The Influence of Problem Construction on Undergraduates’ Success with Stoichiometry Problems
Abstract
:1. Introduction
2. Methods
2.1. Research Questions
- How does the problem-construction intervention affect student’s performance with problem solving?
- How do high- and low- achieving students benefit from the implementation of problem-construction intervention? Do their performances with stoichiometry problems change differently?
2.2. Participants
2.3. Design and Instrument
2.4. Data Analysis
3. Results and Discussions
3.1. Examining the Effect of Problem Construction on Student’s Problem-Solving Performance
3.1.1. Evaluating the Changes in the Complete Success Rate (CSR) Scores
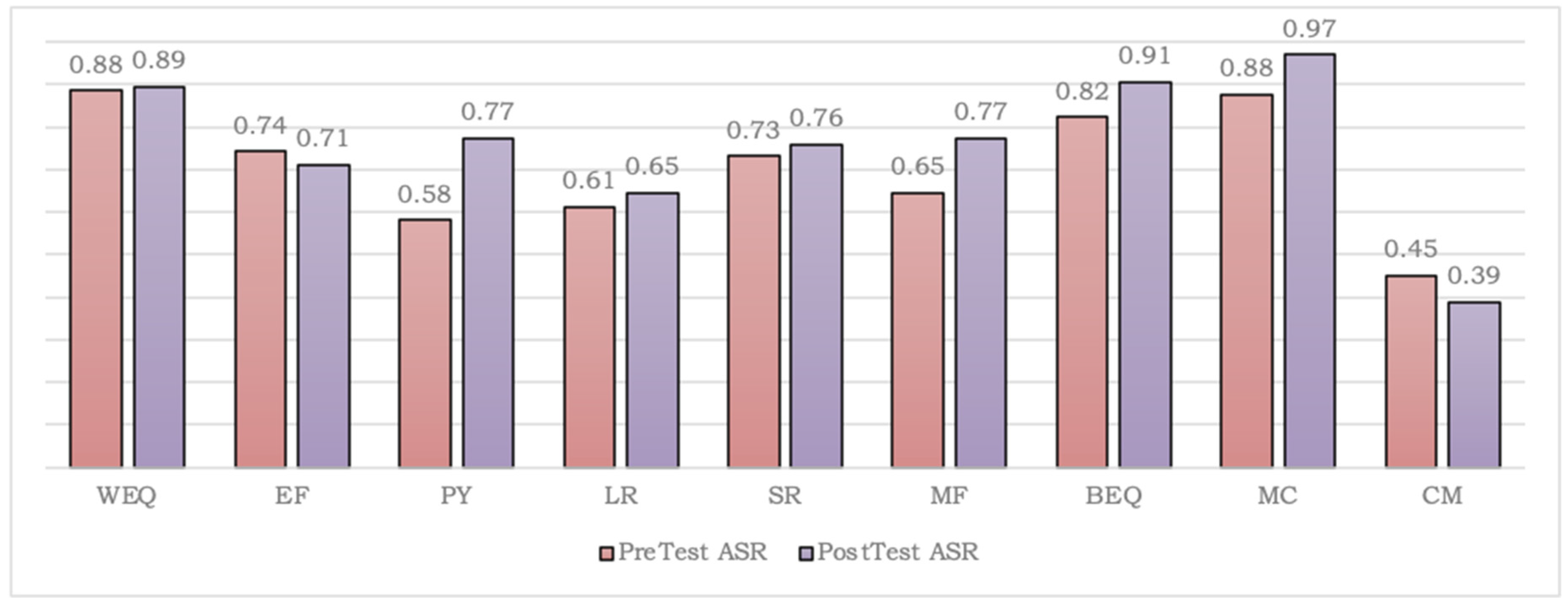
3.1.2. Interpreting the Variations in the Attempt Success Rate (ASR) Scores
3.1.3. Bringing Hidden Differences to Daylight: Inspecting the Changes in the Codes
3.2. Examining the Influence of the Intervention on High and Low Achieving Students
3.2.1. An In-Depth Analysis of Changes in CSR and ASR Scores
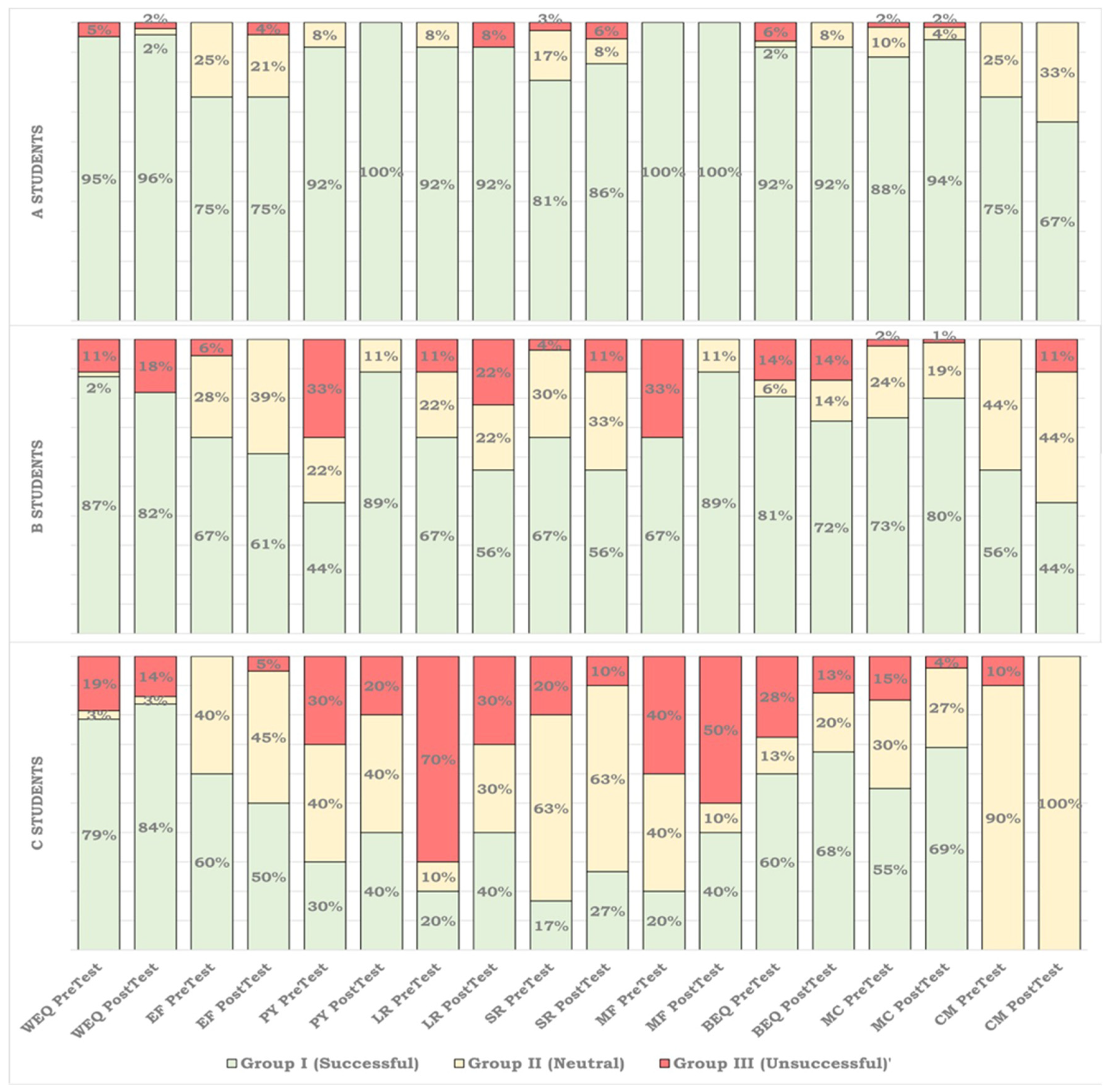
3.2.2. Looking into the Distribution of Unsuccessful, Neutral, and Successful Codes
4. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogilvie, C. Changes in students’ problem-solving strategies in a course that includes context-rich, multifaceted problems. Phys. Rev. Spec. Top. -Phys. Educ. Res. 2009, 5, 020102. [Google Scholar] [CrossRef] [Green Version]
- Ederer, P.; Nedelkoska, L.; Patt, A.; Castellazzi, S. What do employers pay for employees’ complex problem solving skills? Int. J. Lifelong Educ. 2015, 34, 430–447. [Google Scholar] [CrossRef]
- Frey, R.F.; Cahill, M.J.; McDaniel, M.A. Students’ Concept-Building Approaches: A Novel Predictor of Success in Chemistry Courses. J. Chem. Educ. 2017, 94, 1185. [Google Scholar] [CrossRef]
- Mikula, B.D.; Heckler, A.F. Framework and implementation for improving physics essential skills via computer-based practice: Vector math. Phys. Review. Phys. Educ. Res. 2017, 13, 010122. [Google Scholar] [CrossRef] [Green Version]
- Gulacar, O.; Eilks, I.; Bowman, C.R. Differences in General Cognitive Abilities and Domain-Specific Skills of Higher- and Lower-Achieving Students in Stoichiometry. J. Chem. Educ. 2014, 91, 961–968. [Google Scholar] [CrossRef]
