Cell Surface Proteomics Reveals Hypoxia-Regulated Pathways in Cervical and Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Biotinylation and Isolation of PMPs
2.3. Sample Preparation for MS Downstream Analysis
2.4. Whole-Cell Lysate Processing
2.5. LC-MS/MS Analytical Methodology
2.5.1. Chromatographic Separation (Loop-Out Injection)
2.5.2. MS Source Parameters
2.5.3. MS/MS Acquisition Settings
2.5.4. Protein Identification and Quantitative Analysis
2.6. In Silico Proteomic Data Analysis
3. Results and Discussion
3.1. Protein Detection in Biotin-Based vs. Whole-Cell Fractions
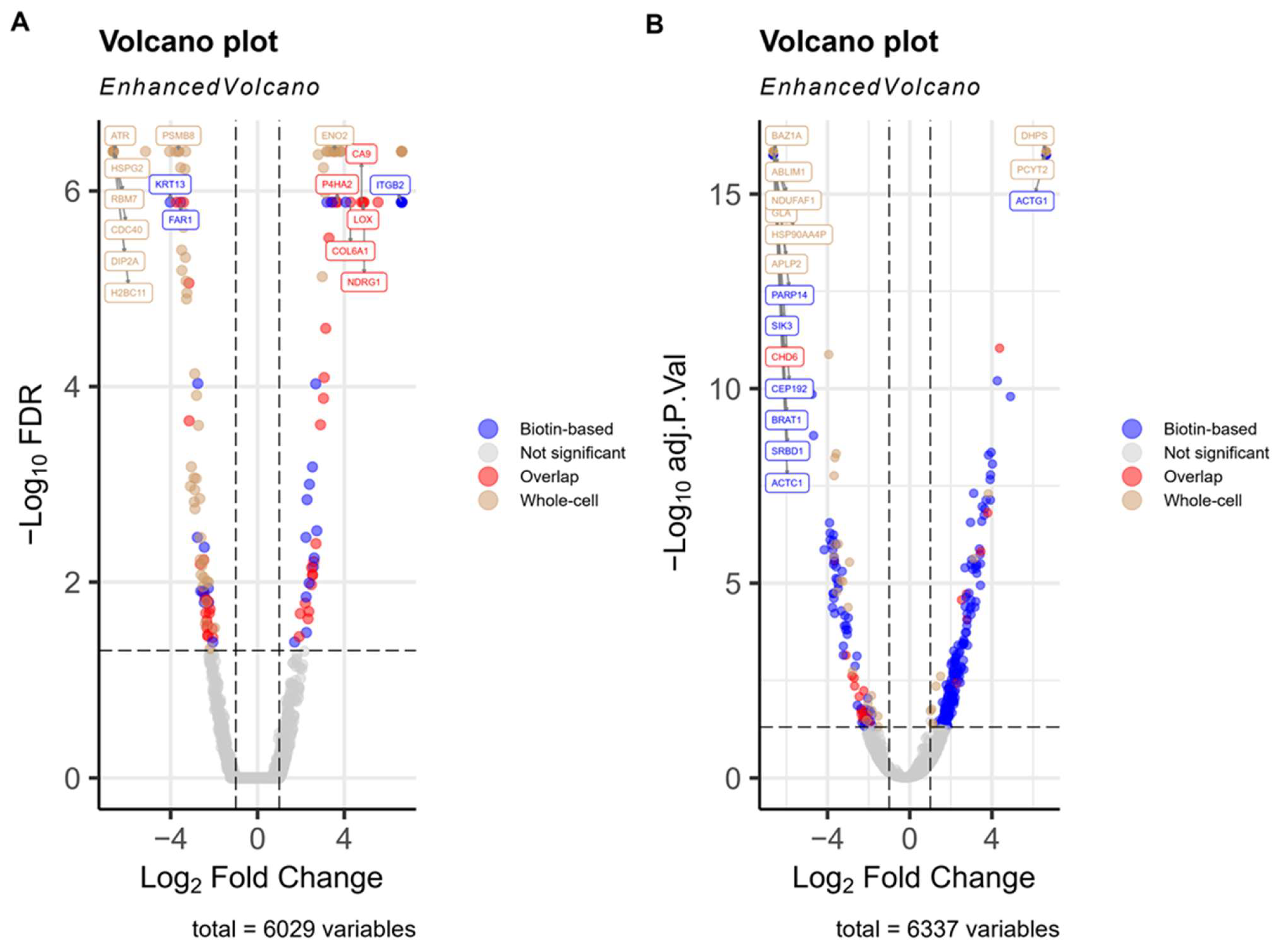
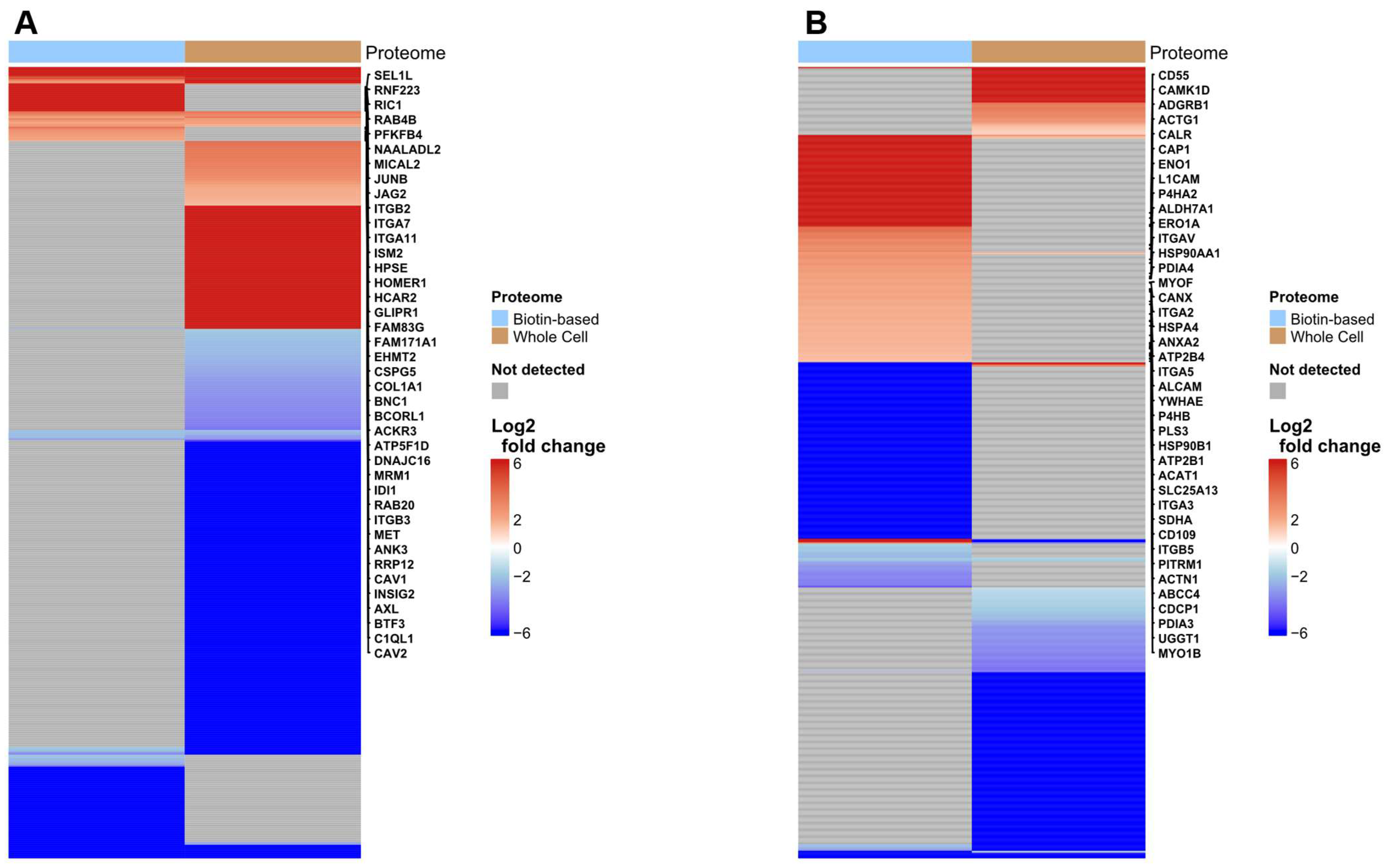
3.2. Hypoxia Induced Change in PMPs Abundance Demonstrated in Biotin-Enriched Fractions
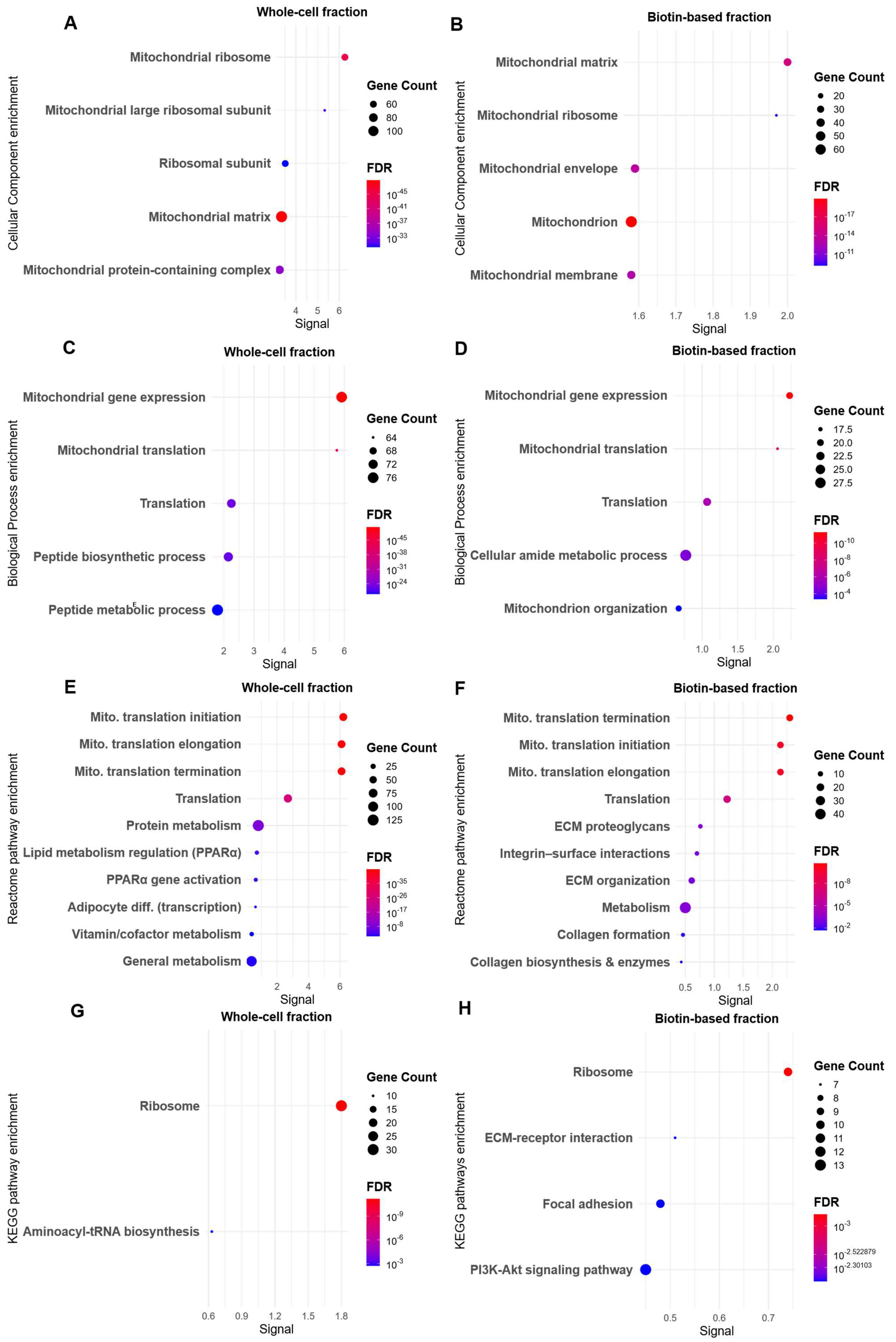
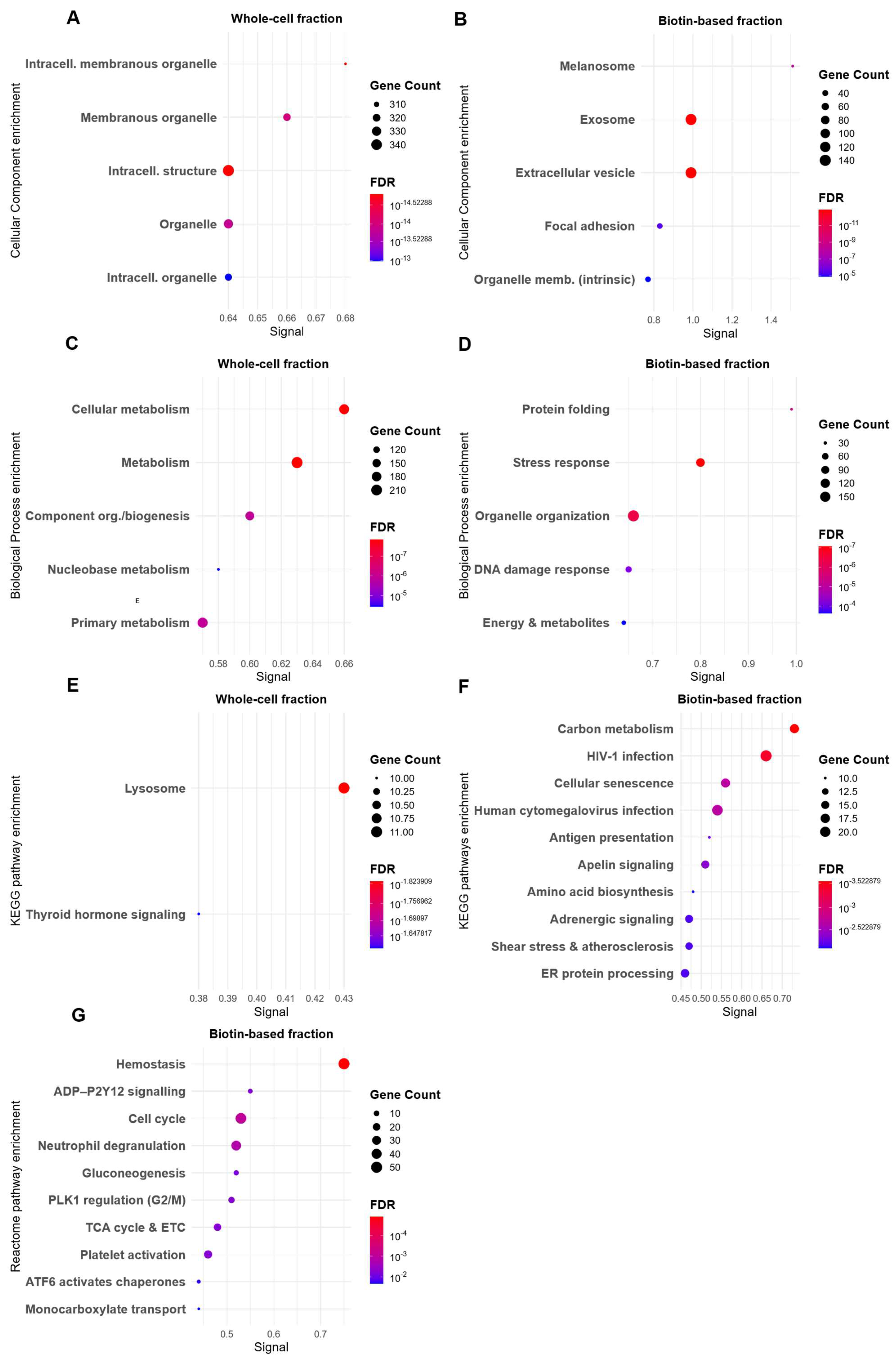
3.3. Biotin-Baed Enricment Revels Additonal Hypoxia-Related Changes in Plasma Membrane-Associated Functional Pathways
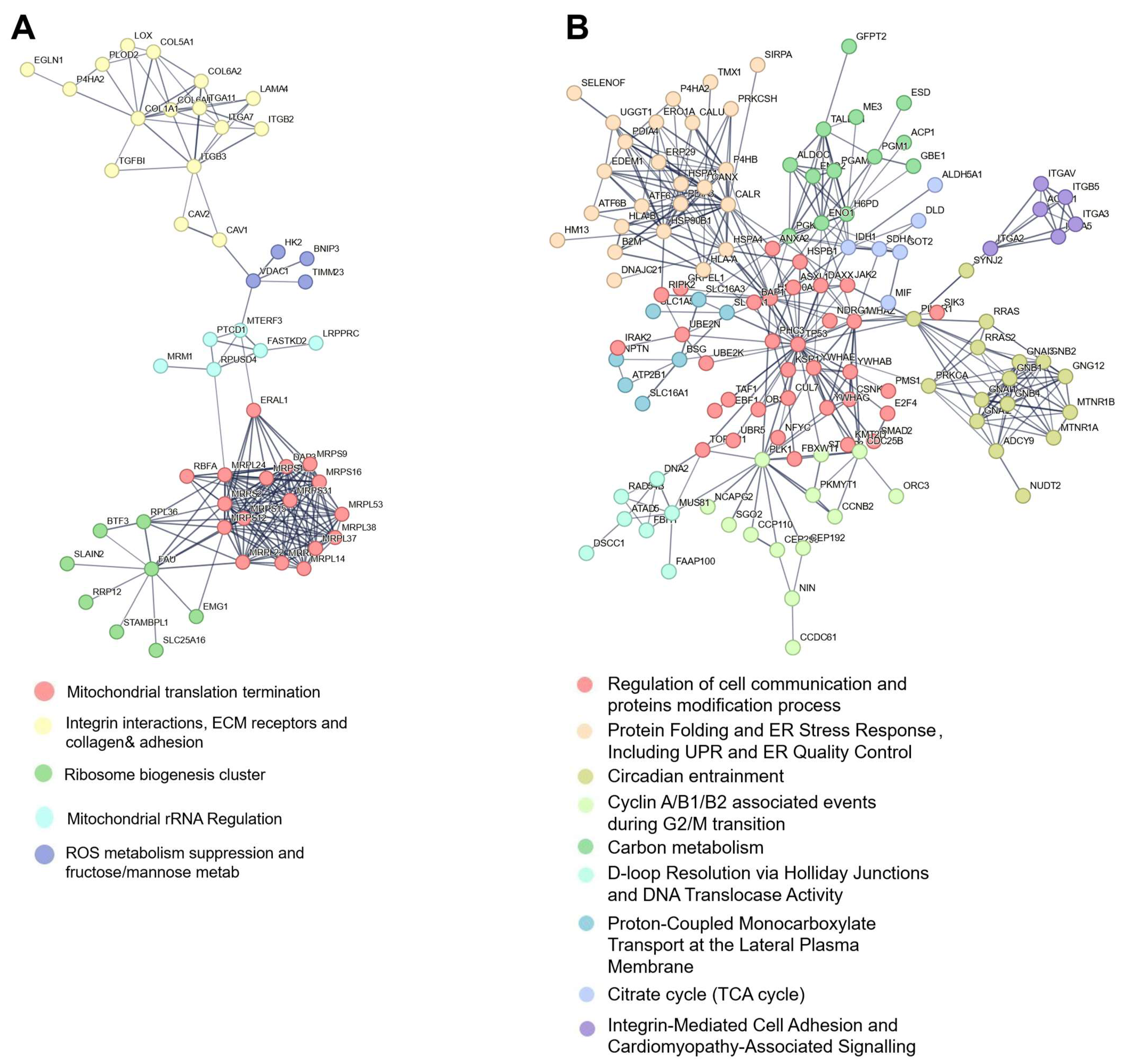
3.4. Protein–Protein Interaction Mapping Reveals ECM–Mitochondrial Crosstalk in Enriched Membrane Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elschenbroich, S.; Kim, Y.; Medin, J.A.; Kislinger, T. Isolation of cell surface proteins for mass spectrometry-based proteomics. Expert Rev. Proteom. 2010, 7, 141–154. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. Exploiting the hypoxic cancer cell: Mechanisms and therapeutic strategies. Mol. Med. Today 2000, 6, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Sibtain, A.; Daley, F.M.; Wilson, G.D. