1. Introduction
The transfer of courses into the digital space by means of asynchronous or synchronous lectures and seminars leads to a strong restriction regarding immediate feedback and communication between students and teachers [
1,
2]. In addition to this limitation, another form of teaching becomes difficult to implement in the digital space, and that is practical/lab courses with educational experiments [
3]. This paper addresses the challenge of conducting a teaching course in the digital space with motivating (hands-on) experiments and achieving student participation by means of audience response systems. The basic question is whether students are able to overlook the demonstrative aspect and perceive the course as a practical experience owing to activating elements (with expectation queries, suggestions for the selection of parameters and the execution of experiments, and peer evaluation) or whether they perceive the video transmission as a distancing barrier.
The initial motivation for the paper was to establish an experimental lecture with interactive elements in the digital space. Digital experiments shall be understood in this context as demonstrated experiments, i.e., performed by an operator, which are (only) accessible to the students viewing it by means of digital broadcast technology. In the specific context of this publication, the term also includes the direct feedback opportunity for students during the synchronous experiment broadcasting through audience response elements.
In order to illustrate the implementation of digital experiments, an extensive collection of experiments from the field of materials science, in particular dental materials, was integrated into the experimental lecture. This second subject-specific part, which is also a component of this publication, concentrates on suitable experiments, which should be easy to implement and, after adaptation, transferable to other subject areas. In addition, the results and discussion suggestions should stimulate imitation and further development of the experiments. Of course, the significance for dentistry and dental material application or examination is also important.
In general, it should be noted that in the course of the digitization of higher education, many lecture forms have been tested and discussed in the literature, with a focus on didactic methods (e.g., polls, group work, teacherbots, artcasting, inverted/flipped classrooms, tweeting book projects, etc.) [
4,
5,
6], technical tools (e.g., Zoom, Prezi, pre-recorded video lectures, etc.) [
7,
8,
9], and social-economic impact (neoliberal transformation of education) [
10]. However, the transfer of experiments into the digital space seems to be underrepresented, which is probably due to the fact that the original self-learning effect is missing and there is a high risk for demonstration only. In order to avoid this, the activation of students before and after the experiments with expectation queries and explanatory discussions is described in detail in this paper.
Audience response systems are an important approach to motivate and engage students when used correctly [
11]. This was found by K. C. Good, who additionally stated that the quantity and quality of different question structures by audience response systems (clickers) influenced student perceptions and supported student learning in presence lectures and might be transferred to digital lectures with ease. Furthermore, her review on the students’ perspective supports the association of audience response systems with terms like “enjoyable” and “fun,” with reduced desire to skip class, and the students desired to remain anonymous [
11]. A more systematic model of the student experiences of using audience response systems was given by Wood and Shirazi, pointing out that students connect the themes of engagement, interaction, anonymity, questioning and discussion, instant feedback and student learning, and technological benefits and limitations to those systems. In order to not carry out the audience response methods for their own sake, K.C. Good as well as Kay and LaSage emphasized the importance of explaining the pedagogical goal [
11,
12]. They described that students generally respond positively to audience response systems, but often express the fear of feeling uncomfortable when responding incorrectly, particularly when a majority of the class responds correctly. This aspect should be taken into account in the use of audience response systems by not focusing the questions in a public (albeit anonymous) knowledge check, but rather on the subject-specific co-creativity of the students and a co-creation of a bigger picture out of individual contributions.
The challenge, as such, is to set an incentive for a particular lecture that goes beyond module grades and assessments. In this particular case, it is the field of dental materials, which represent an economically significant but nevertheless very special subgroup in the field of biomaterials, which in turn are located in the far more comprehensive part of materials science. One might think that the fact that we all come into contact with dentists and the materials they use sooner or later should already generate intrinsic motivation. However, the existing threshold between dentist and patient, which is reflected, e.g., in the language (characterized by Latin technical terms as well as uncommon orientation terms), seems problematic here. Furthermore, it must be urgently emphasized that the subject matter of dental materials is very different from the actual medical processes and background. Thus, starting from the natural model, i.e., the tooth and periodontium, the predominantly artificial materials for the treatment of dental defects are discussed on the basis of their chemical and physical properties. Thus, material selection, mechanical characteristics, testing of biocompatibility with the various tissues of the mouth, corrosion effects and types, and degradation and wear processes are in the focus of this subject area and only indirectly provide a reference to specific treatment procedures.
This dichotomy between the expectations of students from the field of materials science and the actual teaching content gave rise to the idea of illustrating materials science issues through experiments. Unfortunately, it is an approach that cannot be transferred into the digital teaching environment with ease. This leads to the question of how an experimental lecture can be structured and which methods and tools it needs in order to give students the feeling of being able to participate in the execution of the lecture—in other words, the question of how to remove the demonstration barrier and how students accept the methods in the digital space. Therefore, first the question arose of how the experiments, which are time-consuming and may not be feasible in a face-to-face lecture format, can be prepared for a continuous broadcast in a synchronous digital lecture and be performed live by the lecturer without making significant cuts that would impair comprehensibility. In this context, the technical aspects of experiment broadcast, time slots for their introduction, and interpretation were queried as the subject of the evaluation.
Secondly, the question arose of which methods and tools to activate students in partaking in the lecture (e.g., by suggesting experimental parameters or speculating on the outcome of experiments) are regarded by students as a suitable way to interact with the lecturer and influence the experiments. The general assessment of experiment selection and implementation was also included in this set of questions in order to obtain feedback on the quality of knowledge transfer.
