Reflections of a First-Year Chemistry Teacher: Intersecting PCK, Responsiveness, and Inquiry Instruction
Abstract
1. Introduction
- (1)
- How does a novice first-year chemistry teacher describe her responsive teaching practices, and how do they impact her implementation of inquiry instruction?
- (2)
- What patterns emerge around responsiveness, inquiry, and topic-specific pedagogical content knowledge (PCK) during the implementation of several chemistry lessons?
2. Theoretical Framework
3. Review of the Literature
3.1. Inquiry Instruction in Science
3.1.1. Impact of Inquiry Instruction
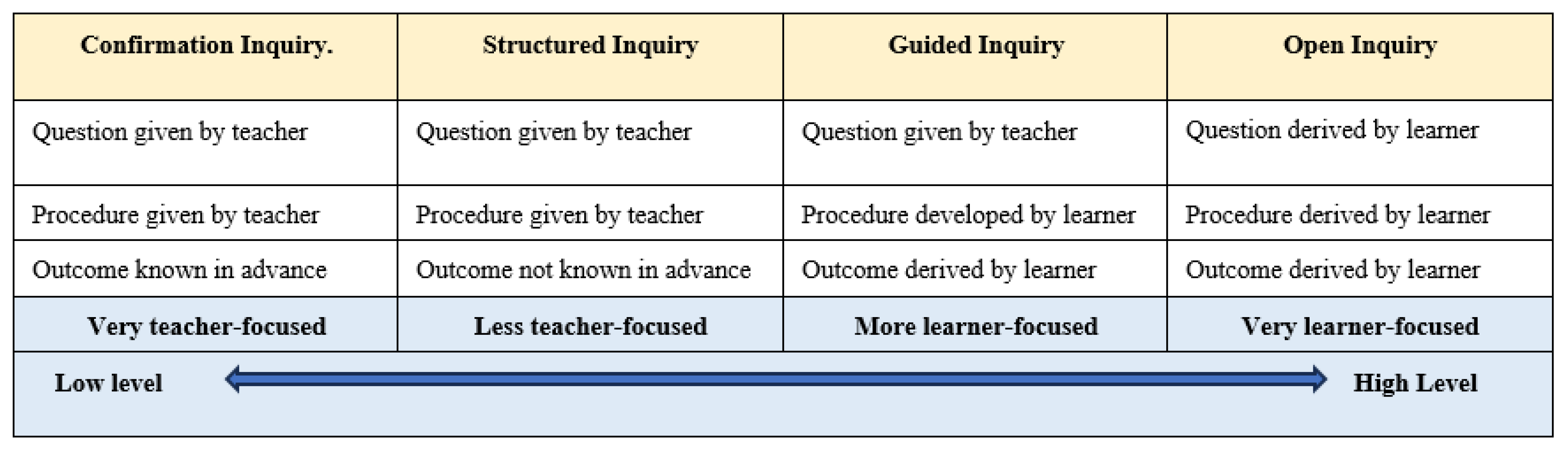
3.1.2. The Continuum of Inquiry Practices
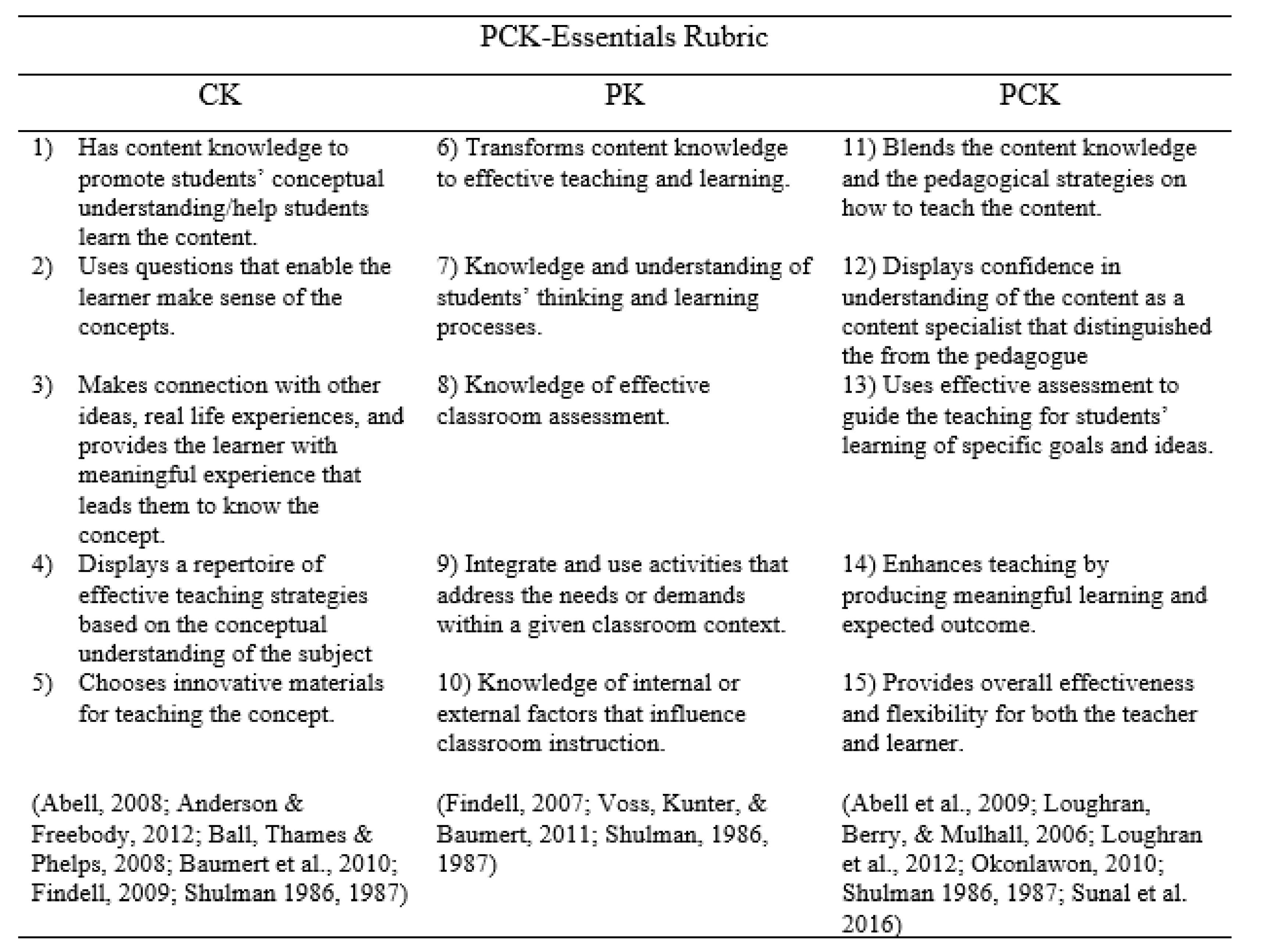
3.2. PCK and Classroom Practice
Teachers must not only be capable of defining for students the accepted truths in a domain. They must also be able to explain why a particular proposition is deemed warranted, why it is worth knowing, and how it relates to other propositions, both within the discipline and without, both in theory and in practice.(p. 9)
3.3. Responsive Teaching
Adaptive teaching in science, technology, engineering, and mathematics (STEM) is a process that teachers initiate when they recognize and gauge their students’ STEM-related conceptual development, inquiry processes, and real-world connections and then maneuver their instruction to further develop these features of students’ learning. An adaptive teacher may engage in this process during planning, while teaching, or after teaching.(p. 217)
4. Materials and Methods
4.1. Background and Context
4.2. Data Collection
4.3. Data Analysis
5. Results
5.1. How Does a Novice First-Year Chemistry Teacher Describe Her Responsive Teaching Practices, and How Do They Impact Her Implementation of Inquiry Instruction?
