Examining the Effects of Supervised Laboratory Instruction on Students’ Motivation and Their Understanding of Chemistry
Abstract
1. Introduction
- (1)
- What is the effect of an SLI teaching approach in improving grade 12 students’ conceptual understandingofacid–base and solution chemistry topics?
- (2)
- What aspects of SLI do the students find motivating and useful for their chemistry learning and which aspects do they find challenging?
2. Theoretical Background and Framework
2.1. Theoretical Background
2.2. Theoretical Framework and the Design of SLI Teaching
3. Method
3.1. Research Design
3.1.1. Participants and Setting
3.1.2. Experimental Group Teachers’ Training
3.2. Interventions
3.2.1. Intervention for the EGs
3.2.2. Intervention for the CGs
3.3. Hardness of Water Experiment
3.4. Unknown Concentration Determination of Cu (II) Solution Experiment
3.5. Data Collection
3.5.1. Chemistry Concept Test (CCT)
3.5.2. Semi-Structured Focus Group Interviews
3.5.3. Classroom Observations
3.6. Data Analysis
3.7. Ethical Issues
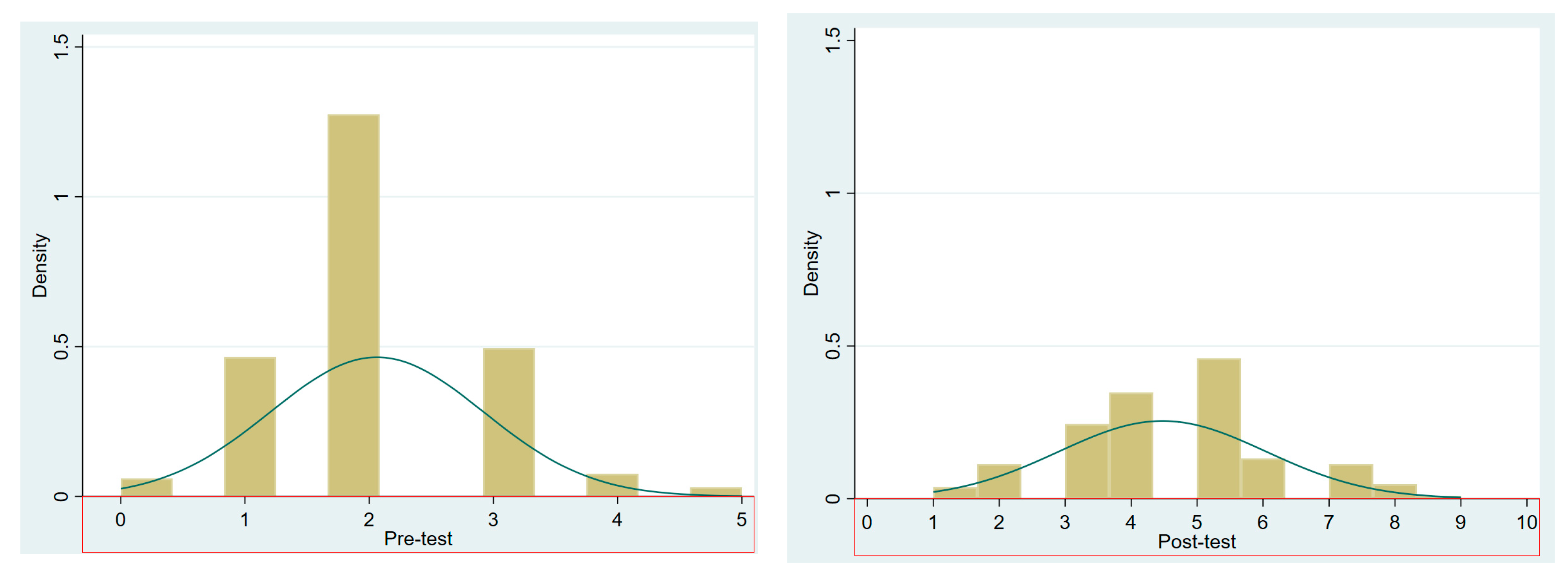
4. Results
4.1. Quantitative Results of the Three-Tier CCT
Pre-Test–Post-Test Improvement on Conceptual Understanding
4.2. Qualitative Results
4.2.1. SLI Increases Students’ Motivation for Learning Chemistry
“…studentsrecollected what they had previously learnt in their biology lessons about the function of epithelial cells on the stomach wall in producing the bicarbonate-rich mucus, which, as they pointed out, was responsible to neutralize the gastric acid. The students seemed to be motivated in the lesson”.(Author 1, notes [20 March 2020])
4.2.2. SLI Is a Multifaceted Learner-Centred Laboratory Instruction
“Students had reflected on a nice smelling musk that is produced by an African civet in the discussion forum and one student asked an interesting question: ‘You guys, do you think that after doing the unknown concentration of Cu (II) experiment we can manage to determine the concentration of the chemical components in the musk of a civet, I mean in the near future? It is awesome! I appreciate the role that chemistry can play in understanding the natural world’”.(Author 1, [19 March 2020])
4.2.3. Laboratory Learning through SLI Is a Demanding Process
5. Discussion
5.1. The Effect of SLI on Students’ Conceptual Understanding of Chemistry (RQ1)
5.2. Aspects of SLI That Students Find Motivating and Useful for Their Chemistry Learning, as Well as Challenging Aspects(RQ2)
5.3. Limitations of the Study
6. Implications for Practice and Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Three-Tier Multiple-Choice Chemistry Concept Test (CCT)
- 1. Which statement is correct about pH?
