Abstract
Early childhood is a time of rapid physiological, cognitive, and social development, affected by various environmental factors. The physical environment, including the environmental microbiome (the entire consortium of microorganisms and their theatre of activity in a given environment), plays an essential role in childhood development and can be shaped in ways to support health and wellbeing. In this Perspective article, we present considerations for early childhood education settings that wish to shape their outdoor and indoor environments to optimise human and ecosystem health. This is done in line with the latest evidence base on optimising health-supporting interactions between humans and environmental microbiota, but also in pedagogically and developmentally appropriate ways. Based on the Microbiome-Inspired Green Infrastructure (MIGI) principles, the considerations presented here not only support health through human–nature interactions and a healthier natural environment, but also promote a closer, reciprocal relationship between children and their natural environments.
1. Introduction
Early childhood is a critical period of development and growth and a unique opportunity to positively influence the developmental trajectory of children. The years between birth and the start of primary education (ranging from 5 to 7 years of age in different countries) represent a window for improving life chances and ensuring all children reach their developmental potential. The importance of this developmental period is acknowledged widely by its inclusion in the United Nations Sustainable Development Goals [1].
A host of environmental factors have been shown to play a role in the physical, psychological, and cognitive development of young children, from motor competence [2], sleep and energy levels [3], obesity [4], and cognitive and psychomotor skills [5] to name a few. One of the environmental factors that has attracted increasing attention is the microbiome of natural and built environments. Growing research links exposure to diverse microbial communities in the environment to our health and wellbeing [6] through interactions with our physiological systems and the microbial communities living on our skin, airways, and gut [7].
The term ‘human microbiome’ describes the whole consortium of microorganisms, such as bacteria, fungi, archaea, and viruses, that live in and on the human body, including in our gut, skin, airways, and other body sites. The term also encompasses the microorganisms’ ecological theatre of activity. These microorganisms have been increasingly linked to a range of health outcomes [8]. As Finnish Immunologist Tari Haahtela said, “we are protected by two nested layers of biodiversity, microbiota of the outer layer (soil, water, plants and animals), and the inner layer (gut, skin, airways). The latter inhabits our body and is colonised from the outer layer” [9]. As such, our own health is intricately connected to the health of our surrounding ecosystems. Crucially, recruiting a diverse microbiome in early childhood, namely in the first 1000 days and beyond, can directly influence growth and development [10]. Exposure to a diverse assemblage of microorganisms in early childhood can play an important role in human health and development, including of the endocrine and immune systems [11,12]. At the same time, biodiversity loss and other features of the urban environment (such as pollution) can limit children’s exposure to a variety of microorganisms that can be beneficial for health, while also exacerbating their exposure to pathogenic bacteria [13].
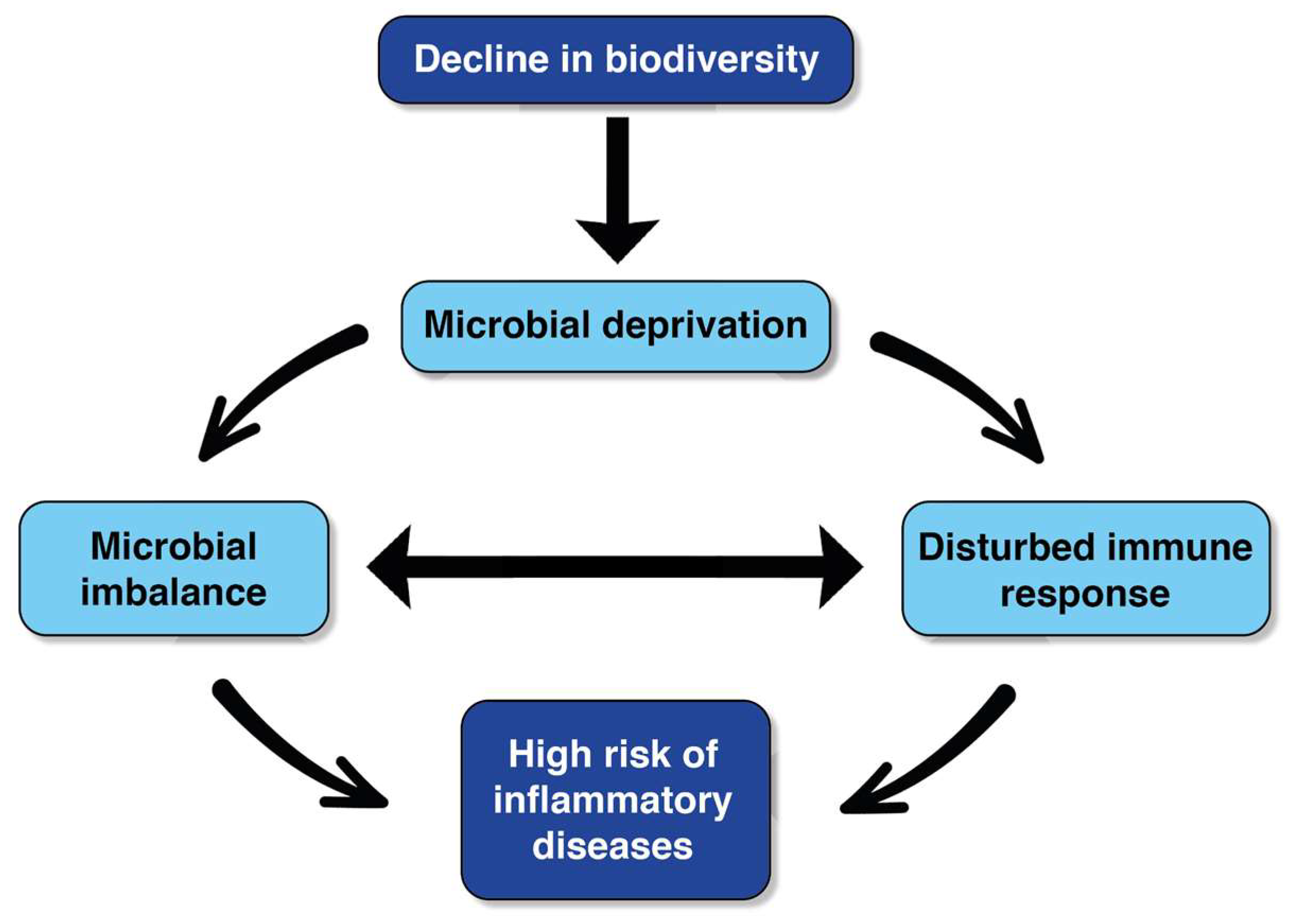
A large percentage of children attend early childhood education settings; for example, 68% of children 0–4 in the UK [14], 51–62% in India [15], and up to 80% of children in the US [16]. Therefore, altering the early childhood education setting in order to optimise the presence of diverse and beneficial microbiota is important from a health equity and utilitarian or ‘democratisation of resources’ perspective. Furthermore, there is a general move towards improving health-promoting interactions between humans and environmental microbiomes in various contexts, including urban infrastructure [6], and previous experimental research suggests that biodiversity interventions in early childhood education settings can enhance children’s microbiota and immunoregulation [17]. For instance, the aforementioned experimental study [17] found positive effects on the skin and gastrointestinal microbiota of children who attended the intervention settings, where part of the day care centre that was previously gravel was covered with forest floor and sod, both of which are rich in microbial organisms. These findings corroborate other research [12] and support the biodiversity hypothesis [18] (Figure 1). Moreover, after 28 days of attending the intervention settings, children were found to have enhanced immune regulation, measured in increased plasma levels of cytokines and T cells. Importantly, similar effects were found after a two-year follow-up [19] and in a separate placebo-controlled double-blinded study [7].
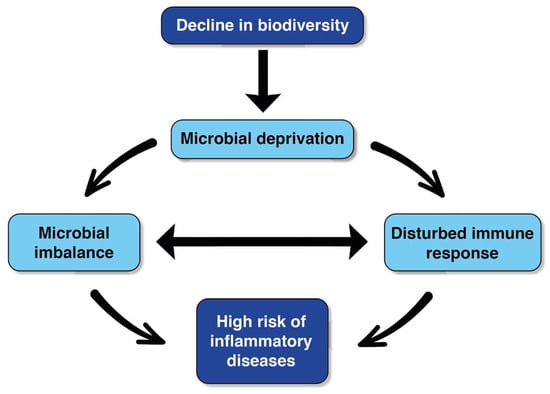
Figure 1.
The biodiversity hypothesis (adapted from Haahtela, 2019).