Big Idea A. Annie: In fourth block, I added the driving question. And so, they were actually more engaged and interested in trying to figure out how many atoms were in the piece of foil. They were trying to figure out how to do it and do research. And they were thinking about “I know this mass on the periodic table is the mass of an atom. So, if we figured out the mass of the foil, we could, you know, like divide it.” And so, they were actually trying to figure it out.
I had a couple of students who are working independently and just kind of flying through it. And then I had a group at the back that was just really interested in figuring out the answer to the foil question. So, I didn’t make them do the whole packet. I showed them how to figure out the number of atoms in the piece of foil. And then I gave them a strip of magnesium from the prep room. And they figured out how many atoms were in that.
It seemed like the driving question and having an actual, “By the end of this class, you’re going to answer this question” and a genuine application for what they were learning helped keep them be more engaged. It gave them more purpose for what they were doing in the lab.
Big Idea D. Annie: So, we didn’t actually get to lab, but we wrote the procedure for it. I don’t know. I think they got the point. I think I care more about them actually thinking through the procedure and getting that skill rather than weighing things out and dissolving things because we did that. We’ve done that now multiple times. I didn’t get everything done that I had wanted to, but that’s okay.
Annie adjusted her lesson from conducting a teacher-focused lab to supporting students in writing a procedure during the first implementation of the Big Idea D lesson. She scaffolded students through the process and supported them in building skills aligning with the NGSS scientific practices. For this big idea, responsiveness led to higher levels of inquiry.
5.2. What Patterns Emerge around Responsiveness, Inquiry, and Topic-Specific Pedagogical Content Knowledge (PCK) during the Implementation of Several Chemistry Lessons?
Big Idea A. Annie: They were actually more engaged and interested in trying to figure out how many atoms were in the piece of foil. I kept having to remind them to do the packet. I kept reminding them that the packet was a scaffold.
Big Idea C. Annie: It was very painful. So, we did a little hands-on lab and discussed expectations before it the lab. I kind of did it with stations. Each one had a different substance that they were going to test to see if that was soluble in water. Since it was very inquiry-based, I have to discuss in order for them to get anything out of the lesson. And they just weren’t interested in discussing. When we tried to have group discussions, they really weren’t giving a lot of input.
The second time, before the lab started, I did put a chart, a data table up on the board with the six substances that they were testing the solubility of. We did a hypothesis before to see if they already knew if they were soluble or not. And we made a hypothesis as a class before we went in the lab and tested them. Afterward, we looked back at the hypotheses that we made and corrected anything that needed to be corrected. The lesson went better, but it wasn’t a better lesson.
Big Idea D. Annie: So, today’s lesson went [pause] not bad, but not good. I didn’t get everything done that I had planned. So, we didn’t actually get to the lab, but we wrote the procedure for it. So, I don’t know.
Big Idea B. Annie (1st implementation): Ok, so I just finished doing first block. It was a mess. I did not do a good job of explaining. I didn’t do the mole map, like I said I wanted to. Or go over it at least. And I feel like they were all confused at the same time, and so I couldn’t help everybody. And it was just [pause] not good. Yeah. Yeah. I was thinking about the question they kept asking me about where to start the calculations. “What should I do first?” I didn’t really get to over the mole map thing. I had copies of it on the lab tables for them to use, but I think I need to spend more time on the fact that the mole is the middleman. And maybe I need to go over the mole map and walk them through how to use it.
Annie (2nd implementation): They still did not love Gas Law ‘stoic,’ but I do think it made it a little bit easier for them to see the actual path. So, they could see how they were connected. Even if maybe, they didn’t necessarily know why you would use both. But they could see the connection and it gave them a pathway. So, like it wasn’t like every single problem they had to start from scratch and figure out what to do. They started to see the pattern and used it as a tool. The first group didn’t have that tool, so they were kind of like all over the place.
