campanharo, C.V.; dos Santos Silveira, L.V.; Meira, D.D.; Casotti, M.C.; Altoé, L.S.C.; Louro, I.D.; Gonçalves, A.F.M.; Machado, A.M.; Paiva, B.S.; de Souza Inocencio, E.; et al. Pan-cancer and multiomics: Advanced strategies for diagnosis, prognosis, and therapy in the complex genetic and molecular universe of cancer. Clin. Transl. Oncol. 2024, 27, 2936–2954. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Debik, J.; Naume, B.; Ohnstad, H.O.; Sahlber, K.K.; Borgen, E.; Børresen-Dale, A.-L.; Engebråten, O.; Fritzman, B.; Garred, Ø.; et al. Comprehensive multi-omics analysis of breast cancer reveals distinct long-term prognostic subtypes. Oncogenesis 2024, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, P.A.; Markey, S.P.; Roth, J.; Mirokhin, Y.; Yan, X.; Tchekhovskoi, D.V.; Edwards, N.J.; Thangudu, R.R.; Ketchum, K.A.; Kinsinger, C.R.; et al. A Description of the Clinical Proteomic Tumor Analysis Consortium (CPTAC) Common Data Analysis Pipeline. J. Proteome Res. 2016, 15, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.M.; Li, J.; Wang, Y.; Akbani, R.; Lu, Y.; Mills, G.B.; Liang, H. TCPA v3.0: An Integrative Platform to Explore the Pan-Cancer Analysis of Functional Proteomic Data. Mol. Cell. Proteom. 2019, 18, S15–S25. [Google Scholar] [CrossRef]
- Geffen, Y.; Anand, S.; Akiyama, Y.; Yaron, T.M.; Song, Y.; Johnson, J.L.; Govindan, A.; Babur, Ö.; Li, Y.; Huntsman, E.; et al. Pan-cancer analysis of post-translational modifications reveals shared patterns of protein regulation. Cell 2023, 186, 3945–3967.e26. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, J.; Marostica, E.; Yuan, W.; Jin, J.; Zhang, J.; Li, R.; Tang, H.; Wang, K.; Li, Y.; et al. A pathology foundation model for cancer diagnosis and prognosis prediction. Nature 2024, 634, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhan, X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018, 9, 77–102. [Google Scholar] [CrossRef] [PubMed]
- van Delft, F.; Schuurbiers, M.; Muller, M.; Burgers, S.; Rossum, H.; Ijzerman, M.; Heuvel, M.; Koffijberg, H. Comparing modeling strategies combining changes in multiple serum tumor biomarkers for early prediction of immunotherapy non-response in non-small cell lung cancer. Tumor Biol. 2023, 46, S269–S281. [Google Scholar] [CrossRef]
- Maroco, J.; Silva, D.; Pina Rodrigues, A.; Guerreiro, M.; Santana, I.; Mendonça, A. Data mining methods in the prediction of Dementia: A real-data comparison of the accuracy, sensitivity and specificity of linear discriminant analysis, logistic regression, neural networks, support vector machines, classification trees and random forests. BMC Res. Notes 2011, 4, 299. [Google Scholar] [CrossRef]
- Moshawrab, M.; Adda, M.; Bouzouane, A.; Ibrahim, H.; Raad, A. Reviewing Federated Machine Learning and Its Use in Diseases Prediction. Sensors 2023, 23, 2112. [Google Scholar] [CrossRef]
- William, C.; Wangmo, C.; Ranjan, A. Unravelling the application of machine learning in cancer biomarker discovery. Cancer Insight 2023, 2, 1–8. [Google Scholar] [CrossRef]
- Qureshi, M.D.A.; Ramzan, M.F.; Amjad, F.; Haider, N. Artificial Intelligence in Metabolomics for Disease Profiling: A Machine Learning Approach to Biomarker Discovery. Indus J. Biosci. Res. 2024, 2, 87–96. [Google Scholar] [CrossRef]