- (A)
- A solution that has a pH of 3 has hundred-fold times greater H+ ions than a solution with a pH of 5
- (B)
- pH is a measure of ‘strength’ and ‘powerfulness’ of a solution
- (C)
- As the number of hydrogen atoms increases in the formula of an acid, its pH values decrease
- (D)
- If the pH = 0, the substance is neither an acid nor a base
- 1.1. What is your reason or explanation for your response above?
- (A)
- Because pH measures the degree to which an acid or base reacts
- (B)
- Because the more hydrogen atoms in the formula of an acid means that there are higher H+ ions in the solution
- (C)
- Because the pH of a solution is the negative logarithm of H+ ion concentration, a solution with a pH of 3 has 100 times greater H+ ions than that of pH 5
- (D)
- Because a substance with pH = 0 contains no H+ and OH- ion concentrations
- 1.2. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
- 2. Which statement is correct about titration?
- (A)
- In acid-base titration, before the end point of the titration there will be no change in pH
- (B)
- In neutralization titration, at the equivalence point of titration, a single drop increase in acidic solution leads to a large decrease in pH
- (C)
- In acid-base titration, acids and bases physically mix together to form a solution
- (D)
- The end point of complexometric titration occurs at acidic pH
- 2.1. What is your reason or explanation for your response above?
- (A)
- Because the dissolution of an acid with the base to give a neutral solution predominantly occurs instead of chemical reactions
- (B)
- Because the metal-EDTA complex will be highly stable at a lower pH and the analyte and titrant are essentially completely reacted at the end point of titration
- (C)
- Because at the equivalence point when approximately all OH- ions are consumed, a small increase in acid leaves the solution with an excess H+ ions
- (D)
- Because it is only at the equivalence point that reaction will start taking place
- 2.2. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
- 3. Which statement is correct about the properties of the solutions?
- (A)
- Sugar dissolved in water conducts electricity
- (B)
- Sugar molecules are mostly present at the top of the sugared water mixture
- (C)
- Electrical conductivity of solutions depends on the availability of only negatively charged ions
- (D)
- Sodium chloride is much more soluble in water than in benzene
- 3.1. What is your reason or explanation for your response above?
- (A)
- Because all solutions conduct electricity and conductivity doesn’t depend on the type of solution
- (B)
- Because the benzene molecules lack a dipole moment, they cannot effectively solvate Na+ and Cl− ions
- (C)
- Because negatively charged ions have a more active role than positively charged ions in supporting electrical conduction
- (D)
- Because the sugar molecules are regularly arranged at the top of the sugared water mixture
- 3.2. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
- 4. Which statement is correct about the concentration of a solution?
- (A)
- The concentration of a solution helps to determine the strengths of acids and bases
- (B)
- If the volume of aqueous ethanol solution in beaker A is twice the volume in beaker B, there should be more particles in beaker B than in A with equal concentration
- (C)
- Adding a saturated solution of a bit more of its solid increases ion concentration
- (D)
- Concentration has a non-linear relationship with absorbance
- 4.1. What is your reason or explanation for your response above?
- (A)
- Because absorbance has an inversely proportional relationship to concentration
- (B)
- Because the concentration of acids and bases affects the degree to which acids and bases dissociate in solutions
- (C)
- Because there should be more particles in a smaller volume than in the more diluted solution with equal concentration
- (D)
- Because the same solute can be dissolved in a saturated solution regardless of the amount of its solute dissolved in the solution
- 4.2. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
- 5. Which statement is correct about the physical and chemical properties of acids and bases?
- (A)
- A neutralization reaction does contain H3O+ and OH- ions
- (B)
- When an acidic solution is ultra-diluted, it will become a basic solution
- (C)
- A strong acid doesn’t dissociate in water solution, because its intra-molecular bonds are very strong
- (D)
- Acid-base reactions result in a solution that does not possess any acidic or basic properties
- 5.1. What is your reason or explanation for your response above?
- (A)
- Because ultra-diluted acidic solutions will have higher pH values that make it a basic solution
- (B)
- Because the neutralization process can contain either acids or bases that are left unreacted in the resulting solution
- (C)
- Because the neutralization process indicates that acids have consumed all bases, and the resulting solution is neutral
- (D)
- Because the strong intramolecular bond in HCl that is hydrogen bond prevents separating its molecules into hydrated anions and cations in solution
- 5.2. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
- 6. Which statement best describes the formation of aqueous solutions?