In this article, we propose considerations for early childhood education settings that want to design or alter their outdoor and indoor environment to optimise human and ecosystem health in pedagogically and developmentally appropriate ways. Some of the suggested interventions utilise features already commonly used in early childhood education settings, such as raised garden beds and sand pits. In addition, the other features we explore have pedagogical value or utility besides optimising microbiome-associated health outcomes, and some of this will be touched on in the discussion. We base our recommendations on the Microbiome-Inspired Green Infrastructure (MIGI) principles [20], which are informed by the best-available evidence in the environmental microbiome–human health discipline. We consider some of the complexities and challenges, as well as some of the most frequently included features of early childhood education settings. Our considerations aim to improve the quality of the environment and enhance the health-promoting potential of the microbiome in such settings. While these can be undertaken individually, they have an additive effect and present a holistic approach to enhancing ecosystem health and promoting pro-environmental behaviours. We hope this article provides a stimulus for designers, providers, and preschool practitioners to consider how simple environmental improvements can promote healthy development in early childhood and contribute to a symbiotic future characterised by reciprocity with nature.
2. The Microbiome-Inspired Green Infrastructure (MIGI) Context
Microbiome-Inspired Green Infrastructure (MIGI) was recently proposed as an integrative framework to enhance urban ecosystem health through multidisciplinary design [20]. Specifically, the MIGI aims to promote nature-centric infrastructure restored or designed to enhance health-supporting interactions between humans and environmental microbiota whilst sustaining microbially-mediated ecosystem functionality and resilience. The MIGI also seeks to stimulate a research agenda that focuses on the importance of microbiota in urban environments, including in early childhood educational settings. Six broad principles based on considerations for microbiota have been developed, with the aim of optimising urban environment design and restoration, including 1. features that promote the compositional and functional diversity of the microbiome, 2. features that reduce the likelihood of a high relative abundance of pathogens, 3. features that promote direct human engagement with diverse microbiota, 4. features that increase the wildlife value of a site, 5. integration of bio-integrated architecture, and 6. practices that promote long-term ecological resilience. More information on the ongoing development of these principles can be found in the supplementary materials. We use these principles to guide the recommendations in this paper.
3. Microbiome-Centric Considerations
3.1. Increase the Diversity and Structural Complexity of Vegetation
Vegetation and soil are primary sources of airborne microbiota (i.e., the ‘aerobiome’) [21]. Vegetation complexity (in species richness and structural diversity) is associated with an increased alpha diversity––the number of different species––of microbiota in the air [22]. Tree proximity and density affect aerobiome assembly, whereby alpha diversity increases and potential pathogen diversity decreases in air samples closer to trees [22]. Reductions in the relative abundance of human-associated pathogens with increasing urban tree density have also been demonstrated [23] and monoculture habitats, such as amenity grasslands/sports fields, likely harbour a higher relative abundance of pathogenic microbiota compared to more complex habitats [22].
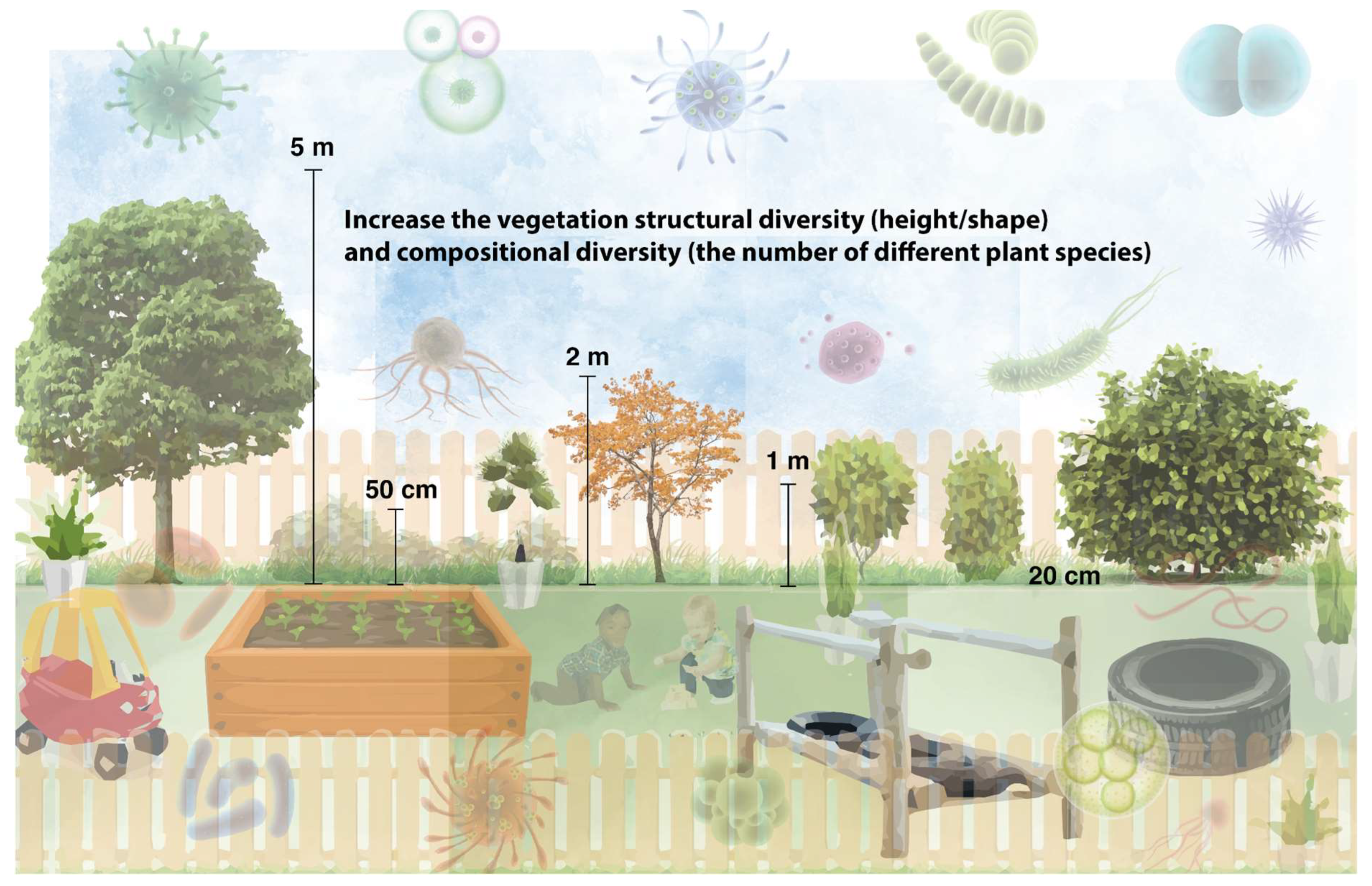
Therefore, emerging evidence suggests that we should increase the vegetation complexity (i.e., diversify the structure and increase the number of species) in a given environment to facilitate a health-promoting aerobiome. Furthermore, due to the critical need to be exposed to a diverse assemblage of environmental microbiota from a young age, we recommend, where possible, planting more vegetation species of different varieties in early childhood educational settings (Figure 2).
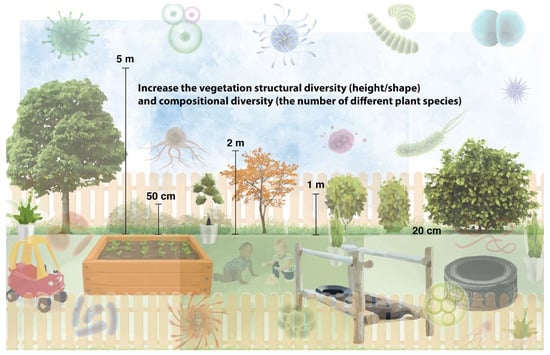
Figure 2.
Increase vegetation species richness and structure in early childhood educational settings to promote a diverse aerobiome. Consult an ecologist to determine the best plants to use in a given location.
3.2. Consult Green Barrier Planting Designs to Reduce Pollution
It is well documented that air pollution (e.g., NO2, O3, and particulate matter) has a detrimental effect on human health by causing severe respiratory illnesses. However, emerging evidence also indicates that air pollution (in addition to anthropogenic noise and artificial light pollution) can negatively impact the microbiome [24]. For instance, lead (Pb)—a highly toxic environmental pollutant—can profoundly affect the composition and functionality of the environmental and human gut microbiomes with various health implications [24]. Moreover, people across socioeconomic gradients are unequally exposed to Pb pollution, with low socioeconomic neighbourhoods often receiving higher Pb exposure [25].