- Wang, F.; Su, Q.; Li, C. Identidication of novel biomarkers in non-small cell lung cancer using machine learning. Sci. Rep. 2022, 12, 16693. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Park, U.-H.; Goh, C.; Park, D.; Lim, Y.; Lee, I.; Do, W.-J.; Lee, K.; Kim, H.; Yun, S.-Y.; et al. Enhancing Lung Cancer Classification through Integration of Liquid Biopsy Multi-Omics Data with Machine Learning Techniques. Cancers 2023, 15, 4556. [Google Scholar] [CrossRef]
- Rigakos, G.; Razis, E.; Koliou, G.-A.; Oikonomopoulos, G.; Tsolaki, E.; Sperinde, J.; Chrisafi, S.; Zarkavelis, G.; Pazarli, E.; Batistatou, A.; et al. Evaluation of the Role of p95 HER2 Isoform in Trastuzumab Efficacy in Metastatic Breast Cancer. Anticancer. Res. 2021, 41, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.W.; Yang, B.; Chi, Y.; Wu, J. p95HER2 expression in HER2-positive breast cancer with primary resistance. Precis. Med. Sci. 2024, 13, 99–106. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 2087204. [Google Scholar] [CrossRef]
- Thomas, D.; Sagar, S.; Liu, X.; Lee, H.-R.; Grunkemeyer, J.A.; Grandgenett, P.M.; Caffrey, T.; O’Connell, K.A.; Swanson, B.; Marcos-Silva, L.; et al. Isoforms of MUC16 activate oncogenic signaling through EGF receptors to enhance the progression of pancreatic cancer. Mol. Ther. 2021, 29, 1557–1571. [Google Scholar] [CrossRef]
- Duncan, K.D.; Pětrošová, H.; Lum, J.J.; Goodlett, D.R. Mass spectrometry imaging methods for visualizing tumor heterogeneity. Curr. Opin. Biotechnol. 2024, 86, 103068. [Google Scholar] [CrossRef]
- McGee, J.P.; Su, P.; Durbin, K.R.; Hollas, M.A.R.; Bateman, N.W.; Maxwell, G.L.; Conrads, T.P.; Fellers, R.T.; Melani, R.D.; Camarillo, J.M.; et al. Automated imaging and identification of proteoforms directly from ovarian cancer tissue. Nat. Commun. 2023, 14, 6478. [Google Scholar] [CrossRef]
- Li, M.; Zuo, J.; Yang, K.; Wang, P.; Zhou, S. Proteomics mining of cancer hallmarks on a single-cell resolution. Mass Spectrom. Rev. 2024, 43, 1019–1040. [Google Scholar] [CrossRef]
- Su, P.-R.; You, L.; Beerens, C.; Bezstarosti, K.; Demmers, J.; Pabst, M.; Kanaar, R.; Hsu, C.-C.; Chien, M.-P. Microscopy-based single-cell proteomic profiling reveals heterogeneity in DNA damage response dynamics. Cell Rep. Methods 2022, 2, 100237. [Google Scholar] [CrossRef] [PubMed]
- King, M.E.; Zhang, J.; Lin, J.Q.; Garza, K.Y.; DeHoog, R.J.; Feider, C.L.; Bensussan, A.; Sans, M.; Krieger, A.; Badal, S.; et al. Rapid diagnosis and tumor margin assessment during pancreatic cancer surgery with the MasSpec Pen technology. Proc. Natl. Acad. Sci. USA 2021, 118, e2104411118. [Google Scholar] [CrossRef]
- Sans, M.; Zhang, J.; Lin, J.Q.; Feider, C.L.; Giese, N.; Breen, M.T.; Sebastian, K.; Liu, J.; Sood, A.K.; Eberlin, L.S. Performance of the MasSpec Pen for Rapid Diagnosis of Ovarian Cancer. Clin. Chem. 2019, 65, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.Y.; King, M.E.; Nagi, C.; DeHoog, R.J.; Zhang, J.; Sans, M.; Krieger, A.; Feider, C.L.; Bensussan, A.V.; Keating, M.F.; et al. Intraoperative Evaluation of Breast Tissues During Breast Cancer Operations Using the MasSpec Pen. JAMA Netw. Open 2024, 7, e242684. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, M.; Gamella, M.; Serafín, V. Chapter 19 Nanomachines and nanorobotics: Improving cancer diagnosis and therapy. In The Detection of Biomarkers; Ozkan, S.A., Bakirhan, N.K., Mollarasouli, F., Eds.; Academic Press: Cambridge, UK, 2022; pp. 503–543. [Google Scholar] [CrossRef]
- Syedmoradi, L.; Norton, M.L.; Omidfar, K. Point-of-care cancer diagnostic devices: From academic research to clinical translation. Talanta 2021, 225, 122002. [Google Scholar] [CrossRef]
