Proteomic Analysis of Mechanical Injury Effects in Papaya Fruit at Two Maturity Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Papaya Ripeness Measurement
2.3. Mechanical Damage Treatment
2.4. Optical Microscopy Analysis
2.5. Protein Extraction Protocol
2.6. Digestion and Tandem Mass Tag (TMT) Labeling
2.7. Protein Fractionation
2.8. Nano-LC-MS/MS and Synchronous Precursor Selection (SPS)-MS3
2.9. Protein Identification and Quantification
2.10. Bioinformatic Analysis
2.11. Statistical Analysis
3. Results
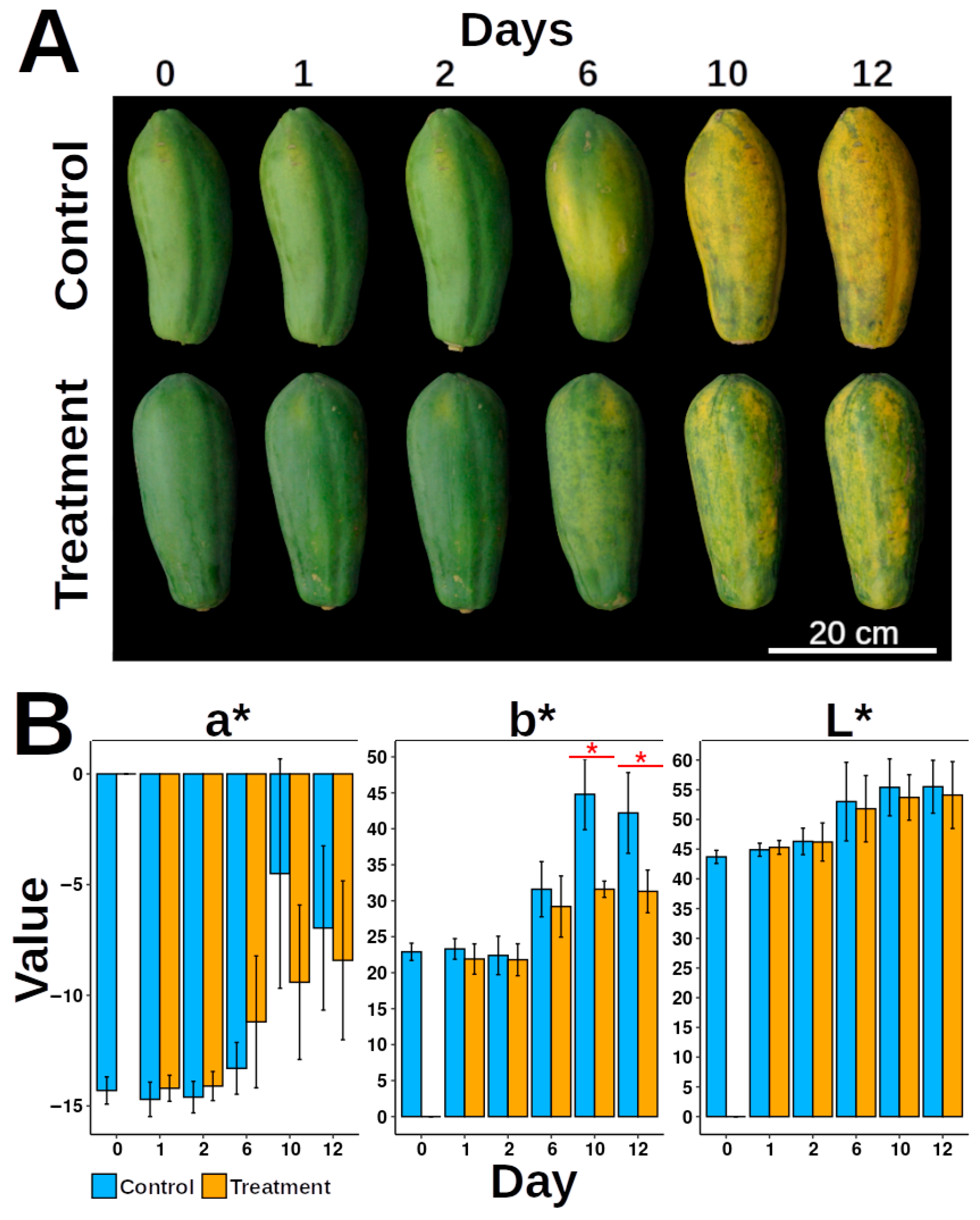
3.1. Epicarp Color Measurement
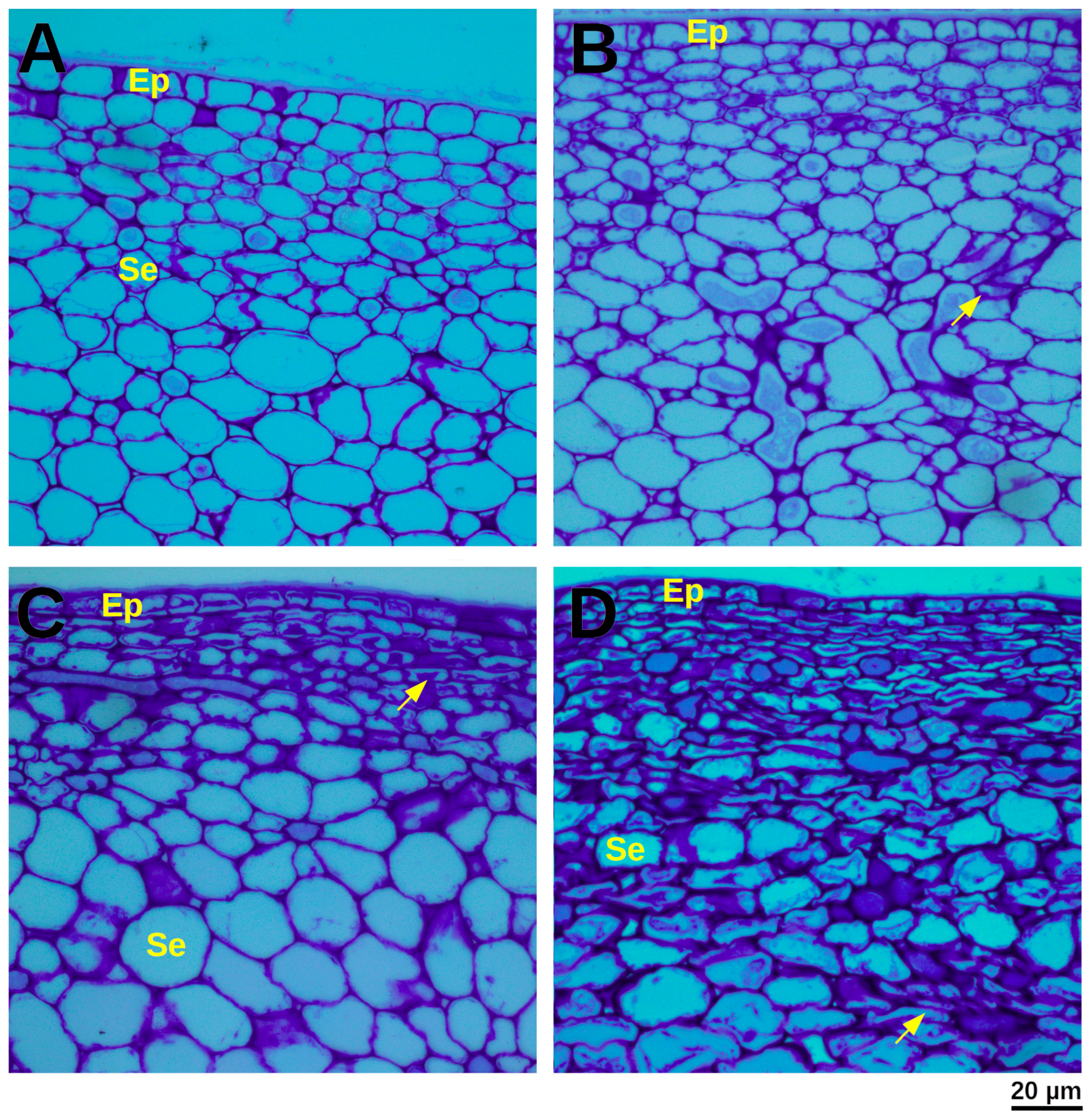
3.2. Assessment of Physical Damage Using Optical Microscopy
3.3. Protein Extraction Protocol
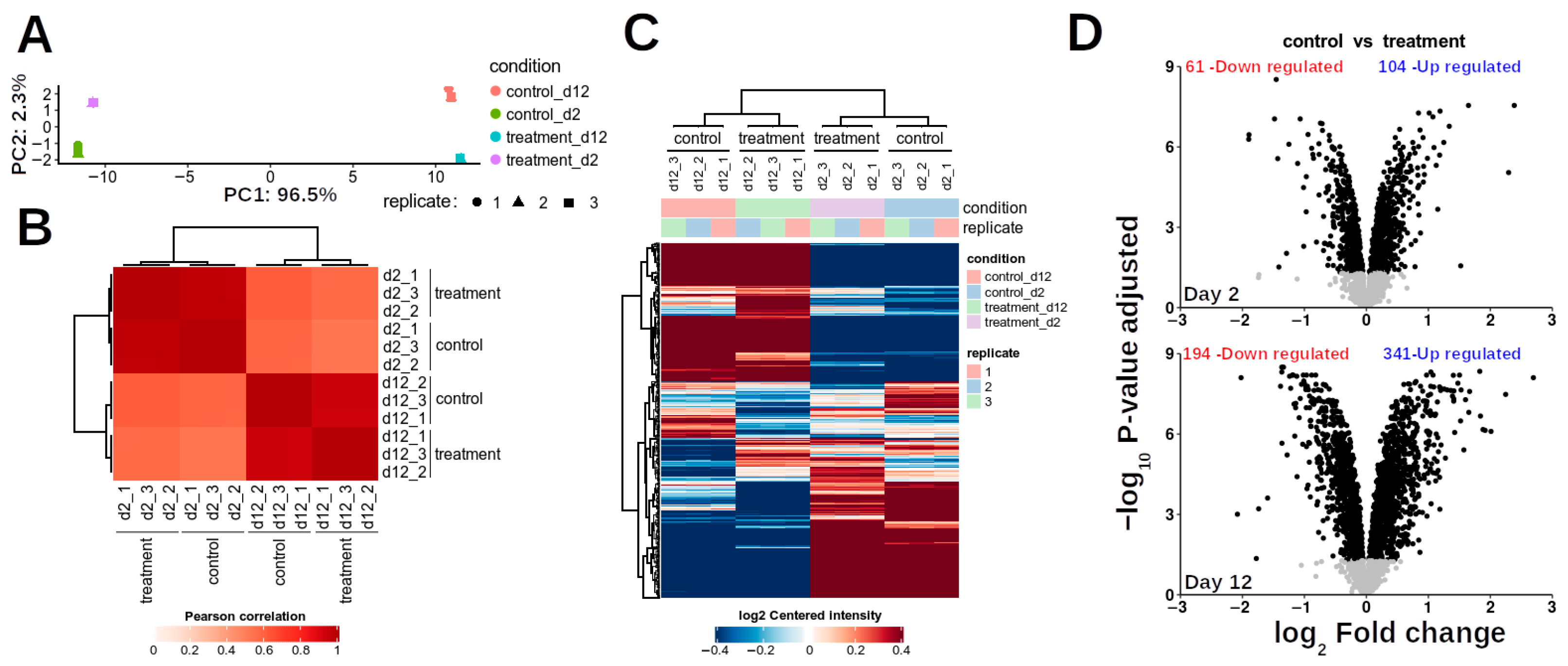
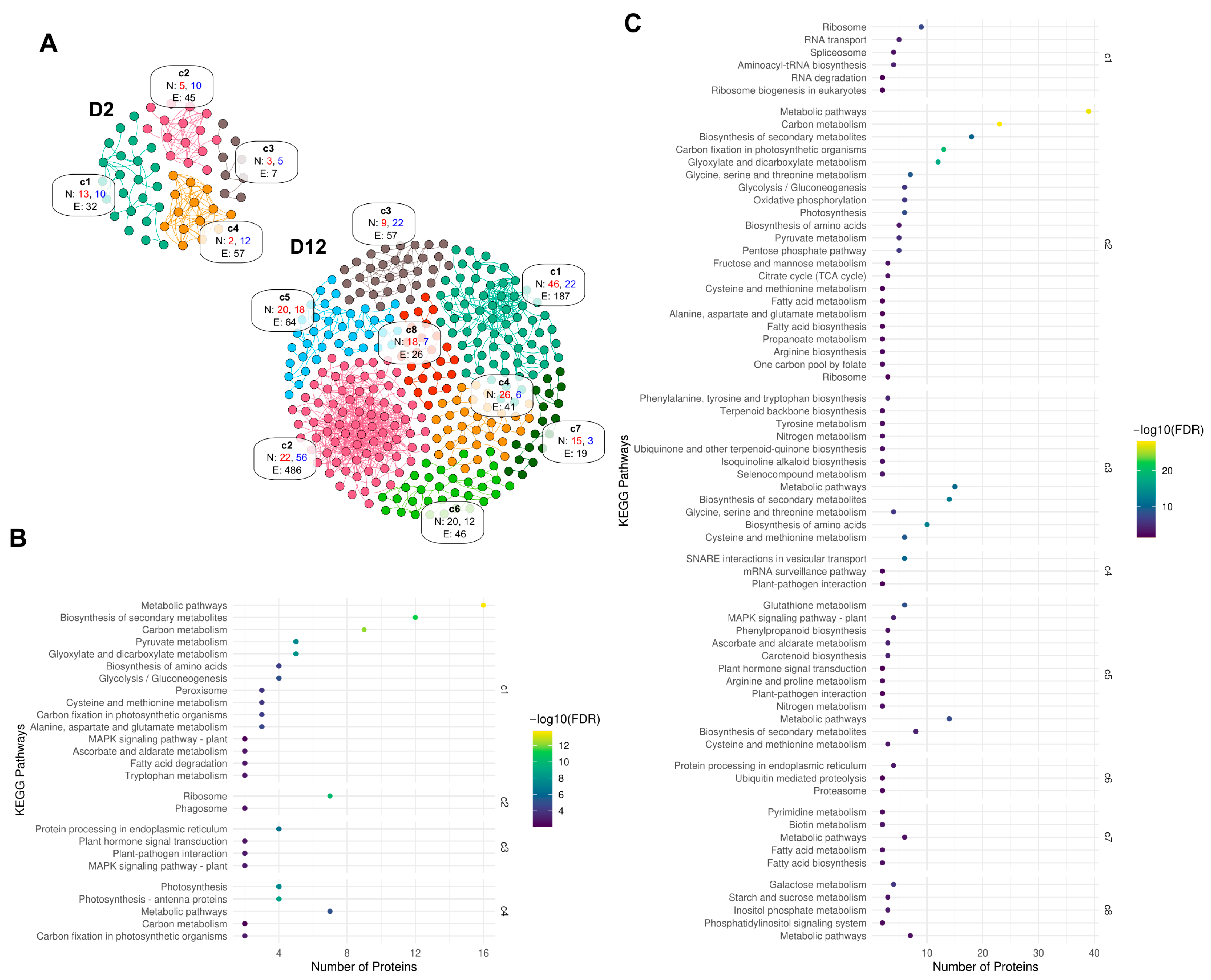
3.4. Bioinformatics Analysis
4. Discussion
4.1. Exocarp Color as Papaya Ripeness Indicator
4.2. Proteomic Insights into Papaya Exocarp Response to Mechanical Damage