The result is presented below, with six selected experiments that are more or less logically coordinated and build on each other, as well as selected results on audience response systems integrated and the overall evaluation by students, giving evidence on the perception of the experimental lecture. In addition to materials science motivation, the transfer of the teaching content and the experiment-supported activation in the digital space are presented. Here, it was possible to rely on experiences from teaching with blended learning [
13].
2. Materials and Methods
First, the process of an experimental lecture in the digital space is described with a focus on technical equipment and execution, student activation, and audience response. Afterwards, the lecture boundary conditions are explained as well as the evaluation questionnaire for students. The latter is intended to provide an insight into how and whether the transfer of an experimental lecture to the digital space was successful from the student perspective.
Second, subject-related experiments from the field of dental materials are explained. All selected experiments were in general planned for a face-to-face lecture with students and could be performed by the students themselves without major laboratory equipment. With high school and basic scientific knowledge, it should be possible to derive a hypothesis for the respective expectations of the experiments. Furthermore, it should be possible for the students as a group to give an explanation for the expectations of the experiment’s outcome.
2.1. Workflow of an Experimental Lecture in the Digital Space
Technical Equipment
For the synchronous lecture, Zoom (version 5.4.6, San José, CA, USA) was used as a digital communication platform. In principle, alternative systems (e.g., BigBlueButton, MS Teams, WebEx, or similar) that allow sharing a camera image and at the same time sharing a presentation or PC screen with the students were also possible. It was also advantageous to have a system that allows breakout sessions.
The computer used was a Microsoft Surface Pro 7 (Intel
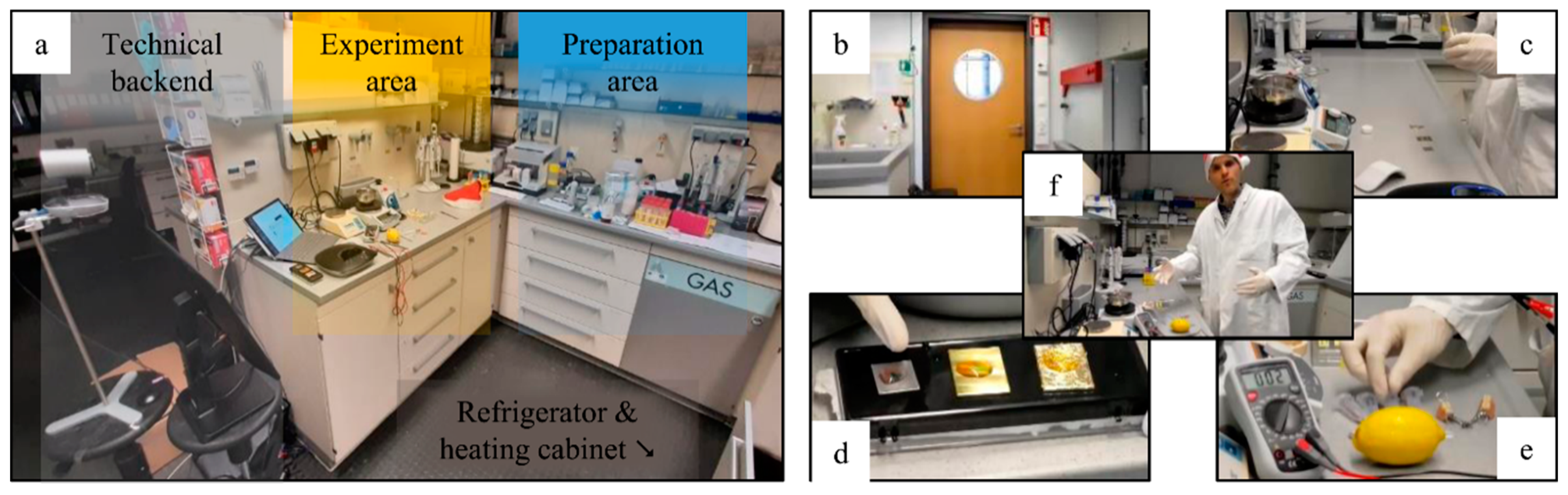
® CoreTM i5-1035G4 CPU @ 1.10 GHz, 1498 MHz, 4 cores, 64-bit, 16 GB RAM; Microsoft, Albuquerque, NM, USA) with Microsoft Windows 10 Education. The presentation was created using Microsoft Office Professional Plus 2016 (64 bit)—PowerPoint. A USB-RJ45 adapter and 1000 Gbit network connection was used for stable internet connection. For easier presentation, a second screen was connected to the computer and the presentation was run in speaker mode during the online lecture. The setup in the lab was divided into the technical backend, the experimentation area, and the preparation area (
Figure 1a).
For flexible video camera control and high freedom of movement, the plug-and-play video conferencing system Logitech Group (Logitech, Lausanne, Switzerland) connected via USB with two extension microphones was used. The system allows pre-saving up to 5 camera positions with up to 10× optical zoom. These positions can be selected by means of a remote control with only one keystroke and are thus automatically moved. The system offers HD video resolution and focuses automatically. The following camera positions were selected and saved in advance (
Figure 1b–e): 2 close-ups (1× for the work area (e), 1× for a storage area of a longer-lasting experiment (d)), a semi-close-up of the experimental area (c), a wide shot of the laboratory working area (f), and a wide shot of the laboratory area with refrigerator and heat cabinet (b).