- Sinapuelas, M.L.S. Why Do Some Students Struggle while others Succeed in Chemistry? A Study of the Influence of Undergraduate Student Beliefs, Perceptions, and Use of Resources on Performance in Introductory Chemistry. Ph.D. Thesis, University of California, Oakland, CA, USA, 2011. Available online: https://escholarship.org/uc/item/7nx2f1fx (accessed on 19 September 2022).
- Larson, L.; Stephen, A.; Bonitz, V.; Wu, T. Predicting Science Achievement in India: Role of Gender, Self-Efficacy, Interests, and Effort. J. Career Assess. 2014, 22, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Gulacar, O. Investigation of Successful and Unsucccessful Students’ Problem Solving in Stoichiometry. Ph.D. Thesis, Western Michigan University, Kalamazoo, MI, USA, 2007. Available online: https://scholarworks.wmich.edu/dissertations/870 (accessed on 11 November 2022).
- Gulacar, O.; Wu, A.; Prathikanti, V.H.; Vernoy, B.J.; Kim, H.; Bacha, T.A.; Oentoro, T.C.; Reedy, K.J.; Navarrete-Pleitez, M. Benefits of Desirable Difficulties: Comparing the Influence of Mixed Practice to that of Categorized Sets of Questions on Students’ Problem-Solving Performance in Chemistry. Chem. Educ. Res. Pract. 2022, 23, 422–435. [Google Scholar] [CrossRef]
- Gulacar, O.; Cox, C.; Fynewever, H. Deconstructing the problem-solving process: Beneath assigned points and beyond traditional assessment. In Problems and Problem Solving in Chemistry Education: Analysing Data, Looking for Patterns and Making Deductions; Tsaparlis, G., Ed.; The Royal Society of Chemistry: London, UK, 2021; pp. 68–92. [Google Scholar]
- Gulacar, O.; Cox, C.; Tribble, E.; Rothbart, N.; Cohen-Sandler, R. Investigation of the correlation between college students’ success with stoichiometry subproblems and metacognitive awareness. Can. J. Chem. 2020, 98, 676–682. [Google Scholar] [CrossRef]
- Gulacar, O.; Tan, A.; Cox, C.T.; Bloomquist, J.; Jimmy, O.; Cao, N. Analyzing Characteristics of Experts in the Context of Stoichiometric Problem-Solving. Educ. Sci. 2019, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Bopegedera, A.M.R.P. Preventing mole concepts and stoichiometry from becoming "gatekeepers" in first year chemistry courses. ACS Symp. Ser. 2019, 1330, 121–136. [Google Scholar]
- Chi, M.T.H.; Feltovich, P.J.; Glaser, R. Categorization and Representation of Physics Problems by Experts and Novices. Cogn. Sci. 1981, 5, 121–152. [Google Scholar] [CrossRef]
- Taasoobshirazi, G.; Glynn, S.M. College students solving chemistry problems: A theoretical model of expertise. J. Res. Sci. Teach. 2009, 46, 1070–1089. [Google Scholar] [CrossRef]