- Fu, L.; Karimi-Maleh, H. Leveraging electrochemical sensors to improve efficiency of cancer detection. World J. Clin. Oncol. 2024, 15, 360–366. [Google Scholar] [CrossRef]
- Saasa, V.; Chibagidi, R.; Ipileng, K.; Feleni, U. Advances in cancer detection: A review on electrochemical biosensor technologies. Sens. Bio-Sens. Res. 2025, 49, 100826. [Google Scholar] [CrossRef]
- Kim, Y.J.; Rho, W.-Y.; Park, S.-m.; Jun, B.-H. Optical nanomaterial-based detection of biomarkers in liquid biopsy. J. Hematol. Oncol. 2024, 17, 10. [Google Scholar] [CrossRef]
- Campuzano, S.; Barderas, R.; Moreno-Casbas, M.T.; Almeida, Á.; Pingarrón, J.M. Pursuing precision in medicine and nutrition: The rise of electrochemical biosensing at the molecular level. Anal. Bioanal. Chem. 2024, 416, 2151–2172. [Google Scholar] [CrossRef]
- O’Brien, C.; Khor, C.K.; Ardalan, S.; Ignaszak, A. Multiplex electrochemical sensing platforms for the detection of breast cancer biomarkers. Front. Med. Technol. 2024, 6, 1360510. [Google Scholar] [CrossRef]
- Dosnon, L.; Rduch, T.; Meyer, C.; Herrmann, I. A Wearable In-Pad Diagnostic for the Detection of Disease Biomarkers in Menstruation Blood. Adv. Sci. 2025, 12, e05170. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, N.; Kashif, A.; Yang, S.-J.; Nam, A.R.; Song, I.-C.; Gong, S.-J.; Hong, M.; Kim, G.; Seok, P.; et al. S100A8 and S100A9 Promote Apoptosis of Chronic Eosinophilic Leukemia Cells. Front. Immunol. 2020, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Baydar, E.; Celikkol, A.; GÜRdal, S.; Seber, S. Predictive Value of Serum Calprotectin Level in Response to Treatment, a New Inflammatory Marker in Patients with Breast Cancer Requesting Neoadjuvant Treatment. Namık Kemal Tıp Derg. 2023, 11, 12–16. [Google Scholar] [CrossRef]
- Argyris, P.P.; Slama, Z.M.; Ross, K.F.; Khammanivong, A.; Herzberg, M.C. Calprotectin and the Initiation and Progression of Head and Neck Cancer. J. Dent. Res. 2018, 97, 674–682. [Google Scholar] [CrossRef]
- Chen, L.; Shu, P.; Zhang, X.; Ye, S.; Tian, L.; Shen, S.; Ma, J.; Ai, F.; Li, X. S100A8-Mediated Inflammatory Signaling Drives Colorectal Cancer Progression via the CXCL5/CXCR2 Axis. J. Cancer 2024, 15, 3452–3465. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chang, J.; Jia, B.; Liu, J. The Blood Biomarkers of Thyroid Cancer. Cancer Manag. Res. 2020, 12, 5431–5438. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, D.; Huang, X.; Cai, M.; Yuan, K.; Huang, P. S100A8 as a Promising Biomarker and Oncogenic Immune Protein in the Tumor Microenvironment: An Integrative Pancancer Analysis. J. Oncol. 2022, 2022, 6947652. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, M.; Li, T.; Wu, Y.; Gao, J.; Yi, M.; Wu, K. S100A8/A9 as a risk factor for breast cancer negatively regulated by DACH1. Biomark. Res. 2023, 11, 106. [Google Scholar] [CrossRef]
- Miller, P.; Kidwell, K.M.; Thomas, D.; Sabel, M.; Rae, J.M.; Hayes, D.F.; Hudson, B.I.; El-Ashry, D.; Lippman, M.E. Elevated S100A8 protein expression in breast cancer cells and breast tumor stroma is prognostic of poor disease outcome. Breast Cancer Res. Treat. 2017, 166, 85–94. [Google Scholar] [CrossRef]
- Hermani, A.; Hess, J.; De Servi, B.; Medunjanin, S.; Grobholz, R.; Trojan, L.; Angel, P.; Mayer, D. Calcium-Binding Proteins S100A8 and S100A9 as Novel Diagnostic Markers in Human Prostate Cancer. Clin. Cancer Res. 2005, 11, 5146–5152. [Google Scholar] [CrossRef] [PubMed]
