Survey of Anti-Pathogen Antibody Levels in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. ME/CFS Case Selection and Sample Acquisition
2.2. Augmenta Serological Testing
2.3. Statistical Analysis
3. Results
3.1. Subject Characteristics
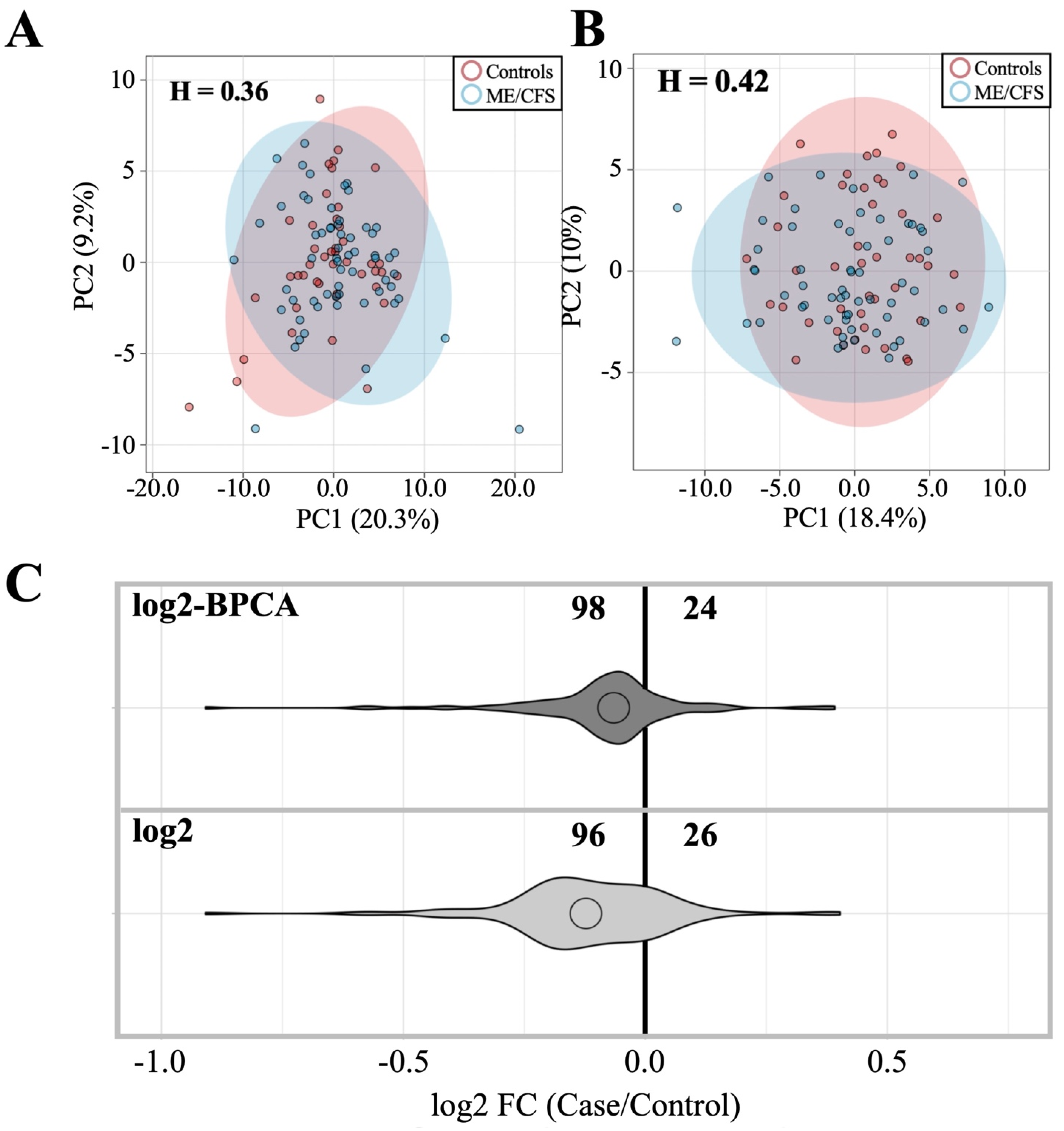
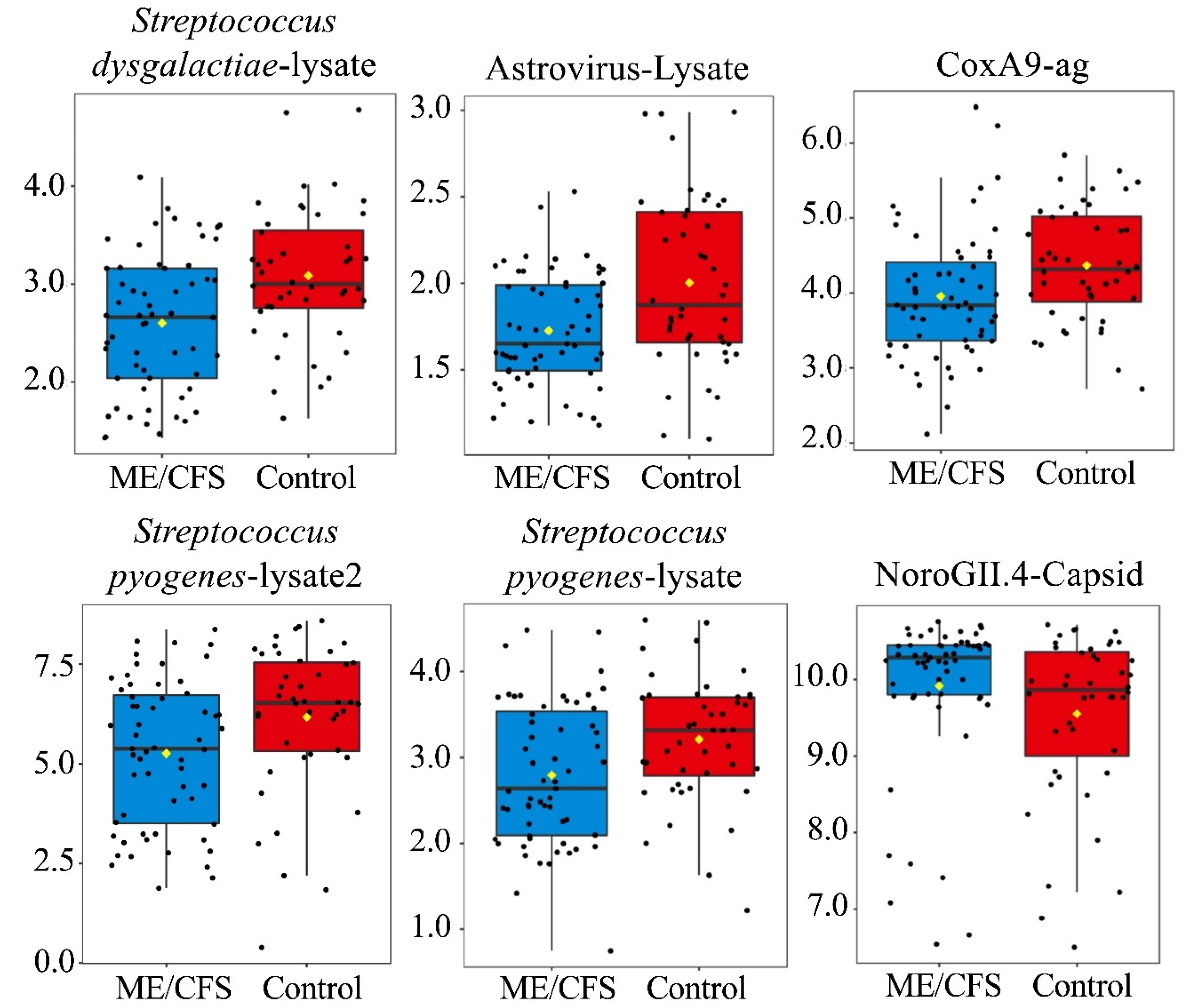
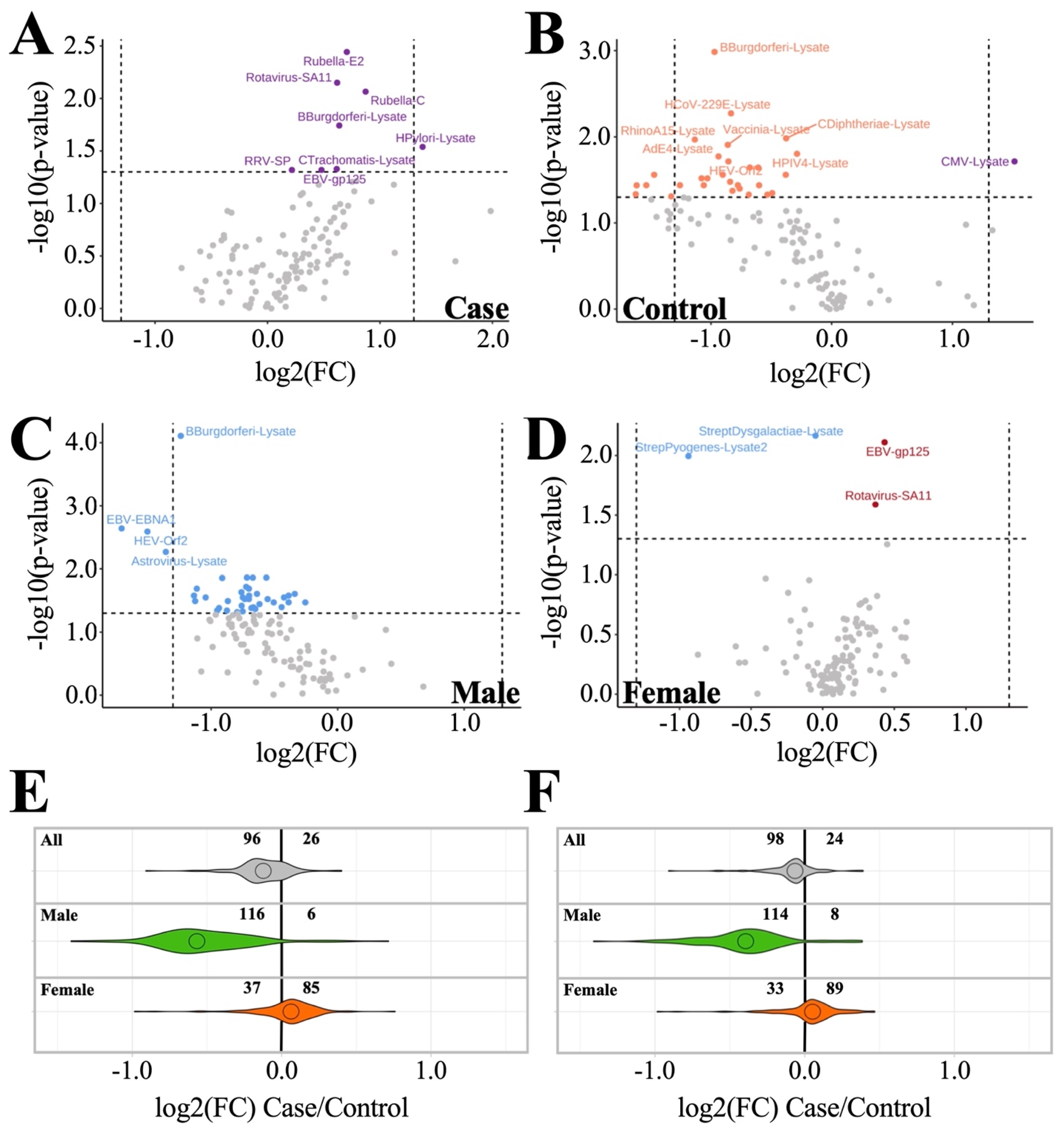
3.2. Anti-Pathogen Antibody Profiles between ME/CFS Cases and Controls