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: Relationship with vascularity and proliferation as predictors of outcome of ARCON. Br. J. Cancer 2003, 89, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, C.; Silva, P.; Torres, V.A. Role of glycosylation in hypoxia-driven cell migration and invasion. Cell Adhes. Migr. 2019, 13, 13–22. [Google Scholar] [CrossRef]
- Gluck, A.A.; Aebersold, D.M.; Zimmer, Y.; Medova, M. Interplay between receptor tyrosine kinases and hypoxia signaling in cancer. Int. J. Biochem. Cell Biol. 2015, 62, 101–114. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Kleinendorst, S.C.; Oosterwijk, E.; Molkenboer-Kuenen, J.; Frielink, C.; Franssen, G.M.; Boreel, D.F.; Tamborino, G.; Gloudemans, M.; Hendrikx, M.; Kroon, D.; et al. Towards effective CAIX-targeted radionuclide and checkpoint inhibition combination therapy for advanced clear cell renal cell carcinoma. Theranostics 2024, 14, 3693–3707. [Google Scholar] [CrossRef]
- Li, Y.; Qin, H.; Ye, M. An overview on enrichment methods for cell surface proteome profiling. J. Sep. Sci. 2020, 43, 292–312. [Google Scholar] [CrossRef]
- Young, J.W.; Wason, I.S.; Zhao, Z.; Rattray, D.G.; Foster, L.J.; Van Hoa, F.D. His-Tagged Peptidiscs Enable Affinity Purification of the Membrane Proteome for Downstream Mass Spectrometry Analysis. J. Proteome Res. 2020, 19, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, L.; Cummins, E.; Samudio, I.; Kislinger, T. Cell-surface proteomics for the identification of novel therapeutic targets in cancer. Expert Rev. Proteom. 