- Ebbing, J.; Mathia, S.; Seibert, F.; Pagonas, N.; Bauer, F.; Erber, B.; Günzel, K.; Kilic, E.; Kempkensteffen, C.; Miller, K.; et al. Urinary calprotectin: A new diagnostic marker in urothelial carcinoma of the bladder. World J. Urol. 2013, 32, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-r.; Pang, Z.; Wang, Q.-z.; Ou-Yang, S. S100A8 promotes the proliferation, migration and invasion in bladder cancer cells. J. Cancer 2025, 16, 1066–1077. [Google Scholar] [CrossRef]
- Miranda-Galvis, M.; Soares, C.C.; Carnielli, C.M.; Buttura, J.R.; de Sá, R.S.; Kaminagakura, E.; Marchi, F.A.; Leme, A.F.P.; Pinto, C.A.L.; Santos-Silva, A.R.; et al. New Insights into the Impact of Human Papillomavirus on Oral Cancer in Young Patients: Proteomic Approach Reveals a Novel Role for S100A8. Cells 2023, 12, 1323. [Google Scholar] [CrossRef]
- Shen, J.; Person, M.D.; Zhu, J.; Abbruzzese, J.L.; Li, D. Protein Expression Profiles in Pancreatic Adenocarcinoma Compared with Normal Pancreatic Tissue and Tissue Affected by Pancreatitis as Detected by Two-Dimensional Gel Electrophoresis and Mass Spectrometry. Cancer Res. 2004, 64, 9018–9026. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, I.D.; Cholidou, K.G.; Kallianidis, K.; Perrea, D.; Antsaklis, A. The role of calprotectin in obstetrics and gynecology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 151, 3–9. [Google Scholar] [CrossRef]
- Walker, J.; Joy, A.A.; Vos, L.J.; Stenson, T.H.; Mackey, J.R.; Jovel, J.; Kao, D.; Madsen, K.L.; Wong, G.K.-S. Chemotherapy-induced weight gain in early-stage breast cancer: A prospective matched cohort study reveals associations with inflammation and gut dysbiosis. BMC Med. 2023, 21, 178. [Google Scholar] [CrossRef]
- Yasar, O.; Akcay, T.; Obek, C.; Turegun, F.A. Significance of S100A8, S100A9 and calprotectin levels in bladder cancer. Scand. J. Clin. Lab. Investig. 2017, 77, 437–441. [Google Scholar] [CrossRef]
- Sahin, Y.; Yucetas, U.; Ates, H.A.; Erkan, E.; Yucetas, E.; Temiz, M.Z.; Toktas, M.G.; Kadihasanoglu, M.; Topkaya, B.C. Improving the diagnosis of high grade and stage bladder cancer by detecting increased urinary calprotectin expression in tumor tissue and tumor-associated inflammatory response. Investig. Clin. Urol. 2019, 60, 343–350. [Google Scholar] [CrossRef]
- Ye, X.; Huai, J.; Ding, J. Diagnostic accuracy of fecal calprotectin for screening patients with colorectal cancer: A meta-analysis. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2018, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Algeciras-Schimnich, A.; Preissner, C.; Theobald, J.; Finseth, M.; Grebe, S. Procalcitonin: A Marker for the Diagnosis and Follow-Up of Patients with Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2008, 94, 861–868. [Google Scholar] [CrossRef]
- Sun, J.; Chen, X.; Wang, Y. Comparison of the diagnostic value of CEA combined with OPN or DKK1 in non-small cell lung cancer. Oncol. Lett. 2020, 20, 3046–3052. [Google Scholar] [CrossRef]
- Sequist, L.V.; Skates, S.J.; Haas, W. A New Era of Protein-Based Assays for Cancer Early Detection. J. Thorac. Oncol. 2021, 16, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Ostrin, E.J.; Bantis, L.E.; Wilson, D.O.; Patel, N.; Wang, R.; Kundnani, D.; Adams-Haduch, J.; Dennison, J.B.; Fahrmann, J.F.; Chiu, H.T.; et al. Contribution of a Blood-Based Protein Biomarker Panel to the Classification of Indeterminate Pulmonary Nodules. J. Thorac. Oncol. 2021, 16, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Nolen, B.M.; Lomakin, A.; Marrangoni, A.; Velikokhatnaya, L.; Prosser, D.; Lokshin, A.E. Urinary Protein Biomarkers in the Early Detection of Lung Cancer. Cancer Prev. Res. 2015, 8, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lavoue, V.; Favier, A.; Franck, S.; Boutet, G.; Azuar, A.-S.; Brousse, S.; Golfier, F.; Uzan, C.; Vaysse, C.; Molière, S.; et al. French college of gynecologists and obstetricians (CNGOF) recommendations for clinical practice: Place of breast self-examination in screening strategies. Breast 2024, 75, 103619. [Google Scholar] [CrossRef] [PubMed]