Big Ideas D, E, F. Annie: I never really remembered learning about solutions that much in my own chemistry class. And so, I didn’t have a good understanding of the scope that I needed to go in to and specifically what I needed to teach. Whereas solutions, I feel like there’s more wiggle room for how in-depth you go and how much you want them to know about everything. Like when I was writing these two paragraphs to summarize the unit, I kept moving things around and taking this out and putting it down here because there’s so many different sequences you could teach it. Since everything’s kind of intertwined. So, I was having trouble figuring out the best logical way to go. It’s like you’re on a circle and at what point on the circle do you start? I think I just needed a better understanding of the overall end goal so that I could plan the activities better.
Big Idea E. So, I had two pitchers of Kool-Aid. One of them was the correct recipe and one of them was twice the amount of Kool-Aid powder and sugar needed for that specific volume of water. And so, I started out with questions about the difference between the two. Will they taste the same? What’s different about them? And so, I just kind of led them to the fact that one of the solutions is more concentrated. Then I asked them, “How do you think concentration is calculated?” And they were actually able to pretty much tell me the, the formula without knowing the units. They knew that it was a ratio of solute to solvent. And so, I added that molarity is moles to liters. And then we calculated the molarity of what Kool-Aid should be if you follow the recipe correctly. And then we calculated the molarity of what the stock solution or the one that I had made super concentrated was.
Big Idea F. It made sense that we know the concentration, so what do I need to do to get it from this high concentration to the one that I want it to be at. Although the drawback is that the M1V1 = M2V2 formula is kind of confusing. It took me and the other teachers like a minute to figure out, okay, what number goes where? And I still have to really think about it. Since I think of it like that, we have our current concentration, and we don’t know what volume it needs to be to make the other one. Or maybe it’s the other way around.
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Research Council. A Framework for k-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- DeBoer, G. A History of Ideas in Science Education; Teachers College Press: New York City, NY, USA, 2019. [Google Scholar]
- Rudolph, J.L. How We Teach Science-What′s Changed, and Why It Matters; Harvard University Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Lederman, J.; Lederman, N.; Bartels, S.; Jimanez, J.J. Understandings of scientific inquiry: An international collaborative investigation of grade seven students. In Bridging Research and Practice in Science Education: Selected Papers from the ESERA 2017 Conference; McLoughlin, E., Finlayson, O., Erduran, S., Childs, P., Eds.; Contributions from Science Education Research; Springer International Publishing: Cham, Switzerland, 2019; pp. 189–201. [Google Scholar] [CrossRef]