4.3. Mechanical Damage Triggers Ripening-Stage-Specific Proteomic Remodeling in Papaya Exocarp
4.4. Calcium and ROS: Key Messengers in Damage Perception and Signal Amplification
4.5. Ethylene Biosynthesis Modulation: A Ripening-Dependent Response
4.6. ABA and Carotenoid Biosynthesis Are Suppressed at Late Ripening Stages
4.7. Photosynthesis and Primary Metabolism Are Down-Regulated During Late Defense Activation
4.8. Plasmodesmata Regulation via Callose Metabolism: Balancing Connectivity and Defense
4.9. Suberization: A Lipid-Based Defense Against Water Loss and Pathogens
4.10. Induction of Pathogenesis-Related Proteins and Vesicular Transport
4.11. Challenges and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| Ca2+ | Calcium Ion |
| DAPs | Differentially Accumulated Proteins |
| EC | Epicarp Color |
| PPI | Protein–Protein Interaction |
| PSL | Postharvest Shelf Life |
| PR | Pathogenesis-Related |
| ROS | Reactive Oxygen Species |
References
- Tan, G.H.; Ali, A.; Siddiqui, Y. Major fungal postharvest diseases of papaya: Current and prospective diagnosis methods. Crop Prot. 2023, 174, 106399. [Google Scholar] [CrossRef]
- Vinod, B.R.; Asrey, R.; Sethi, S.; Prakash, J.; Meena, N.K.; Menaka, M.; Mishra, S.; Shivaswamy, G. Recent advances in physical treatments of papaya fruit for postharvest quality retention: A review. eFood 2023, 4, e79. [Google Scholar] [CrossRef]
- Pasini, J.; Bender, R.; Antoniolli, L. Methods to evidence mechanical injuries on ‘Packham’s Triumph’ pears. Acta Hortic. 2016, 1120, 311–316. [Google Scholar] [CrossRef]
- Sigalingging, R.; Nasution, H.; Rindang, A.; Ayu, P. Effect of Packaging Fillers Materials on the Quality of Papaya Fruit (Carica papaya I.). IOP Conf. Ser. Earth Environ. Sci. 2019, 260, 012036. [Google Scholar] [CrossRef]
- Workneh, T.S.; Azene, M.; Tesfay, S.Z. A review on the integrated agro-technology of papaya fruit. Afr. J. Biotechnol. 2012, 11, 15098–15110. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Rodríguez-Hernández, M.J.; Hernández-Hernández, H.M.; Delgado-Sánchez, L.F.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Effect of an edible coating based on chitosan and oxidized starch on shelf life of Carica papaya L., and its physicochemical and antimicrobial properties. Coatings 2018, 8, 318. [Google Scholar] [CrossRef]
- Quintana, M.E.G.; Paull, R.E.; Way, M. Mechanical injury during postharvest handling of ‘Solo’ papaya fruit. J. Am. Soc. Hortic. Sci. 1993, 118, 618–622. [Google Scholar] [CrossRef]
- Parven, A.; Sarker, M.R.; Megharaj, M.; Meftaul, I.M. Prolonging the shelf life of Papaya (Carica papaya L.) using Aloe vera gel at ambient temperature. Sci. Hortic. 2020, 265, 109228. [Google Scholar] [CrossRef]
- Santamaría Basulto, F.; Sauri Duch, E.; Espadas y Gil, F.; Díaz Plaza, R.; Larqué Saavedra, A.; Santamaría, J.M. Postharvest ripening and maturity indices for maradol papaya. Interciencia 2009, 34, 583–588. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-18442009000800012&lng=es&tlng=en (accessed on 23 June 2025).