- Vincent, Z.; Hornby, S.; Ball, S.; Sanders, G.; Ayling, R.M. Faecal calprotectin as a marker for oesophago-gastric cancer. Ann. Clin. Biochem. 2015, 52, 660–664. [Google Scholar] [CrossRef]
- Martinez-Garcia, E.; Lesur, A.; Devis, L.; Cabrera, S.; Matias-Guiu, X.; Hirschfeld, M.; Asberger, J.; van Oostrum, J.; Casares de Cal, M.d.l.Á.; Gómez-Tato, A.; et al. Targeted Proteomics Identifies Proteomic Signatures in Liquid Biopsies of the Endometrium to Diagnose Endometrial Cancer and Assist in the Prediction of the Optimal Surgical Treatment. Clin. Cancer Res. 2017, 23, 6458–6467. [Google Scholar] [CrossRef]
- Ngan, T.T.; Nguyen, N.T.Q.; Van Minh, H.; Donnelly, M.; O’Neill, C. Effectiveness of clinical breast examination as a ‘stand-alone’ screening modality: An overview of systematic reviews. BMC Cancer 2020, 20, 1070. [Google Scholar] [CrossRef]
- Fenton, J.J.; Rolnick, S.J.; Harris, E.L.; Barton, M.B.; Barlow, W.E.; Reisch, L.M.; Herrinton, L.J.; Geiger, A.M.; Fletcher, S.W.; Elmore, J.G. Specificity of Clinical Breast Examination in Community Practice. J. Gen. Intern. Med. 2007, 22, 332–337. [Google Scholar] [CrossRef][Green Version]
- Chen, F.K.; de Abreu, A.L.C.; Palmer, S.L. Utility of Ultrasound in the Diagnosis, Treatment, and Follow-up of Prostate Cancer: State of the Art. J. Nucl. Med. 2016, 57 (Suppl. S3), 13S–18S. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Diagnostic Performance of Prostate Imaging Reporting and Data System Version 2 for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-analysis. Eur. Urol. 2017, 72, 177–188. [Google Scholar] [CrossRef]
| Features | Histology | LB |
|---|---|---|
| Limitations | limited for certain types of cancer | potential for detection across multiple cancer types |
| limited to one or few biomarkers by multiplex detection in IHC | panel/multiple biomarkers assignment | |
| time consuming, labor-intensive | detection of low-abundant proteins can be challenging; faster results | |
| Invasiveness | invasive | non-invasive or minimally invasive |
| multiple biopsies are not feasible due to their invasive nature | can be performed more frequently with fewer risks to patient | |
| Accuracy | high specificity, depends on sample quality, interpretation variability and human error | high specificity and sensitivity, especially with advanced technologies |
| Risk to patient | infections, bleeding, pain, and prolonged recovery | minimal to no risk |
| Sample type | tissue sample, usually from biopsy or surgical resection | detect CTCs, DNA, EVs, proteins, metabolites from multiple tumor regions |
| Monitoring and fellow-up | provides a snapshot of the tumor at the time of biopsy | allows for real-time monitoring of cancer |
| Molecular insights | inability to monitor dynamic changes, provides limited molecular insights, focusing on histological features | dynamic insight, provides a broader range of molecular biomarkers |
| Early cancer detection | risk of missing early or small lesions, limitation in detection of early-stage cancer or micrometastases | allows for early detection of cancer and minimal residual disease |