There is also an emerging threat from tyre wear particulates and the chemicals embedded in tyres, i.e., 6PPD-quinone, which has a demonstrated acute toxic effect in wildlife populations [26]. Other similar environmental pollutants are linked to dysbiosis (a negative imbalance in the microbiome) [27]. According to some reports, a child born in Krakow in Poland today may inhale air pollution, equivalent to smoking 11 cigarettes a day due to the burning of fossil fuels. In Shenyang, China, where the worst-ever levels of air pollution were recorded, this figure can reach 63 cigarettes per day [28]. More emphasis should be placed on preventing air pollution; however, in the meantime, we must protect children, their microbiomes, and general health through adaptation measures such as green barriers in outdoor childhood educational settings––particularly those in urban areas or where pollution levels are high. Children are considered to be at increased risk from air pollution [29]. This is exacerbated by children inhaling more air per unit of body weight than their adult counterparts, and their respiratory systems are immature.
Recent work by researchers at the University of Sheffield and others has guided the design and installation of green barriers around school playgrounds to prevent air pollution from entering the school environment and harming the children’s health [30]. The researchers found that relatively simple planting interventions can be implemented to significantly reduce air pollution (a 13% reduction in NO2 in their study) in these settings. These include: (a) consider planting directly into the ground or using raised beds, (b) aim for a minimum planting height of 2 m, which will protect up to 3 m downwind, (c) aim to create a wide a barrier as possible, >2 m, (d) plant multiple species to increase the functional value—this also complements the recommendation of increasing vegetation diversity to enhance the aerobiome, (e) plants that create compact barriers with low porosity are preferred, (f) avoid species with a reputation of producing large amounts of allergenic pollen, and (g) given the childhood context, avoid poisonous and spiky species. In addition, it is recommended that modelling tools such as i-Tree Eco and the GI4RAQ platform are used to model air pollution deposition and dispersion. This will help to determine the benefits of a green barrier and where it should be installed. Consult the Green Barrier Guide for more information [30].
Considering these green barrier designs in combination with consideration #1 (i.e., increasing vegetation complexity and diversity) will help to optimise early childhood educational settings to give children the best possible physiological and cognitive development opportunities.
3.3. Consider the Use of Microbial Inoculants in Sand Pits
In addition to aerobiome exposure, microbiota can transfer to a child’s body and alter their microbiome via direct physical contact with materials. It is possible to alter materials in the landscape that children touch in order to transfer beneficial microbiota to their bodies [31]. Actively augmenting the microbial communities in a given material to achieve a beneficial outcome is called ‘microbial inoculation’. The concept of microbial inoculation has been applied in other contexts for several years. For instance, the agricultural sector has cultured specific beneficial microbiota to improve the growth and health of plants and has inoculated the soil to enhance crop yield [32]. Ecologists have also reintroduced soil microbiota that may be lacking in disturbed ecosystems to enhance the health of the soil and vegetation communities [33]. In terms of inoculating materials to improve human health, a Finnish research group developed a microbial inoculant from forest and agricultural materials that resembles microbiota in organic soils [31]. They inoculated different sand materials (sieved, safety, and sandbox) commonly used in playgrounds. After children touched these materials, their skin’s bacterial diversity and richness increased, and the relative abundance of opportunistic pathogens significantly decreased. More recently, Roslund et al. (2022) built on this work and inoculated sandboxes in preschool yards with microbiologically diverse materials to test the effects on the child microbiome and immune system response [7]. They found that microbiologically rich soil can be used to rewild urban playgrounds with the benefit of promoting immunomodulation among urban children. In this study, shifts in skin microbiota were associated with interleukin-10 and T cell frequencies, supporting the biodiversity hypothesis and its premise of (micro)biodiversity-mediated immunoregulation.
Other studies have shown that transporting small amounts of local forest floor materials into the schoolyard environment can significantly enhance the children’s microbiome with similar immunoregulation effects as those stated above [17,19]. For example, sandpits can be enriched with a ‘biodiversity powder’ (1:1 ratio) containing sieved composted materials comprising agricultural stack, gardening soils, deciduous leaf litter, and Sphagnum moss [7]. To adhere to the study protocols, the inoculant should be saturated with ultra-pure mQ water and hand-squeezed over an ethanol-cleaned 250 μm sieve. The extract is then collected and freeze-dried for 48 h. Microbial ecologists should be consulted prior to creating a microbial inoculant. The inoculant will likely need replacing after several months; however, understanding the inoculant’s ‘shelf life’ is an ongoing area of research.
3.4. Create an Undulating Topography in the Outdoor Environment
As soil is one of the most biodiverse habitats on the planet, soil health and soil-borne microbial inputs to the aerobiome are crucial to a health-promoting aerobiome. Highly degraded and disturbed environments can harbour more pathogens [34]. Poor-quality soils and monoculture habitats provide less diverse microbial assemblages to help competitively exclude opportunistic pathogens and provide various functional roles [22]. Indeed, healthy soils can contain hundreds of millions of microbial cells per gram [35], which feed the aerobiome.
A recent study showed vertical stratification in urban green space aerobiomes occurs, whereby the alpha diversity of bacteria decreases as altitude increases (i.e., the further away from the soil, the fewer microbial species) [36]. As such, we recommend that, where possible, broadly undulating topography is included in outdoor educational settings. This will likely improve the evenness of microbial assemblages in the aerobiome. We say ‘broadly’ to minimise health and safety risks (e.g., omitting trip hazards). Gentle gradients that increase the diversity of contours in the local landscape have the potential to create this undulation. Additionally, such gently undulating gradients offer opportunities for diverse physical movement of the children within the setting, including rolling and crawling and can support children’s motor development [37].
Another way to potentially increase the evenness of microbial input from the soil to the air is to install raised beds or include plant pots of different heights. This will be more appropriate for ‘retrofit scenarios’ and where it is impractical to conduct earthworks. Diversity is key, and increasing the topographical/substrate diversity along with vegetation structural and compositional diversity will likely create a more even distribution of microbiota in the aerobiome. It is also important to consider the potential of soil pollution. For instance, some soils contain high levels of lead, which, as described in consideration #2, can have an adverse effect on a child’s health. Consulting a microbial ecologist to explore microbial bioremediation options and including a lead-free layer of mulch/topsoil could help eliminate risks associated with lead toxicity. Soil geochemistry mapping services such as https://mapapps2.bgs.ac.uk/ukso/home.html can help early childhood educational setting managers understand local soil toxicity risks.
3.5. Include Foraging and Plant Cultivation Opportunities
Despite our coevolutionary relationship with a myriad of plants and fungi, many humans, particularly in Western societies and others in high-income countries, no longer possess the tacit ecological knowledge to forage with confidence. A recent survey of children in the UK suggested that 50% could not identify the common stinging nettle Urtica dioica—a ubiquitous and edible (albeit with the ability to sting prior to preparation) plant in the Northern Hemisphere [38]. As Parsley (2020) wrote, “The general public largely does not notice plants in their environment and therefore do not appreciate how important they are to the biosphere and society”. This phenomenon even has an official scientific term, ‘plant awareness disparity’ [39].
The process of losing this knowledge and experience undermines our nature connectedness, which is seen as a distinct goal of early childhood education [40]. It also erodes our relatedness with plants and the emotional and physical bonds that foster reciprocal benefits for humans and the land. Moreover, it reduces our exposure to biodiversity—including health-promoting microbiota—the phenomenon underscoring the biodiversity hypothesis.
Developing strategies to re-engage humans with plants and their microbial partners is imperative. Implementing such strategies during the window of early life is optimal for building and strengthening the neurological synapses involved in sustaining proecological behaviours. In addition to the psychoneuro development importance, this early-life window is also the critical period when a child’s microbiome is most plastic and when establishing a diverse and robust microbiome by recruiting environmental microbiota from the ‘outer layer of nested biodiversity’ is essential for downstream immune health.