- Levin, D.; Hammer, D.; Elby, A.; Coffey, J. Becoming a Responsive Science Teacher: Focusing on Student Thinking in Secondary Science; National Science Teachers Association Arlington: Arlington, VA, USA, 2012. [Google Scholar]
- Richards, J.; Elby, A.; Luna, M.J.; Robertson, A.D.; Levin, D.M.; Nyeggen, C.G. Reframing the Responsiveness Challenge: A Framing-Anchored Explanatory Framework to Account for Irregularity in Novice Teachers’ Attention and Responsiveness to Student Thinking. Cogn. Instr. 2020, 38, 116–152. [Google Scholar] [CrossRef]
- Allen, M.H.; Matthews, C.E.; Parsons, S.A. A second-grade teacher’s adaptive teaching during an integrated science-literacy unit. Teach. Teach. Educ. 2013, 35, 114–125. [Google Scholar] [CrossRef]
- Hatano, G.; Inagaki, K.; Stevenson, H.W.; Azuma, H.; Hakuta, K. Child development and education in Japan. In Two Courses of Expertise; American Psychological Association: Worcester, MA, USA, 1986; pp. 262–272. [Google Scholar]
- Wiske, M. Teaching for Understanding. Linking Research with Practice; The Jossey-Bass Education Series; ERIC, Jossey-Bass Inc.: San Francisco, CA, USA, 1998. [Google Scholar]
- Shulman, L.S. Those Who Understand: Knowledge Growth in Teaching. Educ. Res. 1986, 15, 4–14. [Google Scholar] [CrossRef]
- Shulman, L. Knowledge and Teaching: Foundations of the New Reform. Harv. Educ. Rev. 1987, 57, 1–23. [Google Scholar] [CrossRef]
- Carlson, J.; Daehler, K. The refined consensus model of pedagogical content knowledge in science education. In Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science; Hume, A., Cooper, R., Borowski, A., Eds.; Springer: Gateway East, Singapore, 2019; pp. 77–94. [Google Scholar]
- Park, S.; Jang, J.-Y.; Chen, Y.-C.; Jung, J. Is Pedagogical Content Knowledge (PCK) Necessary for Reformed Science Teaching?: Evidence from an Empirical Study. Res. Sci. Educ. 2010, 41, 245–260. [Google Scholar] [CrossRef]
- Carpendale, J.; Hume, A. Investigating practising science teachers’ pPCK and ePCK development as a result of collaborative CoRe design. In Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science; Springer: Gateway East, Singapore, 2019; pp. 223–250. [Google Scholar]
- Kavanagh, S.S.; Metz, M.; Hauser, M.; Fogo, B.; Taylor, M.W.; Carlson, J. Practicing Responsiveness: Using Approximations of Teaching to Develop Teachers’ Responsiveness to Students’ Ideas. J. Teach. Educ. 2019, 71, 94–107. [Google Scholar] [CrossRef]
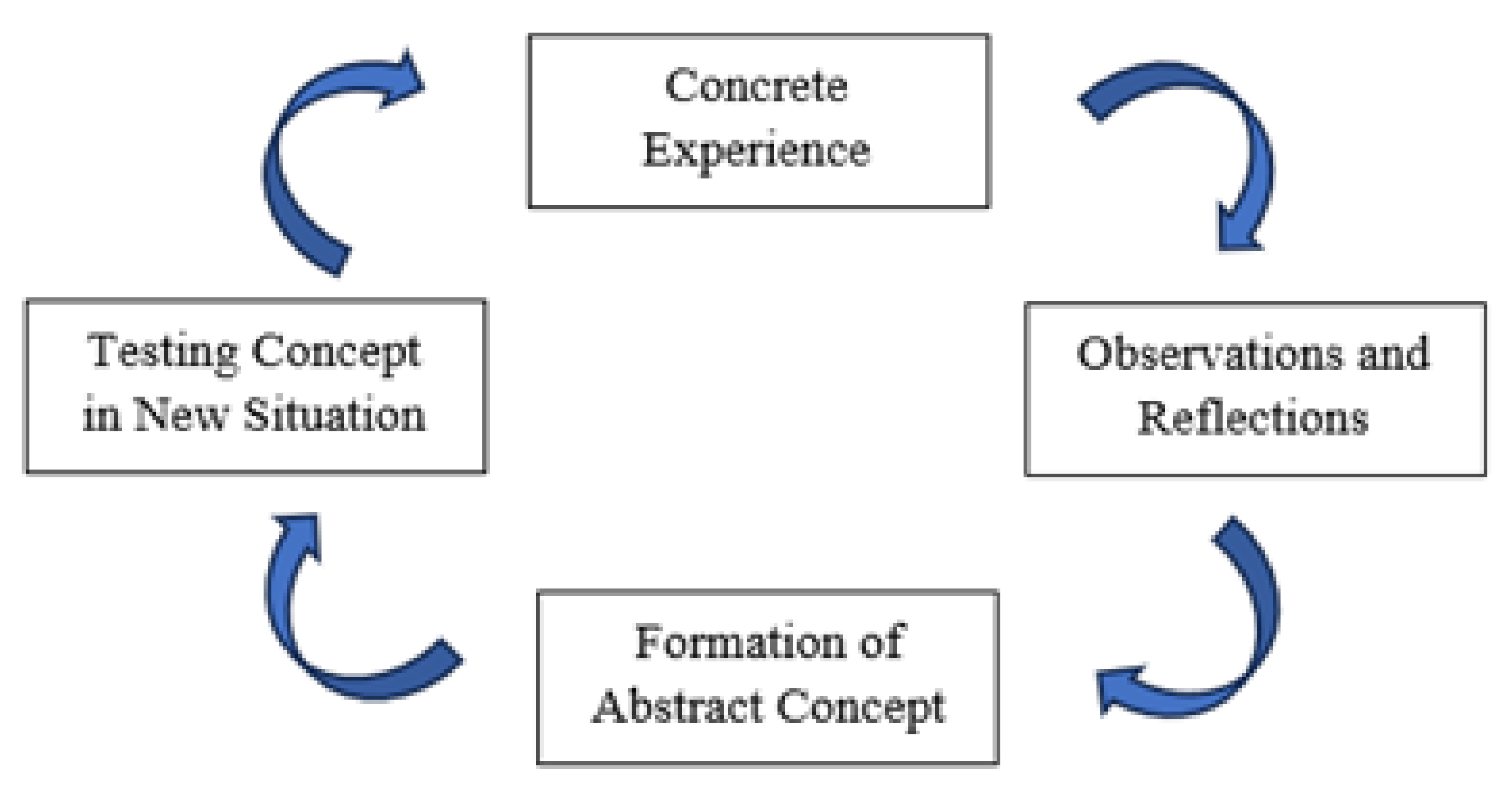
- Kolb, D.A. Experiential Learning: Experience as the Source of Learning and Development; FT Press: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Lehane, L. Experiential Learning—David A. Kolb. In Science Education in Theory and Practice; Akpan, B., Kennedy, T.J., Eds.; Springer Texts in Education; Springer International Publishing: Cham, Switzerland, 2020; pp. 241–257. [Google Scholar] [CrossRef]
- Barber, J.; Cervetti, G. No More Science Kits or Texts in Isolation: Teaching Science and Literacy Together; Heinemann: Portsmouth, NH, USA, 2019. [Google Scholar]
- Windschitl, M.; Thompson, J.; Braaten, M. Ambitious Science Teaching; Harvard Education Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Wilcox, J.; Kruse, J.; Clough, M. Teaching Science Through Inquiry. Sci. Teach. 2015, 82, 62–67. [Google Scholar] [CrossRef]