| Detection of tumor heterogeneity | limited; may not capture the clonal and molecular diversity within the tumor | can detect molecular variations across the entire tumor mass, including mutations and PTMs of proteins |
| Cost | generally higher | generally lower |
| Treatment decision-making | delayed decision-making for treatment | accelerate decision-making for treatment |
| Features | CT | LB |
|---|---|---|
| Principle | considered non-invasive, possible negative effects due to dose-cumulative effects of X-rays | non-invasive/minimally invasive |
| Diagnostic ability | high-resolution images for detecting tumors, distant metastasis, and anatomical changes | detects molecular biomarkers such as mutations, gene expression, and tumor-related proteins |
| Sensitivity | high sensitivity for detecting structural changes, particularly in large tumors | depends on the biomarker used and the cancer stage but can detect early-stage cancers |
| Specificity | varies among tumor types, size, location | depends on the biomarker panel |
| Tumor monitoring | effective for monitoring tumor size and response to treatment | monitors continuously and non-invasive the response to therapy |
| Costs | can be expensive, necessitates imaging equipment | less expensive than conventional imaging, the costs depends on the complexity of the test |
| Clinical applications | commonly use in cancer detection, staging, monitoring, and evaluating metastasis | used for early-stage cancer detection, monitoring minimal residual disease, and assessing treatment efficiency; earlier detection of metastasis at molecular level |
| Use in early detection | detects tumors that have reached a certain size | detects cancer even before clinical signs are present |
| Advantages | high spatial resolution accuracy, effective for visualizing tumor anatomy | provides molecular insights into tumors, can be done repeatedly, and is non- or minimally invasive |
| Disadvantages | limited in early-stage cancer detection, X-ray exposure is disputable; not detects molecular characteristics | many methods require additional validation, can have lower sensitivity for certain tumor types |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, A.-N.; Bruno, P.S.; Josan, C.-L.; Waterman, N.; Morrissiey, H.; Njoku, V.T.; Darie, C.C. In Search of Ideal Solutions for Cancer Diagnosis: From Conventional Methods to Protein Biomarkers in Liquid Biopsy. Proteomes 2025, 13, 47. https://doi.org/10.3390/proteomes13040047
Neagu A-N, Bruno PS, Josan C-L, Waterman N, Morrissiey H, Njoku VT, Darie CC. In Search of Ideal Solutions for Cancer Diagnosis: From Conventional Methods to Protein Biomarkers in Liquid Biopsy. Proteomes. 2025; 13(4):47. https://doi.org/10.3390/proteomes13040047
Chicago/Turabian StyleNeagu, Anca-Narcisa, Pathea S. Bruno, Claudiu-Laurentiu Josan, Natalie Waterman, Hailey Morrissiey, Victor T. Njoku, and Costel C. Darie. 2025. "In Search of Ideal Solutions for Cancer Diagnosis: From Conventional Methods to Protein Biomarkers in Liquid Biopsy" Proteomes 13, no. 4: 47. https://doi.org/10.3390/proteomes13040047
APA StyleNeagu, A.-N., Bruno, P. S., Josan, C.-L., Waterman, N., Morrissiey, H., Njoku, V. T., & Darie, C. C. (2025). In Search of Ideal Solutions for Cancer Diagnosis: From Conventional Methods to Protein Biomarkers in Liquid Biopsy. Proteomes, 13(4), 47. https://doi.org/10.3390/proteomes13040047