- (A)
- Dissolving sugar molecules in water is a chemical process
- (B)
- Powdered detergent soap dissolves in water and forms bubbles on top, so it’s an exothermic reaction
- (C)
- In completely dissolved aqueous solutions, there are molecules of ionic compounds
- (D)
- Sodium chloride that is completely dissolved in water will have no undissociated NaCl units in solution
- 6.1. What is your reason or explanation for your response above?
- (A)
- Because once ionic compounds are dissolved in water, hydration destabilizes ions in solution and helps to recombine anions with cations
- (B)
- Because the sodium chloride that enters the aqueous solution is disintegrated into Na+ and Cl− ions
- (C)
- Because reaction between sugar and water molecules can result in a new substance-sweet water
- (D)
- Because the formation of bubbles shows the dissolution of detergent soap in water by releasing energy and is thus an exothermic reaction
- 6.2. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
- 7. Which statement best describes the right practical laboratory knowledge/practices about the preparation of solid–liquid solutions?
- (A)
- During carrying out the given task of preparing solid-liquid solution, female students need not to use a hair clip to tie their hair back
- (B)
- Reading the top of the meniscus instead of the bottom gives precise volume measurement
- (C)
- When precise measurement of liquid is needed using graduated cylinder is the right equipment instead of volumetric flask
- (D)
- To prepare 1 M NaCl solution, we should not combine 1 mole of NaCl with 1 L of water
- 7.1. What is your reason or explanation for your response above?
- (A)
- Because the probability of having an accident on the hair is very low and it is not considered necessary to act up on it
- (B)
- Because the top of the meniscus provides a more precise volume measurement than the bottom
- (C)
- Because a graduated cylinder is easy to measure out volume, and it is the right equipment to get precise measurement of volume
- (D)
- Because the resulting solution would have a total volume exceeding 1 L and therefore a molarity of less than 1 M
- 7.2. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
- 8. Which statement is correct about the indicators?
- (A)
- Indicators are used to measure the strength of acids and bases
- (B)
- Indicators neutralize acids and change colour
- (C)
- All indicators change colour at the same pH value and this is invariably at pH 7
- (D)
- Indicators provide a clear indication by a colour change in the presence of neutral, acidic or basic solution
- 8.1. What is your reason or explanation for your response above?
- (A)
- Because indicators can define the degree to which acids and bases react to each other
- (B)
- Because indicators are basic organic compounds that can react with acids to show the end point of the neutralization reaction
- (C)
- Because the role of indicators is to show the colour change of neutralization reaction, which always results in neutral solution
- (D)
- Because indicators are acidic or basic organic compounds that indicate whether the titration process ends up with neutral, acidic or neutral solution
- 8.2. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
- 9. Temporary hardness of tap water occurs when Ca2+ ions come from chlorite compound, Ca(OCl)2, but not from the hardness of ground water. This can be removed when tap water is heated.
- 9.1. Which statement is correct about the chemical phenomena that occur when tap water is heated?
- (A)
- The CaCO3 will precipitate after tap water is heated and the water evaporates
- (B)
- Coagulation of CaCO3 will occur after tap water is heated
- (C)
- The density of crystals of CaCO3 is greater than the solution of tap water, and then they precipitate
- (D)
- The CaCO3 will precipitate after ionization of chlorite compound and dissolution of CO2gas
- 9.2. What is your reason or explanation for your response above?
- (A)
- Because when tap water is heated, it will evaporate and CaCO3will be precipitated at the bottom of the container
- (B)
- Because heating of tap water triggers the coagulation process and CaCO3will be removed through filtration
- (C)
- Because heating tap water increases the precipitation of CaCO3since it has a higher density than tap water
- (D)
- Because CaCO3will be formed through the chemical process that occurs between the chlorite compound found in tap water and the CO2 gas in the environment
- 9.3. Are you confident/sure that the responses given above are correct?
- (A)
- Yes
- (B)
- No
Appendix B. Interview Questions Employed in the Semi-Structured Focus-Group Interviews
- Semi-structured interview questions
|
|
|
|
|
References
- Ministry of Education [MoE]. Ethiopian Education Development Roadmap (2018–30): An Integrated Executive Summary (Draft); Ministry of Education, Education Strategy Center: Addis Ababa, Ethiopia, 2018.
- Ministry of Education [MoE]. Education Statistics Annual Abstract September 2019–March 2020; Ministry of Education, Education Management Information System (EMIS) and ICT Directorate: Addis Ababa, Ethiopia, 2020.
