1. Introduction
Tuberculosis (TB) is an infectious disease in humans that is caused by
Mycobacterium tuberculosis bacteria (Mtb), which usually affects lungs [
1]. TB is clinically diagnosed as active or latent TB where patients with active TB are symptomatic (e.g., productive and often bloody sputum), while patients with latent TB are asymptomatic [
2]. According to the World Health Organization, there were 10.8 million cases of active TB worldwide and 1.25 million people died due to active TB in 2023 [
3]. Furthermore, antibiotic treatment remains lengthy and the sole TB vaccine, BCG, is ineffective in preventing TB in most populations [
4]. Therefore, investigating TB is a pressing need to prevent further human suffering and death. Potential clues may lie in the sites of infection wherein Mtb reside.
During lung infection, Mtb is localized in granulomas, which are organized cellular structures consisting primarily of macrophages alongside various other immune cells (e.g., T cells, B cells, etc.) [
5]. As the infection and disease advance, neutrophils are recruited in excess, causing necrosis of granulomas and accumulation of neutrophil debris in granulomas [
5,
6]. Necrotic granulomas are enriched in a neutrophil protein called calprotectin (CP) [
6]. Importantly, granuloma necrosis often precipitates lung cavitation where the lung tissue is degraded and the contents of granulomas are released into the lung airways [
7]. Thus, the composition of the lung environment reflects disease progression and could aid in diagnosis.
The diagnosis of active TB is based on chest X-ray and identification of Mtb from sputum, either directly by microscopy and PCR, or analysis after cultivation (e.g., DNA sequencing for identification) [
3]. Typically, the sputum is treated with a mucolytic (i.e., reducing) agent and sodium hydroxide to break down and decontaminate the sputum, respectively. Following this step, the sputum is centrifuged to pellet and recover the bacteria (i.e., Mtb). Since the sputum supernatant contains proteins (and other molecules) that are not pelleted during centrifugation, they could be used for further analysis instead of discarding them. The proteomic composition of the supernatant could reflect the lung environment and therefore have insights into the stages of TB disease progression. In this study, it was hypothesized that the proteomes of sputum supernatants in TB patients will be sufficiently different to be used to discriminate pulmonary TB versus (vs.) other (non-TB) lung diseases. We also segregated patients with high CP (more inflammation/lung damage) vs. patients with low CP (less inflammation/lung damage) to dissect proteomes that depend on disease severity. We found distinct sets of differentially abundant proteins in pairwise comparisons between groups with high or low CP and in TB vs. non-TB patients that could facilitate discovery of TB biomarkers; and identified TB-specific pathways/processes that could aid in better understanding of TB pathogenesis.
2. Materials and Methods
Collection of sputum samples:
This study was conducted in collaboration with the Hawaii Department of Health and Diagnostics Laboratory Services, Inc. (DLS). Sputum samples were collected from suspected active TB patients from Hawaii and the U.S.-affiliated Pacific Island jurisdictions of Guam, Commonwealth of Northern Mariana Islands, American Samoa, Republic of Palau, Republic of the Marshall Islands, and/or Federated States of Micronesia. The 219 samples were de-identified (except for the results of the TB tests), and therefore this study was not considered human subject research by the University of Hawaii Institutional Review Board (protocol 217-00213) and Queen’s Medical Center. The samples were analyzed by DLS under contract with the Hawaii Department of Health, Centers for Disease Control and Prevention, or private clients to determine the smear grade (0–4+ number of acid-fast bacilli in a sputum smear as determined by microscopy), detect Mtb DNA as well as rifampin resistance by GeneXpert MTB/RIF (Cepheid, Sunnyvale, CA, USA), and culturing (
Table S1). Briefly, the sputum samples were treated with NAC-PAC
® RED (AlphaTec, Vancouver, WA, USA). Equal volume of NAC-PAC
® RED reagent was added to the sputum samples, mixed for 30 s, and incubated for 15–20 min to digest and decontaminate the sample. A neutralizing buffer was added until the color of the sputa changed from red to colorless. The sputum samples were centrifuged at 3000×
g for 15 min, the supernatants were collected and heat inactivated at 80 °C for 30 min, transported to the University of Hawaii at Mānoa, and stored at −80 °C until analysis.
Confirmation of heat inactivation:
Ten microliters of undiluted supernatant samples were inoculated on Middlebrook 7H9 (BD Difco, Franklin Lakes, MI, USA) supplemented with 10% albumin, dextrose, and catalase (ADC) enrichment (BD Difco). Agar medium was incubated at 37 °C for >3 weeks to confirm that Mtb were heat-inactivated prior to manipulation in a Biosafety Level 2 laboratory.