3.2.1. Dataset Amendment for Outlier Influence Does Not Significantly Alter Findings Related to Antibody Profile Differences
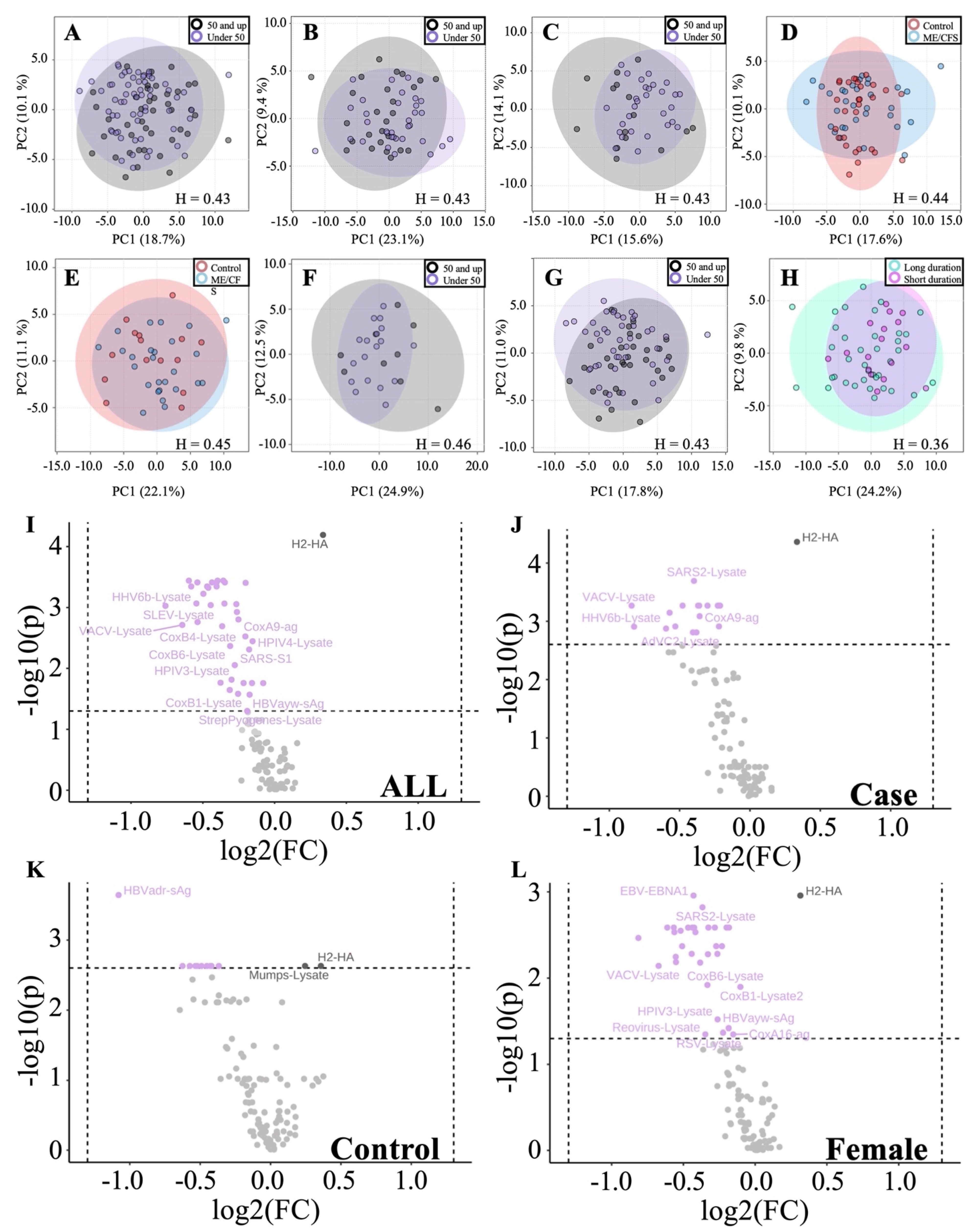
3.2.2. Sex-Based Subgrouping Reveals Differences between Male and Female Antibody Profiles
3.2.3. Age and Illness Duration Subgroup Analyses Reveals Additional Insights into Antibody Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic. Fatigue. Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Hyde, B.M.; Goldstein, J.; Levine, P. (Eds.) The Clinical and Scientific Basis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Nightingale Research Foundation: Ottawa, ON, Canada, 1992. [Google Scholar]
- Sydenham, T. The Works of Thomas Sydenham, M.D., on Acute and Chronic Diseases: With Their Histories and Modes of Cure; Benjamin & Thomas Kite: Philadelphia, PA, USA, 1809. [Google Scholar]
- Manningham, R. The Symptoms, Nature, Causes, and Cure of the Febricula, or Little Fever: Commonly Called the Nervous or Hysteric Fever; the Fever on the Spirits, Vapours, Hypo, or Spleen; Printed for J. Robinson; Golden-Lion in Ludgate-Fleet: London, UK, 1750. [Google Scholar]
- Gilliam, A.G. Epidemiological Study of an Epidemic, Diagnosed as Poliomyelitis, Occurring among the Personnel of the Los Angeles County General Hospital durring the Summer of 1934; Public Health Service, for Sale by the Supt. of Docs.; US Government Printing Office: Washington, DC, USA, 1938.