2018, 15, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Tan, H.T.; Chung, M.C. Membrane proteins and membrane proteomics. Proteomics 2008, 8, 3924–3932. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Tucholski, T.; Alpert, A.J.; Eken, C.; Wesemann, L.; Kyrvasilis, A.; Jin, S.; Ge, Y. Top-Down Proteomics of Endogenous Membrane Proteins Enabled by Cloud Point Enrichment and Multidimensional Liquid Chromatography-Mass Spectrometry. Anal. Chem. 2020, 92, 15726–15735. [Google Scholar] [CrossRef]
- Elia, G. Biotinylation reagents for the study of cell surface proteins. Proteomics 2008, 8, 4012–4024. [Google Scholar] [CrossRef]
- Liu, G.; Choi, M.H.; Ma, H.; Guo, X.; Lo, P.C.; Kim, J.; Zhang, L. Bioorthogonal Conjugation-Assisted Purification Method for Profiling Cell Surface Proteome. Anal. Chem. 2022, 94, 1901–1909. [Google Scholar] [CrossRef]
- de Boer, E.; Rodriguez, P.; Bonte, E.; Krijgsveld, J.; Katsantoni, E.; Heck, A.; Grosveld, F.; Strouboulis, J. Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA 2003, 100, 7480–7485. [Google Scholar] [CrossRef]
- Massobrio, R.; Bianco, L.; Campigotto, B.; Attianese, D.; Maisto, E.; Pascotto, M.; Redda, M.G.R.; Ferrero, A. New Frontiers in Locally Advanced Cervical Cancer Treatment. J. Clin. Med. 2024, 13, 4458. [Google Scholar] [CrossRef]
- Gennigens, C.; De Cuypere, M.; Hermesse, J.; Kridelka, F.; Jerusalem, G. Optimal treatment in locally advanced cervical cancer. Expert Rev. Anticancer Ther. 2021, 21, 657–671. [Google Scholar] [CrossRef]