- Persky, A.M.; Robinson, J.D. Moving from Novice to Expertise and Its Implications for Instruction. Am. J. Pharm. Educ. 2017, 81, 6065. [Google Scholar] [CrossRef] [PubMed]
- Bransford, J.D.; Brown, A.L.; Cocking, R.R. How experts differ from novices. In How People Learn: Brain, Mind, Experience, and School; Bransford, J.D., Brown, A.L., Cocking, R.R., Eds.; National Academy Press: Washington, DC, USA, 1999; pp. 31–50. [Google Scholar]
- Day, E.L.; Tang, H.; Kendhammer, L.K.; Pienta, N.J. Sequence Analysis: Use of scanpath patterns for analysis of students’ problem-solving strategies. In Eye Tracking for the Chemistry Education Researcher; American Chemical Society: New York, NY, USA, 2018; Volume 1292, pp. 73–97. [Google Scholar]
- Mehta, J. Search of Deeper Learning: The Quest to Remake the American High School; Fine, S.M., Ed.; Harvard University Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Koretz, D.M. Measuring up What Educational Testing Really Tells Us; Harvard University Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Dixson, D.D.; Worrell, F.C. Formative and Summative Assessment in the Classroom. Theory Into Pract. 2016, 55, 153–159. [Google Scholar] [CrossRef]
- Smith, K.J.; Metz, P.A. Evaluating Student Understanding of Solution Chemistry through Microscopic Representations. J. Chem. Educ. 1996, 73, 233–235. [Google Scholar] [CrossRef]
- Gilbert, J. Chemical Education: Towards Research-Based Practice; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA, 2002. [Google Scholar]
- diSessa, A.A.; Gillespie, N.M.; Esterly, J.B. Coherence versus fragmentation in the development of the concept of force. Cogn. Sci. 2004, 28, 843–900. [Google Scholar] [CrossRef]
- Vosniadou, S. Capturing and modeling the process of conceptual change. Learn. Instr. Learn. Instr. 1994, 4, 45–69. [Google Scholar] [CrossRef]
- Lee, C.; Rooney, P.; Parada, R.H. Fostering intentional learning with systems dynamic modeling. Aust. J. Educ. 2014, 58, 89–103. [Google Scholar] [CrossRef]
- Chen, X.; Mitrovic, A.; Mathews, M. Learning From Worked Examples, Erroneous Examples, and Problem Solving: Toward Adaptive Selection of Learning Activities. IEEE Trans. Learn. Technol. 2020, 13, 135–149. [Google Scholar] [CrossRef]
- Angawi, R.F. Using a problem solving-cooperative learning approach to improve students’ skills for interpreting 1H NMR spectra of unknown compounds in an organic spectroscopy course. J. Chem. Educ. 2014, 91, 823–829. [Google Scholar] [CrossRef]