In order to obtain parameters for the experiments and test procedures to be selected from the students themselves, two online blackboards, namely, padlets (
www.padlet.com, 14 December 2020), were created in advance of the experimental lecture to allow students to give direct feedback or suggestions for the experiments. Furthermore, one padlet was used for the expectation query during another experiment. The structure of the respective padlet pages is explained in more detail in the corresponding experiments. Students could access the padlets via QR code (on PowerPoint slides) or hyperlink (posted in a Zoom chat) at the corresponding time. Furthermore, audience response was obtained through the voting function of Zoom as well as via direct questions to the auditorium and direct requests to speak after unmuting the respective students. Following the lecture, an evaluation was conducted with the online platform for academic teaching and learning used by TU-Dresden (
https://bildungsportal.sachsen.de/opal, 14 December 2020).
2.2. Study Design
A lecture from the Materials Science course at Technische Universität Dresden served as the experimental unit. The lecture on dental materials, including a seminar, is an obligatory part of the course and is scheduled for the 9th semester of diploma studies or the 5th semester of the bachelor’s degree. During the experimental lecture 14 students (and 3 co-workers) were present as participants of a Zoom meeting the whole time. This corresponded to a proportion of 54% of the students registered at the beginning of the semester, of which 2 were enrolled in mechanical engineering, 1 was enrolled in dental medicine, and the rest were enrolled in materials science. The final exams were taken by 18 students. From this point of view 78% of the students’ partaking in the exams visited the experimental lecture at the halfway point of the semester.
A midterm and a final evaluation of the lecture series were submitted anonymously by 7 students. The broadcasted experimental lecture was evaluated by a separate questionnaire, which was answered anonymously by 6 students in the period right after the end of the event and the following 2 weeks. The questions asked allowed free text (FT) answers or grade assignments (GA) according to the German school grading system: very good, good, satisfactory, sufficient, poor, insufficient. The following questions were asked in the evaluation:
Please rate the technical implementation of the experimental lecture in the digital space. (GA)
Which comments do you have on the technical implementation? (FT)
Rate the activation of students (padlet, chat, surveys, feedback via microphone, etc.). (GA)
What did you particularly like about the experimental lecture? (FT)
What did you not like about the experimental lecture? (FT)
How would you rate the selection of experiments in general? (GA)
How would you rate the relationship of the experiments to the respective explanation of the fundamentals? (GA)
What comments do you have on individual experiments? (GA)
The evaluation results were analyzed by the OPAL internal analysis tool, which calculates mean values from the grade assignments and visualizes the number of students and their responses for each question. Emphasis was put on the verbal feedback of the students in order to assess the subjective effect of the experimental lecture as a substitute for a real self-learning experience.
2.3. Subject-Related Experiments from the Field of Dental Materials
In the following, the subject-related experiments from the field of dental materials are explained with a detailed description of the procedure.
2.3.1. Experiment I—Bye, Bye Teeth—Teeth in Non-Aggressive and Aggressive Liquids
Materials
Animal teeth (especially suitable incisors from buffalo with a length of 4–6.5 cm; purchased from
www.animalskeletons.de 14 September 2020, Gladbeck, Germany)
50 mL reaction tubes with screw cap
Reference liquids and solutions:
- o
ddH2O (arium® pro, Satroius Lab Instruments GmbH & Co KG, Göttingen, Germany)
- o
Ethanol (VWR Chemicals, Darmstadt, Germany)
- o
Artificial saliva (according to DIN EN ISO 10993-15 [
14])
- o
Simulated body fluid (mSBF according to Kokubo et al. [
15])
(Presumably) aggressive solutions:
- o
1 M HCl (Sigma-Aldrich, St. Luis, MO, USA)
- o
1 M NaOH (Roth, Karlsruhe, Germany)
- o
1 M Citric acid (Sigma-Aldrich, St. Luis, MO, USA)
- o
Coca Cola ® (The Coca-Cola Company, Atlanta, GA, USA)
Fine balance (+/− 10 mg; YA102, OHAUS Coop., Parsippany, NJ, USA)
pH-indicator strips (MQuant, non-bleeding, pH 0–14; Merck KGaA, Darmstadt, Germany)
Photo documentation, ruler
Optional:
- o
Hardness meter (Shore D LX-D digital durometer, gainexpress, To Kwa Wan, Hong Kong)
Procedure and Duration
To carry out the experiment, it is essential to start storing the teeth a few weeks (in the present case for 6 weeks) before the final evaluation. It is suitable, for example, to use this experiment as a prelude to the lecture series for motivation and to carry out the final measurements in the middle or at the end of the semester.
In face-to-face lectures, it is suitable to let the students carry out the measurements of the initial mass of the teeth and pH values of the solutions themselves. The optional hardness measurement at various points (enamel or dentin) can also be carried out by the students themselves using a portable hardness tester. In addition, it is suitable to have the students make a photo documentation.
