1. Introduction
Oxidation-reduction reaction (redox reaction) is an important science concept, as it underpins innumerable biological and chemical reactions. Photosynthesis, electrochemical cell reactions, and multiple reactions in the human body, such as ATP production, are all examples of redox reactions. However, research has shown that the topic of redox is both difficult to teach and to learn in secondary chemistry courses [
1,
2]. Teachers find it difficult to demonstrate the mechanism of redox reactions, and students struggle to identify redox reactions, assign oxidation numbers, and balance redox reaction equations. Most of these difficulties relate to the molecular representations characterizing the redox processes [
3].
Johnstone [
4] proposed that there were three levels of representation in chemistry: macroscopic, submicroscopic (molecular), and symbolic. Although researchers have adapted and reinterpreted this framework to understand the nature of chemical knowledge, it is generally agreed that students need to seamlessly move among all the three levels of representation to have a deep understanding of chemical concepts [
5] and that failure to connect these three representations leads to students’ learning difficulties in chemistry [
3,
6]. Previous research on redox instruction has focused on supporting students’ understanding of the symbolic level representation by introducing chemical equations and symbols [
7,
8]. However, researchers found that merely teaching students the symbolic level concepts caused learning difficulties in terms of conceptualizing the surface features of chemical equations and oxidation numbers [
3,
9].
To address this issue, teachers started to employ laboratory activities to teach redox reactions either by performing demonstrations or by having students conduct experiments. Laboratory experiences are an integral part of chemistry instruction, providing students the opportunity of experiencing the macroscopic nature of redox reactions [
10], examples of which include reactions between steel wool and copper sulfate, copper (II) and zinc [
11,
12], and iron nail with copper sulfate and zinc sulfate [
8]. These lab experiences provide students the opportunity of observing some sort of phenomena with their naked eyes, such as the color change of a solution or the formation of a new metal. Such laboratory instruction is typically delivered in two steps: a laboratory experience wherein students observe the effects of redox reactions, and a follow-up discussion or a worksheet portion where students try to make sense of what they observed in the lab. As such, students typically leave the lab, the place where the changes take place, to delve into the mechanism driving the reactions. This disconnection between macroscopic and symbolic representations thus contributes to students’ learning difficulties [
8].
More recently, researchers have employed animations and visualizations to present the molecular level of representation. Depicting the particulate properties of redox reactions benefited students’ learning of different concepts related to oxidation and reduction processes [
13,
14,
15,
16]. However, students experienced learning difficulties when other levels of representation were missing, because it was hard for them to understand “that the animations are representing the submicroscopic level of the macroscopic events” [
14]. In addition, only observing the visual interactions among particles resulted in a higher cognitive load, which may have prevented students from interpreting more complex concepts related to redox [
13,
16]. Therefore, researchers suggested partnering the animations and/or visualizations with laboratory experiences to promote student learning of chemical concepts [
14].
Collectively, pedagogical literature tends to present the concept of redox only with one or two levels of representation. Although the significance of connecting all three levels of representation in chemistry learning has been well documented [
3], limited learning opportunities have been offered with all three levels to promote students’ understanding of the mechanism of redox reaction.
