An Exploration of How Multimodally Designed Teaching and the Creation of Digital Animations can Contribute to Six-Year-Olds’ Meaning Making in Chemistry
Abstract
1. Introduction
- -
- What happens and what is possible when six-year-olds participate in multimodally designed learning activities and create digital animations in chemistry?
1.1. Semiotic Resources in Chemistry Education
…the actions and artefacts we use to communicate, whether they are produced physiologically—with our vocal apparatus; with the muscles we use to create facial expressions and gestures, etc.—or by means of technologies—with pen, ink and paper; with computer hardware and software; with fabrics, scissors and sewing machines, etc.[19] (p. 3).
1.2. The Creation of Digital Animations
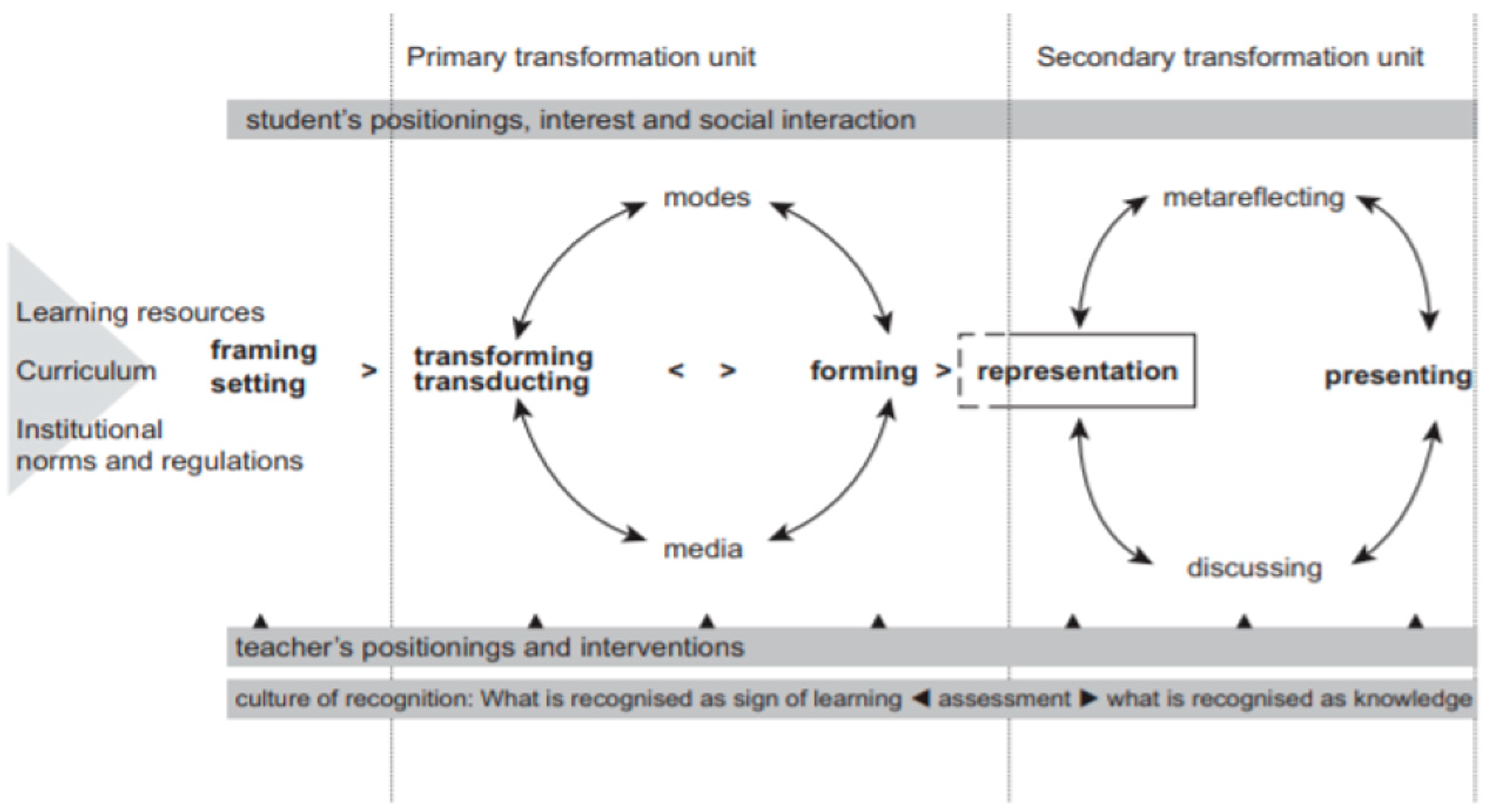
1.3. Designs for Learning Theory and the Learning Design Sequence Model
the concept of “designs for learning” highlights the material and temporal conditions for learning as well as the learning activity itself. The use of modes and media in processes of interpretation and identity construction is here central for the understanding of learning activities.