Quantification of calprotectin:
CP subunit S100A8 was quantified in sputum supernatant samples using a sandwich-based Enzyme-Linked Immunosorbent Assay (ELISA) that was designed and used to quantify CP in sputum supernatant samples in our previous study [
8]. Sheep polyclonal anti-S100A8 (R&D Systems, Minneapolis, MN, USA) and mouse monoclonal anti-S100A8 (Santa Cruz Biotechnology, Dallas, TX, USA) were used as the capture and detection antibodies, respectively. Recombinant human CP (from Dr. Walter Chazin) was used to prepare the standards. The capture antibody was incubated in high antibody-binding 96-well white plates (Thermo Fisher Scientific, Waltham, MA, USA) for 16 h at 4 °C. The plates were washed using phosphate-buffered saline with 0.05% Tween 20 (PBST) and blocked using 3% bovine serum albumin (BSA) in PBST at 22 °C for 2 h. The CP standards and sputum samples were prepared in 3% BSA in PBST and added to the plate and incubated at 22 °C for 2 h. The plates were washed using PBST and detection antibody was added and incubated at 22 °C for 2 h. The plates were washed using PBST and incubated with goat anti-mouse IgG antibody with horseradish peroxidase (Thermo Fisher Scientific) at 22 °C for 1 h. The plates were washed to remove excess secondary antibody and Super Signal
TM ELISA Pico Substrate (Thermo Fisher Scientific) was added. Chemiluminescence was measured using a BioTek plate reader. The mass of CP was converted into picomoles (pmol) using its molecular mass of 24,077 Da and normalized against the total amount of protein (μg), as measured using the Bradford assay (Thermo Fisher Scientific). Note that the range of CP concentrations in TB-positive samples were published previously [
8].
Preparation of proteins for mass spectrometry (MS):
A total of 12 samples were selected for proteomics analysis: “TB” and “non-TB” with high or low concentrations of CP, with 3 samples in each group. The sample size was limited to 3 due to the limited size of the cohort and the high cost of the analysis. Samples were selected based on the amount of CP relative to their cohort, i.e., samples with top 3 highest and 3 lowest CP concentrations in TB and non-TB groups were selected. Other mycobacterial infections were excluded and only samples with 0.1 mg/mL total protein were considered for proteomics. Supernatant proteins were precipitated using trichloroacetic acid with 0.1% sodium deoxycholate method [
9] and resuspended in 9.5 M urea and 2% CHAPS at pH 8.5 buffer. Protein concentrations were quantified using the Bradford assay (Thermo Fisher Scientific). Each sample was processed in triplicate, resulting in 36 samples in total. The proteins were prepared for mass spectroscopy by the filter-aided sample preparation (FASP) method [
10], with some modifications [
11]. Proteins were reduced using a solution of 250 mM Tris(2-carboxyethyl)phosphine (TCEP) and 500 mM TEAB (ThermoFisher). TCEP-TEAB was added at a ratio of 1:5 to the protein samples (e.g., 1 µL TCEP-TEAB per 5 µL sample), and incubated at 55 °C for 1 h. Reduced samples were loaded onto Microcon-10 kDa filter units with Ultracell-10 membranes (MilliporeSigma, Burlington, MA, USA) that were pre-wet with 9.5 M urea in 100 mM Tris-HCl buffer at pH 8.5 (urea solution). Loaded columns were treated with 100 mM iodoacetamide (Bio-Rad, Hercules, CA, USA) in urea solution for 30 min at room temperature, washed using urea solution followed by 50 mM Tris-HCl at pH 8.0, and digested using Trypsin-LysC (Promega, Madison, WI, USA) in 50 mM Tris-HCl at pH 8.0 and at 37 °C for 16 h. Peptides were eluted in two sequential steps using 50 mM Tris-HCl followed by 0.5 M NaCl, which were pooled. The pooled peptides were acidified using freshly prepared 10% formic acid (~1% final concentration). The peptides were desalted using Pierce Graphite Spin Columns (Thermo Fisher Scientific) and eluted in 0.1% formic acid in 50% acetonitrile following the manufacturer’s protocol. The peptides were quantified using the Pierce Quantitative Fluorometric Peptide Assay (Thermo Fisher Scientific). Ten micrograms of peptides per sample were dried in a speed vacuum and sent to the proteomics facility at the University of California, Davis. The peptides were analyzed by liquid chromatography–tandem MS (LC-MS/MS) on a Q Exactive™ Plus Orbitrap Mass spectrometer (Thermo Fisher Scientific).