- Teixeira da Silva, J.A.; Rashid, Z.; Nhut, D.T.; Sivakumar, D.; Gera, A.; Teixeira, M.; Tennant, P.F. Papaya (Carica papaya L.) biology and biotechnology. Tree For. Sci. Biotechnol. 2007, 1, 47–73. [Google Scholar]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Preharvest factors influencing bruise damage of fresh fruits-A review. Sci. Hortic. 2018, 229, 45–58. [Google Scholar] [CrossRef]
- Li, Z.; Thomas, C. Quantitative evaluation of mechanical damage to fresh fruits. Trends Food Sci. Technol. 2014, 35, 138–150. [Google Scholar] [CrossRef]
- de Godoy-Beltrame, A.E.; Jacomino, A.P.; Costa Cerqueira-Pereira, E.; Almeida, A.C. Mechanical injuries and their effects on the physiology of ‘Golden’ papaya fruit. Rev. Iberoam. Tecnol. Postcosecha 2015, 16, 49–57. [Google Scholar]
- Opara, U.L.; Pathare, P.B. Bruise damage measurement and analysis of fresh horticultural produce-A review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Pedreschi, R. Postharvest proteomics of perishables. In Proteomics in Food Science: From Farm to Fork; Colgrave, M.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–16. [Google Scholar] [CrossRef]
- Mathabe, P.M.K.; Belay, Z.A.; Ndlovu, T.; Caleb, O.J. Progress in proteomic profiling of horticultural commodities during postharvest handling and storage: A review. Sci. Hortic. 2020, 261, 108996. [Google Scholar] [CrossRef]
- Rocco, M.; d’Ambrosio, C.; Arena, S.; Faurobert, M.; Scaloni, A.; Marra, M. Proteomic analysis of tomato fruits from two ecotypes during ripening. Proteomics 2006, 6, 3781–3791. [Google Scholar] [CrossRef]
- Palma, J.M.; Corpas, F.J.; del Río, L.A. Proteomics as an approach to the understanding of the molecular physiology of fruit development and ripening. J. Proteom. 2011, 74, 1230–1243. [Google Scholar] [CrossRef]
- Prinsi, B.; Negri, A.S.; Fedeli, C.; Morgutti, S.; Negrini, N.; Cocucci, M.; Espen, L. Peach fruit ripening: A proteomic comparative analysis of the mesocarp of two cultivars with different flesh firmness at two ripening stages. Phytochemistry 2011, 72, 1251–1262. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Belghazi, M.; Job, D.; Manganaris, G.A.; Molassiotis, A.; Vasilakakis, M. Physiological and proteomic approaches to address the active role of ozone in kiwifruit post-harvest ripening. J. Exp. Bot. 2012, 63, 2449–2464. [Google Scholar] [CrossRef]
- Camacho-Vázquez, C.; Ruiz-May, E.; Guerrero-Analco, J.A.; Elizalde-Contreras, J.M.; Enciso-Ortiz, E.J.; Rosas-Saito, G.; López-Sánchez, L.; Kiel-Martínez, A.L.; Bonilla-Landa, I.; Monribot-Villanueva, J.L.; et al. Filling gaps in our knowledge on the cuticle of mangoes (Mangifera indica) by analyzing six fruit cultivars: Architecture/structure, postharvest physiology, and possible resistance to fruit fly (Tephritidae) attack. Postharvest Biol. Technol. 2019, 148, 83–96. [Google Scholar] [CrossRef]
- Chin, C.F.; Teoh, E.Y.; Chee, M.J.; Al-Obaidi, J.R.; Rahmad, N.; Lawson, T. Comparative proteomic analysis on fruit ripening processes in two varieties of tropical mango (I). Protein J. 2019, 38, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Ocampo, J.Á.; Osuna-Castro, J.A.; Lino-López, G.J.; Barrera-Pacheco, A.; Mendoza-Hernández, G.; De León-Rodríguez, A.; Barba de la Rosa, A.P. Proteomic analysis of differentially accumulated proteins during ripening and in response to 1-MCP in papaya fruit. J. Proteom. 2012, 75, 2160–2169. [Google Scholar] [CrossRef]
- Nogueira, S.B.; Labate, C.A.; Gozzo, F.C.; Pilau, E.J.; Lajolo, F.M.; Oliveira do Nascimento, J.R. Proteomic analysis of papaya fruit ripening using 2DE-DIGE. J. Proteom. 2012, 75, 1428–1439. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.; Li, H.; Chen, K.; Lu, L.; Shen, C.; Zheng, X. A qualitative proteome-wide lysine crotonylation profiling of papaya (Carica papaya L.). Sci. Rep. 2018, 8, 8230. [Google Scholar] [CrossRef]
- Jiang, B.; Ou, S.; Xu, L.; Mai, W.; Ye, M.; Gu, H.; Zhang, T.; Yuan, C.; Shen, C.; Wang, J.; et al. Comparative proteomic analysis provides novel insights into the regulation mechanism underlying papaya (Carica papaya L.) exocarp during fruit ripening process. BMC Plant Biol. 2019, 19, 238. [Google Scholar] [CrossRef]