2. Methods
2.1. Participants, Settings, and Ethical Considerations
2.2. Data Collection
Video-Stimulated Recall Interviews in Pairs and Groups
2.3. Analytical Methods
3. Results
3.1. Framing and Setting
3.2. The Primary Transformation Unit
3.2.1. Activity 1—Tasting Water to Arouse Curiosity and Connect to the Pupils’ Everyday Experiences
3.2.2. Activity 2—Exploring the Submicroscopic Level of Water with Three-Dimensional Ball and Stick Models
3.2.3. Activity 3—Looking at Pictures of Water Molecules and Different States of Water to Connect the Submicroscopic Level to the Macroscopic Level
3.2.4. Activity 4—Exploring the Motion of Water Molecules through a Combination of Modes
3.2.5. Activity 5—Exploring the Motion of Water Molecules through Bodily Action
3.2.6. Activity 6—Exploring the Motion of Water Molecules by Watching a Video Clip
3.2.7. Activity 7—Reconnecting to the Macroscopic Level by Holding an Ice Cube
3.2.8. Activity 8—Using Previous Resources to Reconnect the Structure and the Movement of Water Molecules
3.2.9. Activity 9—Building with Ball and Stick Models to Explore the Three-Dimensional Structure of Water Molecules
3.2.10. Activity 10—Pupils Creating Digital Animations to Transform the Content and Represent Their Experience
3.3. The Secondary Transformation Unit
Meta-Reflection
4. Discussion
4.1. A Multimodal Design can Increase Pupils’ Meaning Making in Chemistry
4.2. The Creation of Digital Animations Increases Pupils’ Participation and Meaning Making
4.3. Meta-Reflection about Pupils’ Representations as an Important Part of the Lesson Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, P. Delaktighet och Digitala Resurser. Ph.D. Thesis, Stockholm University, Göteborg, Sweden, 2020. [Google Scholar]
- Ainsworth, S.; Tytler, R.; Prain, V. Learning by construction of multiple representations. In Handbook of Learning from Multiple Representations and Perspectives; Van Meter, P., List, A., Lombardi, D., Kendeou, P., Eds.; Routledge: New York, NY, USA, 2020; pp. 92–106. [Google Scholar]
- Danielsson, K.; Jeppsson, F.; Nestlog, E.B.; Tang, K.-S. Representations of science content in a primary classroom: Combining long and short timescales for multimodal analysis. Sci. Educ. 2023, 107, 1561–1592. [Google Scholar] [CrossRef]
- Bezemer, J.; Kress, G. Writing in multimodal texts—A social semiotic account of designs for learning. Writ. Commun. 2008, 25, 166–195. [Google Scholar] [CrossRef]
- National Agency for Education. Curriculum for the Compulsory School, Preschool Class and Leisure-Time Centre: Lgr22; Skolverket: Stockholm, Sweden, 2022. [Google Scholar]
- Statens Medieråd. Småungar & Medier 2019; Statens Medieråd: Stockholm, Sweden, 2019. [Google Scholar]
- Bezemer, J.; Cowan, K. Exploring reading in social semiotics: Theory and methods. Education 2021, 49, 107–118. [Google Scholar] [CrossRef]
- Livingstone, S.; Sefton-Green, J. The Class: Living and Learning in the Digital Age; New York University Press: New York, NY, USA, 2016. [Google Scholar]
- Berättarministeriet. Man lär av varandra och är aldrig fullärd—Rapport om lärares Behov av Professionsutveckling i Områden med hög Arbetslöshet. 2020. Available online: https://www.berattarministeriet.se/wp-content/uploads/2020/10/Man_la%CC%88r_av_varandra_och_a%CC%88r_aldrig_fulla%CC%88rd___.pdf (accessed on 22 August 2022).