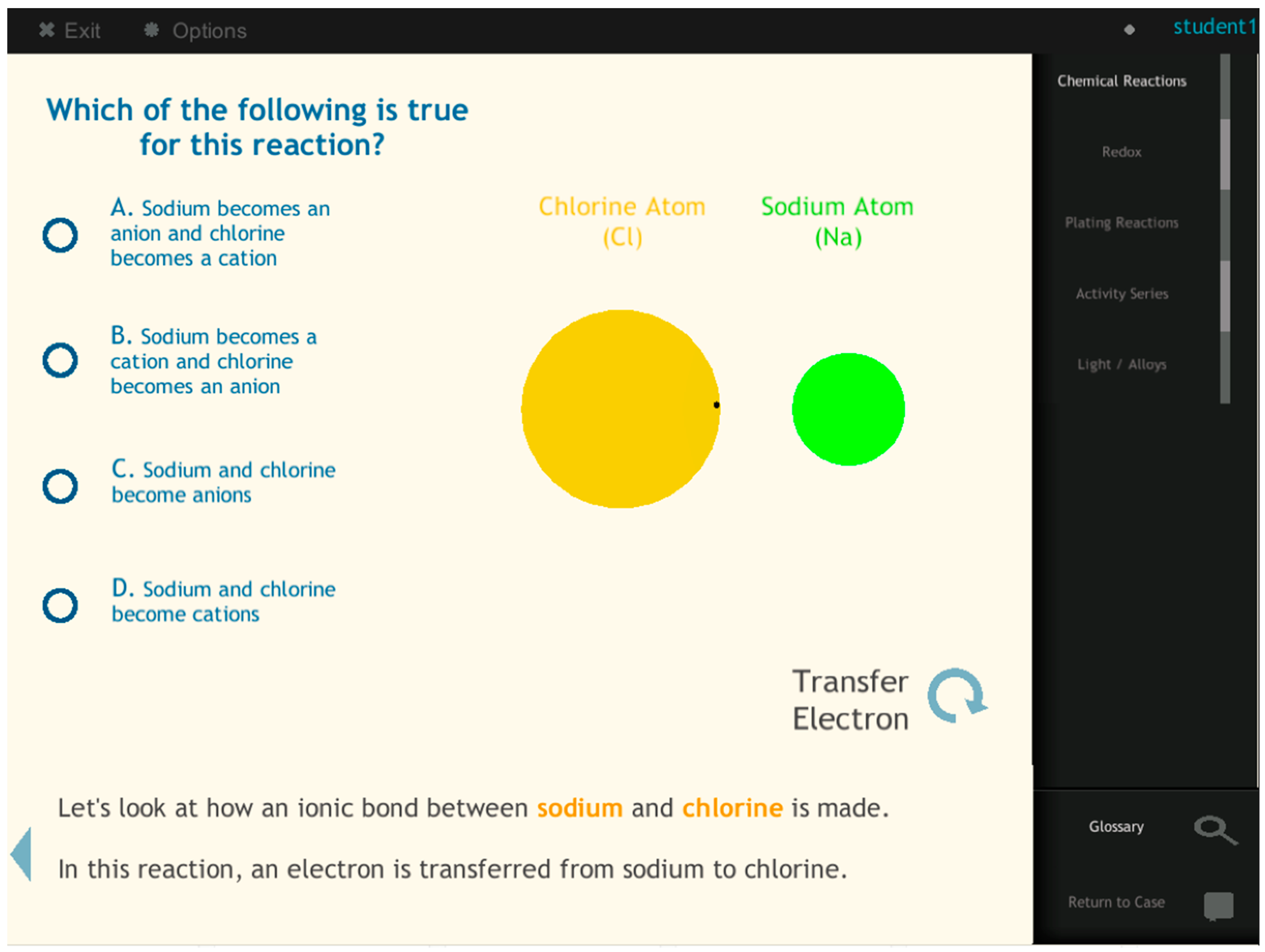
To bridge this gap, we designed a blended-reality immersive learning environment (BRE), integrating the macroscopic, molecular, and symbolic representations of redox reactions. The macroscopic representation is provided through a laboratory, which is commonly used by high school teachers in the state where this research was conducted. Students observe chemical phenomena such as color change within the lab. Redox equations and symbols are presented in an interactive serious educational game setting with immersive technologies. We integrated the molecular level of representation by including the electron transfer concept and hypothesized that integrating the electron transfer topic in BRE could effectively demonstrate the molecular level representation of a redox reaction. Compared to other models through which redox reactions are conceptualized, such as the oxygen model, whose title can be misleading because students might be inclined to believe that every redox reaction involves oxygen (as the name OX suggests), understanding the nature of redox reactions as electron transfer captures the mechanism of the reaction [
17,
18]. Facilitated by immersive technologies, the BRE demonstrates the dynamic interactions of particles through the concept of chemical bonds. Therefore, the molecular representation of a redox reaction is made visible. The BRE model seamlessly blends the hands-on laboratory experience with a serious educational game supported by immersive technologies.
The authors reported the effectiveness of the BRE model in supporting student learning of redox reaction in a different paper [
19]. The treatment-group students who experienced the BRE model significantly outperformed the control group. However, the previous study did not explore how the BRE model facilitated students’ understanding of the different concepts related to redox reactions, or whether the inclusion of the electron transfer drove the learning gains. The objectives of this manuscript are to investigate these two questions. Two research questions guided this inquiry:
Compared with the control group, how does the BRE model facilitate student learning of redox reactions?
Does including the electron transfer concept support student learning of redox reactions? If so, in what ways?