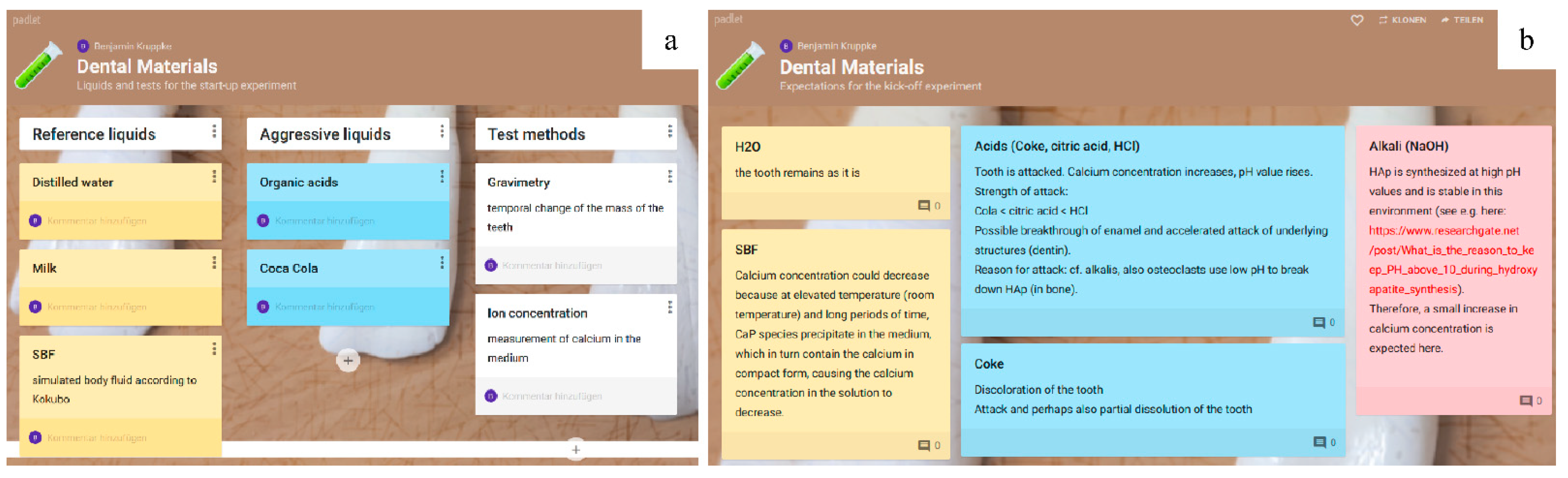
Prior to the experiment, a padlet can be used to query suitable examination methods and parameters with which the effects of the liquids on teeth can be observed. In addition, the query of fluids that serve as reference and fluids that are expected to cause a visible or measurable change can be carried out via a padlet or other web-based survey software.
The opening experiment is documented, e.g., by video, or might be part of a synchronous lecture as well. All teeth are weighed and photographed from the front and back before being incubated in the liquid. During the course of the semester, documentation by means of photographs is advisable.
The evaluation requires approx. 3–5 days before the synchronous experimental lecture to remove the teeth with tweezers from the liquids in order to dry them to mass constancy. This is when masses of the dried teeth after incubation can be determined in the live lecture. Photo documentation and optional hardness measurements can be performed with ease live as well. The pH values of the solutions after removal of the teeth can also be quickly analyzed. If these values are entered into an Excel spreadsheet that is integrated into the PowerPoint presentation, a comparison of the change in mass, pH, and hardness can be made immediately.
Here it is advisable to let the students formulate the evaluation or hypotheses for the outcome of the experiment themselves and let them discuss as a group first. Depending on the desired depth of explanation, the background for the results can be explained subsequently in one or two PowerPoint slides according to the previous discussion.
2.3.2. Experiment II—Brush or Away—Protective Toothpaste
Materials
Egg (from a grocery store)
Pencil and screw-cap vessel in which the egg fits
Toothpaste (fluoride containing; e.g., Mara expert Fluorid Gelee (containing Natriumfluorid (12,300 ppm F-; Hager & Werken GmbH & CoKG, Duisburg, Germany)
Acetic acid (vinegar essence 25% acid from a grocery store) or a concentrated acetic acid (≥99%; Sigma-Aldrich, St. Louis, MO, USA) diluted to 5 v/v-%
Procedure and Duration
First, a line is drawn with a pencil along the smaller circumference of the egg’s circumference. Even after the experiment, when the eggshell is (partially) dissolved, the two halves (treated and untreated) should still be identifiable. Then one half of the egg, in this case the low (rounder) part, is covered with the fluoride-containing toothpaste (fluoride gel). The egg is stored for 24 h this way. In case of drying out, the toothpaste can be replaced from time to time. The toothpaste is rinsed off carefully under running water so that no toothpaste remains. The egg is placed afterwards in a vessel and completely covered with 5
v/
v-% acetic acid [
16]. The most visually attractive part is from 5 min to 1 h after starting incubation. The experiment may proceed with up to 24 h of storing the egg in acetic acid. The live lecture may be used to start incubation or may take place when the egg is removed from the acid. In both cases the condition of the shell at the toothpaste-treated and untreated sites can be discussed together with the students and the translation to the teeth can be deduced.
As an add-on, it is possible to use an egg whose shell has been completely dissolved (about 48 h storage in acetic acid) and clean it with water and dry it with a tissue. Subsequently, a drop of fluoride toothpaste (transparent fluoride gels are most suitable) is placed locally on the egg skin and stored for another 24–48 h. To prevent drying, the gel drop can be replaced from time to time. Finally, the gel is rinsed thoroughly. Re-mineralization can be discussed in a following lecture, for example.
2.3.3. Experiment III—Very Impressive—Dental Impression and Model Materials
Materials
Screw-cap vessels with approx. 150 mL capacity
Teeth for taking impressions (particularly suitable: equine molars approx. 4 × 4 × 2 cm
3, purchased from
www.animalskeletons.de 14 September 2020, Gladbeck, Germany).