- Zimmerman, B.J. Becoming a self-regulated learner: An overview. Theory Pract. 2002, 41, 64–70. [Google Scholar] [CrossRef]
- Anderson, R.D. Reforming science teaching: What research says about inquiry. J. Sci. Teach. Educ. 2002, 13, 1–12. [Google Scholar] [CrossRef]
- Asheim, J.; Kvittingen, E.V.; Kvittingen, L.; Verley, R. A simple, small-scale Lego colorimeter with a light-emitting diode (LED) used as detector. J.Chem.Educ. 2014, 91, 1037–1039. [Google Scholar] [CrossRef]
- Ali, M.T.; Lykknes, A. In-service chemistry teachers’ reflections on and experiences with supervised laboratory instruction in Ethiopia. Afr. J. Chem. Educ. 2022, 12, 60–95. [Google Scholar]
- Childs, P.E.; Hayes, S.M.; O’dwyer, A. Chemistryand everyday life: Relating secondary school chemistry to the current and future lives of students. In Relevant Chemistry Education; Eilks, I., Hofstein, A., Eds.; Brill Sense: Rotterdam, The Netherlands, 2013; pp. 33–54. [Google Scholar]
- Jiménez-Liso, M.R.; López-Banet, L.; Dillon, J. Changing how we teach acid-base chemistry. Sci. Educ. 2020, 29, 1291–1315. [Google Scholar] [CrossRef] [PubMed]
- Calik, M.; Kolomuç, A.; Karagölge, Z. The effect of conceptual change pedagogy on students’ conceptions of rate of reaction. J. Sci. Educ. Technol. 2010, 19, 422–433. [Google Scholar] [CrossRef]
- Duis, J.M. Organic chemistry educators’ perspectives on fundamental concepts and misconceptions: An exploratory study. J. Chem. Educ. 2011, 88, 346–350. [Google Scholar] [CrossRef]
- Barke, H.-D.; Hazari, A.; Yitbarek, S. Misconceptions in Chemistry. Addressing Perceptions in Chemical Education; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Hoe, K.Y.; Ramanathan, R. On the prevalence of alternative conceptions on acid–base chemistry among secondary students: Insights from cognitive and confidence measures. Chem. Educ. Res. Pract. 2016, 17, 263–282. [Google Scholar] [CrossRef]
- Sheppard, K. High school students’ understanding of titrations and relatedacid-base phenomena. Chem. Educ. Res. Pract. 2006, 7, 32–45. [Google Scholar] [CrossRef]
- Ebenezer, J.V.; Erickson, G.L. Chemistry students’ conceptions of solubility: A phenomenography. Sci. Educ. 1996, 80, 181–201. [Google Scholar] [CrossRef]
- Çalik, M. A cross-age study of different perspectives in solution chemistry from junior to senior high school. Int. J. Sci. Math. 2005, 3, 671–696. [Google Scholar] [CrossRef]
- Pinarbasi, T.; Canpolat, N. Students’ understanding of solution chemistry concepts. J. Chem. Educ. 2003, 80, 1328–1332. [Google Scholar] [CrossRef]
- Özden, M. Prospective science teachers’ conceptions of the solution chemistry. J. Balt. Sci. Educ. 2009, 8, 69–78. [Google Scholar]
- Amin, T.G.; Smith, C.L.; Wiser, M. Student conceptions and conceptual change. In Handbook of Research on Science Education; Lederman, N.G., Abell, S.K., Eds.; Routledge: New York, NY, USA; London, UK, 2014; pp. 57–81. [Google Scholar]
- Özdemir, G.; Clark, D.B. An overview of conceptual change theories. Eurasia J. Math. Sci. Technol. Educ. 2007, 3, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Potvin, P.; Malenfant-Robichaud, G.; Cormier, C.; Masson, S. Coexistence of misconceptions and scientific conceptions in chemistry professors: A mental chronometry and fMRI study. Front. Educ. 2020, 5, 542458. [Google Scholar] [CrossRef]
- Limón, M. On the cognitive conflict as an instructional strategy for conceptual change: A critical appraisal. Learn Instr. 2001, 11, 357–380. [Google Scholar] [CrossRef]
- Demircioğlu, G.; Ayas, A.; Demircioğlu, H. Conceptual change achieved through a new teaching program on acids and bases. Chem. Educ. Res. Pract. 2005, 6, 36–51. [Google Scholar] [CrossRef]
- Asterhan, C.S.; Dotan, A. Feedback that corrects and contrasts students’ erroneous solutions with expert ones improves expository instruction for conceptual change. Instr. Sci. 2018, 46, 337–355. [Google Scholar] [CrossRef]
- Abrahams, I.; Millar, R. Does practical work really work? A study of the effectiveness of practical work as a teaching and learning method in school science. Int. J. Sci. Educ. 2008, 30, 1945–1969. [Google Scholar] [CrossRef]
- Abrahams, I. Does practical work really motivate? A study of the affective value of practical work in secondary school science. Int. J. Sci. Educ. 2009, 31, 2335–2353. [Google Scholar] [CrossRef]
- Abrahams, I.; Reiss, M.J. Practical work: Its effectiveness in primary and secondary schools in England. J. Res. Sci. Teach. 2012, 49, 1035–1055. [Google Scholar] [CrossRef]