3. Results and Discussion
We began by assessing students’ overall performances on pre- and post-tests to investigate their learning of all three concepts integrated into the BRE model. We then analyzed the treatment and control groups of students’ learning for the three learning objectives respectively to explore the areas in which learning took place. To further understand whether electron transfer was the key topic that helped students grasp the concept of redox reactions, we analyzed BRE students’ performances on the embedded questions related to chemical bonds. The results of students’ misconceptions regarding redox reactions and interview data are also presented in the following sections.
3.1. Students’ Overall Performances on Pre- and Post-Tests
Students overall understanding of redox was measured through pre- and post-tests. We have reported the same data in a different paper [
19], but we will present the students’ overall performances on the pre- and post-tests again here to aid the subsequent discussions. The treatment (BRE) and control (BAU) groups of students’ levels of knowledge before participating this learning experience were similar (
Table 2). After the learning experience, the BRE group surpassed the BAU group, as their post-test scores increased by 14.8%.
Since students’ pre-test results were similar between the treatment and control groups, we could compare their learning gains. The gain scores were calculated by subtracting the pre-test scores from the post-test scores (i.e., gain scores = post-test total score − pre-test total score). A one-way analysis of variance (ANOVA) was conducted to evaluate the effects of treatment on students’ gain scores. The means and standard deviations for the gain scores are presented in
Table 3.
When the two groups were compared, the mean gain score of the BRE group (mean = 1.12) was larger than that of the BAU group (mean = −0.56). The results of ANOVA indicated a significant effect of treatment, F (1350) = 32.44, p < 0.05, ηp2 = 0.09.
3.2. Treatment Effects on Different Learning Objectives
Since we also included atomic structure (LO1) and chemical bonds (LO2) in the BRE model, the pre- and post-assessments contained items that were related to the two learning objectives (in addition to redox-related items in LO3). To understand whether the above significant difference occurred for the redox-related items, we divided the pre- and post-test items into three groups based on the different learning objectives and conducted three ANOVA analyses (