LC-MS/MS data searches and differential abundance analysis:
The LC-MS/MS data files were converted from .RAW format to .mzXML format using MSConvert (ProteoWizard 3.0) [
12]. Protein identification and label-free quantification was performed using X! Tandem (The GPM, thegpm.org; version X! Tandem Alanine (2017.2.1.4)) [
13] with the following search parameters: human proteome (
Homo sapiens), precursor mass tolerance at 20 ppm, fragment mass tolerance at 10 ppm, trypsin digestion, fixed modification of cysteine (carbamidomethylation), and variable modifications of oxidation (Met), deamidation (Asn and Gln), phosphorylation (Ser, Thr, Tyr), and acetylation (Lys). The protein spectral counts were exported as .csv files. Differential abundance analyses were performed using edgeR v4.0.16 and limma v3.58.1, as we did in a previous study [
8,
14]. edgeR was used to normalize the unfiltered raw data (i.e., total spectral counts) to logCPM, and limma was used to determine the common dispersion using the Quantile-adjusted conditional maximum likelihood linear modeling approach [
8,
14]. The following groups were compared: TB vs. non-TB, TB with low CP vs. non-TB with low CP, TB with high CP vs. non-TB with high CP, non-TB with high CP vs. non-TB with low CP, and TB with high CP vs. TB with low CP. Proteins with log2 fold changes > 1 and <−1 and adjusted
p-values (Hochberg false discovery rates) < 0.05 were called differentially abundant.
Pathway enrichment analyses:
The differentially abundant proteins were used to do pathway enrichment analyses. The KEGG and GO enrichment analyses were performed using DAVID 2021 (
https://david.ncifcrf.gov/) [
15,