- Agustian, H.Y.; Seery, M.K. Reasserting the role of pre-laboratory activities in chemistry education: A proposed framework for their design. Chem. Educ. Res. Pract. 2017, 18, 518–532. [Google Scholar] [CrossRef]
- Sesen, B.A.; Tarhan, L. Promoting active learning in high school chemistry: Learning achievement and attitude. Procedia Soc. Behav. Sci. 2010, 2, 2625–2630. [Google Scholar] [CrossRef]
- Tarhan, L.; Sesen, B.A. Investigation the effectiveness of laboratory works related to “acids and bases” on learning achievements and attitudes toward laboratory. Procedia Soc. Behav. Sci. 2010, 2, 2631–2636. [Google Scholar] [CrossRef]
- Priyambodo, E.; Fitriyana, N.; Primastuti, M.; Artistic, F.A.D. The role of collaborative learning based STSE in acid base chemistry: Effects on students’ motivation. In Proceedings of the 7th International Conference on Research, Implementation, and Education of Mathematics and Sciences (ICRIEMS 2020), Yogyakarta, Indonesia, 25–26 September 2020; Atlantis Press: Dordrecht, The Netherlands, 2021; pp. 253–263. [Google Scholar]
- Wong, J.; Baars, M.; de Koning, B.B.; Paas, F. Examining the use of prompts to facilitate self-regulated learning in massive open online courses. Comput. Hum. Behav. 2021, 115, 106596. [Google Scholar] [CrossRef]
- Zumbrunn, S.; Tadlock, J.; Roberts, E.D. Encourage Self-Regulated Learning in the Classroom; Metropolitan Educational Research Consortium (MERC), Virginia Commonwealth University: Richmond, VA, USA, 2011; pp. 1–29. [Google Scholar]
- Carneiro, R.; Lefrere, P.; Steffens, K.; Underwood, J. Self-Regulated Learning in Technology Enhanced Learning Environments: A European Perspctive; Sense: Roterdam, The Netherlands, 2012; Volume 5. [Google Scholar]
- Cera, R.; Mancini, M.; Antonietti, A. Relationships between metacognition, self-efficacy and self-regulation in learning. J. Educ. Cult. Psychol. Stud. 2013, 4, 115–141. [Google Scholar] [CrossRef]
- Labuhn, A.S.; Bögeholz, S.; Hasselhorn, M. LernförderungdurchAnregung der Selbst regulation imnaturwissenschaftlichen, [Fostering learning through stimulation of self-regulation in science lessons]. Unterr. Z. Pa¨dagogische Psychol. 2008, 22, 13–24. [Google Scholar] [CrossRef]
- Cleary, T.J.; Platten, P.; Nelson, A.C. Effectiveness of the self-regulation empowerment program with urban high school students. J. Adv. Acad. 2008, 20, 70–107. [Google Scholar] [CrossRef]
- Williams, A.E.; Aguilar-Roca, N.M.; Tsai, M.; Wong, M.; Beaupr’e, M.M.; O’Dowd, D.K. Assessment of learning gains associated with independent exam analysis in introductory biology. CBE Life Sci. Educ. 2011, 10, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.J.; Moylan, A.R. Self-regulation: Where metacognition and motivation intersect. In Handbook of Metacognition in Education; Hacker, D.J., Dunlosky, J., Graesser, A.C., Eds.; Routledge: New York, NY, USA, 2009; pp. 299–315. [Google Scholar]
- Hullinger, A.M.; DiGirolamo, J.A.; Tkach, J.T. Reflective practice for coaches and clients: An integrated model for learning. Philos. Coach Int. J. 2019, 4, 5–34. [Google Scholar] [CrossRef]
- Zimmerman, B.J. Investigating self-regulation and motivation: Historical background, methodological developments, and future prospects. Am. Educ. Res. J. 2008, 45, 166–183. [Google Scholar] [CrossRef]
- Gericke, N.; Högström, P.; Wallin, J. A systematic review of research on laboratory work in secondary school. Stud. Sci. Educ. 2022, 59, 245–285. [Google Scholar] [CrossRef]
- Smith, C.J. Improving the school-to-university transition: Using a problem-based approach to teach practical skills whilst simultaneously developing students’ independent study skills. Chem. Educ. Res. Pract. 2012, 13, 490–499. [Google Scholar] [CrossRef]
- Hofstein, A.; Mamlok-Naaman, R. The laboratory in science education: The state of the art. Chem. Educ. Res. Pract. 2007, 8, 105–107. [Google Scholar] [CrossRef]
- National Research Council. A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas; National Academies Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Kakisako, M.; Nishikawa, K.; Nakano, M.; Harada, K.S.; Tatsuoka, T.; Koga, N. Stepwise inquiry into hard water in a high school chemistry laboratory. J. Chem. Educ. 2016, 93, 1923–1928. [Google Scholar] [CrossRef]
- Mandler, D.; Mamlok-Naaman, R.; Blonder, R.; Yayon, M.; Hofstein, A. High-school chemistry teaching through environmentally oriented curricula. Chem. Educ. Res. Pract. 2012, 13, 80–92. [Google Scholar] [CrossRef]
- World Health Organization (WHO).Calcium and Magnesium in Drinking-Water: Public Health Significance. 2009. Available online: http://apps.who.int/iris/bitstream/10665/43836/1/9789241563550_eng.pdf (accessed on 16 December 2019).