- Chin, C.; Brown, D.E. Student-generated questions: A meaningful aspect of learning in science. Int. J. Sci. Educ. 2002, 24, 521–549. [Google Scholar] [CrossRef]
- Gilbert, G.L. How do I get the answer? Problem solving in chemistry. J. Chem. Educ. 1980, 57, 79–81. [Google Scholar] [CrossRef]
- Scott, F.J. A simulated peer-assessment approach to improving student performance in chemical calculations. Chem. Educ. Res. Pract. 2014, 15, 568–575. [Google Scholar] [CrossRef]
- Hardy, J.; Bates, S.P.; Casey, M.M.; Galloway, K.W.; Galloway, R.K.; Kay, A.E.; Kirsop, P.; McQueen, H.A. Student-Generated Content: Enhancing learning through sharing multiple-choice questions. Int. J. Sci. Educ. 2014, 36, 2180–2194. [Google Scholar] [CrossRef]
- Botelho, M.; Lam, O.; Watt, R.; Leung, D.; Kember, D. Evaluation of peer-generated MCQ s to assess and support learning in a problem-based learning programme. Eur. J. Dent. Educ. 2018, 22, 358–363. [Google Scholar] [CrossRef]
- Arasasingham, R.D.; Taagepera, M.; Potter, F. Assessing the Effect of Web-Based Learning Tools on Student Understanding of Stoichiometry Using Knowledge Space Theory. J. Chem. Educ. 2005, 82, 1251–1262. [Google Scholar] [CrossRef]
- Okanlawon, A.E. Teaching reaction stoichiometry: Exploring and acknowledging Nigerian chemistry teachers’ pedagogical content knowledge. Cypriot J. Educ. Sci. 2010, 5, 107–129. [Google Scholar]
- Gulacar, O.; Overton, T.L.; Bowman, C.R.; Fynewever, H. A novel code system for revealing sources of students’ difficulties with stoichiometry. Chem. Educ. Res. Pract. 2013, 14, 507–515. [Google Scholar] [CrossRef]
- Krippendorff, K. Reliability in Content Analysis: Some Common Misconceptions and Recommendations. Hum. Commun. Res. 2004, 30, 411–433. [Google Scholar] [CrossRef]
- Agung, S.; Schwartz, M.S. Students’ Understanding of Conservation of Matter, Stoichiometry and Balancing Equations in Indonesia. Int. J. Sci. Educ. 2007, 29, 1679–1702. [Google Scholar] [CrossRef]
- Ault, A. How to Say How Much: Amounts and Stoichiometry. J. Chem. Educ. 2001, 78, 1347. [Google Scholar] [CrossRef]
- Chandrasegaran, A.L.; Treagust, D.F.; Waldrip, B.G.; Chandrasegaran, A. Students’ Dilemmas in Reaction Stoichiometry Problem Solving: Deducing the Limiting Reagent in Chemical Reactions. Chem. Educ. Res. Pract. 2009, 10, 14–23. [Google Scholar] [CrossRef]
- Ralph, V.R.; Lewis, S.E. Chemistry topics posing incommensurate difficulty to students with low math aptitude scores. Chem. Educ. Res. Pract. 2018, 19, 867–884. [Google Scholar] [CrossRef]