- Alfred Witjes, J.; Max Bruins, H.; Carrion, A.; Cathomas, R.; Comperat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2024, 85, 17–31. [Google Scholar] [CrossRef]
- Tian, J.; Sun, J.; Fu, G.; Xu, Z.; Chen, X.; Shi, Y.; Jin, B. Population-based outcome of muscle-invasive bladder cancer following radical cystectomy: Who can benefit from adjuvant chemotherapy? Transl. Androl. Urol. 2021, 10, 356–373. [Google Scholar] [CrossRef]
- Li, S.; Sampson, C.; Liu, C.; Piao, H.L.; Liu, H.X. Integrin signaling in cancer: Bidirectional mechanisms and therapeutic opportunities. Cell Commun. Signal. 2023, 21, 266. [Google Scholar] [CrossRef]
- Parton, R.G. Caveolae: Structure, Function, and Relationship to Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 111–136. [Google Scholar] [CrossRef]
- Comoglio, P.M.; Trusolino, L.; Boccaccio, C. Known and novel roles of the MET oncogene in cancer: A coherent approach to targeted therapy. Nat. Rev. Cancer 2018, 18, 341–358. [Google Scholar] [CrossRef]
- Bharti, R.; Dey, G.; Khan, D.; Myers, A.; Huffman, O.G.; Saygin, C.; Braley, C.; Richards, E.; Sangwan, N.; Willard, B.; et al. Cell surface CD55 traffics to the nucleus leading to cisplatin resistance and stemness by inducing PRC2 and H3K27 trimethylation on chromatin in ovarian cancer. Mol. Cancer 2024, 23, 121. [Google Scholar] [CrossRef]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774. [Google Scholar] [CrossRef]