16]. Ensembl protein accession identifiers were used to conduct the searches [
17]. GOplot was used to visualize the results [
18].
4. Discussion
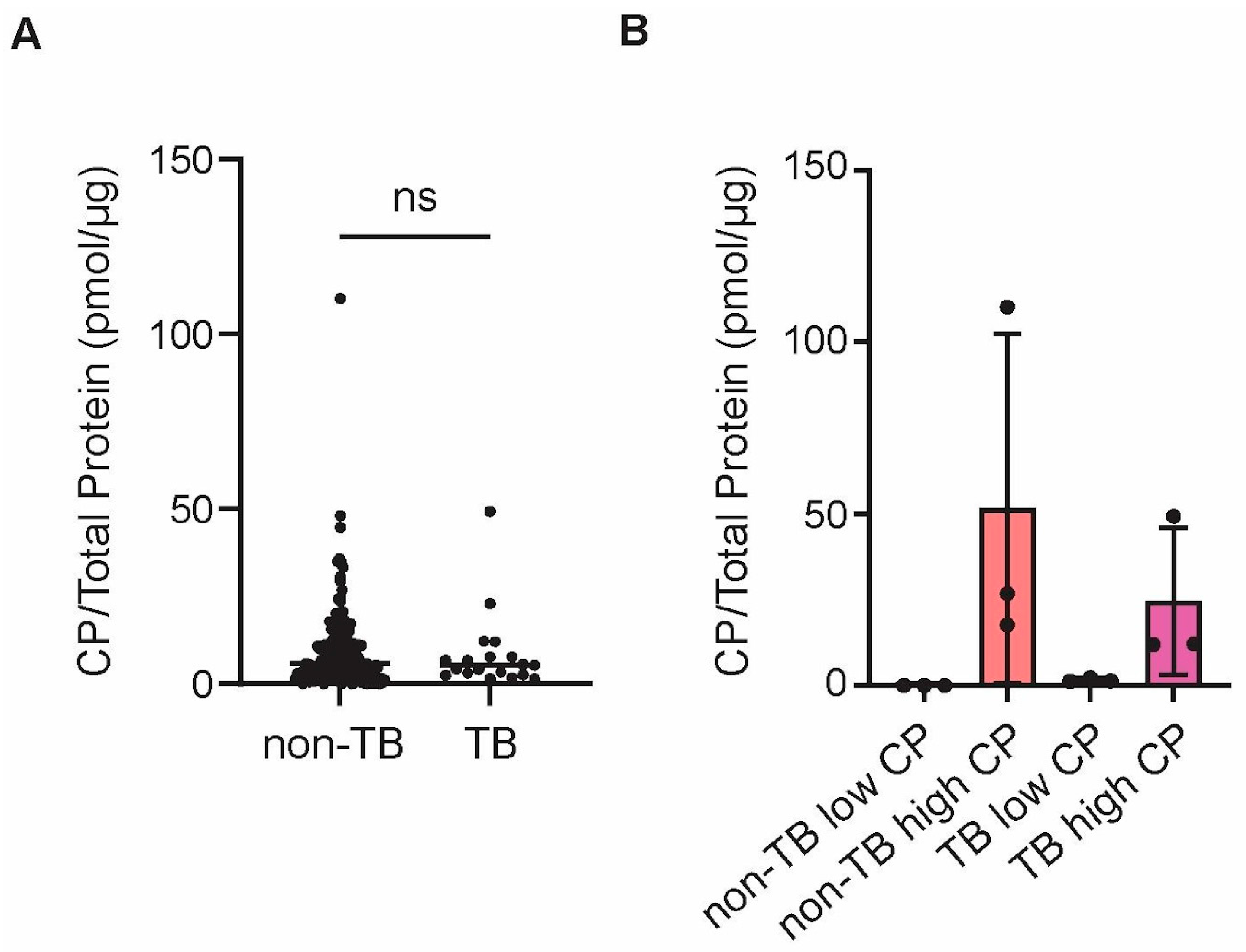
The purpose of this study was to compare proteomes from sputum samples from patients with active pulmonary TB and from those with other lung diseases. We also investigated if CP could be used as a biomarker for active TB, when compared to other lung diseases, and if the proteomes of TB vs. non-TB sputa could be delineated when CP was high or low. The ultimate goal was to determine if TB sputa have different amounts of CP and other proteins that can serve as biomarkers for active pulmonary TB.
Over 200 sputa were collected from suspected TB patients, which were later shown to contain less than 10% of TB-positive samples. CP was quantified in all samples and there was no statistically significant difference between TB and non-TB samples regarding the abundance of CP. However, no additional information was available for these patients and, therefore, it is not clear what could have caused elevated CP in non-TB samples or if TB patients were already treated with antibiotics, or not, at the time of sputum collection, which could have affected CP levels as well [
24]. Considering that CP is a general marker of inflammation [
25] and all patients were suspects for TB, likely because they had lung conditions that triggered sputum formation and other typical symptoms of lung inflammation, it is not surprising that CP cannot be used as a specific marker for TB. However, future studies could determine if sputum CP can be used as a biomarker for successful treatment of pulmonary TB.