- O’Neal, A.J.; Hanson, M.R. The enterovirus theory of disease etiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A critical review. Front. Med. 2021, 8, 688486. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D. Seasonality of viral infections: Mechanisms and unknowns. Clin. Microbiol. Infect. 2012, 18, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.G. Early outbreaks of ‘epidemic neuromyasthenia’. Postgrad. Med. J. 1978, 54, 711–717. [Google Scholar] [CrossRef]
- Chia, J.K.S. The role of enterovirus in chronic fatigue syndrome. J. Clin. Pathol. 2005, 58, 1126–1132. [Google Scholar] [CrossRef]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef]
- Evengard, B.; Briese, T.; Lindh, G.; Lee, S.; Lipkin, W.I. Absence of evidence of Borna disease virus infection in Swedish patients with Chronic Fatigue Syndrome. J. Neurovirol. 1999, 5, 495–499. [Google Scholar] [CrossRef][Green Version]
- Nowotny, N.; Kolodziejek, J. Demonstration of Borna disease virus nucleic acid in a patient with chronic fatigue syndrome. J. Infect. Dis. 2000, 181, 1860–1862. [Google Scholar] [CrossRef][Green Version]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Evidence for an autoimmune disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Brenu, E.W.; Huth, T.K.; Hardcastle, S.L.; Fuller, K.; Kaur, M.; Johnston, S.; Ramos, S.B.; Staines, D.R.; Marshall-Gradisnik, S.M. Role of adaptive and innate immune cells in chronic fatigue syndrome/myalgic encephalomyelitis. Int. Immunol. 2014, 26, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S. (Ed.) The Doctor’s Guide to Chronic Fatigue Syndrome. Understanding, Treating, and Living with CFIDS; Addison-Wesley Publishing Company: Reading, MA, USA, 1994. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.G.; Jurs, P.C. New index for clustering tendency and its application to chemical problems. J. Chem. Inform. Comput. Sci. 1990, 30, 36–41. [Google Scholar] [CrossRef]
- Banerjee, A.; Dave, R.N. (Eds.) Validating clusters using the Hopkins statistic. In Proceedings of the 2004 IEEE International Conference on Fuzzy Systems (IEEE Cat No04CH37542), Budapest, Hungary, 25–29 July 2004. [Google Scholar]
- Pickering, J.W.; Martins, T.B.; Schroder, M.C.; Hill, H.R. Comparison of a Multiplex Flow Cytometric Assay with Enzyme-Linked Immunosorbent Assay for Quantitation of Antibodies to Tetanus, Diphtheria, and Haemophilus influenzae Type b. Clin. Vaccine Immunol. 2002, 9, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.; Chandler, D.J.; Dunbar, S.A. The genesis and evolution of bead-based multiplexing. Methods 2019, 158, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Rizwan, M.; Böhlin-Wiener, A.; Elfaitouri, A.; Julin, P.; Zachrisson, O.; Rosén, A.; Gottfries, C.-G. Antibodies to Human Herpesviruses in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Front. Immunol. 2019, 10, 1946. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2019, 6, 412. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E5472–E5480. [Google Scholar] [CrossRef]
- Anderson, E.J.; Weber, S.G. Rotavirus infection in adults. Lancet Infect. Dis. 2004, 4, 91–99. [Google Scholar] [CrossRef]
- Ojobor, C.D.; Olovo, C.V.; Onah, L.O.; Ike, A.C. Prevalence and associated factors to rotavirus infection in children less than 5 years in Enugu State, Nigeria. Virusdisease 2020, 31, 316–322. [Google Scholar] [CrossRef] [PubMed]