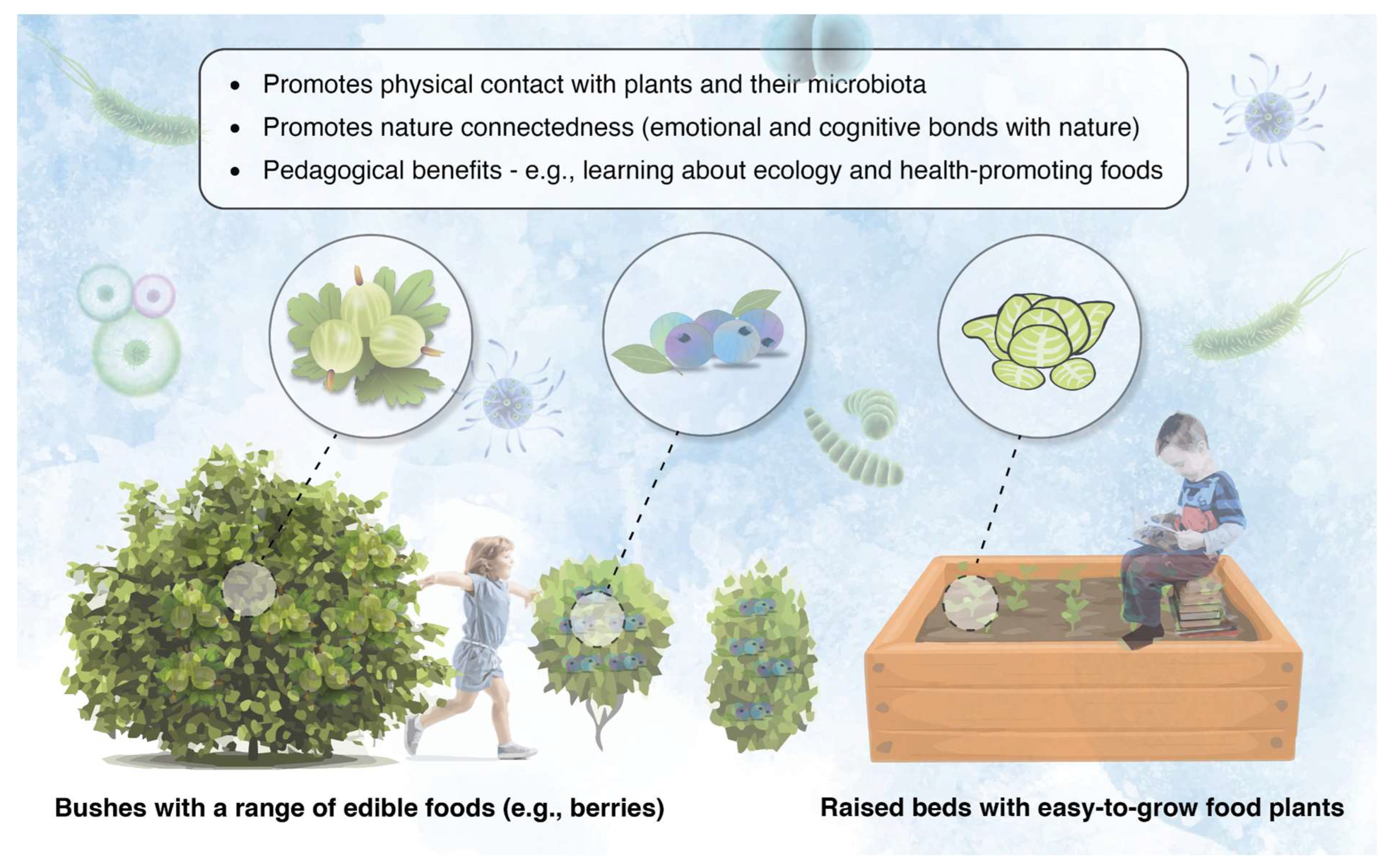
Diverse microbiota in soil and plant material readily transfer to a person’s skin microbiome through touch [41]. Therefore, relatively simple and cost-effective strategies can be implemented in early childhood outdoor educational settings to re-establish the connections between children, plants, and their resident microbiota. For example, opportunities to forage safely and cultivate fresh produce can be developed by (a) planting edible food plants only (e.g., blueberries, blackcurrants, etc.), which can be managed by having a demarcated ‘foraging zone’ so the children can be supervised if necessary, and (b) install raised beds with easy-to-grow (low-maintenance) food plants, such as lettuce and spinach (Figure 3). The children could even plant and tend to the raised beds themselves (under supervision if needed) and take their produce home, thereby promoting reciprocity with the land.
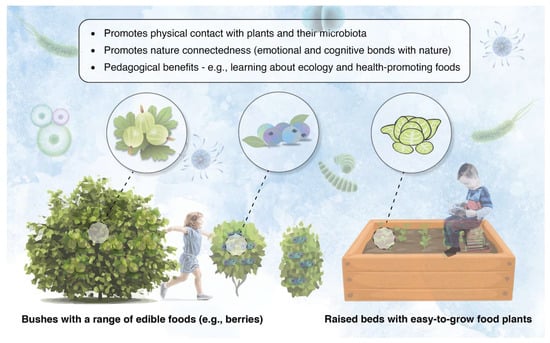
Figure 3.
Foraging and plant growing areas (such as raised beds) have the potential to promote beneficial interactions between children, plants, and their microbiota, with important pedagogical benefits.
3.6. Increase the Wildlife Value of a Site
Animal–plant–microbiota interactions are vital for ecosystem health. Animals contribute to seed dispersal, pollination, and nutrient cycling, facilitating the balanced organic matter required by diverse and complex microbial communities [20]. The functional roles that microbiota play is vital for plant health, which is, in turn, vital for animal health [42] and sustaining a quality aerobiome. Nested symbioses between microbiota, animals, and plants occur all around us, but ecosystem degradation and grey infrastructure inhibit these natural processes [43]. Increasing the wildlife value of an early childhood outdoor educational setting has several important co-benefits. In addition to animals contributing to the diversity of the environmental microbiome, they may also contribute to a child’s nature connectedness, which is linked to enhanced wellbeing [44] and proenvironmental behaviours [45].
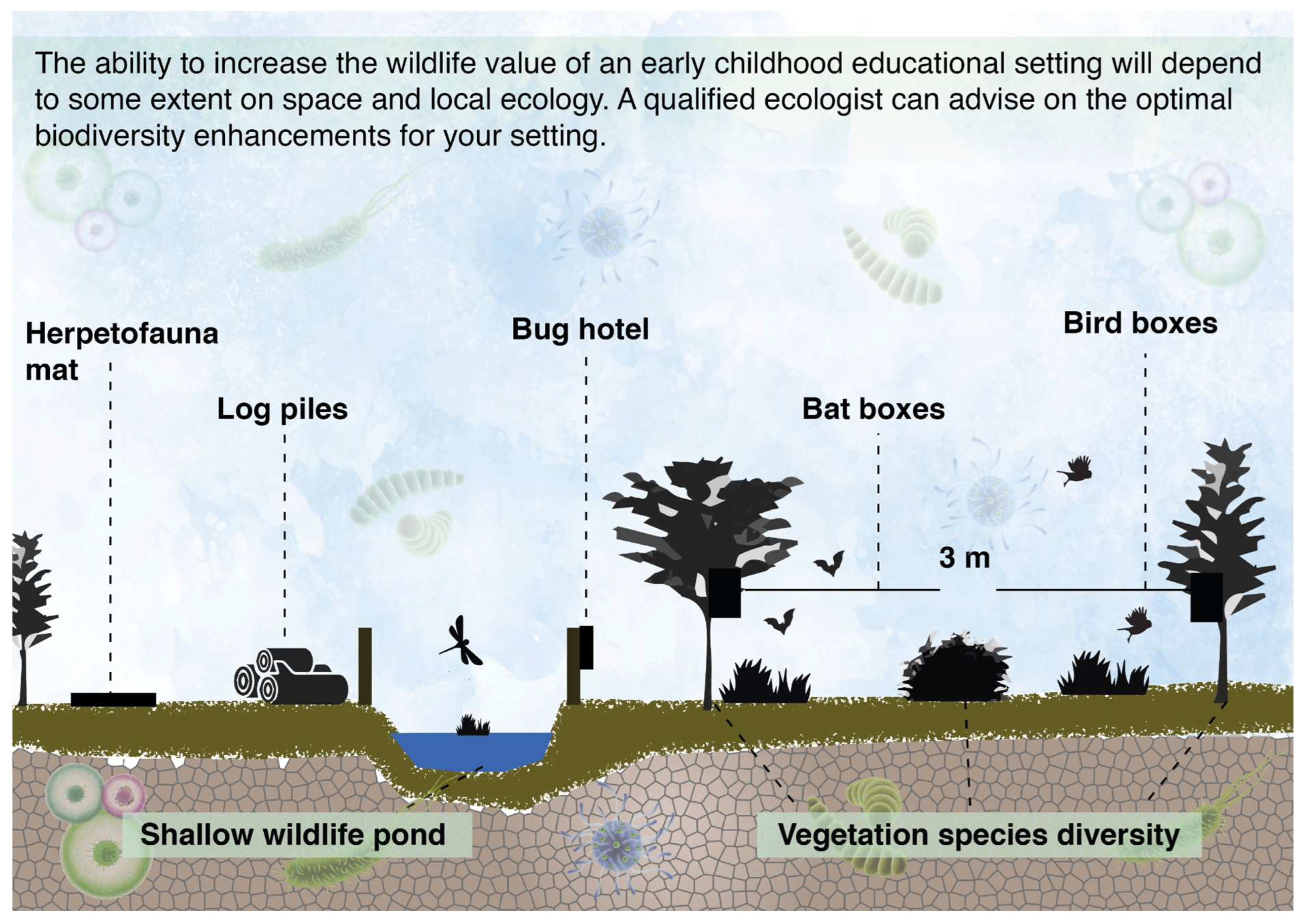
Simple wildlife enhancement measures can be implemented in most outdoor educational settings. These include installing bird and bat boxes on appropriate features such as trees and walls (bird and bat boxes should be placed >3 m from the ground, facing somewhere between north and east for bird boxes and east to south for bat boxes), log piles and bug hotels to support invertebrate species that play vital roles in ecosystem health, refugia (such as large logs, rubble, and corrugated mats as per Figure 4) for amphibians, reptiles, and small mammals, and if safety measures are taken, a wildlife pond––often considered the single most valuable intervention to enhance the wildlife value of a site. When it is possible to include a wildlife pond, small ecological floating beds of aquatic plants can be included, which float like mats on the surface of water. The plant roots hang beneath the floating mat and provide a large surface area for biofilm growth [46]. This biofilm mat can provide important bioremediation benefits, i.e., reducing the pollutants that gather in the waterbody, thereby improving the quality of the local environment.