- Dyachok, J.; Shao, M.R.; Vaughn, K.; Bowling, A.; Facette, M.; Djakovic, S.; Clark, L.; Smith, L. Plasma membrane-associated SCAR complex subunits promote cortical F-actin accumulation and normal growth characteristics in Arabidopsis roots. Mol. Plant 2008, 1, 990–1006. [Google Scholar] [CrossRef] [PubMed]
- Volpin, V.; Michels, T.; Sorrentino, A.; Menevse, A.N.; Knoll, G.; Ditz, M.; Milenkovic, V.M.; Chen, C.-Y.; Rathinasamy, A.; Griewank, K.; et al. CAMK1D Triggers Immune Resistance of Human Tumor Cells Refractory to Anti-PD-L1 Treatment. Cancer Immunol. Res. 2020, 8, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.K.; Boxer, L.D.; Ransohoff, J.D.; Siprashvili, Z.; Qu, K.; Lopez-Pajares, V.; Hollmig, S.T.; Khavari, P.A. CALML5 is a ZNF750- and TINCR-induced protein that binds stratifin to regulate epidermal differentiation. Genes Dev. 2015, 29, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Vitriol, E.A.; Shim, S.; Wise, A.L.; Velayutham, R.P.; Zheng, J.Q. Dynamic localization of G-actin during membrane protrusion in neuronal motility. Curr. Biol. 2013, 23, 1046–1056. [Google Scholar] [CrossRef]
- Suresh, R.; Diaz, R.J. The remodelling of actin composition as a hallmark of cancer. Transl. Oncol. 2021, 14, 101051. [Google Scholar] [CrossRef]
- Tang, Y.; Peng, X.; Huang, X.; Li, J. Actin gamma 1 is a critical regulator of pancreatic ductal adenocarcinoma. Saudi J. Gastroenterol. 2022, 28, 239–246. [Google Scholar] [CrossRef]
- He, Q.; Li, Z. The dysregulated expression and functional effect of CaMK2 in cancer. Cancer Cell Int. 2021, 21, 326. [Google Scholar] [CrossRef]