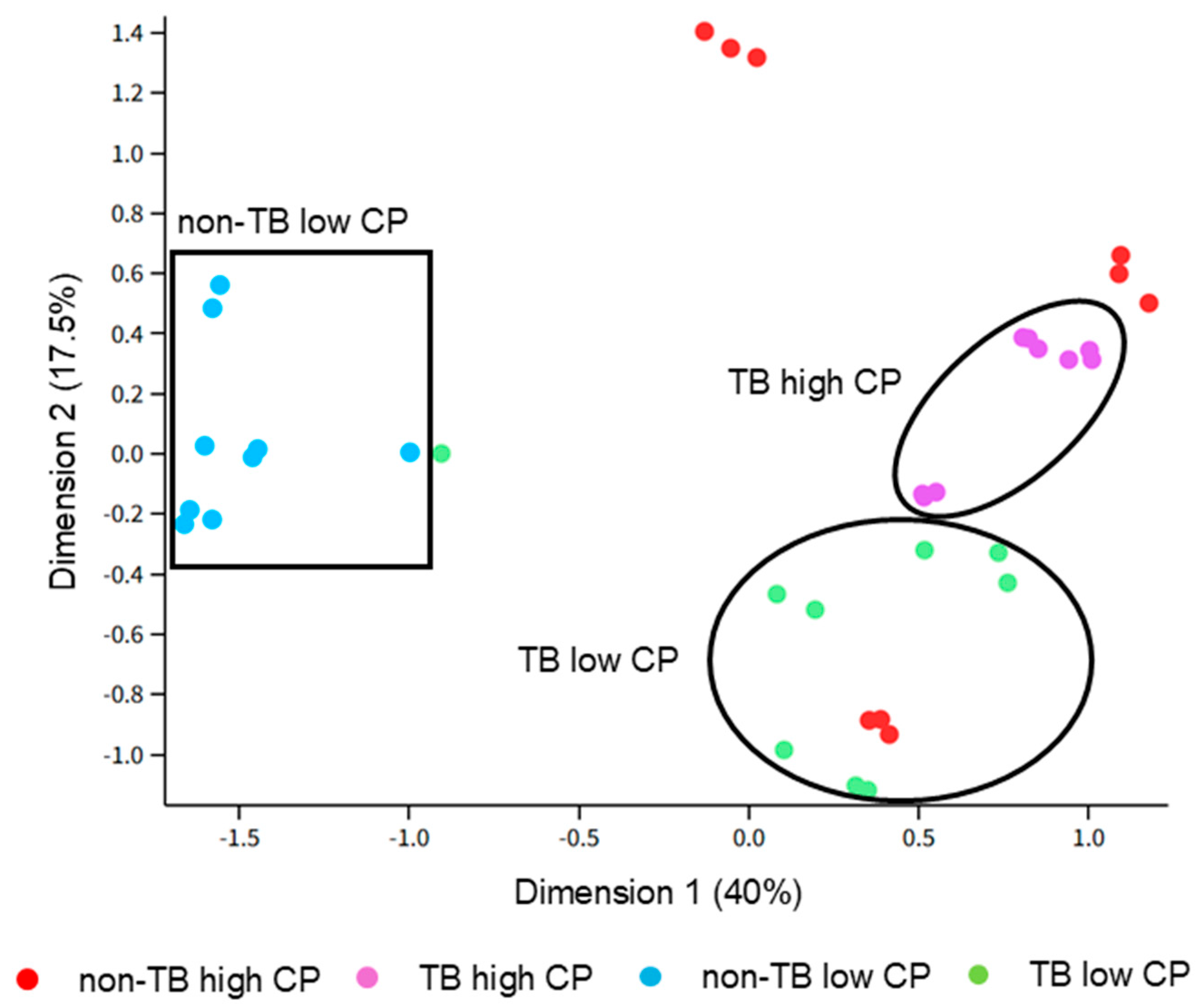
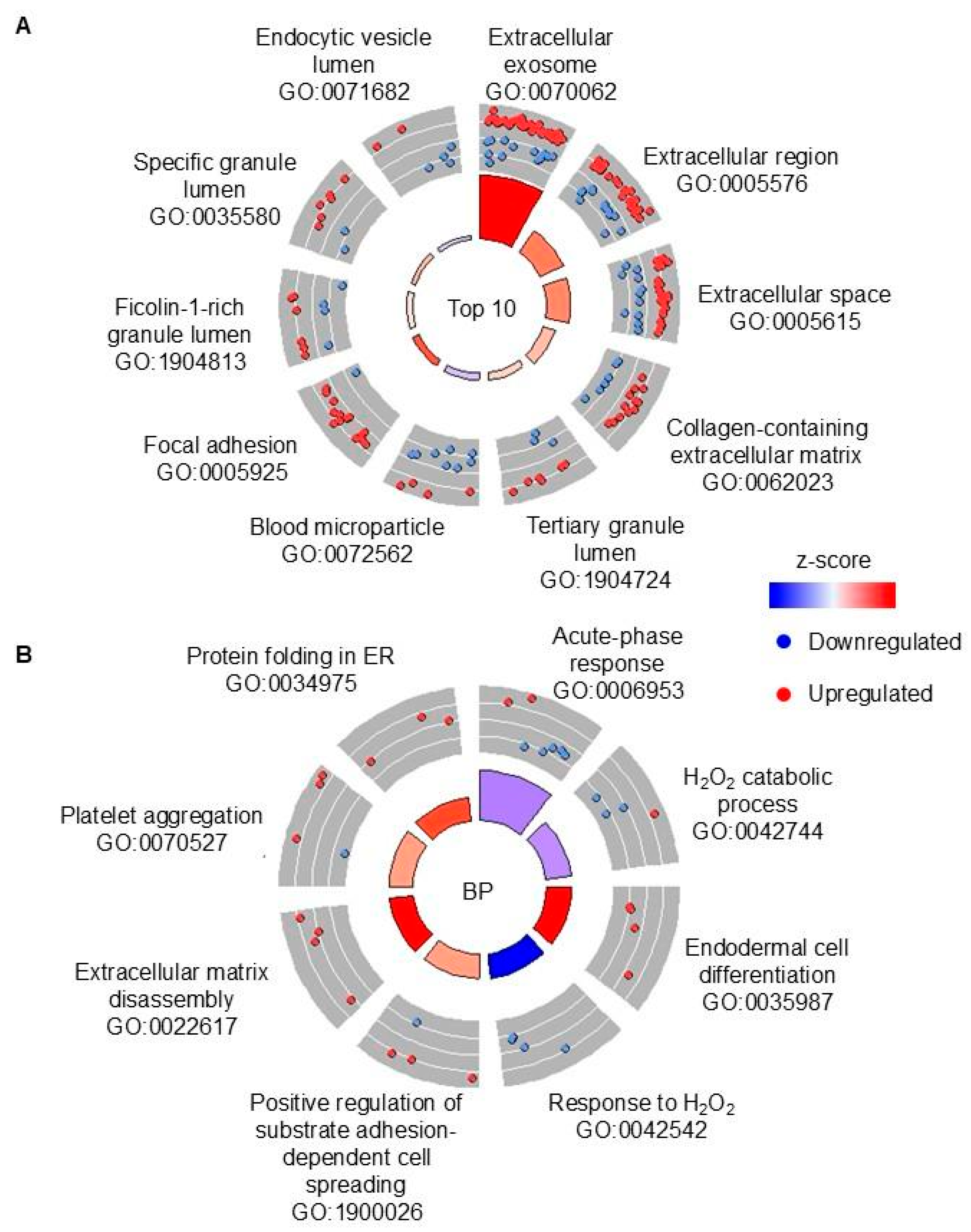
There were four groups of samples that were selected for further analysis using proteomics: (1) TB with high CP, (2) TB with low CP, (3) non-TB with high CP, and (4) non-TB with low CP. We first compared TB vs. non-TB samples, regardless of the CP amount, in order to identify potential biomarkers of pulmonary TB that would distinguish it from other lung diseases. Although blood in sputum is one of the typical symptoms of TB, blood-associated proteins were less abundant in TB samples, when compared to non-TB samples, which was also confirmed with pathway analyses. The lung conditions that non-TB patients had were not disclosed, so it is likely that they had symptoms severe enough to cause bloody sputum. In contrast, several proteins that are associated with neutrophils were shown to be more abundant (e.g., MPO and LCN2), as well as neutrophil-associated processes being over-represented in the pathway analyses, although CP, which is the most abundant neutrophil-derived protein, was not more abundant in TB vs. non-TB samples, as stated above. Similarly, the results from this study suggest that matrix metalloproteinases (i.e., MMP8 and MMP9) are upregulated in TB sputum vs. non-TB sputum, which is in agreement with previous reported studies that implicated these enzymes in active pulmonary TB (reviewed in [
26]). Therefore, although markers of neutrophil-driven inflammation may not be specific for TB, certain neutrophil-derived proteins may be useful biomarkers.
There is a small number of published studies describing sputum proteomics in TB and they show a limited overlap with each other [