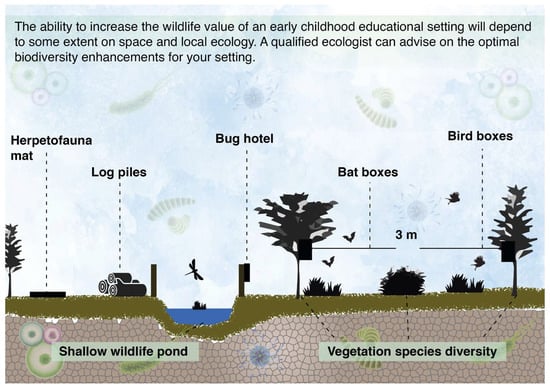
Figure 4.
Examples of different wildlife enhancement measures that can be implemented in the outdoor space of nursery settings. This is an ideal scenario; however, the ability to include features such as a wildlife pond will sometimes depend on the amount of space, the type of setting and health and safety protocols.
Increasing the site’s vegetation compositional and structural complexity (as per consideration #1) will also provide a diversity of ecological niches to support a range of taxonomic groups. Reversing ecosystem degradation in urban environments by planting and nurturing vegetation communities also has the potential to improve the microbiome of wildlife populations. Indeed, urbanisation has been shown to reduce the diversity of the passerine bird microbiome and potentially increase the abundance of pathogens in these birds [47]. Therefore, regreening the outdoor parts of early childhood educational settings and reducing the negative impacts of urbanisation has an important—albeit small—role to play in improving the health of local wildlife. If we view this from a One Health perspective (i.e., that human, non-human animal, and environmental health are intimately connected), it also has an indirect role in helping to secure human health and wellbeing.
3.7. Avoid the Use of Harsh Pesticides, Fertilisers, and Artificial Grass
Pesticides and synthetic fertilisers are well marketed as being a gardener’s friend. However, when we modify the nutrient environment that plants are in, we fundamentally change natural plant–microbiota interactions and the microbiome-mediated protection of these interactions. Indeed, studies have shown that harsh fertilisers can inhibit a plant microbiome’s ability to protect against diseases [48]. Some pesticides contain anti-microbial compounds, which may harm the environmental microbiome. Pesticides may indirectly change the trajectory of host–microbiome coevolution in honey bees and alter their social behaviours, with potential implications for plant–pollinator symbioses [49]. Herbicides such as glyphosate have been shown to affect neuronal communication, resulting in altered behaviours and gut microbiota in rodent models [50]. Glyphosate can also alter the gut microbiome and antioxidant activity in birds [51]. Research also suggests that glyphosate could potentially alter the human microbiome, with serious health implications [52]. Therefore, whilst acknowledging that further research is needed, we recommend that harsh pesticides/herbicides and fertilisers are avoided in early childhood education settings as a measure to protect the environmental and human microbiome.
The artificial grass industry has boomed in the last decade. Based on revenue figures and average price per m2, an annual increase of 60,000 ha in artificial grass coverage is expected by 2030 (calculated by the authors). Artificial grass is considered convenient and low maintenance [53]; however, we argue that it also causes considerable plastic waste/pollution, has the potential to reduce nature connectedness, is detrimental to wildlife, and has the potential to reduce the diversity of the aerobiome (by creating a non-natural monoculture habitat). In addition, some evidence suggests it is associated with higher loads of Methicillin-resistant Staphylococcus aureus (MRSA), a group of bacteria often called ‘superbugs’, which can cause difficult-to-treat human infections due to their resistance to antibiotics [54]. Therefore, until further comprehensive research is performed to indicate otherwise, we recommend not including artificial lawns in early childhood educational settings.
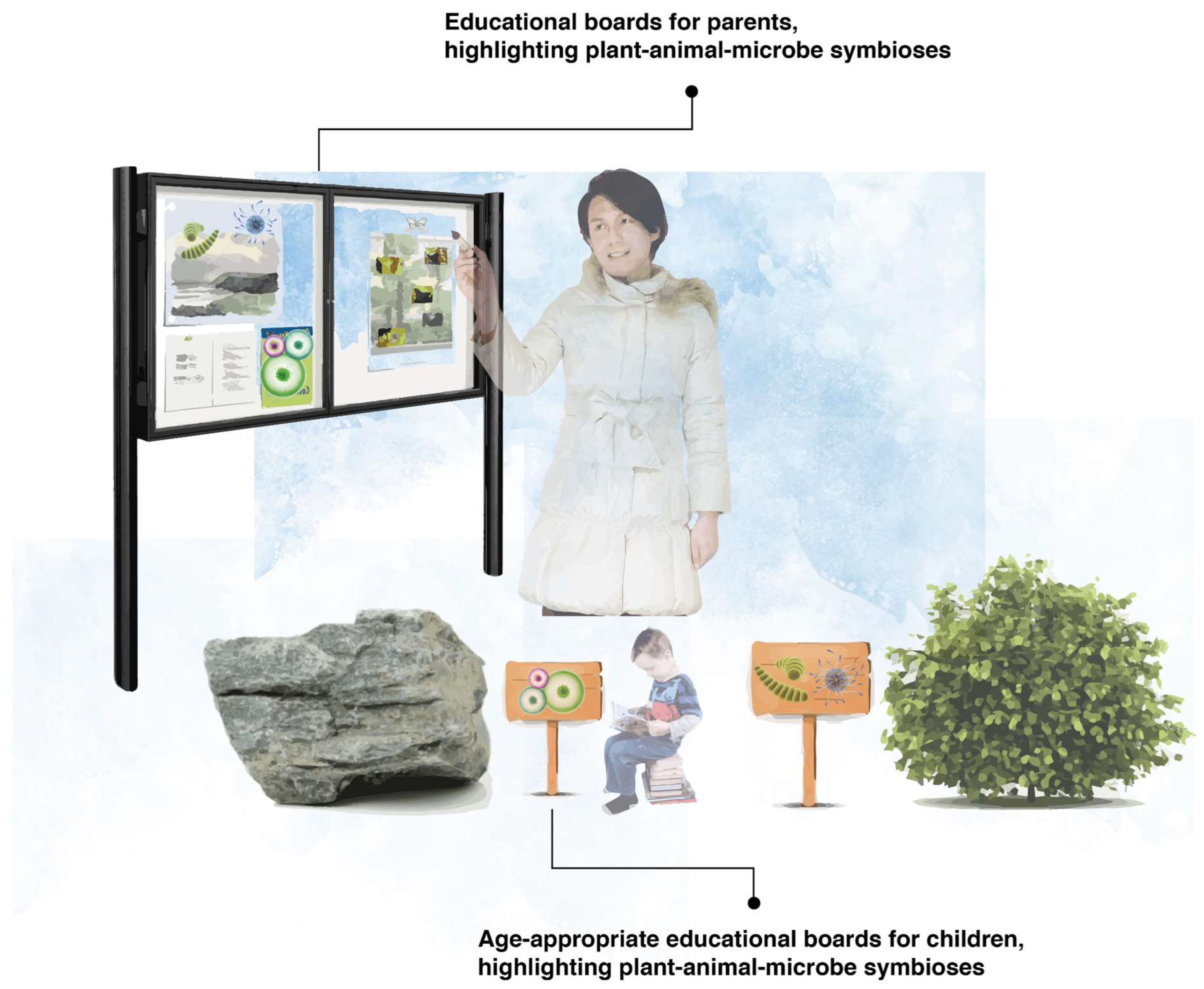
3.8. Educational Interpretation Boards to Make the Invisible Visible
Educational interpretation boards are widely used in nature reserves and national parks to convey important learning, behavioural and emotional messages and insight to visitors. Interpretation is a form of communication that helps people understand elements of the place being visited, bridging the gap between what a person already knows and what they want or need to know. Interpretation boards are an important tool to improve visitor knowledge and understanding of a given topic. Introducing educational interpretation boards with engaging graphics has been implemented before to engage communities with visible biodiversity [55].