- Buron-Moles, G.; Torres, R.; Amoako-Andoh, F.; Vinas, I.; Teixido, N.; Usall, J.; Keulemans, W.; Davey, M.W. Analysis of changes in protein abundance after wounding in ‘Golden Delicious’ apples. Postharvest Biol. Technol. 2014, 87, 51–60. [Google Scholar] [CrossRef]
- Tosetti, R.; Tardelli, F.; Tadiello, A.; Zaffalon, V.; Giorgi, F.M.; Guidi, L.; Trainotti, L.; Bonghi, C.; Tonutti, P. Molecular and biochemical responses to wounding in mesocarp of ripe peach (Prunus persica L. Batsch) fruit. Postharvest Biol. Technol. 2014, 90, 40–51. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Klíma, M.; Renaut, J. Plant Proteoforms Under Environmental Stress: Functional Proteins Arising From a Single Gene. Front. Plant Sci. 2021, 12, 793113. [Google Scholar] [CrossRef]
- Barragán-Iglesias, J.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Ripeness indexes and physicochemical changes of papaya (Carica papaya L. cv. Maradol) during ripening on-tree. Sci. Hortic. 2018, 236, 272–278. [Google Scholar] [CrossRef]
- Piven, N.M.; Barredo-Pool, F.A.; Borges-Argáez, I.C.; Herrera-Alamillo, M.A.; Mayo-Mosqueda, A.; Herrera-Herrera, J.L.; Robert, M.L. Reproductive biology of henequén (Agave fourcroydes) and its wild ancestor Agave angustifolia (Agavaceae). I. Gametophyte development. Am. J. Bot. 2001, 88, 1966–1976. [Google Scholar] [CrossRef]
- Faurobert, M.; Pelpoir, E.; Chaïb, J. Phenol extraction of proteins for proteomic studies of recalcitrant plant tissues. In Plant Proteomics; Thiellement, H., Zivy, M., Damerval, C., Méchin, V., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 9–14. [Google Scholar] [CrossRef]
- Rivera-Pastrana, D.M.; Yahia, E.M.; González-Aguilar, G.A. Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage. J. Sci. Food Agric. 2010, 90, 2358–2365. [Google Scholar] [CrossRef]
- McAlister, G.C.; Nusinow, D.P.; Jedrychowski, M.P.; Wühr, M.; Huttlin, E.L.; Erickson, B.K.; Rad, R.; Haas, W.; Gygi, S.P. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 2014, 86, 7150–7158. [Google Scholar] [CrossRef]
- Ting, L.; Rad, R.; Gygi, S.P.; Haas, W. MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat. Methods 2011, 8, 937–940. [Google Scholar] [CrossRef]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Dorfer, V.; Pichler, P.; Stranzl, T.; Stadlmann, J.; Taus, T.; Winkler, S.; Mechtler, K. MS Amanda, a Universal Identification Algorithm Optimized for High Accuracy Tandem Mass Spectra. J. Proteome Res. 2014, 13, 3679–3684. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Storey, J.D.; MacCoss, M.J.; Noble, W.S. Assigning significance to peptides identified by tandem mass spectrometry using decoy databases. J. Proteome Res. 2008, 7, 29–34. [Google Scholar] [CrossRef]
- Yao, S.; You, R.; Wang, S.; Xiong, Y.; Huang, X.; Zhu, S. NetGO 2.0: Improving large-scale protein function prediction with massive sequence, text, domain, family and network information. Nucleic Acids Res. 2021, 49, W469–W475. [Google Scholar] [CrossRef]
- Zhang, X.; Smits, A.H.; van Tilburg, G.B.; Ovaa, H.; Huber, W.; Vermeulen, M. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat. Protoc. 2018, 13, 530–550. [Google Scholar] [CrossRef] [PubMed]
- Claudius, M.; Ludivine, T. Proteomic as a tool to study fruit ripening. In Proteomics in Food Science: From Farm to Fork, 1st ed.; Colgrave, M.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 127–141. [Google Scholar] [CrossRef]
- Vázquez-García, E.; Chávez-Franco, S.; Ariza-Flores, R.; Yahia-Kazuz, E.; Salazar-Zazueta, A.; Saucedo-Veloz, C.; Colinas-León, M.T. Propiedades mecánicas de frutos de papaya ‘Maradol Roja’ bajo compresión estática. Rev. Chapingo Ser. Hortic. 2003, 9, 345–355. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Dai, M.S.; Cai, D.Y.; Zhang, S.; Shi, Z.B. A review for the molecular research of russet/semi-russet of sand pear exocarp and their genetic characters. Sci. Hortic. 2016, 210, 138–142. [Google Scholar] [CrossRef]