Alginate impression material (algistar classic, Müller-Omicron GmbH & Co. KG, Lindlar, Germany)
Two-component silicone (Silon 25 ABT, Schuhmann-Silikone.de, Dresden, Germany)
Commercially available plaster (gypsum) and wax (candle wax) from retail stores
Tap water (no ddH2O, otherwise the setting reactions are delayed)
A crucible and a heating plate (Hei-Tec, Heidolph, Schwabach, Germany) for liquefying the wax
Optional:
- o
A heating cabinet to accelerate silicone crosslinking
- o
A refrigerator to accelerate wax hardening
- o
NaCl (Merck KGaA, Darmstadt, Germany) to accelerate the plaster-setting reaction
Procedure and Duration
First, the alginate impression material is mixed with water according to the instructions (in the present case: 2 spoons of water (40 g) were added to 2 spoons of powder (19 g)). It is important to pay attention to the exact time when the teeth are pressed in so that the silicone and the alginate are not yet too solid. A short phase of curing must then take place (for alginate approx. 1 min). The tooth can be removed once the impression material is cross-linked. Now the impressions, i.e., the negatives, can be filled with a model material to produce a positive of the tooth. For this purpose, the candle wax is heated to approx. 70 °C on a hotplate and poured into the solidified impression material. The candle wax solidifies according to the model size within approx. 15 min. This process can be accelerated by storing it in a refrigerator. The two-component silicone is mixed in the ratio of components of A:B = 10:1. The time of silicone polymerization is approx. 30 min and can be significantly reduced by storing in a heating cabinet at approx. 40–65 °C. The plaster is mixed with tap water according to the manufacturer’s instructions and poured into the alginate impression mold. The setting reaction takes approx. 60 min and can be accelerated by adding table salt to the tap water.
The wax, silicone, and plaster models can then be removed from the alginate impression material and compared with the tooth used for the impression. It is also useful to store the alginate impression at ambient conditions (for a few weeks) to illustrate the shrinkage owing to the loss of liquid.
2.3.4. Experiment IV—When the Spark Jumps Over—Galvanic Elements
Materials
Lemon (from a grocery store)
Metal samples
- o
Aluminum plate or foil, zinc nail (or galvano-zinc-plated steel nail), steel nail, brass nail, (all available at a hardware store)
- o
Titanium dental implant (may be obtained as a sample from a manufacturer)
- o
Partial denture with stainless steel framework (may be obtained as a sample from a manufacturer)
Multimeter (20–2000 mV, Voltcraft VC 130, Conrad, Hirschau, Germany)
Procedure and Duration
In advance of the experiment, the students can use a padlet (timeline) to create a hypothesis about the order of the different materials from base to noble. The joint editing option in the padlet allows corrections to be made or comments to be added (e.g., on different states of the materials, such as aluminum with and without a passive layer). Subsequently, the potentials of the metals are measured in relation to each other. Here, care must be taken to ensure a high-impedance voltage measurement, since any reactions that occur can falsify the potential measurement. The potential measurement is best started with the least noble material at the negative pole of the multimeter and any other metal at the positive pole. Keeping the least noble metal at the negative pole, all further potentials are indicated as positive values and a practical galvanic series can be easily determined. The students can add the measured values directly in the padlet for the individual materials as comments and correct the order if necessary. Subsequently, the reactions occurring at the anode and cathode as well as the sequence of the materials can be discussed. Special attention can be drawn to any deviations from the electrochemical voltage series under standard conditions.
2.3.5. Experiment V—No Need for Contact—Evans Drop
Procedure and Duration
The solution should be prepared fresh (on the day of the experiment). To do this, 3 g NaCl, 0.1 g K
3[Fe(CN)
6]
rotes Blutlaugensalz and 1 drop of phenolphthalein is added to 100 mL distilled water. The solution is pipetted onto the plate specimens as a larger drop in each case (drop diameter approx. 10–15 mm) [
17]. Subsequently, the color change of the pH indicator, the color change of the potassium hexacyanoferrate(III) to form the Prussian blue complex, and the rust ring formation can be observed over a few minutes. The result can be seen particularly clearly after about 60 min and can be discussed together with the students via a live discussion and an explanatory PowerPoint slide. Of particular interest here is the observation of the type of corrosion in contrast to experiment IV.
2.3.6. Experiment VI—Give Me Candy. Give Me Cake. Give Me Something Sour to Make!—Bacteria as Acid Manufacturers
Materials
Sterile device for tacking a saliva sample
15 mL reaction vessels with screw cap (6×)
SSL broth for the cultivation of acid-tolerant microorganisms (e.g., lactic acid bacteria, acetic acid bacteria, yeasts, and molds from soft drinks) (Döhler, Darmstadt, Germany)
Glucose (Sigma-Aldrich, St. Louis, MO, USA)
Optional:
- o
A cookie or other sugar-containing cake (finely crushed)
Procedure and Duration
The experiment is performed with slight changes to a TV broadcasted experiment [
18] in accordance with the bacterial broth user manual [
19]. It requires a preparation of about 3 days before the actual evaluation. First, the SSL broth is divided among six reaction vessels. Then, a saliva sample is added to 3 of the 6 solutions. Subsequently, the samples are stored in a heating cabinet at 25–29 °C for the proliferation of bacteria from the oral cavity for 48 h. Afterwards, about 1 g of glucose and 1 g of ground cookie are added to one broth with and without saliva sample, respectively, and stored in the heating cabinet for an additional 24 h. During the experimental lecture, the saliva sampling part and the addition of the sugar-containing materials can be shown, and then the prepared samples are used for evaluation. Finally, the pH value of all six liquids is determined with the pH measuring strips and the result is discussed with the students. Here, too, an explanatory PowerPoint slide can be used for the sugar reactions that occur in the presence of lactic acid bacteria.