- Wang, S.; Tan, J.; Miao, Y.; Zhang, Q. Mitochondrial Dynamics, Mitophagy, and Mitochondria-Endoplasmic Reticulum Contact Sites Crosstalk Under Hypoxia. Front. Cell Dev. Biol. 2022, 10, 848214. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, W.W.; Chandel, N.S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Cell Physiol. 2011, 300, C385–C393. [Google Scholar] [CrossRef] [PubMed]
- Tragni, V.; Primiano, G.; Tummolo, A.; Cafferati Beltrame, L.; La Piana, G.; Sgobba, M.N.; Cavalluzzi, M.M.; Paterno, G.; Gorgoglione, R.; Volpicella, M.; et al. Personalized Medicine in Mitochondrial Health and Disease: Molecular Basis of Therapeutic Approaches Based on Nutritional Supplements and Their Analogs. Molecules 2022, 27, 3494. [Google Scholar] [CrossRef]
- Yates, J.R.; Ruse, C.I.; Nakorchevsky, A. Proteomics by mass spectrometry: Approaches, advances, and applications. Annu. Rev. Biomed. Eng. 2009, 11, 49–79. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Dong, P.; Konno, Y.; Watari, H.; Hosaka, M.; Noguchi, M.; Sakuragi, N. The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J. Transl. Med. 2014, 12, 231. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Lemasters, J.J. Warburg revisited: Regulation of mitochondrial metabolism by voltage-dependent anion channels in cancer cells. J. Pharmacol. Exp. Ther. 2012, 342, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Shteinfer-Kuzmine, A.; Verma, A. VDAC1 at the Intersection of Cell Metabolism, Apoptosis, and Diseases. Biomolecules 2020, 10, 1485. [Google Scholar] [CrossRef]