- Stevens, F.C. Calmodulin: An introduction. Can. J. Biochem. Cell Biol. 1983, 61, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Ichida, A.; Wang, Y.; Gens, J.S.; Pickard, B.G.; Harper, J.F. Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 1999, 119, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: One protein, one gene, many functions. Biochem. J. 1999, 344, 281–292. [Google Scholar] [CrossRef]
- Joshi, R.; Paul, M.; Kumar, A.; Pandey, D. Role of calreticulin in biotic and abiotic stress signalling and tolerance mechanisms in plants. Gene 2019, 714, 144004. [Google Scholar] [CrossRef]
- Sparke, M.A.; Wünsche, J.N. Mechanosensing of plants. In Horticultural Reviews; Warrington, I., Ed.; Wiley: Hoboken, NJ, USA, 2020; Volume 47. [Google Scholar] [CrossRef]
- Askerlund, P. Calmodulin-stimulated Ca2+-ATPases in the vacuolar and plasma membranes in cauliflower. Plant Physiol. 1997, 114, 999–1007. [Google Scholar] [CrossRef]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef]
- Pacheco-Coeto, R.; Cárdenas-Torres, L.; Hernández-Rosas, F.; Hidalgo-Contreras, J.V.; Aquino-Pérez, G. Callose and reactive oxygen species expressed in sugarcane leaves by mechanical damage of spittlebugs. Rev. Mex. Cienc. Agrícolas 2019, 10, 105–114. [Google Scholar] [CrossRef]
- Vo, K.T.X.; Rahman, M.M.; Rahman, M.M.; Trinh, K.T.T.; Kim, S.T.; Jeon, J.S. Proteomics and metabolomics studies on the biotic stress responses of rice: An update. Rice 2021, 14, 30. [Google Scholar] [CrossRef]
- Yan, S.; Bhawal, R.; Yin, Z.; Thannhauser, T.W.; Zhang, S. Recent advances in proteomics and metabolomics in plants. Mol. Hortic. 2022, 2, 17. [Google Scholar] [CrossRef]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef]
- Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Adams, D.O.; Yang, S.F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 1979, 76, 170–174. [Google Scholar] [CrossRef]
- Houben, M.; Van de Poel, B. 1-Aminocyclopropane-1-carboxylic acid oxidase (ACO): The enzyme that makes the plant hormone ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef]
- España, L.; Heredia-Guerrero, J.A.; Reina-Pinto, J.J.; Fernández-Muñoz, R.; Heredia, A.; Domínguez, E. Transient silencing of CHALCONE SYNTHASE during fruit ripening modifies tomato epidermal cells and cuticle properties. Plant Physiol. 2014, 166, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Xie, X.; Chen, K.; Grierson, D. Ethylene and fruit softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- González-Guzmán, M.; Apostolova, N.; Bellés, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodríguez, P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 2002, 14, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Gampala, S.S.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14 (Suppl. S1), S15–S45. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Murillo, C.; Wurtzel, E.T. Maize Y9 encodes a product essential for 15-cis-ζ-carotene isomerization. Plant Physiol. 2007, 144, 1181–1189. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Wurtzel, E.T. Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol. 2010, 153, 66–79. [Google Scholar] [CrossRef]
- Shen, Y.H.; Yang, F.Y.; Lu, B.G.; Zhao, W.W.; Jiang, T.; Feng, L.; Chen, X.J.; Ming, R. Exploring the differential mechanisms of carotenoid biosynthesis in the yellow peel and red flesh of papaya. BMC Genom. 2019, 20, 49. [Google Scholar] [CrossRef]
- Meng, C.; Yang, D.; Ma, X.; Zhao, W.; Liang, X.; Ma, N.; Meng, Q. Suppression of tomato SlNAC1 transcription factor delays fruit ripening. J. Plant Physiol. 2016, 193, 88–96. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588. [Google Scholar] [CrossRef]
- Little, D.; Gouhier-Darimont, C.; Bruessow, F.; Reymond, P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2007, 143, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; Delucia, E.H. Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef]
- Cipollini, D.; Walters, D.; Voelckel, C. Costs of resistance in plants: From theory to evidence. Annu. Plant Rev. Online 2017, 47, 263–307. [Google Scholar] [CrossRef]