3. Results
3.1. Workflow of an Experimental Lecture in the Digital Space, Students’ Participation, and Students’ Perception
The carrying out of the course from the laboratory did not cause any major difficulties, but actually allowed a slightly more flexible delivery than would have been possible with students in an auditorium. The lecture was carried out according to the scheduled time schedule. The camera positions stored in advance were selected with the remote control according to the area of interest (preparation, experiment, detailed observation, explanation by lecturer). In addition to the continuously broadcast video image, a screen was shared on which either the PowerPoint presentation with a short teaser for each experiment or a slide for explanation was shown, or the current status of padlets in a web browser was presented. The padlets were used to allow students to work together in one document, flow chart, or sketch board. This way it was always possible for the students to follow both the lecturer and the experiment and to follow the slides or to see the padlet as an audience response tool.
Since the students were not made aware in advance that a survey would be conducted after the event, participation was not biased. It should be noted, however, that some experiments had already begun before the day of the experimental lecture and/or suggestions for carrying them out had already been requested, which might have built up a certain degree of suspense.
The question of the perception of the digital experimental lecture (as a demonstration lecture or as an activating practical course without physical experience) was investigated by means of a questionnaire. The evaluation survey among the students (using OPAL) showed that the technical implementation of the experimental lecture in the digital space was rated as very good by 67% of the participants and as good by 33% of the evaluation participants. As a remark, the use of QR codes and additional classical hyperlinks was pointed out, because in one case the QR codes were not readable with a smartphone.
Furthermore, the technical methods for activating the students and broadcasting the lecture were evaluated as follows: “The technical implementation was really super, also with the panning camera and the pre-programmed settings,” “It was really incredible how well the switching between cameras worked—chapeau. Internet was also stable,“ and ”I found everything well solved. Nobody can do anything about the stability of the internet connection.” In response to the free-text questions about what the students liked or did not like about the experimental lecture, they responded as can be seen in
Table 1. No answers were given to the question of what advice the students had about individual experiments.
The selection of experiments and the ratio of experiments to explanations were also rated as very good by 50% of the students, as good but with too much explanation by 33.3%, and as good but with too many experiments by 16.7% of the students. The evaluation of the participation of the students by means of padlet, chat, surveys, and feedback by microphone was rated by 50% of the students as very good and 50% as good.
In the final teaching evaluation, four of the seven participating students explicitly referred to the experimental lecture, with three citing the experimental lecture as a particular strength of the course. Here, reference was made to the aspect of the experiments as a combination of video and live events, the built-up nature, and the integration into the theoretical teaching context. Furthermore, a detailed feedback on the experimental lecture was given, which highlighted the transfer of the practical teaching unit into a digital demonstration lecture:
“Another big positive point is the implementation of the demonstration experiments. Again, you have managed to convert the demonstration experiments into a very enjoyable digital format where we can learn a lot and at the same time not get bored because it is just fun to watch them being performed. Yes, it’s a shame not to do our own experiments, but this version is almost as good as it would usually be.”
In addition, from colleagues partaking as listeners, there was a short assessment from experienced teachers on the Chair of Biomaterials of TU Dresden, which was shortened and translated as follows:
“This is how a lecture should be. You structured the experiments very well and conveyed the learning content in a sympathetic manner. The ratio of experiments and explanations was optimal. It was easy to follow and I think that the students will remember it for a long time. It was also great to see what is technically possible! These various pre-programmed camera settings actually make an assistant obsolete, but also shows how well you planned and thought through the entire process in advance.”
3.2. Subject-Related Experiments from the Field of Dental Materials
In the following, the results of the experiments are presented very briefly. In many cases, similar setups and results can be found on the internet, which can be adapted in many ways. Thus, setups can be chosen that provide results that can be used as incentives and motivational questions for many disciplines. In the following, the focus was on the field of scientific material characterization and biomedical properties of teeth and dental materials.
3.2.1. Experiment I—Bye, Bye Teeth—Teeth in Non-Agressive and Aggressive Liquids
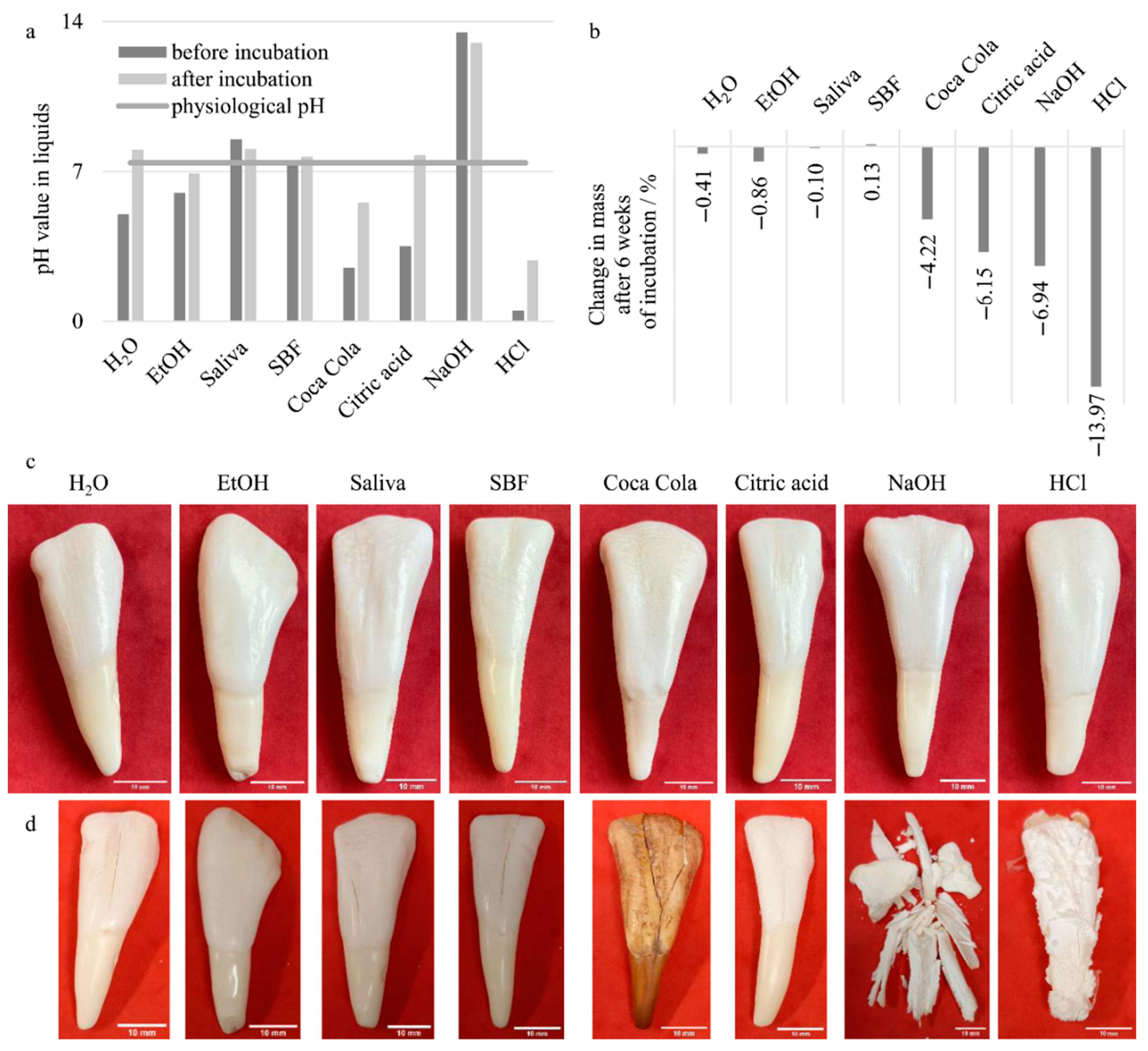
In the case of the four selected reference liquids, there was hardly any effect on the pH before and after incubation or on the change in mass (
Figure 2a,b). It is worth noting that storage in modified SBF led to an increase of the mass, while in all other cases the dry mass after incubation was lower than the initial mass of the teeth. In the photographs of the teeth, hardly any changes were visible to the naked eye (
Figure 2c,d). The only noticeable change was that after drying (following the incubation) the teeth showed increased cracks in the enamel (
Figure 2d).
Incubation of the teeth in the aggressive liquids showed mass decreases of 4 to 14 wt.%, whereby the pH values, which were quite low in the case of the acids, were clearly raised as a consequence of the incubation. They even reached the range of the physiological pH value of saliva of approx. 7.1 [
20] in the case of citric acid. The strong changes in the masses were also reflected in the photographs of the teeth (
Figure 2d). In the case of Coca Cola
®, no erosion of the enamel or dentin was visible to the naked eye, but the discoloration was very marked and could not be removed by rinsing simply with water. With citric acid and HCl, the destruction of the enamel was very evident, and with HCl, a coarse, very solid crystalline phase was formed on the surface. Particularly striking was the severe destruction of the tooth by incubation in NaOH (
Figure 2d). This was already apparent after 1 week of incubation and finally led to complete destruction, which started from the dentin, as the internal part of the tooth.
3.2.2. Experiment II—Brush or Away—Protective Toothpaste
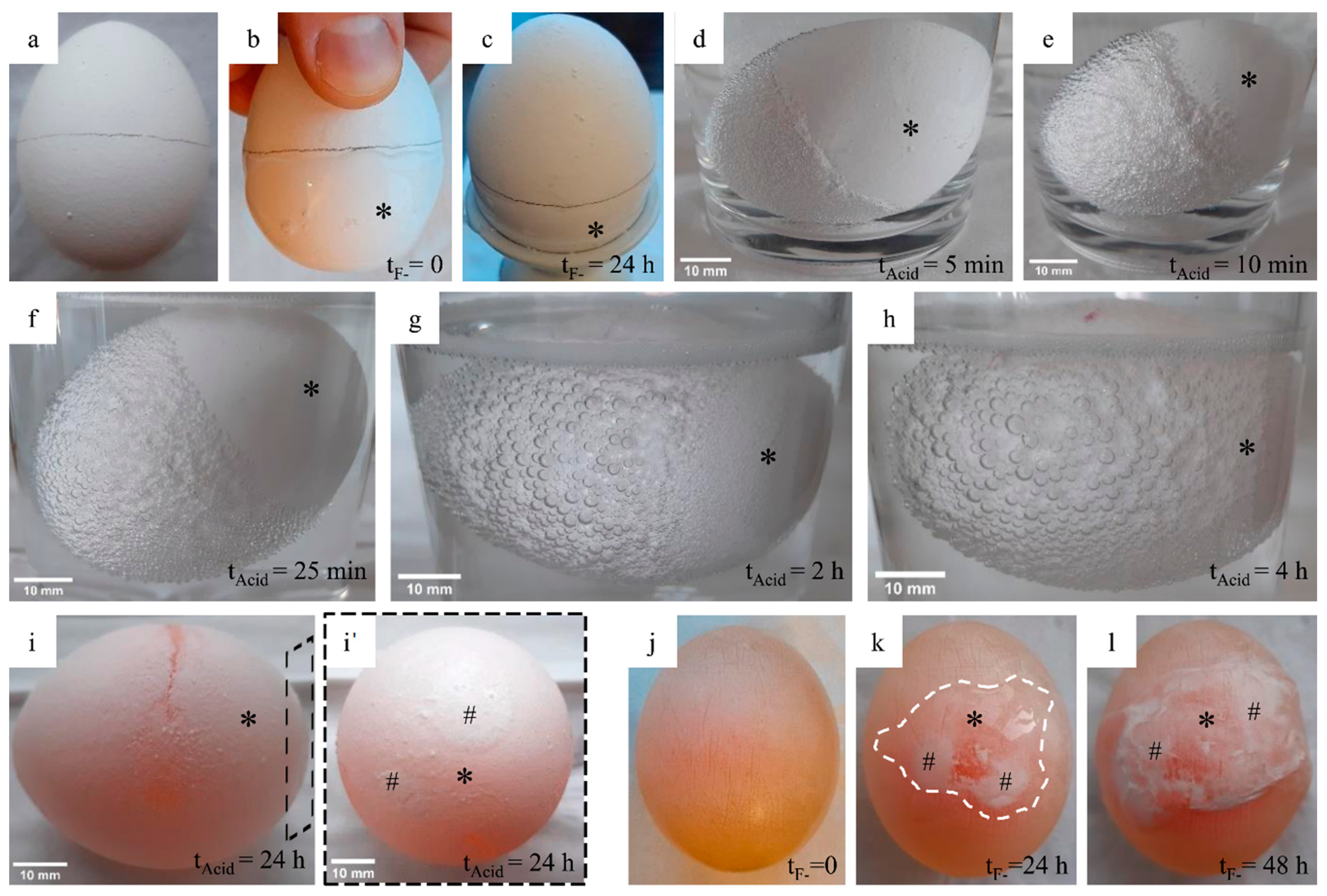
Treatment of an egg with fluoride gel did not cause any change in the eggshell visible to the naked eye (
Figure 3a–c). After rinsing off the gel, it was not possible to distinguish between the two sides or to determine the exact border (except for the drawn line). When the egg was placed in the acetic acid, gas bubbles could quickly be seen on the surface of the egg, which were very clearly delineated over the first 25–30 min and thus only visible on the untreated side (