- Bapu Ramesh, V.; Selvam, A.A.A.; Kulkarni, S.; Dattatreya Manganahalli, A.; Bettadapur, K.R. Designing and Using an Atomic Model Kit with H, C, N, and O Model Atoms Having a Mass Ratio of 1:12:14:16 to Teach the Concept of Mole and Associated Stoichiometric Relationships. J. Chem. Educ. 2020, 97, 986–991. [Google Scholar] [CrossRef]
- Chonkaew, P.; Sukhummek, B.; Faikhamta, C. STEM Activities in Determining Stoichiometric Mole Ratios for Secondary-School Chemistry Teaching. J. Chem. Educ. 2019, 96, 1182–1186. [Google Scholar] [CrossRef]
- Diab, S.; Sartawi, B. Classification of Questions and Learning Outcome Statements (LOS) Into Blooms Taxonomy (BT) By Similarity Measurements Towards Extracting Of Learning Outcome from Learning Material. Int. J. Manag. Inf. Technol. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Chandran, S.; Treagust, D.F.; Tobin, K.G. The role of cognitive factors in chemistry achievement. J. Res. Sci. Teach. 1987, 24, 145–160. [Google Scholar] [CrossRef]
- Guthrie, J.M. Proportional Reasoning in the Solution of Problems in High School Chemistry and Its Impact on Developing Critical Thinking Skills; ERIC: Washington, DC, USA, 1991. [Google Scholar]
- Kilner, W.C. The Chem-Math Project: Enhancing Success in General Chemistry through the Integration of Mathematics, Problem-Solving and Conceptual Understanding. An Action-Research Study. Ph.D. Thesis, University of New Hampshire, Durham, NH, USA, 2014. [Google Scholar]
- Darby, K.P.; Sloutsky, V.M. The Cost of Learning: Interference Effects in Memory Development. J. Exp. Psychology. Gen. 2015, 144, 410–431. [Google Scholar] [CrossRef]
- Simonsmeier, B.A.; Mayer, A.-K.; Rosman, T.; Gorges, J.; Flaig, M.; Schneider, M. Conceptual change and knowledge integration as learning processes in higher education: A latent transition analysis. Learn. Individ. Differ. 2018, 62, 49–61. [Google Scholar]
- Gulacar, O.; Sinan, O.; Bowman, C.; Yildirim, Y. Exploring the Changes in Students’ Understanding of the Scientific Method Using Word Associations. Res. Sci. Educ. 2015, 45, 717–726. [Google Scholar] [CrossRef]
- DiSessa, A.A. A History of Conceptual Change Research: Threads and Fault Lines; Cambridge University Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Jeon, K.; Huffman, D.; Noh, T. The Effects of Thinking Aloud Pair Problem Solving on High School Students’ Chemistry Problem-Solving Performance and Verbal Interactions. J. Chem. Educ. 2005, 82, 1558. [Google Scholar]