- Poderoso, C. The Science Experience: The Relationship between an Inquiry-Based Science Program and Student Outcomes; California State University: Fullerton, CA, USA, 2013; Available online: https://search.proquest.com/openview/1dd8a3aea652343ffc18c74387b51bd6/1?pq-origsite=gscholar&cbl=18750 (accessed on 8 October 2023).
- Twahirwa, J.N.; Ntivuguruzwa, C.; Wizeyimana, E.T.; Nyirahagenimana, J. Teachers’ Perceptions of Inquiry-based Learning in Science Education: A Case of Selected Secondary Schools in Kirehe District, Rwanda. East Afr. J. Educ. Soc. Sci. 2022, 3, 29–38. [Google Scholar] [CrossRef]
- Harlen, W. Inquiry-Based Learning in Science: Assessment and Content Implications; LAMBERT Academic Publishing: Saarbrücken, Germany, 2015. [Google Scholar]
- Wei, L.; LeSage-Clements, T. Science Teacher Preparation: Themes of Exemplary STEM Inquiry Instruction. Int. J. Contemp. Educ. 2018, 2, 72–77. [Google Scholar] [CrossRef][Green Version]
- Areepattamannil, S.; Cairns, D.; Dickson, M. Teacher-Directed Versus Inquiry-Based Science Instruction: Investigating Links to Adolescent Students’ Science Dispositions Across 66 Countries. J. Sci. Teach. Educ. 2020, 31, 675–704. [Google Scholar] [CrossRef]
- Oliver, M.; McConney, A.; Woods-McConney, A. The Efficacy of Inquiry-Based Instruction in Science: A Comparative Analysis of Six Countries Using PISA 2015. Res. Sci. Educ. 2019, 51, 595–616. [Google Scholar] [CrossRef]
- Kirschner, P.A.; Sweller, J.; Clark, R.E. Why Minimal Guidance During Instruction Does Not Work: An Analysis of the Failure of Constructivist, Discovery, Problem-Based, Experiential, and Inquiry-Based Teaching. Educ. Psychol. 2006, 41, 75–86. [Google Scholar] [CrossRef]
- Dobber, M.; Zwart, R.; Tanis, M.; van Oers, B. Literature review: The role of the teacher in inquiry-based education. Educ. Res. Rev. 2017, 22, 194–214. [Google Scholar] [CrossRef]
- Nicol, C.B. An Overview of Inquiry-Based Science Instruction Amid Challenges. Eurasia J. Math. Sci. Technol. Educ. 2021, 17, em2042. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Hume, A. Towards a consensus model: Literature review of how science teachers’ pedagogical content knowledge is investigated in empirical studies. In Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science; Springer: Gateway East, Singapore, 2019; pp. 3–76. [Google Scholar]
- Robertson, A.D.; Scherr, R.; Hammer, D. Responsive Teaching in Science and Mathematics; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Richards, J.; Robertson, A.D. A review of the research on responsive teaching in science and mathematics. In Responsive Teaching in Science and Mathematics; Routledge: New York, NY, USA, 2015; pp. 36–55. [Google Scholar]
- Franke, M.L.; Webb, N.M.; Chan, A.G.; Ing, M.; Freund, D.; Battey, D. Teacher Questioning to Elicit Students’ Mathematical Thinking in Elementary School Classrooms. J. Teach. Educ. 2009, 60, 380–392. [Google Scholar] [CrossRef]
- Pierson, J.L. The Relationship between Patterns of Classroom Discourse and Mathematics Learning. Ph.D. Thesis, University of Texas, Austin, TX, USA, 2008. [Google Scholar]