Table 4).
The ANOVA results revealed significant effects of treatment on LO2 and LO3, suggesting that the significant differences between the two groups were arising at the concepts of chemical bonds and redox reaction.
When we examined the curriculum, we found that both the topics—atomic structure (LO1) and chemical bonds (LO2)—were addressed before the teachers started the unit of redox reaction and that students learned the concept of atomic structure before learning about chemical bonds. As the two groups did not show any significant difference in performance on LO1, we asserted that the time when they were taught the concepts did not influence their performances. As we mentioned above, we included the concept of chemical bonds because we believed that it was an effective way to illustrate how electrons interacted with particles. Since the mechanism of redox reactions involves the movement of electrons, we hypothesized that including the topic of chemical bonds could benefit students in learning about redox. Considering that BRE students showed a significant difference in performance on both LO2 and LO3, we inferred that including the electron transfer concept benefited students in learning about redox.
Several studies have documented the difficulties encountered by students when they try to connect macroscopic representations with microscopic meaning, especially when explaining findings from lab observations and particle interactions [
2,
7,
8]. The inclusion of molecular representation—in this case, the illustration of electrons’ interactions with particles through chemical bonds in the BRE model—facilitates students in making connections to promote their learning of redox.
3.3. Students’ Performances on Embedded Questions of LO2
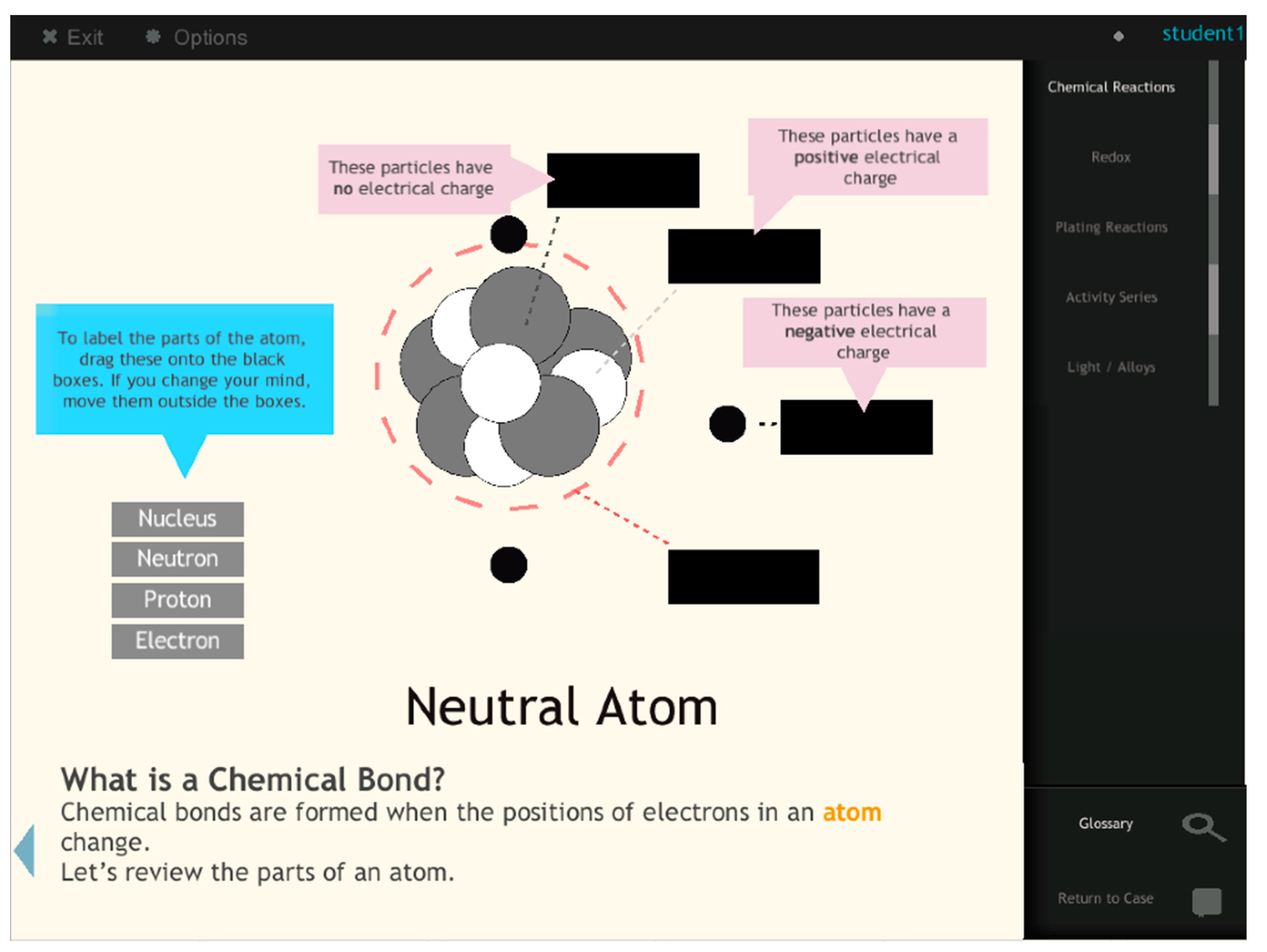
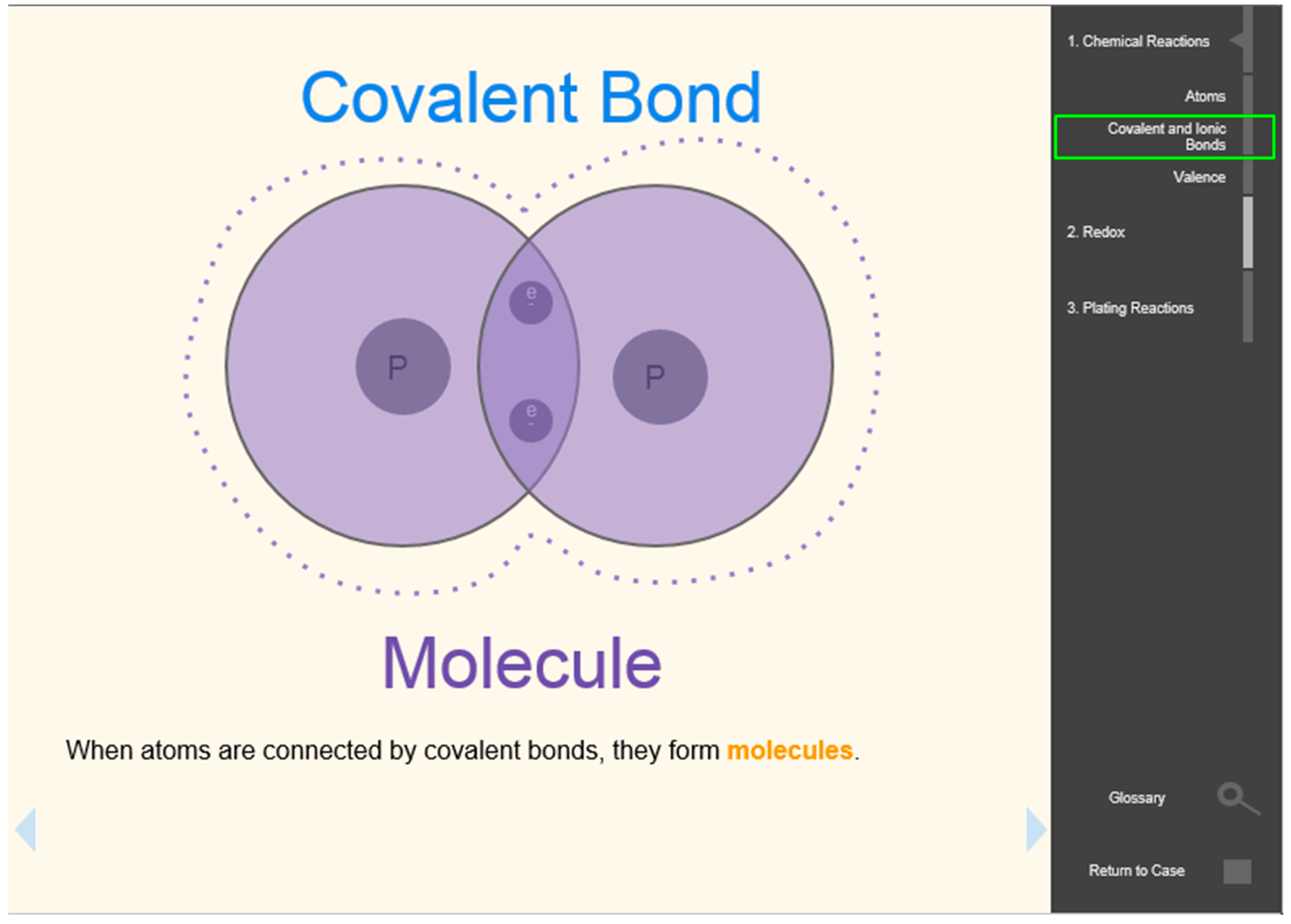
To understand how the BRE model might scaffold students’ learning of LO2 (chemical bonds), we examined students’ performances on three embedded questions (EQ3, EQ4, and EQ5) within the BRE, all of which measured students’ knowledge of chemical bonds. The concept assessed by each question and the proportion of students in the BRE group who answered the questions correctly are depicted in