27]. Using sputum samples from 10 TB patients from Ethiopia to compare their proteomes to latently infected or community controls, the authors identified 50 DAPs [
28]. Only one protein, MMP8, was also implicated as a potential biomarker of active TB (
Table S18). The follow-up study by the same group with a larger cohort identified 103 DAPs in pulmonary TB vs. latent TB infection, with 9 (including again MMP8) matching our findings and noting the neutrophil-driven inflammation, as we did in our study [
29] (
Table S18). Another previously reported proteomics analysis of sputum samples from India identified 25 DAPs, 5 of which are also found in our study, although the authors did not report if they were increased or decreased [
30]. Surprisingly, this study reported decreased MMP9 in active TB [
30] (
Table S18). Next, sputum and salivary samples from 9 TB patients from Spain were compared to latent and uninfected controls to show DAPs in pairwise comparisons between these three groups [
31]. There were 21 and 19 DAPs that overlapped with our findings in active TB vs. latent TB or TB vs. uninfected control group, respectively [
31] (
Table S18). These include proteins associated with neutrophils such as MMP8, GCA, and LCN2 [
20,
32]. Interestingly, MMP9 was increased in TB vs. latent TB, but decreased in TB vs. uninfected [
31]. Since, in our study, it was not disclosed if individuals from non-TB group were latently infected (e.g., if they were PPD-tested), we cannot tell if this was the reason for this difference in comparisons. Regardless, some, but not all, neutrophil-derived proteins, such as MMP8, could be more universal biomarkers for pulmonary TB.