- O’Donoghue, J. Simplified low-cost colorimetry for education and public engagement. J. Chem. Educ. 2019, 96, 1136–1142. [Google Scholar] [CrossRef]
- Dooling, K.; Bodenstedt, K.; Page, M.F. A caffeinated boost on UV spectrophotometry: A lab for high school chemistry or an introductory university chemistry course. J. Chem. Educ. 2013, 90, 914–917. [Google Scholar] [CrossRef]
- Liampa, V.; Malandrakis, G.N.; Papadopoulou, P.; Pnevmatikos, D. Development and evaluation of a three-tier diagnostic test to assess undergraduate primary teachers’ understanding of ecological foot print. Res. Sci. Educ. 2019, 49, 711–736. [Google Scholar] [CrossRef]
- Cetin-Dindar, A.; Geban, O. Development of a three-tier test to assess high school students’ understanding of acids and bases. Procedia Soc. Behav. Sci. 2011, 15, 600–604. [Google Scholar] [CrossRef]
- Khairani, A.Z.; Shamsuddin, H. Assessing Item Difficulty and Discrimination Indices of Teacher-Developed Multiple-Choice Tests. In Assessment for Learning within and beyond the Classroom; Tang, S.F., Logonnathan, L., Eds.; Springer: Singapore, 2016; pp. 417–426. [Google Scholar] [CrossRef]
- Dilshad, R.M.; Latif, M.I. Focus group interview as a tool for qualitative research: An analysis. Pak. J. Soc. Sci. 2013, 33, 191–198. [Google Scholar]
- Gill, P.; Stewart, K.; Treasure, E.; Chadwick, B. Methods of data collection in qualitative research: Interviews and focus groups. Br. Dent. J. 2008, 204, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V.; Hayfield, N.; Terry, G. Thematic analysis. In Handbook of Research Methods in Health Social Sciences; Liamputtong, P., Ed.; Springer: Singapore, 2019; pp. 843–860. [Google Scholar]
- Braun, V.; Clarke, V. Can I use TA? Should I use TA? Should I not use TA? Comparing reflexive thematic analysis and other pattern-based qualitative analytic approaches. Couns. Psychother. Res. 2021, 21, 37–47. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Conceptual and design thinking for thematic analysis. Qual. Psychol. 2022, 9, 3–26. [Google Scholar] [CrossRef]
- Damanhuri, M.I.M.; Treagust, D.F.; Won, M.; Chandrasegaran, A.L. High school students’ understanding of acid-base concepts: An ongoing challenge for teachers. Int. J. Environ. Sci. Educ. 2016, 11, 9–27. [Google Scholar]
- Karslı-Baydere, F.; Ayaş, A.; Çalık, M. Effects of a 5Es learning model on the conceptual understanding and science process skills of pre-service science teachers: The case of gases and gas laws. J. Serb. Chem. Soc. 2020, 85, 559–573. [Google Scholar] [CrossRef]
- Baydere, F.K. Effects of a context-based approach with prediction–observation–explanation on conceptual understanding of the states of matter, heat and temperature. Chem. Educ. Res. Pract. 2021, 22, 640–652. [Google Scholar] [CrossRef]
- Spagnoli, D.; Wong, L.; Maisey, S.; Clemons, T.D. Prepare, do, review: A model used to reduce the negative feelings towards laboratory classes in an introductory chemistry undergraduate unit. Chem. Educ. Res. Pract. 2017, 18, 26–44. [Google Scholar] [CrossRef]
- Aflalo, E.; Raviv, A. Characteristics of classroom discourse in physics lessons. Res. Sci. Technol. Educ. 2022, 40, 168–188. [Google Scholar] [CrossRef]
- Çetin, P.S.; Kaya, E.; Geban, Ö. Facilitating conceptual change in gases concepts. J. Sci. Educ. Technol. 2009, 18, 130–137. [Google Scholar] [CrossRef]
- Kusurkar, R.A.; Ten Cate, O.T.J.; Vos, C.M.P.; Westers, P.; Croiset, G. How motivation affects academic performance: A structural equation modeling analysis. Adv. Health Sci. Educ. Theory Pract. 2013, 18, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Vaino, K.; Holbrook, J.; Rannikmäe, M. Stimulating students’ intrinsic motivation for learning chemistry through the use of context-based learning modules. Chem. Educ. Res. Pract. 2012, 13, 410–419. [Google Scholar] [CrossRef]
- Nakata, Y.; Nitta, R.; Tsuda, A. Understanding motivation and classroom modes of regulation in collaborative learning: An exploratory study. Innov. Lang. Learn. Teach. 2020, 16, 14–28. [Google Scholar] [CrossRef]