- Lineback, J.E. Methods to assess teacher responsiveness in situ. In Responsive Teaching in Science and Mathematics; Routledge: New York, NY, USA, 2015; pp. 203–226. Available online: https://api.taylorfrancis.com/content/chapters/edit/download?identifierName=doi&identifierValue=10.4324/9781315689302-10&type=chapterpdf (accessed on 8 October 2023).
- Allen, M.; Webb, A.W.; Matthews, C.E. Adaptive Teaching in STEM: Characteristics for Effectiveness. Theory Pract. 2016, 55, 217–224. [Google Scholar] [CrossRef]
- Creswell, J.; Poth, C. Qualitative Inquiry and Research Design: Choosing among Five Approaches, 4th ed.; Sage Publications: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Merriam, S.; Tisdell, E. Qualitative Research: A Guide to Design and Implementation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Morris, D.L. Exploring Coach-Mediated Reflection: Developing an Early-Career Chemistry Teacher’s Pedagogical Content Knowledge. Ph.D. Thesis, Baylor University, Waco, TX, USA, 2022. [Google Scholar]
- Wilson, C.; Stuhlsatz, M.; Hvidsten, C.; Gardner, A. Analysis of practice and teacher PCK: Inferences from professional development research. In Pedagogical Content Knowledge in STEM; Springer: Cham, Switzerland, 2018; pp. 3–16. [Google Scholar]
- Ogodo, J.A. Comparing Advanced Placement Physics Teachers Experiencing Physics-Focused Professional Development. J. Sci. Teach. Educ. 2019, 30, 639–665. [Google Scholar] [CrossRef]
| Label | Big Idea | Initial PCK Score |
|---|---|---|
| Big Idea A | The mole concept | 53 |
| Big Idea B | Gas law stoichiometry | 43 |
| Big Idea C Big Idea D | “Like dissolves like” Types of solutions | 38 38 |
| Big Idea E Big Idea F | Molarity Dilutions | 37 34 |
| Big Idea (PCK/60) | Response/Adaptation | Timing of Response | Student Reaction | Movement on Continuum |
|---|---|---|---|---|
| A (53) | Began the second lesson with the driving question about the piece of aluminum foil | Between Implementations | Increased engagement | Away from very student-focused |
| Did not require all students to use the packet/let them focus on the aluminum foil from the driving question/provided an application/extension using a magnesium strip for the independent groups. | During 2nd Implementation | Student thinking visible Student questions driving investigation | Toward more student-focused | |
| Begin the lesson with the aluminum foil, have them guess how many atoms are in it, and brainstorm how to figure it out | For Future Implementations | NA | Toward more student-focused | |
| B (43) | Began the 2nd lesson by explaining the mole map (Direct Instruction) | Between Implementations | Increased engagement | Not on continuum |
| C (38) | Allowed flexibility in stations | Between Implementations | Increased engagement | Toward more student-focused |
| Gave clearer instructions/added a printed procedures for students to take to lab/put data chart on the board | Between Implementations | Increased engagement | Toward more teacher-focused | |
| Class worked collectively to develop a hypothesis before entering the lab | Between Implementations | Student thinking visible | Toward more student-focused | |