Next, we were interested in dissecting the host response using CP levels as a proxy for disease severity caused by neutrophil accumulation. There were more DAPs when comparing samples with low CP (181), with only 10 DAPs when comparing TB vs. non-TB samples with high concentration of CP. Six out of these 10 DAPs in high-CP samples are also DAPs in low-CP samples, including MMP8, but not MMP9, which was not elevated in TB vs. non-TB sputum samples with high CP. If all three comparisons are considered, i.e., regardless of CP, low CP or high CP, mucin 5AC is the only other protein (in addition to MMP8) that is significantly more abundant in TB vs. non-TB. Therefore, these two proteins (MMP8 and MUC5AC) may be insensitive to disease severity. However, MUC5AC is found to be less abundant in TB sputa in several other studies [
29,
31], while MMP8 is consistently shown to be more abundant in active TB across multiple studies [
28,
29,
31]. The discrepancy with MUC5AC may have been due to differences in the patient populations. This present study analyzed samples collected from the Pacific Basin, whereas the other studies used samples from Ethiopia and Spain [
29,
31]. In addition, we only had samples from six patients in each group. Hence, the increased abundance of MUC5AC could have been due to population diversity and/or small sample size. It does not seem probable that our sample decontamination protocol specifically contributed to this discrepancy as well. However, it is possible that our sample treatment affected the overall results compared to other studies because we subjected our samples to strong alkaline treatment, whereas other studies did not [
29,
31]. In general, efforts to improve reproducibility between sputum proteomic studies should be made. Sample collection is usually performed by clinical providers (as was performed in this study and introduces some variability) and also prioritizes pathogen isolation over preservation of host proteins. Current proteomics analysis requires specialized expertise and equipment and is therefore complex and costly. Accordingly, sputum processing should be standardized to allow analysis of host proteins and proteomics protocols should be designed to be more affordable and accessible to researchers in low-income countries where TB burden is the highest.
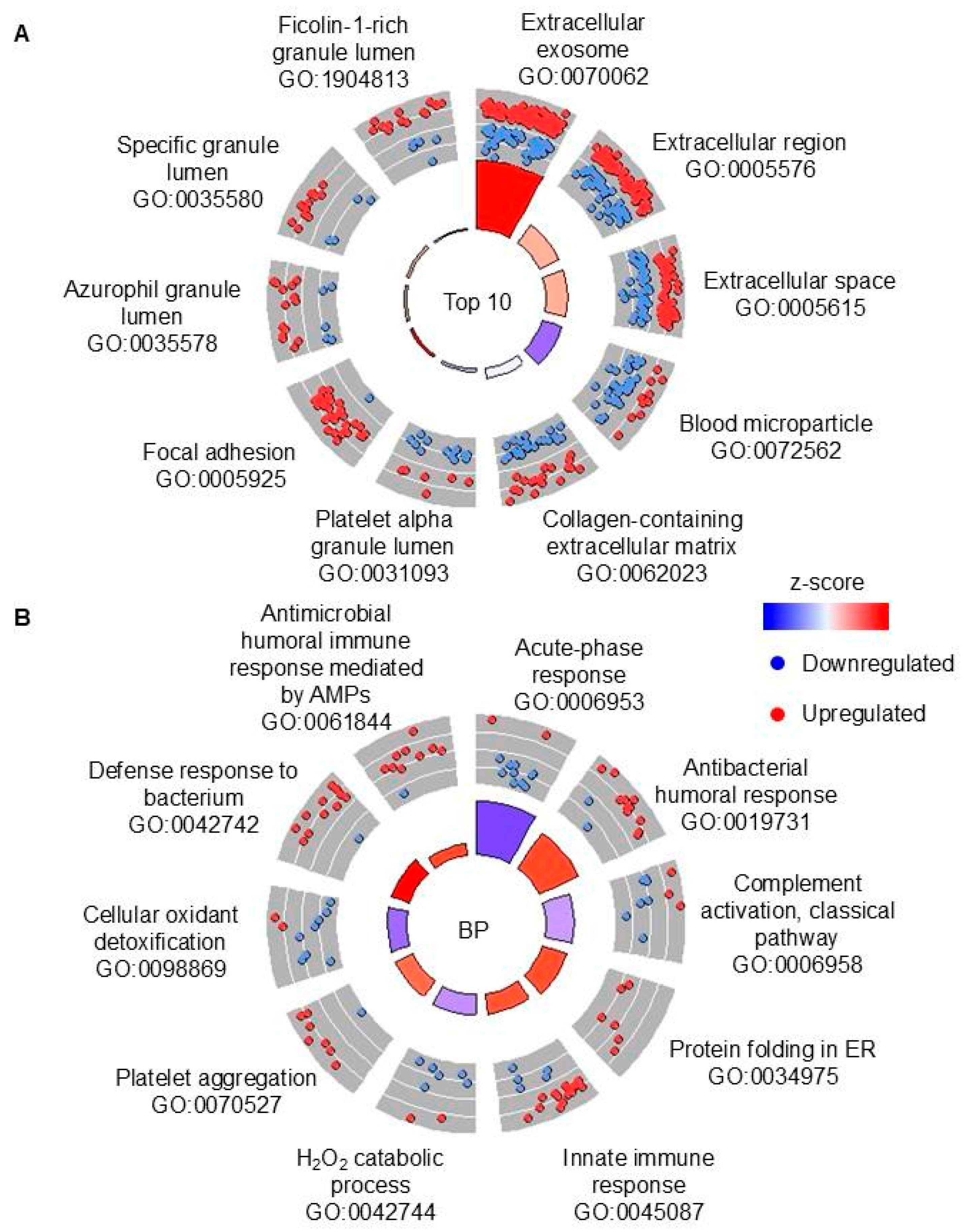
Finally, we wanted to compare TB samples with high vs. low CP. There were 83 DAPs in this comparison, but 48 out of these DAPs are also found in non-TB high vs. low CP comparisons, so they are likely caused by higher inflammation in high-CP samples. Thirty-five proteins that are uniquely DAPs in high vs. low CP TB samples (including LTF that is a DAP in the opposite direction in the non-TB comparison) may provide potential biomarkers for disease severity or treatment success. Since other studies did not compare samples with high or low CP, as we did, we looked for studies in which proteomics analysis was performed during treatment, which would presumably decrease neutrophil inflammation. In one such study from South Africa, 266 DAPs were identified when proteins from bronchoalveolar lavage (BAL) from TB patients with active disease and clinically cured individuals [