- Chen, X.Y.; Kim, J.Y. Callose synthesis in higher plants. Plant Signal. Behav. 2009, 4, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, M.; Epel, B.L. Macromolecular transport and signaling through plasmodesmata. Int. Rev. Cytol. 2004, 235, 93–164. [Google Scholar] [CrossRef]
- De Storme, N.; Geelen, D. Callose homeostasis at plasmodesmata: Molecular regulators and developmental relevance. Front. Plant Sci. 2014, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, L.J.; Feng, D.; Jiang, W.; Miu, W.; Li, N. Plasmodesmata-related structural and functional proteins: The long sought-after secrets of a cytoplasmic channel in plant cell walls. Int. J. Mol. Sci. 2019, 20, 2946. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Höfer, R.; Briesen, I.; Beck, M.; Pinot, F.; Schreiber, L.; Franke, R. The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω-hydroxylase involved in suberin monomer biosynthesis. J. Exp. Bot. 2008, 59, 2347–2360. [Google Scholar] [CrossRef]
- Chaves, I.; Pinheiro, C.; Paiva, J.A.P.; Planchon, S.; Sergeant, K.; Renaut, J.; Graça, J.A.; Costa, G.; Coelho, A.V.; Ricardo, C.P.P. Proteomic evaluation of wound-healing processes in potato (Solanum tuberosum L.) tuber tissue. Proteomics 2009, 9, 4154–4175. [Google Scholar] [CrossRef]
- Dasgupta, A.; Urquidi Camacho, R.A.; Enganti, R.; Cho, S.K.; Tucker, L.L.; Torreverde, J.S.; Abraham, P.E.; von Arnim, A.G. A phosphorylation-deficient ribosomal protein eS6 is largely functional in Arabidopsis thaliana, rescuing mutant defects from global translation and gene expression to photosynthesis and growth. Plant Direct. 2024, 8, e566. [Google Scholar] [CrossRef]
- Macnee, N.C.; Rebstock, R.; Hallett, I.C.; Schaffer, R.J.; Bulley, S.M. A review of current knowledge about the formation of native peridermal exocarp in fruit. Funct. Plant Biol. 2020, 47, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Wick, P.; Gansel, X.; Oulevey, C.; Page, V.; Studer, I.; Dürst, M.; Sticher, L. The expression of the t-SNARE AtSNAP33 is induced by pathogens and mechanical stimulation. Plant Physiol. 2003, 132, 343–351. [Google Scholar] [CrossRef]
- Momo, J.; Rawoof, A.; Kumar, A.; Islam, K.; Ahmad, I.; Ramchiary, N. Proteomics of reproductive development, fruit ripening, and stress responses in tomato. J. Agric. Food Chem. 2023, 71, 65–95. [Google Scholar] [CrossRef]
- Pratyusha, D.S.; Sarada, D.V.L. Rapid Alkalinization Factor—A cryptide regulating developmental and stress responses. Plant Sci. 2025, 359, 112600. [Google Scholar] [CrossRef] [PubMed]
- Sansebastiano, G.D.; Piro, G. The SNARE proteins (in plants) beyond the Nobel Prize. J. Plant Biochem. Physiol. 2014, 2, e122. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Soria, F.A.; Ruiz-May, E.; Castaño, E.; Herrera-Alamillo, M.Á.; Elizalde-Contreras, J.M.; Gamboa-Tuz, S.D.; Huerta-Nuñez, L.F.E.; Zamora-Briseño, J.A.; Rodríguez-Zapata, L.C. Proteomic Analysis of Mechanical Injury Effects in Papaya Fruit at Two Maturity Stages. Proteomes 2025, 13, 44. https://doi.org/10.3390/proteomes13030044
Reyes-Soria FA, Ruiz-May E, Castaño E, Herrera-Alamillo MÁ, Elizalde-Contreras JM, Gamboa-Tuz SD, Huerta-Nuñez LFE, Zamora-Briseño JA, Rodríguez-Zapata LC. Proteomic Analysis of Mechanical Injury Effects in Papaya Fruit at Two Maturity Stages. Proteomes. 2025; 13(3):44. https://doi.org/10.3390/proteomes13030044
Chicago/Turabian StyleReyes-Soria, Francisco Antonio, Eliel Ruiz-May, Enrique Castaño, Miguel Ángel Herrera-Alamillo, José Miguel Elizalde-Contreras, Samuel David Gamboa-Tuz, Lidia F. E. Huerta-Nuñez, Jesús Alejandro Zamora-Briseño, and Luis Carlos Rodríguez-Zapata. 2025. "Proteomic Analysis of Mechanical Injury Effects in Papaya Fruit at Two Maturity Stages" Proteomes 13, no. 3: 44. https://doi.org/10.3390/proteomes13030044
APA StyleReyes-Soria, F. A., Ruiz-May, E., Castaño, E., Herrera-Alamillo, M. Á., Elizalde-Contreras, J. M., Gamboa-Tuz, S. D., Huerta-Nuñez, L. F. E., Zamora-Briseño, J. A., & Rodríguez-Zapata, L. C. (2025). Proteomic Analysis of Mechanical Injury Effects in Papaya Fruit at Two Maturity Stages. Proteomes, 13(3), 44. https://doi.org/10.3390/proteomes13030044