Table 5. The depth of knowledge levels on chemical bonds increased from EQ3 to EQ5. The high percentages of correct answers in EQ3 and EQ4 indicate that BRE students gained a good understanding of how electrons interact with particles and applied this knowledge to the concept of chemical bonds. When triangulated with the pre- and post-test analysis results, these data confirmed that the BRE group’s better performance on LO3 was due to their knowledge of electron transfer. This also indicates that students need to understand electron movement among particles before mastering the concept of redox.
By incorporating the concept of electron interaction, which is a molecular representation, the BRE supported students in learning the topic of redox by building connections between the macroscopic and symbolic levels of representation. In other words, the addition of molecular representation helped students in connecting the color changes of the penny from the lab (macro representation) with the chemical equations representing what was happening in the lab (micro representation).
3.4. Comparison within LO3 (Redox Reaction)
Our BRE model contains four major concepts pertaining to redox reactions: associating the oxidation and reduction reactions with electron transfer, identifying the reduced and oxidized agents, identifying the direction of electron transfer, and utilizing the activity series to determine whether a redox reaction can occur [
8]. To further examine where the significant difference occurred within the concept of redox, we divided the items under the redox reaction learning objective into four clusters—LO3-1, LO3-2, LO3-3, and LO3-4—and employed ANOVA to investigate the effects of treatment (
Table 6).
When the performances between the BRE and BAU groups under the LO of a redox reaction were compared, we found significant differences arising from the learning objective of associating the oxidation and reduction reactions with electron transfer. These data confirm our above assertion that the inclusion of electron transfer facilitates students’ understanding of redox.
None of the other three LOs indicated significant differences. Since this study was conducted within the settings of introductory chemistry classes, it is possible that, although learning about redox reactions was appropriately supported, the additional goal of using the activity series might have been too ambitious for students to accomplish within one learning experience.