We propose that early childhood educational settings should incorporate interpretation boards, as per Figure 5, within their grounds to convey the importance of microbiota and the symbiotic relationship they form with plants and animals; after all, everything you can see intimately depends on everything you cannot see. We suggest that, where possible, two types of educational interpretation boards be included in early childhood educational settings: (1) interpretation boards for adults (e.g., the parents of the children), which can provide vital biodiversity knowledge to the parents, allowing them to understand the invisible world and the myriad benefits of microbiota, with important downstream impacts on parent–child interactions, and (2) age-appropriate interpretation boards for children attending the early childhood educational settings. We also recommend other age-appropriate media, such as videos, songs, or storybooks. These can include less text-based information and more engaging pictures describing the symbiotic relationships between the visible and invisible world. We recommend further research to better understand the potential implications of these interpretation boards, e.g., for enhancing a child’s microbiology literacy (which is associated with a lower risk of ‘germophobia’ or the fear of germs and ‘dirt’) [56], and a child’s nature connectedness by stimulating nature engagement.
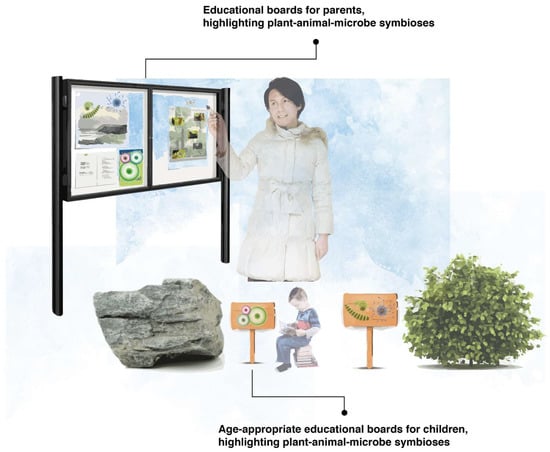
Figure 5.
Examples of microbiome-centric educational boards, illuminating the unseen and the different symbiotic relationships that occur all around us––to both children and their parents.
4. Naturing the Indoor Environment and Future Possibilities
This article has focused on the outdoor environment of early childhood educational settings. However, some of the principles can be applied to indoor settings. For instance, increasing the abundance and diversity of indoor plant species is likely to provide aerobiome benefits and reduce air pollutants [57]. Indoor ‘foraging zones’ could also bring value, i.e., having small growing spaces where children can interact with vegetation and their microbiota. Optimising the ventilation and daylight availability in the indoor space will likely enhance the air quality and reduce the abundance of opportunistic pathogens [58].
In terms of future possibilities, microbial inoculants will become more targeted as research progresses. By this, we mean that instead of creating large mixture of microbial inoculants, specific health-promoting taxa may become available to inoculate materials that children touch (similar to the probiotic concept, e.g., drinks with high Lactobacilli content and other bacteria to promote gut health). Another near-future possibility is incorporating bio-integrated designs within early childhood educational settings. Bio-integrated design involves designing architectural materials that integrate biology within them. For instance, a wall or a building façade can be designed, and 3D printed to include cryptogams (such as algae, lichens, and mosses) or designed to promote colonisation by these organisms from the environment—also known as bioreceptivity [59]. These bio-integrated designs will likely improve the quality of the local environment by providing important pollution-reducing functions and feeding the aerobiome. For example, a trial by the Bio-ID Lab at the Bartlett School of Architecture, University College London, is currently underway in London to see if moss-based bioreceptive wall panels can improve the air quality of a school yard. In the near future, combining the more traditional green barriers described in this article with modern bio-integrated architecture could significantly improve our urban environments and our children’s health.
5. Discussion
Interactions with natural environments are known to benefit humans [60] and are associated with optimised developmental trajectories in young children [61]. More specifically, previous research has suggested that ‘greener’ educational and living environments can positively influence different aspects of development and have beneficial outcomes, including improved cognitive development [62], social behaviour [5], emotional development [63], nature connection [64], and immune development via child–environmental microbiota interactions [17].
The above considerations offer an opportunity to address several aspects of development in children. For instance, creating diverse vegetation cover and undulating topography not only improves the diversity and evenness of microbial assemblages in the aerobiome, but can also provide opportunities for different physical and cognitive activities. Indeed, diverse features in the early childhood education setting can promote different types of play and learning [65]. Another example of co-beneficial outcomes is how the inclusion of nursery gardens can provide opportunities for foraging and cultivation opportunities, but can also have a positive effect on other indicators of physiological and mental health [66].
Overall, the recommendations in this article offer an opportunity to mobilise resources and continue the drive of integrating health promotion in early childhood education settings [67]. The guidance also helps practitioners to avoid siloed approaches to health and wellbeing in early childhood [68]. Moreover, improving early childhood settings to optimise child health, as proposed above, can provide an additional stimulus to enhance health equity through early care and education [69].
This is not to say that such improvements come without challenges. Indeed, it is likely that early childhood education setting staff lack the expertise to independently enact some of the recommendations. In addition, the design and construction of educational spaces do not historically consider the complex needs of children [70]. However, steps have been taken in the right direction, for example, in Scotland [71]. Including specialists (such as microbial ecologists and specialised landscape architects) during the design, construction, and maintenance of education settings would be valuable to optimise those settings for child health. Teaming up with such specialists from local universities is one way to achieve these goals.
Another challenge is the practitioner and parent education aspect, which we alluded to in our last outdoor recommendation. Previous research suggests that people with limited microbial literacy are less likely to engage with nature in outdoor environments [56]. As such, it could be possible that a lack of education on the positive potential of microbial diversity can be a barrier to the uptake of our recommendations. Moreover, it may affect the optimal use of the environments we have recommended. This, as well as the possibilities of bespoke pre-service and in-service education programmes for practitioners, should be explored by further research.
6. Conclusions
Early childhood setting interventions have been increasingly used to improve health outcomes in children and enhance developmental trajectories [72]. In this article, we outline a set of nine recommendations, eight in the outdoor environment and one generally pertaining to naturing the indoor spaces, that can optimise child health by improving the functional diversity of the children’s microbiota. These recommendations can be used singly, as space and expertise permit, although there is an additive effect when used together. Aside from single settings adopting these recommendations, ideally, they would be adopted by local and national government bodies and other authorities responsible for the design, construction, and maintenance of early childhood education settings. We recommend adopting the One Health perspective that recognises the interconnectedness of human health and that of the non-human animals and environments around us [73]. The need to foster mutually advantageous relationships between humans, starting from an early age, and the wider communities of life has never been more salient. As reciprocal restoration and nature-based solutions for the challenges facing humanity and our planet are increasingly valued, we urge relevant bodies to consider incorporating our recommendations in the design of early childhood education settings.