- Ma, Y.C. Using Participatory Teaching in Hands-On Courses: Exploring the Influence of Teaching Cases on Learning Motivation. Educ. Sci. 2023, 13, 547. [Google Scholar] [CrossRef]
- Araújo, J.L.; Morais, C.; Paiva, J.C. Student participation in a coastal water quality citizen science project and its contribution to the conceptual and procedural learning of chemistry. Chem. Educ. Res. Pract. 2022, 23, 100–112. [Google Scholar] [CrossRef]
- Gilbert, J.K.; Bulte, A.M.; Pilot, A. Concept development and transfer in context-based science education. Int. J. Sci. Educ. 2011, 33, 817–837. [Google Scholar] [CrossRef]
- Ucan, S.; Webb, M. Social regulation of learning during collaborative inquiry learning in science: How does it emerge and what are its functions? Int. J. Sci. Educ. 2015, 37, 2503–2532. [Google Scholar] [CrossRef]
- Varadarajan, S.; Ladage, S. Exploring the role of scaffolds in problem-based learning (PBL)inan undergraduate chemistry laboratory. Chem. Educ. Res. Pract. 2022, 23, 159–172. [Google Scholar] [CrossRef]
- National Research Council [NRC]. America’s Lab Report: Investigations in High School Science; National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Reid, J.W.; Gunes, Z.D.K.; Fateh, S.; Fatima, A.; Macrie-Shuck, M.; Nennig, H.T. Investigating patterns of student engagement during collaborative activities in undergraduate chemistry courses. Chem. Educ. Res. Pract. 2022, 23, 173–188. [Google Scholar] [CrossRef]
- Yalcin-Celik, A.; Kadayifci, H.; Uner, S.; Turan-Oluk, N. Challenges faced by pre-service chemistry teachers teaching in a laboratory and their solution proposals. Eur. J. Teach. Educ. 2017, 40, 210–230. [Google Scholar] [CrossRef]
- Nivalainen, V.; Asikainen, M.A.; Sormunen, K.; Hirvonen, P.E. Preservice and inservice teachers’ challenges in the planning of practical work in physics. J. Sci. Teach. Educ. 2010, 21, 393–409. [Google Scholar] [CrossRef]
- Cossa, E.F.R.; Uamusse, A.A. Effects of an in-service program on biology and chemistry teachers’ perception of the role of laboratory work. Procedia Soc. Behav. Sci. 2015, 167, 152–160. [Google Scholar] [CrossRef]
- Hamidu, M.Y.; Ibrahim, A.I.; Mohammed, A. The use of laboratory method in teaching secondary school students: A key to improving the quality of education. Int. J. Sci. Eng. Res. 2014, 5, 81–86. [Google Scholar]
- Peeters, J.; De Backer, F.; Reina, V.R.; Kindekens, A.; Buffel, T.; Lombaerts, K. The role of teachers’ self-regulatory capacities in the implementation of self-regulated learning practices. Procedia Soc. Behav. Sci. 2014, 116, 1963–1970. [Google Scholar]
- Akani, O. Laboratory teaching: Implication on students’ achievement in chemistry in secondary schools in Ebonyi State of Nigeria. J. Educ. Pract. 2015, 6, 206–213. [Google Scholar]
- Wigfield, A.; Klauda, S.L.; Cambria, J. Influences on the Development of Academic Self-Regulatory Processes. In Handbook of Self-Regulation of Learning and Performance; Zimmerman, B.J., Schunk, D.H., Eds.; Routledge/Taylor & Frncis Group: New York, NY, USA, 2011; pp. 33–48. [Google Scholar]
- Hofstein, A.; Hugerat, M. Teaching and Learning in the School Chemistry Laboratory, Advances in Chemistry Education Series; Royal Society of Chemistry: London, UK, 2022; Volume 8. [Google Scholar]
- Montesdeoca, K.K. Middle grades math with ice cream sundaes: Connecting math to the real world. Educ. Sci. 2023, 13, 615. [Google Scholar] [CrossRef]
| Types of Instruction (Groups) | Main Features of the Intervention | Supplementary Features of the Intervention | Teaching—Learning Strategies |
|---|---|---|---|
| Regular teaching method (CG) | Lectures |
|
|
| Asking and answering questions |
|
| |
| Take-home assignments |
|
| |
| SLI (EG) | Pre-laboratory activities |
|
|
| Teachers’ scaffolding |
|
| |
| Reflective group discussions |
|
|
| Themes | Codes | Sample Excerpt |
|---|---|---|
| SLI increases students’ motivation for learning chemistry | SLI is a motivating method to learn with | SLI is an essential and motivational instruction, which could redress the problems of the lecture method (Abdi, FG6). |