- Clair-Thompson, H.S.; Overton, T.; Bugler, M. Mental capacity and working memory in chemistry: Algorithmic versus open-ended problem solving. Chem. Educ. Res. Pract. 2012, 13, 484–489. [Google Scholar] [CrossRef]
- Fayol, M.; Abdi, H.; Gombert, J.-E. Arithmetic Problems Formulation and Working Memory Load. Cogn. Instr. 1987, 4, 187–202. [Google Scholar] [CrossRef]
- Wagner, E.P. A Study Comparing the Efficacy of a Mole Ratio Flow Chart to Dimensional Analysis for Teaching Reaction Stoichiometry. Sch. Sci. Math. 2001, 101, 10–22. [Google Scholar] [CrossRef]
- Giuliodori, M.J.; Lujan, H.L.; DiCarlo, S.E. Collaborative group testing benefits high- and low-performing students. Adv. Physiol. Educ. 2008, 32, 274–278. [Google Scholar] [CrossRef]
- Gulacar, O.; Fynewever, H. A Research Methodology for Studying What Makes Some Problems Difficult to Solve. Int. J. Sci. Educ. 2010, 32, 2167–2184. [Google Scholar] [CrossRef]
- Romine, W.L.; Todd, A.N.; Clark, T.B. How Do Undergraduate Students Conceptualize Acid-Base Chemistry? Measurement of a Concept Progression. Sci. Educ. 2016, 100, 1150–1183. [Google Scholar] [CrossRef]
- Kousathana, M.; Demerouti, M.; Tsaparlis, G. Instructional Misconceptions in Acid-Base Equilibria: An Analysis from a History and Philosophy of Science Perspective. Sci. Educ. 2005, 14, 173–193. [Google Scholar] [CrossRef]
- Rodriguez, J.-M.G.; Bain, K.; Hux, N.P.; Towns, M.H. Productive features of problem solving in chemical kinetics: More than just algorithmic manipulation of variables. Chem. Educ. Res. Pract. 2019, 20, 175–186. [Google Scholar] [CrossRef]
- Marzabal, A.; Delgado, V.; Moreira, P.; Barrientos, L.; Moreno, J. Pedagogical Content Knowledge of Chemical Kinetics: Experiment Selection Criteria To Address Students’ Intuitive Conceptions. J. Chem. Educ. 2018, 95, 1245–1249. [Google Scholar] [CrossRef]

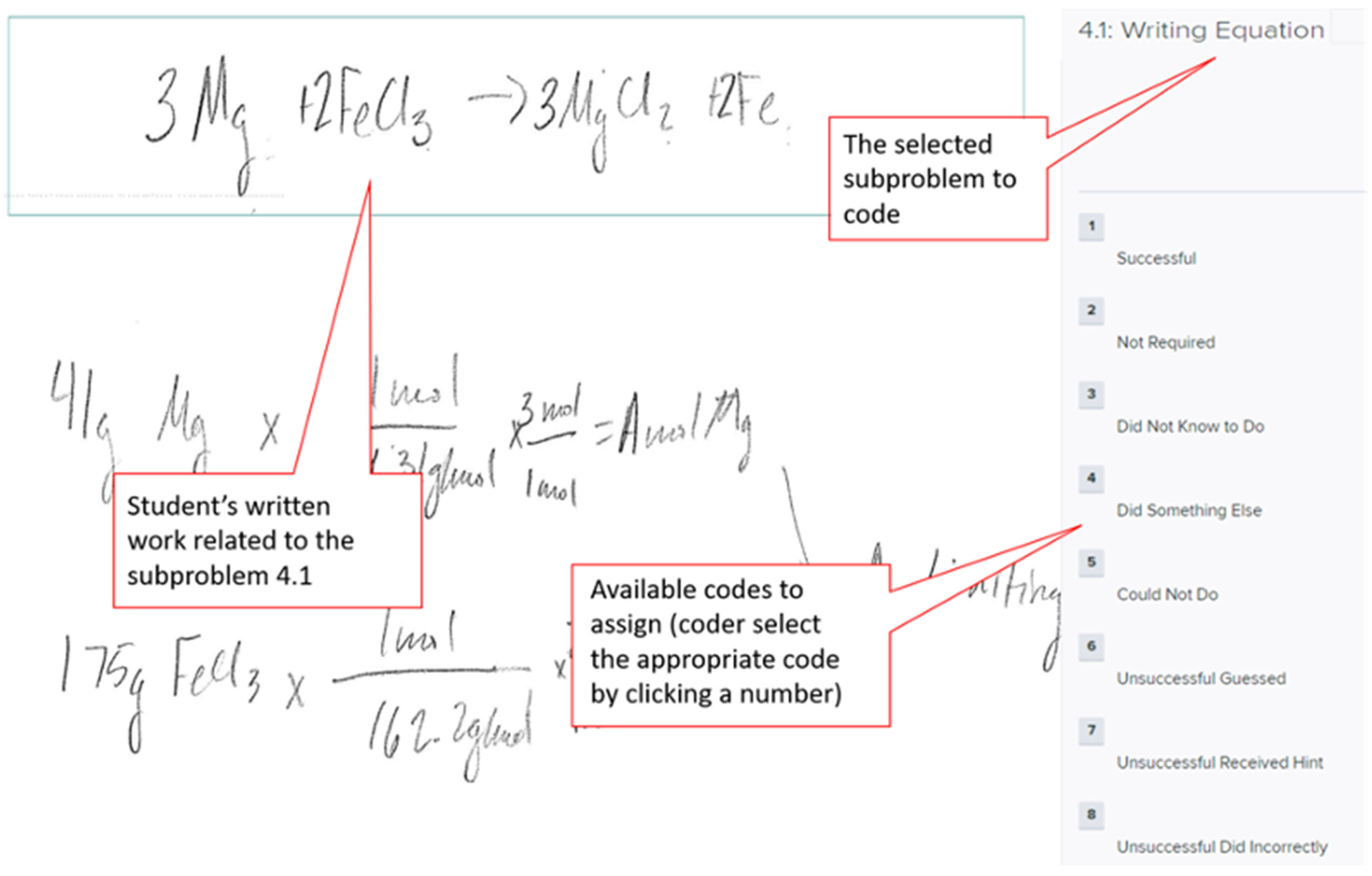


| Codes | Subtopic | Students’ Thinking Process | Student Work ** |
|---|---|---|---|
| S | MC (Finding Moles of Carbon) | In this solution, the participant correctly identifies that he needs to use stoichiometry to go from grams to moles. The participant says, “I am going to convert everything I see here into moles. So, 40 g of carbon, I am going to use stoichiometry. Carbon is 12.011 g and then that equals to 1 mole. I am going to take 40 and divide that by 12.01 to get 3.33 moles of Carbon.” | 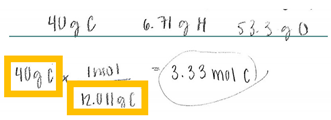 |