- De Paschale, M.; Clerici, P. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World J. Virol. 2012, 1, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Loebel, M.; Eckey, M.; Sotzny, F.; Hahn, E.; Bauer, S.; Grabowski, P.; Zerweck, J.; Holenya, P.; Hanitsch, L.G.; Wittke, K.; et al. Serological profiling of the EBV immune response in Chronic Fatigue Syndrome using a peptide microarray. PLoS ONE 2017, 12, e0179124. [Google Scholar] [CrossRef]
- Domingues, T.D.; Grabowska, A.D.; Lee, J.S.; Ameijeiras-Alonso, J.; Westermeier, F.; Scheibenbogen, C.; Cliff, J.M.; Nacul, L.; Lacerda, E.M.; Mouriño, H.; et al. Herpesviruses Serology Distinguishes Different Subgroups of Patients From the United Kingdom Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Biobank. Front. Med. 2021, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- De Ory, F.; Guisasola, M.E.; Sanz, J.C.; García-Bermejo, I. Evaluation of Four Commercial Systems for the Diagnosis of Epstein-Barr Virus Primary Infections. Clin. Vaccine Immunol. 2011, 18, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Cliff, J.M.; King, E.C.; Lee, J.S.; Sepúlveda, N.; Wolf, A.S.; Kingdon, C.; Bowman, E.; Dockrell, H.M.; Nacul, L.; Lacerda, E.; et al. Cellular Immune Function in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Immunol. 2019, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, N.; Carneiro, J.; Lacerda, E.; Nacul, L. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome as a Hyper-Regulated Immune System Driven by an Interplay Between Regulatory T Cells and Chronic Human Herpesvirus Infections. Front. Immunol. 2019, 10, 2684. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, L.; Moreni, G.; Wolthers, K.C.; Pajkrt, D. World-wide prevalence and genotype distribution of enteroviruses. Viruses 2021, 13, 434. [Google Scholar] [CrossRef]
- Goldman, L. Enteroviruses. In Goldman-Cecil Medicine, 26th ed.; Goldman LaS, A.I., Ed.; Elsevier: Philadelphia, PA, USA, 2020; p. 2664. [Google Scholar]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef]
| ME/CFS | Controls | Mann–Whitney U-Test | |
|---|---|---|---|
| Age | 46.1 ± 10.5 | 42.1 ± 14.2 | p = 0.11 |
| Gender | |||
| Female | 47 | 29 | NA |
| Male | 12 | 15 | NA |
| BMI (kg/m2) | 26.5 ± 5.7 | 27.5 ± 5.0 | p = 0.30 |
| Ethnicity | |||
| Hispanic or Latino | 9 | 6 | NA |
| Not Hispanic or Latino | 46 | 37 | NA |
| Unknown | 4 | 1 | NA |
| Race | |||
| American Indian or Alaska Native | 0 | 1 | NA |
| Asian | 2 | 5 | NA |
| Black or African American | 0 | 3 | NA |
| Native Hawaiian or other Pacific Islander | 1 | 0 | NA |
| White | 51 | 32 | NA |
| Unknown | 5 | 3 | NA |
| Onset of disease | |||
| Gradual | 44% | NA | NA |
| Sudden | 56% | NA | NA |
| Illness Duration (years) | 12.1 ± 9.6 | NA | NA |
| Bell Score | 34.6 ± 12.2 | 95.5 ± 8.5 | p < 0.001 |
| SF-36 | |||
| Physical function | 38.6 ± 19.3 | 94.2 ± 9.3 | p < 0.001 |
| Role physical | 16.1 ± 18.8 | 98.3 ± 4.5 | p < 0.001 |
| Pain | 41.9 ± 22.0 | 83.9 ± 16.7 | p < 0.001 |
| General health | 22.9 ± 11.6 | 81.2 ± 13.9 | p < 0.001 |
| Vitality | 17.6 ± 14.0 | 70.3 ± 17.8 | p < 0.001 |
| PCS a | 27.2 ± 7.2 | 56.1 ± 4.7 | p < 0.001 |
| Social function | 26.3 ± 20.7 | 97.7 ± 11.5 | p < 0.001 |
| Role emotional | 81.5 ± 24.8 | 97.0 ± 7.9 | p < 0.001 |
| Mental health | 66.4 ± 18.8 | 83.9 ± 10.1 | p < 0.001 |