Author Contributions
J.M.R. and A.B. conceived of the idea, wrote the first draft and edited the final version. J.M.R. produced all the figures from scratch. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Britto, P.R.; Lye, S.J.; Proulx, K.; Yousafzai, A.K.; Matthews, S.G.; Vaivada, T.; Perez-Escamilla, R.; Rao, N.; Ip, P.; Fernald, L.C.H.; et al. Nurturing Care: Promoting Early Childhood Development. Lancet 2017, 389, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Venetsanou, F.; Kambas, A. Environmental Factors Affecting Preschoolers’ Motor Development. Early Child. Educ. J. 2010, 37, 319–327. [Google Scholar] [CrossRef]
- McDonald, L.; Wardle, J.; Llewellyn, C.H.; van Jaarsveld, C.H.M.; Fisher, A. Predictors of Shorter Sleep in Early Childhood. Sleep Med. 2014, 15, 536–540. [Google Scholar] [CrossRef]
- Silventoinen, K.; Rokholm, B.; Kaprio, J.; Sørensen, T.I.A. The Genetic and Environmental Influences on Childhood Obesity: A Systematic Review of Twin and Adoption Studies. Int. J. Obes. 2010, 34, 29–40. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, B.; Xia, W.; Cao, Z.; Zhang, Y.; Liang, S.; Hu, K.; Xu, S.; Li, Y. Residential Exposure to Green Space and Early Childhood Neurodevelopment. Environ. Int. 2019, 128, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Fumagalli, S.; Ghisleni, G.; Labra, M. The Microbiome of the Built Environment: The Nexus for Urban Regeneration for the Cities of Tomorrow. Microorganisms 2022, 10, 2311. [Google Scholar] [CrossRef]
- Roslund, M.I.; Parajuli, A.; Hui, N.; Puhakka, R.; Grönroos, M.; Soininen, L.; Nurminen, N.; Oikarinen, S.; Cinek, O.; Kramná, L.; et al. A Placebo-Controlled Double-Blinded Test of the Biodiversity Hypothesis of Immune-Mediated Diseases: Environmental Microbial Diversity Elicits Changes in Cytokines and Increase in T Regulatory Cells in Young Children. Ecotoxicol. Environ. Saf. 2022, 242, 113900. [Google Scholar] [CrossRef] [PubMed]
- Relman, D.A. The Human Microbiome: Ecosystem Resilience and Health. Nutr. Rev. 2012, 70, S2–S9. [Google Scholar] [CrossRef]
- Haahtela, T. A Biodiversity Hypothesis. Allergy 2019, 74, 1445–1456. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth – First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Williams, C.L.; Garcia-Reyero, N.; Martyniuk, C.J.; Tubbs, C.W.; Bisesi, J.H. Regulation of Endocrine Systems by the Microbiome: Perspectives from Comparative Animal Models. Gen. Comp. Endocrinol. 2020, 292, 113437. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Skevaki, C. Early Life Microbial Exposures and Allergy Risks: Opportunities for Prevention. Nat. Rev. Immunol. 2021, 21, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, A.; Grönroos, M.; Siter, N.; Puhakka, R.; Vari, H.K.; Roslund, M.I.; Jumpponen, A.; Nurminen, N.; Laitinen, O.H.; Hyöty, H.; et al. Urbanization Reduces Transfer of Diverse Environmental Microbiota Indoors. Front. Microbiol. 2018, 9, 84. [Google Scholar] [CrossRef]
- Childcare and Early Years Survey of Parents, Reporting Year. 2021. Available online: https://explore-education-statistics.service.gov.uk/find-statistics/childcare-and-early-years-survey-of-parents (accessed on 1 December 2022).
- Early Childhood Education. Available online: https://www.unicef.org/india/what-we-do/early-childhood-education (accessed on 4 December 2022).
- Ansari, A.; Gottfried, M.A. Early Childhood Educational Settings and School Absenteeism for Children With Disabilities. AERA Open 2018, 4. [Google Scholar] [CrossRef]
- Roslund, M.I.; Puhakka, R.; Grönroos, M.; Nurminen, N.; Oikarinen, S.; Gazali, A.M.; Cinek, O.; Kramná, L.; Siter, N.; Vari, H.K.; et al. Biodiversity Intervention Enhances Immune Regulation and Health-Associated Commensal Microbiota among Daycare Children. Sci. Adv. 2020, 6, eaba2578. [Google Scholar] [CrossRef]
- Haahtela, T.; Holgate, S.; Pawankar, R.; Akdis, C.A.; Benjaponpitak, S.; Caraballo, L.; Demain, J.; Portnoy, J.; von Hertzen, L. The Biodiversity Hypothesis and Allergic Disease: World Allergy Organization Position Statement. World Allergy Organ. J. 2013, 6, 3. [Google Scholar] [CrossRef]
- Roslund, M.I.; Puhakka, R.; Nurminen, N.; Oikarinen, S.; Siter, N.; Grönroos, M.; Cinek, O.; Kramná, L.; Jumpponen, A.; Laitinen, O.H.; et al. Long-Term Biodiversity Intervention Shapes Health-Associated Commensal Microbiota among Urban Day-Care Children. Environ. Int. 2021, 157, 106811. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Watkins, H.; Man, I.; Liddicoat, C.; Cameron, R.; Parker, B.; Cruz, M.; Meagher, L. Microbiome-Inspired Green Infrastructure: A bioscience roadmap for urban ecosystem health. Archit. Res. Q. 2021, 25, 292–303. [Google Scholar] [CrossRef]
- Rai, S.; Singh, D.K.; Kumar, A. Microbial, Environmental and Anthropogenic Factors Influencing the Indoor Microbiome of the Built Environment. J. Basic Microbiol. 2021, 61, 267–292. [Google Scholar] [CrossRef]
- Robinson, J.M.; Cando-Dumancela, C.; Antwis, R.E.; Cameron, R.; Liddicoat, C.; Poudel, R.; Weinstein, P.; Breed, M.F. Exposure to Airborne Bacteria Depends upon Vertical Stratification and Vegetation Complexity. Sci. Rep. 2021, 11, 9516. [Google Scholar] [CrossRef]
- Li, H.; Wu, Z.-F.; Yang, X.-R.; An, X.-L.; Ren, Y.; Su, J.-Q. Urban Greenness and Plant Species Are Key Factors in Shaping Air Microbiomes and Reducing Airborne Pathogens. Environ. Int. 2021, 153, 106539. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chi, L.; Mahbub, R.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Multi-Omics Reveals That Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem. Res. Toxicol. 2017, 30, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kwon, H.; Ha, M.; Lim, J.-A.; Lim, M.; Yoo, S.-J.; Paik, K. How Does Low Socioecon. Status Increase Blood Lead Levelsin KoreanChildren? IJERPH 2018, 15, 1488. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Gora, A.H.; Siriyappagouder, P.; Kiron, V.; Olsvik, P.A. Toxicological Effects of 6PPD and 6PPD Quinone in Zebrafish Larvae. J. Hazard. Mater. 2022, 424, 127623. [Google Scholar] [CrossRef]
- Fang, L.; Fang, C.; Di, S.; Yu, Y.; Wang, C.; Wang, X.; Jin, Y. Oral Exposure to Tire Rubber-Derived Contaminant 6ppd and 6ppd-Quinone Induced Hepatotoxicity in Mice. Sci. Total. Environ. 2023, 869, 161836. [Google Scholar] [CrossRef]
- Muller, R.; Muller, E. Air Pollution and Cigarette Equivalence. Berkeley Earth. 2015. Available online: https://berkeleyearth.org/air-pollution-and-cigarette-equivalence/ (accessed on 19 December 2022).
- Peled, R. Air pollution exposure: Who is at high risk? Atmos. Environ. 2011, 45, 1781–1785. [Google Scholar] [CrossRef]
- Redondo Bermúdez, M.d.C.; Kanai, J.M.; Astbury, J.; Fabio, V.; Jorgensen, A. Green Fences for Buenos Aires: Implementing Green Infrastructure for (More than) Air Quality. Sustainability 2022, 14, 4129. [Google Scholar] [CrossRef]
- Hui, N.; Grönroos, M.; Roslund, M.I.; Parajuli, A.; Vari, H.K.; Soininen, L.; Laitinen, O.H.; Sinkkonen, A. The ADELE Research Group Diverse Environmental Microbiota as a Tool to Augment Biodiversity in Urban Landscaping Materials. Front. Microbiol. 2019, 10, 536. [Google Scholar] [CrossRef]
- Qiu, Z.; Egidi, E.; Liu, H.; Kaur, S.; Singh, B.K. New Frontiers in Agriculture Productivity: Optimised Microbial Inoculants and in Situ Microbiome Engineering. Biotechnol. Adv. 2019, 37, 107371. [Google Scholar] [CrossRef]
- Dadzie, F.A.; Moles, A.T.; Erickson, T.E.; Slavich, E.; Muñoz-Rojas, M. Native Bacteria and Cyanobacteria Can Influence Seedling Emergence and Growth of Native Plants Used in Dryland Restoration. J. Appl. Ecol. 2022, 59, 2983–2992. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Chen, S.; Qi, G.; He, Z.; Zhao, X. Microbial Taxa and Functional Genes Shift in Degraded Soil with Bacterial Wilt. Sci. Rep. 2017, 7, 39911. [Google Scholar] [CrossRef]
- Roesch, L.F.W.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.M.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.O.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing Enumerates and Contrasts Soil Microbial Diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Cando-Dumancela, C.; Liddicoat, C.; Weinstein, P.; Cameron, R.; Breed, M.F. Vertical Stratification in Urban Green Space Aerobiomes. Env. Health Perspect. 2020, 128, 117008. [Google Scholar] [CrossRef] [PubMed]
- Fjørtoft, I.; Sageie, J. The Natural Environment as a Playground for Children. Landsc. Urban Plan. 2000, 48, 83–97. [Google Scholar] [CrossRef]
- The Shocking State of Biodiversity: Education and Mass Extinction. Available online: https://www.earlham.ac.uk/articles/shocking-state-biodiversity-education-and-mass-extinction (accessed on 10 December 2022).