| Planned to create an inquiry lesson for the future | For Future Implementations | NA | Toward more student-focused | |
| D (38) | Did not get to the lab but had students collectively write the procedure during the 1st and 2nd lesson | During Implementation | Increased engagement Student thinking visible | Toward more student-focused |
| E (37) | Guiding students through developing concentration formula by thinking aloud | Kept Guided Inquiry Strategy | Student thinking visible | Remained highly student-focused |
| F (34) | Worked mathematics problems | Direct Instruction | Confusion | Not on continuum |
| Examples of Narrative Codes | Categories | Themes and Subthemes |
|---|---|---|
| We created a collaborative hypothesis before the lab so it would flow better, looked back at hypothesis after lab, discussed and corrected thinking. I didn’t get everything done I wanted to, we didn’t actually get to the lab, I care more about them getting the skill, they were intrigued, I wanted them to make the connection, I kept reminding them to do the packet, it scaffolded everything for them to get to the answer, The packet told them exactly what to do, and I gave them the resources, I didn’t really like the lesson because it needs to be more inquiry, It was very inquiry based so they needed to do a lot of discussion, We did the first two together, so I didn’t think I was asking a lot, I put a data table up on the board with the six substances that they were testing | Teaching focus Learning focus Lesson completion mindset Inquiry promotes understanding Conducting investigations emphasis Hands-on equals inquiry More scaffolding is better | Teaching Beliefs Stated Beliefs Demonstrated Beliefs |
| I don’t know if that was the best way to do it, It was very confusing, They are not actually separating when they dissolve, I don’t remember doing that in school, I was not as confident with that idea, It was very inquiry based [we were just dissolving salt in water], Students were initially engaged in the lesson but then got confused during calculations, but it took me a minute to figure it out, We got the math out of the way in one day, They didn’t make the connection because it was math on more math | Science content Science pedagogy Mathematics content Mathematics pedagogy Mathematics/Science connections | Teacher Knowledge Competence Self-Efficacy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, D.L. Reflections of a First-Year Chemistry Teacher: Intersecting PCK, Responsiveness, and Inquiry Instruction. Educ. Sci. 2024, 14, 93. https://doi.org/10.3390/educsci14010093
Morris DL. Reflections of a First-Year Chemistry Teacher: Intersecting PCK, Responsiveness, and Inquiry Instruction. Education Sciences. 2024; 14(1):93. https://doi.org/10.3390/educsci14010093
Chicago/Turabian StyleMorris, Dana Lynn. 2024. "Reflections of a First-Year Chemistry Teacher: Intersecting PCK, Responsiveness, and Inquiry Instruction" Education Sciences 14, no. 1: 93. https://doi.org/10.3390/educsci14010093
APA StyleMorris, D. L. (2024). Reflections of a First-Year Chemistry Teacher: Intersecting PCK, Responsiveness, and Inquiry Instruction. Education Sciences, 14(1), 93. https://doi.org/10.3390/educsci14010093