- Halverson, R.R. Games and the future of education research. In Games, Learning and Society; Steinkuehler, C., Squire, K., Barab, S., Eds.; Cambridge University Press: London, UK, 2012. [Google Scholar]
- Gabel, D. Improving teaching and learning through chemistry education research: A look to the future. J. Chem. Educ. 1999, 76, 548–554. [Google Scholar] [CrossRef]
- Johnstone, A.H. You can’t get there from here. J. Chem. Educ. 2010, 87, 22–29. [Google Scholar] [CrossRef]
- Sibanda, D.; Hobden, P. Planning a teaching sequence for the teaching of chemical bonding. Afr. J. Res. Math. Sci. Technol. Educ. 2015, 19, 23–33. [Google Scholar] [CrossRef]
- Eilam, B.; Gilbert, J.K. The significance of visual representations in the teaching of science. In Science Teachers’ Use of Visual Representations; Eilam, B., Gilbert, J.K., Eds.; Springer: Cham, Switzerland, 2014; pp. 3–28. [Google Scholar]
- Treagust, D.F.; Chittleborough, G.; Mamiala, T.L. The role of submicroscopic and symbolic representations in chemical explanations. Int. J. Sci. Educ. 2003, 25, 1353–1368. [Google Scholar] [CrossRef]
- Uttal, D.H.; O’Doherty, K. Comprehending and learning from ‘visualizations’: A developmental perspective. In Visualization: Theory and Practice in Science Education; Gilbert, J.K., Reiner, M., Nakhleh, M., Eds.; Springer: New York, NY, USA, 2008; pp. 53–72. [Google Scholar]
- Fleer, M.; Hoban, G. Using ‘slowmation’ for intentional teaching in early childhood centers: Possibilities and imaginings. Australas. J. Early Child. 2012, 37, 61–70. [Google Scholar] [CrossRef]
- Kress, G.; Jewitt, C.; Ogborn, J.; Charalampos, T. Multimodal Teaching and Learning: The Rhetorics of the Science Classroom; Bloomsbury Publishing: London, UK, 2001; ISBN 9781441109965. [Google Scholar]
- Van Leeuwen, T. Introducing Social Semiotics; Routledge: London, UK, 2005. [Google Scholar]
- Airey, J.; Linder, C. A disciplinary discourse perspective on university science learning: Achieving fluency in a critical constellation of modes. J. Res. Sci. Teach. 2009, 46, 27–49. [Google Scholar] [CrossRef]
- Airey, J.; Linder, C. Social semiotics in university physics education. In Multiple Representations in Physics Education; Treagust, D.F., Fischer, H.E., Duit, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 95–122. ISBN 978-3-319-58912-1. [Google Scholar]
- Lemke, J. Multimedia literacy demands of the scientific curriculum. Linguist. Educ. 1998, 10, 247–271. [Google Scholar] [CrossRef]
- Jewitt, C.; Bezemer, J.; O’Halloran, K. Introducing Multimodality; Routledge: London, UK, 2016; ISBN 9780415639262. [Google Scholar]
- Kress, G. Reading images: Multimodality, representation and new media. Inf. Des. J. 2004, 12, 110–119. [Google Scholar] [CrossRef]
- Patron, E. Exploring the Role that Visual Representations Play When Teaching and Learning Chemical Bonding: An Approach Built on Social Semiotics and Phenomenography. Ph.D. Thesis, Linnaeus University, Kalmar, Sweden, 2022. [Google Scholar]
- Patron, E.; Linder, C.; Wikman, S. Qualitatively different ways of unpacking visual representations when teaching intermolecular forces in upper secondary school. Sci. Educ. 2021, 105, 1173–1201. [Google Scholar] [CrossRef]
- Ainsworth, S. Deft: A conceptual framework for considering learning with multiple representations. Learn. Instr. 2006, 16, 183–198. [Google Scholar] [CrossRef]