| DD | LR (Identifying Limiting Reactant) | The participant states he does not know how to do this problem and he also does not realize that he needed to calculate the limiting reactant using the two compounds. He thinks both would equally contribute the overall mass of N2. He states, “I started by writing down the two compounds given […] the question says it wants you to find the grams of N2 gas […] and now I am trying to find the grams of Nitrogen in this compound, so the 100 g is the total mass of N2H4 and I am trying to find Nitrogen [from it]. I am dividing the mass of nitrogen by the total mass to get the percentage of the mass of nitrogen in N2H4”. “I don’t know how to do this, but with my math knowledge it makes sense to say that there is the same amount of nitrogen in both of them [the two compounds].” | 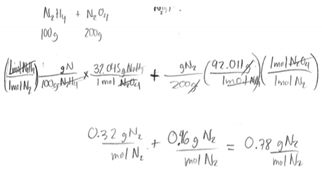 |
| DSE | EF (Empirical Formula Ratio) | The participant multiplies the mole ratios by a common multiple to change the fraction into a whole number. His reasoning for manipulating the mole quantities is not the way specified in the traditional method. In doing so, he does not realize that he is changing the empirical formula he calculated. As they state, “carbon turned out to be 2.67 moles, we know that that is equal to two and two thirds, so we multiply by three to get rid of the fraction. So, you have the formula should be C8H12O12.” |  |
| Codes | Subtopic | Students’ Thinking Process | Student Work ** |
|---|---|---|---|
| CD | MC (Finding Moles of N2H4) | The participant clearly recognizes that this is a limiting reactant problem by stating “limiting reactant” when comprehending the question. She says she needs to “figure out which is the limiting” however she has a hard time recalling the exact steps. It is observed that she knows what needs to be done and as she mentions how there was a “proper procedure” however it “does not stick in my head.” | 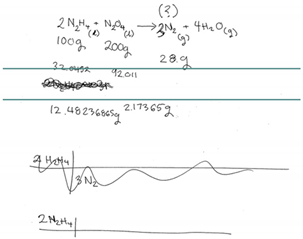 |
| UG | PY (Calculating Percent Yield) | In this subproblem, the participant clearly guesses. He states, “I don’t know, I’m just gonna say 100% yield”. |  |
| URH | WEQ (Writing the Equation) | The participant is struggling to write the formula for strontium halide and the interviewer actually provides it. The interviewer states, “But I’ll just help you a little bit. […] the charge of strontium is plus two so in the halide it is a negative one so it’ll be a SrX2.” |  |