| MCS a | 45.1 ± 9.5 | 55.3 ± 5.1 | p < 0.001 |
| log2 Dataset | log2-BPCA Dataset | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathogen | ME/CFS | Control | p | q | Pathogen | ME/CFS | Control | p | q |
| Streptococcus dysgalactiae-lysate | 2.7 ± 0.9 | 3.1 ± 0.9 | 0.003 | 0.39 | Streptococcus dysgalactiae-lysate | 2.6 ± 0.7 | 3.1 ± 0.7 | 0.001 | 0.18 |
| Streptococcus pyogenes-lysate2 | 5.3 ± 1.8 | 6.2 ± 1.9 | 0.008 | 0.49 | Astrovirus-Lysate | 1.7 ± 0.3 | 2.0 ± 0.5 | 0.003 | 0.18 |
| Streptococcus pyogenes-lysate | 2.8 ± 0.8 | 3.1 ± 0.9 | 0.016 | 0.59 | CoxA9 | 4.0 ± 0.8 | 4.4 ± 0.8 | 0.007 | 0.20 |
| CoxA9 | 4.1 ± 1.1 | 4.4 ± 0.8 | 0.019 | 0.59 | Streptococcus pyogenes-lysate2 | 5.3 ± 1.8 | 6.2 ± 1.9 | 0.008 | 0.20 |
| RhinoA15-Lysate | 2.6 ± 0.6 | 2.9 ± 0.8 | 0.040 | 0.76 | Streptococcus pyogenes-lysate | 2.8 ± 0.8 | 3.2 ± 0.7 | 0.008 | 0.20 |
| Astrovirus-Lysate | 2.0 ± 0.9 | 2.1 ± 0.7 | 0.042 | 0.76 | NoroGII.4-Capsid | 9.9 ± 1.0 | 9.6 ± 1.1 | 0.027 | 0.55 |
| NoroGII.4-Capsid | 9.4 ± 2.1 | 9.0 ± 1.9 | 0.044 | 0.76 | |||||
| Male (n = 42: Top 9 Ranked by p-Value + Rotavirus-SA11) | Female (n = 4) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathogen | ME/CFS | Control | p | q | Pathogen | ME/CFS | Control | p | q |
| B.burgdorferi-lysate | 1.0 ± 0.6 | 2.0 ± 0.7 | 0.000 | 0.01 | Streptococcus dysgalactiae-lysate | 2.6 ± 0.7 | 3.2 ± 0.6 | 0.003 | 0.39 |
| EBV-EBNA1 | 3.0 ± 0.9 | 4.4 ± 1.2 | 0.002 | 0.12 | Streptococcus pyogenes-lysate2 | 5.2 ± 1.7 | 6.2 ± 1.9 | 0.010 | 0.47 |
| RhinoA15-lysate | 2.3 ± 0.5 | 2.9 ± 0.5 | 0.005 | 0.12 | EBV-gp125 | 7.1 ± 0.8 | 6.7 ± 0.9 | 0.012 | 0.47 |
| Astrovirus-lysate | 1.6 ± 0.3 | 2.2 ± 0.6 | 0.005 | 0.12 | Rotavirus-SA11 | 2.9 ± 0.6 | 2.6 ± 0.5 | 0.033 | 0.98 |
| HEV-Orf2 | 3.2 ± 0.8 | 4.1 ± 0.8 | 0.007 | 0.12 | |||||
| RhinoB14-lysate | 2.1 ± 0.4 | 2.5 ± 0.3 | 0.010 | 0.12 | |||||
| SARS2-lysate | 1.3 ± 0.4 | 1.9 ± 0.5 | 0.010 | 0.12 | |||||
| CoxB2-lysate | 0.9 ± 0.2 | 1.4 ± 0.5 | 0.012 | 0.12 | |||||
| C. trachomatis-lysate | 0.8 ± 0.3 | 1.3 ± 0.6 | 0.014 | 0.12 | |||||
| Rotavirus-SA11 | 2.4 ± 0.5 | 2.9 ± 0.7 | 0.025 | 0.12 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neal, A.J.; Glass, K.A.; Emig, C.J.; Vitug, A.A.; Henry, S.J.; Shungu, D.C.; Mao, X.; Levine, S.M.; Hanson, M.R. Survey of Anti-Pathogen Antibody Levels in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Proteomes 2022, 10, 21. https://doi.org/10.3390/proteomes10020021
O’Neal AJ, Glass KA, Emig CJ, Vitug AA, Henry SJ, Shungu DC, Mao X, Levine SM, Hanson MR. Survey of Anti-Pathogen Antibody Levels in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Proteomes. 2022; 10(2):21. https://doi.org/10.3390/proteomes10020021
Chicago/Turabian StyleO’Neal, Adam J., Katherine A. Glass, Christopher J. Emig, Adela A. Vitug, Steven J. Henry, Dikoma C. Shungu, Xiangling Mao, Susan M. Levine, and Maureen R. Hanson. 2022. "Survey of Anti-Pathogen Antibody Levels in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome" Proteomes 10, no. 2: 21. https://doi.org/10.3390/proteomes10020021
APA StyleO’Neal, A. J., Glass, K. A., Emig, C. J., Vitug, A. A., Henry, S. J., Shungu, D. C., Mao, X., Levine, S. M., & Hanson, M. R. (2022). Survey of Anti-Pathogen Antibody Levels in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Proteomes, 10(2), 21. https://doi.org/10.3390/proteomes10020021