- Parsley, K.M. Plant Awareness Disparity: A Case for Renaming Plant Blindness. Plants People Planet 2020, 2, 598–601. [Google Scholar] [CrossRef]
- Barrable, A. The Case for Nature Connectedness as a Distinct Goal of Early Childhood Education. Int. J. Early Child. 2019, 6, 59–70. [Google Scholar]
- Grönroos, M.; Parajuli, A.; Laitinen, O.H.; Roslund, M.I.; Vari, H.K.; Hyöty, H.; Puhakka, R.; Sinkkonen, A. Short-term Direct Contact with Soil and Plant Materials Leads to an Immediate Increase in Diversity of Skin Microbiota. MicrobiologyOpen 2019, 8, e00645. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Edlinger, A.; Garland, G.; Hartman, K.; Banerjee, S.; Degrune, F.; García-Palacios, P.; Hallin, S.; Valzano-Held, A.; Herzog, C.; Jansa, J.; et al. Agricultural Management and Pesticide Use Reduce the Functioning of Beneficial Plant Symbionts. Nat. Ecol. Evol. 2022, 6, 1145–1154. [Google Scholar] [CrossRef]
- Barrable, A.; Booth, D.; Adams, D.; Beauchamp, G. Enhancing Nature Connection and Positive Affect in Children through Mindful Engagement with Natural Environments. Int. J. Environ. Res. Public Health 2021, 18, 4785. [Google Scholar] [CrossRef]
- Martin, L.; White, M.P.; Hunt, A.; Richardson, M.; Pahl, S.; Burt, J. Nature Contact, Nature Connectedness and Associations with Health, Wellbeing and pro-Environmental Behaviours. J. Environ. Psychol. 2020, 68, 101389. [Google Scholar] [CrossRef]
- Samal, K.; Kar, S.; Trivedi, S. Ecological Floating Bed (EFB) for Decontamination of Polluted Water Bodies: Design, Mechanism and Performance. J. Environ. Manag. 2019, 251, 109550. [Google Scholar] [CrossRef] [PubMed]
- Maraci, Ö.; Corsini, M.; Antonatou-Papaioannou, A.; Jünemann, S.; Sudyka, J.; Di Lecce, I.; Caspers, B.A.; Szulkin, M. Changes to the Gut Microbiota of a Wild Juvenile Passerine in a Multidimensional Urban Mosaic. Sci. Rep. 2022, 12, 6872. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Koskella, B. Nutrient- and Dose-Dependent Microbiome-Mediated Protection against a Plant Pathogen. Curr. Biol. 2018, 28, 2487–2492.e3. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Chernyshova, A.M.; Thompson, G.J.; Allen-Vercoe, E. Deteriorating Microbiomes in Agriculture—the Unintended Effects of Pesticides on Microbial Life. MRR 2022, 1, 6. [Google Scholar] [CrossRef]
- Dechartres, J.; Pawluski, J.L.; Gueguen, M.M.; Jablaoui, A.; Maguin, E.; Rhimi, M.; Charlier, T.D. Glyphosate and glyphosate-based herbicide exposure during the peripartum period affects maternal brain plasticity, maternal behaviour and microbiome. Journ Neuroend 2019, 31, e12731. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Rainio, M.J.; Gómez-Gallego, C.; Selenius, O.; Salminen, S.; Collado, M.C.; Saikkonen, K.; Saloniemi, I.; Helander, M. Glyphosate-Based Herbicides Influence Antioxidants, Reproductive Hormones and Gut Microbiome but Not Reproduction: A Long-Term Experiment in an Avian Model. Environ. Pollut. 2020, 266, 115108. [Google Scholar] [CrossRef]
- Puigbò, P.; Leino, L.I.; Rainio, M.J.; Saikkonen, K.; Saloniemi, I.; Helander, M. Does Glyphosate Affect Hum. Microbiota? Life 2022, 12, 707. [Google Scholar] [CrossRef]
- Watterson, A. Artificial Turf: Contested Terrains for Precautionary Public Health with Particular Reference to Europe? Int. J. Environ. Res. Public Health 2017, 14, 1050. [Google Scholar] [CrossRef]
- Keller, M.; Turco, R.F.; Gray, M.B.; Sigler, V. The Fate of Methicillin-Resistant Staphylococcus Aureus in a Synthetic Turf System. Sport. Health 2020, 12, 263–270. [Google Scholar] [CrossRef]
- Mutiara, M.M.; Rachmawati, E.; Sunkar, A. Effectivity Assessment of Interpretive Signs for Biodiversity Conservation. IOP Conf. Ser. Earth Environ. Sci. 2021, 739, 012066. [Google Scholar] [CrossRef]
- Robinson, J.M.; Cameron, R.; Jorgensen, A. Germaphobia! Does Our Relationship With and Knowledge of Biodiversity Affect Our Attitudes Toward Microbes? Front. Psychol. 2021, 12, 678752. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Deng, Q. The Basic Roles of Indoor Plants in Human Health and Comfort. Env. Sci. Pollut. Res. 2018, 25, 36087–36101. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, T.; Ahmad, D.; Serey, N.; Jouhara, H. Review of Ventilation Strategies to Reduce the Risk of Disease Transmission in High Occupancy Buildings. Int. J. Thermofluids 2020, 7–8, 100045. [Google Scholar] [CrossRef]
- Benjamin, D. Adaptation: Bio-Receptive Materials with a New Outlook on Performativity and Sustainability. In The Routledge Companion to Paradigms of Performativity in Design and Architecture; Routledge: Abingdon, UK, 2019; pp. 362–369. [Google Scholar]
- Keniger, L.E.; Gaston, K.J.; Irvine, K.N.; Fuller, R.A. What are the Benefits of Interacting with Nature? Int. J. Environ. Res. Public Health 2013, 10, 913–935. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.F.; Kuo, F.E. Is contact with nature important for healthy child development? State of the evidence. In Children and Their Environments: Learning, Using and Designing Spaces; Spencer, C., Blades, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 124–158. [Google Scholar] [CrossRef]
- Dadvand, P.; Nieuwenhuijsen, M.J.; Esnaola, M.; Forns, J.; Basagaña, X.; Alvarez-Pedrerol, M.; Rivas, I.; López-Vicente, M.; De Castro Pascual, M.; Su, J.; et al. Green Spaces and Cognitive Development in Primary Schoolchildren. Proc. Natl. Acad. Sci. USA 2015, 112, 7937–7942. [Google Scholar] [CrossRef]
- Richardson, E.A.; Pearce, J.; Shortt, N.K.; Mitchell, R. The role of public and private natural space in children’s social, emotional and behavioural development in Scotland: A longitudinal study. Environ. Res. 2017, 158, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Barrable, A.; Booth, D. Nature Connection in Early Childhood: A Quantitative Cross-Sectional Study. Sustainability 2020, 12, 375. [Google Scholar] [CrossRef]
- Sandseter, E.B.H. Affordances for Risky Play in Preschool: The Importance of Features in the Play Environment. Early Child. Educ. J. 2009, 36, 439–446. [Google Scholar] [CrossRef]
- Skelton, K.R.; Lowe, C.; Zaltz, D.A.; Benjamin-Neelon, S.E. Garden-Based Interventions and Early Childhood Health: An Umbrella Review. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 121. [Google Scholar] [CrossRef]
- Early Childhood Development and Health. Available online: https://www.unicef.org/serbia/en/early-childhood-development-and-health (accessed on 10 December 2022).
- Nagle, G.A.; Usry, L.R. Using Public Health Strategies to Shape Early Childhood Policy. Am. J. Orthopsychiatry 2016, 86, 171–178. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Applying Neurobiological and Socio-Behavioral Sciences from Prenatal Through Early Childhood Development: A Health Equity Approach; Negussie, Y.; Geller, A.; DeVoe, J.E. (Eds.) Promoting Health Equity Through Early Care and Education; National Academies Press (US): Washington, DC, USA, 2019. [Google Scholar]
- Woolner, P. Building Schools for the Future through a participatory design process: Exploring the issues and investigating ways forward. BERA 2009. Available online: https://www.ncl.ac.uk/media/wwwnclacuk/cflat/files/Woolner2009Building%20Schools%20for%20the%20Future%20through%20a%20participatory%20design%20process.pdf (accessed on 15 February 2023).
- Building Better Schools: Investing in Scotland’s Future; ANNEX E Sources of Further Information. Available online: http://www.gov.scot/publications/building-better-schools-investing-scotlands-future/pages/14/ (accessed on 10 December 2022).
- Matwiejczyk, L.; Mehta, K.; Scott, J.; Tonkin, E.; Coveney, J. Characteristics of Effective Interventions Promoting Healthy Eating for Pre-Schoolers in Childcare Settings: An Umbrella Review. Nutrients 2018, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- One Health Basics | One Health | CDC. Available online: https://www.cdc.gov/onehealth/basics/index.html (accessed on 10 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).