- Ainsworth, S. The educational value of multiple representations when learning complex scientific concepts. In Visualisation: Theory and Practice in Science Education; Gilbert, J.K., Reiner, M., Nakhleh, M., Eds.; Springer: New York, NY, USA, 2008; pp. 191–208. [Google Scholar]
- Gilbert, J.; Treagust, D.F. Multiple Representations in Chemical Education; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-8871-1. [Google Scholar]
- Kozma, R.; Russell, J. Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena. J. Res. Sci. Teach. 1997, 34, 949–968. [Google Scholar] [CrossRef]
- Prain, V.; Waldrip, B. An exploratory study of teachers’ and students’ use of multi-modal representations of concepts in primary science. Int. J. Sci. Educ. 2006, 28, 1843–1866. [Google Scholar] [CrossRef]
- Waldrip, B.; Prain, V.; Carolan, J. Using multi-modal representations to improve learning in junior secondary science. Res. Sci. Educ. 2010, 40, 65–80. [Google Scholar] [CrossRef]
- Selander, S.; Kress, G. Design för Lärande: Ett Multimodalt Perspektiv; Norstedt: Stockholm, Sweden, 2010; ISBN 9789144155951. [Google Scholar]
- Prain, V.; Tytler, R. Learning through constructing representations in science: A framework of representational construction affordances. Int. J. Sci. Educ. 2012, 34, 2751–2773. [Google Scholar] [CrossRef]
- Prain, V.; Xu, L.; Speldewinde, C. Guiding science and mathematics learning when students construct representations. Res. Sci. Educ. 2023, 53, 445–461. [Google Scholar] [CrossRef]
- Ainsworth, S.E.; Scheiter, K. Learning by drawing visual representations: Potential, purposes, and practical implications. Curr. Dir. Psychol. Sci. 2021, 30, 61–67. [Google Scholar] [CrossRef]
- Waldrip, B.; Prain, V.; Carolan, J. Learning junior secondary science through multi-modal representations. Electron. J. Sci. Educ. 2006, 11, 87–107. [Google Scholar]
- Selander, S. Designs for learning—A theoretical perspective. Des. Learn. 2008, 1, 4–22. [Google Scholar] [CrossRef]
- Kress, G.R. Multimodality: A Social Semiotic Approach to Contemporary Communication; Routledge: New York, NY, USA, 2010; ISBN 9780415320610. [Google Scholar]
- Björklund Boistrup, L.; Selander, S. Designs for Research, Teaching and Learning. A Framework for Future; Routledge: London, UK, 2022. [Google Scholar]
- Kress, G.; Selander, S. Multimodal design, learning and cultures of recognition. Internet High. Educ. 2012, 15, 265–268. [Google Scholar] [CrossRef]
- Bryman, A. Social Research Methods, 4th ed.; Oxford University Press: Oxford, UK, 2012; ISBN 9780199588053. [Google Scholar]
- Wernholm, M.; Danielsson, K.; Ebbelind, A.; Palmér, H.; Patron, E. Young pupils’ joint creation of multimodal fairy tales using analogue and digital resources. Educ. Sci. 2023, 13, 568. [Google Scholar] [CrossRef]
- Walford, G. The nature of educational ethnography. In How to Do Educational Ethnography; Walford, G., Ed.; The Tufnell Press: London, UK, 2008. [Google Scholar]
- Swedish Research Council. Good Research Practice; Swedish Research Council: Stockholm, Sweden, 2017. [Google Scholar]
- Wernholm, M. Children’s Learning at Play in a Hybrid Reality. Ph.D. Thesis, Linnaeus University, Kalmar, Sweden, 2020. [Google Scholar]
- Wernholm, M. Metodologiska och etiska utmaningar med att utforska barns perspektiv på deltagande i en hybrid verklighet. Barn 2021, 39, 63–78. [Google Scholar] [CrossRef]
- Heath, C.; Hindmarsch, J.; Luff, P. Video in Qualitative Research; Sage: London, UK, 2011. [Google Scholar]