33]. Surprisingly, both CP subunits (S100A8 and S100A9) were not DAPs in this comparison, and 7 DAPs that are found in our study, i.e., in high vs. low CP in TB sputum samples and in BAL from patients with active TB vs. cured, are showing the opposite trend. This study was not an appropriate match for our study possibly due to different sample types being used (i.e., sputum vs. BAL) and CP amounts in BAL not being significantly different before and after treatment. The differences between our study and other studies likely reflect the highly heterogeneous nature of the disease, the study populations, sample preparation and analysis, and various other factors that influence human biology. In addition, the high heterogeneity of our non-TB cohort affected our data. To decrease the variability, future studies should use non-TB samples from patients who are not sick or stratify the non-TB samples according to the type of illness (e.g., pneumonia, chronic obstructive pulmonary disease, influenza, etc.). Overall, our study must be validated in other population cohorts due to our small sample size.
If this study were to be repeated, several changes must be made to improve the experimental design. First, the sample size should be increased to account for the intrinsic biological variability of human samples and to increase the statistical power of the analyses. Second, the samples had relatively low spectral counts (~15,000 counts per sample), and much higher numbers can be achieved with newer instruments. The low sample complexity may have contributed to the relatively small number of identified proteins, as some of the most abundant proteins were blood-associated proteins (e.g., hemoglobin subunits, albumin, immunoglobulins, etc.). This could be improved by their removal to improve the detection of proteins with lower abundances, which can be achieved using commercially available kits (e.g., Pierce™ Albumin Serum Depletion Kits from Thermo Fisher Scientific). We also used 10 kDa filters to prepare samples, which might have contributed to the low sample complexity because the low-molecular-weight proteins were not captured by the filter for digestion. Alternatively, using targeted proteomics would be more appropriate as a follow-up study, in which case low complexity will be less problematic. Third, it was initially assumed that samples with high CP had increased neutrophilic inflammation and were from patients with more advanced disease progression compared to samples with lower concentrations of CP. While neutrophils are strongly associated with TB disease progression [
22,
23], and serum CP is as well [
19], the patient information was unknown in this study (i.e., disease progression was unknown). The lack of patient information also prevented us from choosing appropriate non-TB controls, which may be the reason for the high variability in “non-TB high-CP” group, as seen in the MDS plot. Therefore, it is possible that other patient characteristics complicated the comparison, especially when “non-TB high-CP” group was used as a control group. Thus, patient information should be known in future studies in order to minimize confounding variables and select better matched groups. Other characteristics of the patient cohort should be known as well (e.g., age, gender, HIV status, illness of non-TB patients). Lastly, even though we included standard posttranslational modifications in our analysis, we did not attempt to differentiate proteoforms and focused on ORF products only. The lack of proteoform differentiation is problematic because this possibly contributed to the low complexity of our search results and, more importantly, different proteoforms for the same genes can have different functions [
34,
35]. Therefore, our functional analyses remain incomplete without proteoform differentiation, which should be addressed by future studies. In summary, with an increased sample size, depletion of common proteins, and more information about the sample cohort, there would be an increased chance to identify proteins that could serve as biomarkers for TB and TB disease progression. However, MMP8 remains the most promising TB biomarker, as shown previously and in this study to be elevated in TB patients.