| SLI encourages chemistry learning | SLI has helped me develop a sense of responsibility for what I should learn about chemistry in the laboratory (Lucas, FG4). | |
| SLI is an enjoyable teaching method | I found it [SLI] enjoyable to learn with (Husnia, FG7). | |
| SLI inspired me to study chemistry further | SLI further increased my motivation towards the pursuit of heading to chemistry-related careers (Linda, FG1). | |
| SLI is a multifaceted learner-centred laboratory instruction | SLI makes chemistry more relevant | …it [SLI] has helped me identify what really exists in the daily-life practices and to find myself immersed in the process of systematic investigations (Brian, FG9). |
| SLI facilitates the development of laboratory skills | I have learned how to practically prepare solutions, which is quite different from solution on paper (Habib, FG6). | |
| SLI encourages collaborative learning through reflective group discussions | SLI allows me to ask questions, reflect on lab tasks, and to discuss with my peers (Tore, FG1). | |
| SLI is a learner-centred laboratory instruction | I participated in lab activities that provide opportunities for collecting data while actively experimenting and to understand behind-the-scenes of the experiments (Nasir, FG2). | |
| Laboratory learning through SLI is a demanding process | SLI is difficult without technical staff and sufficient laboratory equipment and consumables SLI takes much time It is difficult to comprehend the SLI teaching–learning materials | A shortage of laboratory materials and lack of laboratory technicians who could relentlessly guide students’ chemistry learning…was seriously a real concern (Selam, FG8) The students may get bored with spending much time in the laboratory until they ensure the lab activities they are assigned to perform are thoroughly examined (Abi, FG3) We failed to understand the nature of SLI teaching–learning materials, and hence we were terrified and anxious about the delicacy of the apparatus and the equipment (Habib, FG6) |
| Group | Mean (SD) | Differences in Mean (Post-Test–Pre-Test) | T-Statistics | |
|---|---|---|---|---|
| Pre-Test | Post-Test | |||
| Control (n = 84) | 2.01 (0.91) | 3.55 (1.17) | 1.54 *** | 9.51 |
| Girls (n = 37) | 2.00 (0.88) | 3.57 (1.19) | 1.57 *** | 6.43 |
| Boys (n = 47) | 2.02 (0.94) | 3.53 (1.16) | 1.51 *** | 6.93 |
| School C1 (n = 42) | 2.07 (1.02) | 3.79 (1.00) | 1.72 *** | 7.77 |
| School C2 (n = 42) | 1.95 (0.79) | 3.31 (1.29) | 1.36 *** | 5.84 |
| Experimental (n = 76) | 2.12 (0.80) | 5.50 (1.30) | 3.38 *** | 19.30 |
| Girls (n = 34) | 2.12 (0.81) | 5.38 (1.44) | 3.26 *** | 11.56 |
| Boys (n = 42) | 2.12 (0.80) | 5.60 (1.19) | 3.48 *** | 15.69 |
| School E1 (n = 20) | 2.55 (0.62) | 5.45 (1.05) | 2.90 *** | 8.95 |
| School E2 (n = 23) | 2.26 (0.62) | 5.39 (1.03) | 3.13 *** | 12.47 |
| School E3 (n = 14) | 1.71 (0.61) | 5.43 (1.74) | 3.72 *** | 7.53 |
| School E4 (n = 19) | 1.79 (0.63) | 5.74 (1.52) | 3.95 *** | 10.45 |
| Mean differences: mean (experimental)—mean (control) | 0.11 | 1.95 *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.T.; Lykknes, A.; Tiruneh, D.T. Examining the Effects of Supervised Laboratory Instruction on Students’ Motivation and Their Understanding of Chemistry. Educ. Sci. 2023, 13, 798. https://doi.org/10.3390/educsci13080798
Ali MT, Lykknes A, Tiruneh DT. Examining the Effects of Supervised Laboratory Instruction on Students’ Motivation and Their Understanding of Chemistry. Education Sciences. 2023; 13(8):798. https://doi.org/10.3390/educsci13080798
Chicago/Turabian StyleAli, Mirtachew T., Annette Lykknes, and Dawit T. Tiruneh. 2023. "Examining the Effects of Supervised Laboratory Instruction on Students’ Motivation and Their Understanding of Chemistry" Education Sciences 13, no. 8: 798. https://doi.org/10.3390/educsci13080798
APA StyleAli, M. T., Lykknes, A., & Tiruneh, D. T. (2023). Examining the Effects of Supervised Laboratory Instruction on Students’ Motivation and Their Understanding of Chemistry. Education Sciences, 13(8), 798. https://doi.org/10.3390/educsci13080798