- Wernholm, M. Children’s out-of-school learning in digital gaming communities. Des. Learn. 2021, 13, 8–19. [Google Scholar] [CrossRef]
- Rowe, V.C. Using video-stimulated recall as a basis for interviews: Some experiences from the field. Music Educ. Res. 2009, 11, 425–437. [Google Scholar] [CrossRef]
- Bloom, B.S. Thought-processes in lectures and discussions. J. Gen. Educ. 1953, 7, 160–169. [Google Scholar]
- Calderhead, J. Stimulated recall: A method for research on teaching. Br. J. Educ. Psychol. 1981, 51, 211–217. [Google Scholar] [CrossRef]
- Haglund, B. Stimulated recall—Några anteckningar om en metod att generera data. Pedagog. Forsk. I Sver. 2003, 8, 145–157. [Google Scholar]
- Lyle, J. Stimulated recall: A report on its use in naturalistic research. Br. Educ. Res. J. 2003, 29, 861–878. [Google Scholar] [CrossRef]
- Haudrup Christensen, P. Children’s participation in ethnographic research: Issues of power and representation. Child. Soc. 2004, 18, 165–176. [Google Scholar] [CrossRef]
- Wilson, A.; Onwuegbuzie, A.; Manning, L. Using paired depth interviews to collect qualitative data. Qual. Rep. 2016, 21, 1549–1573. [Google Scholar] [CrossRef]





| Lessons | Video Recordings |
|---|---|
| Lesson 1. Teacher’s introduction | Recording from tablet on tripod, 22.09 min |
| Lesson 2 Pupils creating a digital animation (video observations) and talking about their digital animation (video-stimulated recall interviews) | Recordings from tablets on tripods: Pupils creating a digital animation. Group 1: a girl and a boy 25.02 min Group 2: two girls 12.06 min Group 3: two boys 17.24 min Group 4: two girls and a boy 32 min Total: 1 h 26 min 32 s Recordings from video-stimulated recall interviews: Group 1: a girl and a boy 6.41 min Group 2: two girls 9.28 min Group 3: their digital animation was not saved. Group 4: two girls and a boy 5.43 min Total: 21 min 52 s |
| Lesson 3 Pupils watching all the digital animations and talking about them (video-stimulated recall interviews) | Recordings from video-stimulated recall interviews: Group 2 and 3: two girls and a boy (one of the boys in group 3 did not want to participate) 12.22 min Group 1 and 4: three girls and a boy 11.33 min Total: 23 min 55 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patron, E.; Wernholm, M.; Danielsson, K.; Palmér, H.; Ebbelind, A. An Exploration of How Multimodally Designed Teaching and the Creation of Digital Animations can Contribute to Six-Year-Olds’ Meaning Making in Chemistry. Educ. Sci. 2024, 14, 79. https://doi.org/10.3390/educsci14010079
Patron E, Wernholm M, Danielsson K, Palmér H, Ebbelind A. An Exploration of How Multimodally Designed Teaching and the Creation of Digital Animations can Contribute to Six-Year-Olds’ Meaning Making in Chemistry. Education Sciences. 2024; 14(1):79. https://doi.org/10.3390/educsci14010079
Chicago/Turabian StylePatron, Emelie, Marina Wernholm, Kristina Danielsson, Hanna Palmér, and Andreas Ebbelind. 2024. "An Exploration of How Multimodally Designed Teaching and the Creation of Digital Animations can Contribute to Six-Year-Olds’ Meaning Making in Chemistry" Education Sciences 14, no. 1: 79. https://doi.org/10.3390/educsci14010079
APA StylePatron, E., Wernholm, M., Danielsson, K., Palmér, H., & Ebbelind, A. (2024). An Exploration of How Multimodally Designed Teaching and the Creation of Digital Animations can Contribute to Six-Year-Olds’ Meaning Making in Chemistry. Education Sciences, 14(1), 79. https://doi.org/10.3390/educsci14010079