- Zhang, J.; Ney, P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009, 16, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Rovini, A. Tubulin-VDAC Interaction: Molecular Basis for Mitochondrial Dysfunction in Chemotherapy-Induced Peripheral Neuropathy. Front. Physiol. 2019, 10, 671. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, X.; Liu, Q.; Wilkins, J.A.; Chapman, H.A. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 1999, 144, 1285–1294. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Ling, J.Y.; Chen, W.C.; Lin, H.H.; Tang, M.J. Mechanotransduction of matrix stiffness in regulation of focal adhesion size and number: Reciprocal regulation of caveolin-1 and beta1 integrin. Sci. Rep. 2017, 7, 15008. [Google Scholar] [CrossRef]
- Abdelmaksoud, N.M.; El-Mahdy, H.A.; Ismail, A.; Elsakka, E.G.E.; El-Husseiny, A.A.; Khidr, E.G.; Ali, E.M.; Rashed, M.H.; El-Demerdash, F.E.-S.; Doghish, A.S. The role of miRNAs in the pathogenesis and therapeutic resistance of endometrial cancer: A spotlight on the convergence of signaling pathways. Pathol. Res. Pract. 2023, 244, 154411. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Rikka, S.; Quinsay, M.N.; Thomas, R.L.; Kubli, D.A.; Zhang, X.; Murphy, A.N.; Gustafsson, A.B. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ. 2011, 18, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.E. Biotin Proximity Labeling for Protein-Protein Interaction Discovery: The BioID Method. Methods Mol. Biol. 2021, 2261, 357–379. [Google Scholar]