3.5. Students’ Common Misconceptions about Redox Reactions
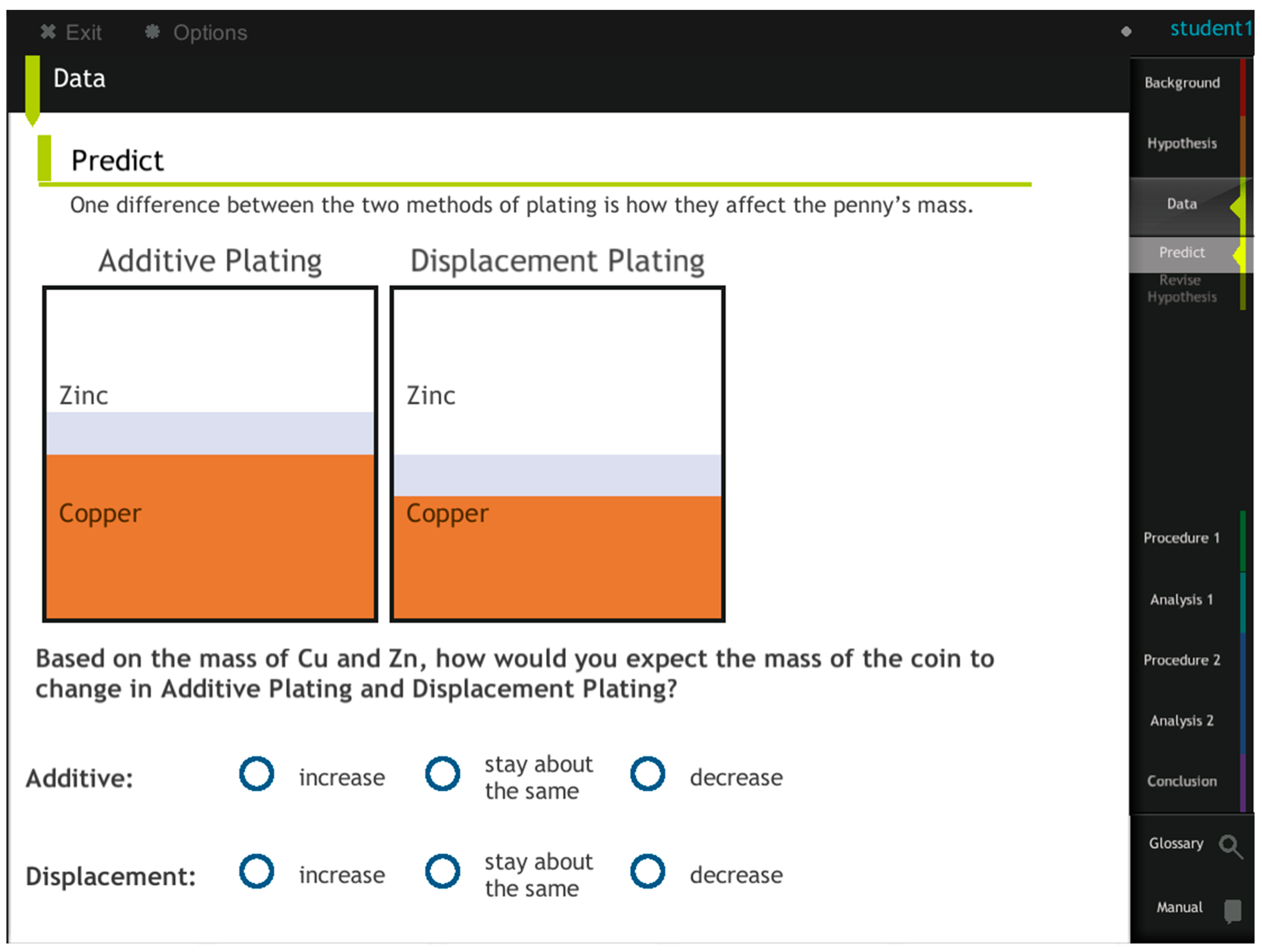
To further explore students’ learning difficulties related to the activity series, we examined students’ answers to one of the embedded questions (QE11). This embedded question asked students to hypothesize which type of chemical reaction caused the penny to turn silver, before they started the experiment. They were prompted to use the information they had just learned to support their hypotheses. Subsequently, after they developed their hypotheses, the BRE provided additional molecular and symbolic support to describe and explain the nature of the redox reaction that could not be obtained through the lab. In addition, after students obtained additional evidence, a hypothesis revision opportunity was offered. Their original and revised hypotheses and the evidence they used were graded to assess their levels of understanding.