| UDI | MC (Finding Moles of Carbon) | The participant does the incorrect stoichiometric calculation to go from grams to moles carbon. Instead of dividing the grams of carbon they state that “I have to multiply the mass by the percentage and then add them up to see if it makes the total mass”. | 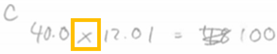 |
| Acronym | Stoichiometry Topics | Acronym | COSINE CODES and Formulas |
|---|---|---|---|
| BEQ | Balancing Chemical Equation | S | Successful |
| WEQ | Writing Chemical Equation | UG | Unsuccessful Guessed |
| MC | Mole Concept | UDI | Unsuccessful Did Incorrectly |
| SR | Stoichiometric Ratio | URH | Unsuccessful Received Hint |
| PY | Percent Yield | CD | Could not Do |
| CM | Conservation of Mass | DSE | Did Something Else |
| EF | Empirical Formula | DD | Did not know to Do |
| MF | Molecular Formula | NR | Not Required |
| LR | Limiting Reagent | ASR | Attempt Success Rate |
| CSR | Complete Success Rate | ||
| Experimental (N = 31) | Control (N = 7) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Min | Max | M | SD | Min | Max | |
| ∆ CSR | 0.04 | 0.12 | −0.17 | 0.44 | −0.07 | 0.08 | −0.19 | 0.00 |
| A Students (N = 12) | B Students (N = 9) | C Students (N = 10) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Min | Max | M | SD | Min | Max | M | SD | Min | Max | |
| ∆ CSR | 0.01 | 0.06 | −0.14 | 0.10 | 0.01 | 0.15 | −0.17 | 0.26 | 0.09 | 0.14 | −0.09 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulacar, O.; Mann, H.K.; Mann, S.S.; Vernoy, B.J. The Influence of Problem Construction on Undergraduates’ Success with Stoichiometry Problems. Educ. Sci. 2022, 12, 867. https://doi.org/10.3390/educsci12120867
Gulacar O, Mann HK, Mann SS, Vernoy BJ. The Influence of Problem Construction on Undergraduates’ Success with Stoichiometry Problems. Education Sciences. 2022; 12(12):867. https://doi.org/10.3390/educsci12120867
Chicago/Turabian StyleGulacar, Ozcan, Harjeet Kaur Mann, Sukhdev Singh Mann, and Brandon James Vernoy. 2022. "The Influence of Problem Construction on Undergraduates’ Success with Stoichiometry Problems" Education Sciences 12, no. 12: 867. https://doi.org/10.3390/educsci12120867
APA StyleGulacar, O., Mann, H. K., Mann, S. S., & Vernoy, B. J. (2022). The Influence of Problem Construction on Undergraduates’ Success with Stoichiometry Problems. Education Sciences, 12(12), 867. https://doi.org/10.3390/educsci12120867