- Hollinshead, M.; Sanderson, J.; Vaux, D.J. Anti-biotin antibodies offer superior organelle-specific labeling of mitochondria over avidin or streptavidin. J. Histochem. Cytochem. 1997, 45, 1053–1057. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, F.; Sharif, A.; Kidd, M.; Keevill, E.-J.; Biolatti, V.; Unwin, R.D.; Hoskin, P.; Choudhury, A.; Smith, T.A.D.; Quiles, C.G. Cell Surface Proteomics Reveals Hypoxia-Regulated Pathways in Cervical and Bladder Cancer. Proteomes 2025, 13, 36. https://doi.org/10.3390/proteomes13030036
Alanazi F, Sharif A, Kidd M, Keevill E-J, Biolatti V, Unwin RD, Hoskin P, Choudhury A, Smith TAD, Quiles CG. Cell Surface Proteomics Reveals Hypoxia-Regulated Pathways in Cervical and Bladder Cancer. Proteomes. 2025; 13(3):36. https://doi.org/10.3390/proteomes13030036
Chicago/Turabian StyleAlanazi, Faris, Ammar Sharif, Melissa Kidd, Emma-Jayne Keevill, Vanesa Biolatti, Richard D. Unwin, Peter Hoskin, Ananya Choudhury, Tim A. D. Smith, and Conrado G. Quiles. 2025. "Cell Surface Proteomics Reveals Hypoxia-Regulated Pathways in Cervical and Bladder Cancer" Proteomes 13, no. 3: 36. https://doi.org/10.3390/proteomes13030036
APA StyleAlanazi, F., Sharif, A., Kidd, M., Keevill, E.-J., Biolatti, V., Unwin, R. D., Hoskin, P., Choudhury, A., Smith, T. A. D., & Quiles, C. G. (2025). Cell Surface Proteomics Reveals Hypoxia-Regulated Pathways in Cervical and Bladder Cancer. Proteomes, 13(3), 36. https://doi.org/10.3390/proteomes13030036