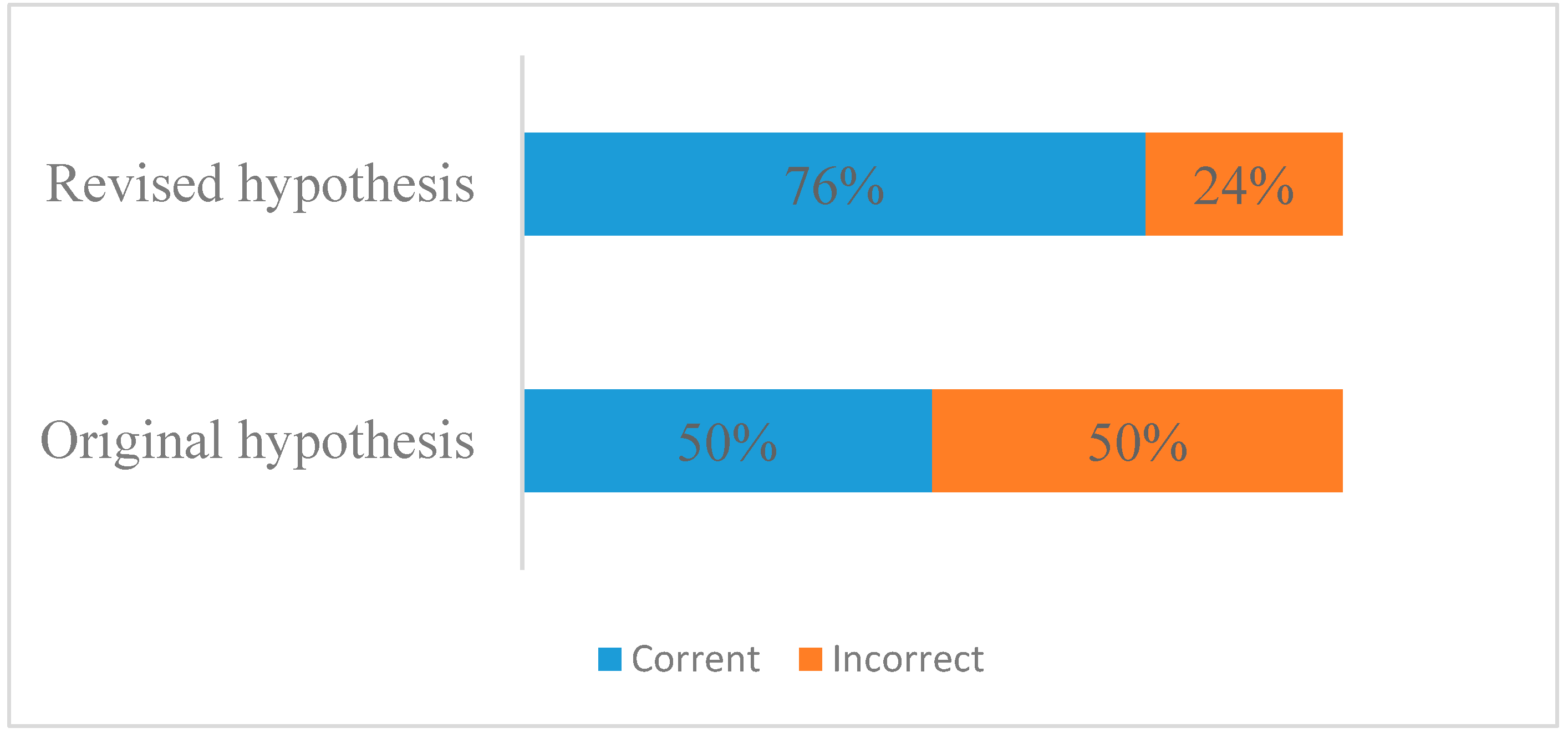
To answer QE11, students needed to provide two pieces of evidence: that the redox reaction could occur according to the activity series, and the mass changes of the penny signifying that the redox reaction took place. After examining students’ responses, we found that 76% of the students made the correct hypothesis before conducting the lab experiments, among which 55% used both to support their claims (
Figure 7). These data show that before conducting lab experiments, students’ learning from the immersive environment benefited their prediction.
To capture the misunderstanding students still had about activity series at this stage, we performed thematic analysis using the responses given by the students who inappropriately used the evidence. Three themes on students’ misconceptions about one of the major concepts within redox reactions emerged from the analysis.
Misconception 1: An atom and ion of the same metal can react to form a neutral metal atom because they occupy the same position in the activity series. The misunderstanding that ions will form neutral ions was found to be difficult to understand by other researchers [
15]. Below is an excerpt from one student in the BRE group:
“… In additive plating the zinc metal and zinc ions reacts which is allowed since they are in the same position on the activity series. Then the new neutral zinc atoms bond metallically with the copper atoms. If the criminlas (criminals) used displacement plating there would be no need for zinc ions.”
In this quote, the student understood that additive plating was involved. However, when providing justification, they pointed to the reaction between the zinc and zinc ion and believed that they could react because they were in the same position in the activity series. This indicates that the student had the fundamental knowledge about the redox reaction occurring in the system but misinterpreted the meaning and use of the activity series to predict what was taking place in the redox reaction.
Misconception 2: When a redox reaction occurs, a low-activity ion and high-activity atom combine to become neutral.
Misconception 3: A low-activity metal replaces a high-activity one.
These themes indicate that the students faced difficulties in understanding the meaning of activity series and its usage to decide whether and how a redox reaction could occur. This confirms the previous findings that the learning objective of the activity series was too ambitious for this group of students.
3.6. Interview Analysis
We have presented our findings of the interview analysis in detail in another paper [
19]. In this study, however, we will only list the themes that emerged from the interview data to illustrate teachers’ perceptions of engaging students in scientific practices within the BRE model. (1) A coherent explanation of scientific phenomena: The immersive environment enabled students to refer to information as needed in the midst of the experiment. This experience allowed students to build their knowledge and apply what they had learned simultaneously while performing experiments in the lab. (2) An authentic science inquiry: Both teachers who implemented the BRE learning environment shared that this model, instead of feeding new information, allowed students to discover things by themselves. (3) A real-time feedback function connects students and teacher in a meaningful way. The two teachers explained that this analysis system involved them as facilitators.
4. Conclusions
This study presents a model of teaching redox reactions to high school students. Based on the results, two conclusions can be drawn.
First, the BRE model promotes students’ learning of redox reactions by connecting macroscopic and symbolic levels of representation, with molecular representation being incorporated and clearly illustrated in the immersive learning environment. By integrating the molecular level of representation in the BRE environment, achieved by adding the chemical bonds concept, this model presents a coherent way of learning redox. Our data indicate that compared with the BAU group, the BRE students showed significant learning gains for both chemical bonds and redox reactions. By performing further analysis of students’ performances on pre- and post-tests and then triangulating these data with the embedded questions, we conclude that the molecular-level representation in the BRE model effectively facilitates student learning of redox by blending the traditional Gold Rush Lab and redox reaction equations and symbols.
Second, electron transfer is identified as a critical concept for students to understand the nature of redox reactions. This model incorporates the electron transfer concept by adding the topic of chemical bonds. The addition of electron transfer enables students to understand the mechanism of redox reactions: the movements of electrons among particles. The BRE emphasizes the dynamic nature of electron movement. This molecular representation helps students connect their observations of color changes in the penny to the chemical equations. These results imply that when teaching redox, teachers can focus on electron interactions to illustrate how redox occurs. In addition, for curriculum developers, redox should not be taught in isolation. Incorporating the concept of chemical bonds in the unit is an effective way of illustrating electron transfer and can help students understand the concept of redox.
The BRE also engages students in scientific practices. The learning experience begins with a real-world problem that students are tasked to solve. To solve the problem, fundamental chemical knowledge of redox is required. Students then build their own hypotheses, are provided materials to conduct laboratory experiments to test these hypotheses, and finally report their results. By following these processes, students learn in an authentic learning environment by investigating a real-world problem. Within this environment, students also experience how to use evidence to support their claims.
Our study is a pilot for future research for supplementing chemistry labs with immersive technologies to help students learn chemistry by providing all three levels of representation. This model converts lab instruction from students following directions into discovery experiences. Future studies can extend the implementation of the BRE model in additional lab experiences.
5. Limitations
One limitation of this study is that we did not collect as much data for the BAU group as we did for the treatment group. Both the BRE and BAU groups of teachers co-planned for the unit, but we did not observe the lab instruction of the BAU group. We neither interviewed teachers nor students about their learning experiences. As a result, learning construction process comparison is limited. Without these observation data, we are unsure whether the BAU teachers reviewed the concepts of both atomic structure and chemical bonds before their students began the lab experiments, whether the symbolic and molecular levels of representations were provided when the BAU students conducted the Gold Rush Lab, or whether formative assessments, which are comparable to the embedded questions in BRE, were administered to make sure students understood each step before proceeding to subsequent steps. We believe that with more data from the BAU group, we will be in a position to better understand the learning experiences of the BAU group and compare the learning construction process between the BRE and BAU groups.