Figure 3d–f). With increasing incubation time, the gas bubble formation became more and more visible also on the treated side (
Figure 3g,h). After removal from storage in acetic acid and rinsing as well as careful drying, only two small areas could be seen (and sensed) on the treated side, where the mineral phase of the shell was still present (
Figure 3i,i’). The rest of the egg was very soft and could be deformed with great elasticity.
An extension of the experiment was prepared by further storage of the egg in acetic acid (for about 48 h in total). The mineral of the eggshell was dissolved completely and the egg was more translucent. After applying a drop of fluoride gel, a re-mineralization could be seen over a period of about 48 h (
Figure 3k,l). This mineral also led to a clearly noticeable local hardening of the egg membrane.
3.2.3. Experiment III—Very Impressive—Dental Impression and Model Materials
The preparation of the impression material was subject to a strict time sequence of only a few minutes, which could be easily observed from the color changes of the alginate-based impression material (described in the instruction manual). The great elasticity of the alginate after gel formation allowed the teeth to be demolded even if undercuts were present to a certain extent (
Figure 4a,a’,a”). In this case, loss of the mold might be expected if the model material was to be demolded without damage. Alginate setting took place in a period of about 5–10 min, which provided an opportunity for explaining the sol-gel transition during the alginate setting. Subsequently to the demolding of the teeth from the alginate negative, the model materials were prepared and filled in the negatives. All three model materials—silicone (
Figure 4b,b’), plaster (
Figure 4c,c’), and wax (
Figure 4d,d’) required a period of about 15–60 min to cure. This time was used to explain the chemical reactions (plaster curing, addition polymerization of silicone) and physical processes (phase transition of the wax). In the case of plaster a significant cooling of the alginate mold was reported to the students. When the wax cured, a shrinkage at the filling position of the alginate mold was clearly visible. It is advisable to carry out other experiments and subsequently perform the demolding of the silicone, wax, and plaster model from the alginate (
Figure 4b”–d”).
An important circumstance for model extraction was derived from a freshly prepared and a dried alginate negative (
Figure 4e,e’) in comparison with the originally molded tooth (
Figure 4e”). Here it can be seen that immediate model preparation from the alginate mold without exposure to elevated temperatures was necessary, since the drying of the alginate mold led to considerable shrinkage and no dimensionally accurate model could be prepared.
3.2.4. Experiment IV—When the Spark Jumps Over—Galvanic Elements
When measuring the potentials between the different metals and alloys, care needed to be taken to achieve a constant value. The aggressive citric acid could have cause corrosion reactions, which in turn could result in distortions (
Figure 5a). Therefore, the measurements needed to be carried out immediately. The measured values could be seen directly by the students in the selected video stream (
Figure 5b,c) The sequence of metals chosen by the students was initially aluminum—zinc—steel—brass—titanium—stainless steel. The first measurement already showed that the lowest potential under the selected conditions was present in zinc, which the students were able to change themselves in the padlet to the correct galvanic order (
Figure 5d), allowing the lecturer to continue the experiment.
3.2.5. Experiment V—No Need for Contact—Evans Drop
The metal plates without surface treatment and with gold sputtering were very smooth at the beginning, whereas the gold leaf could not be applied as an ideal single layer and thus showed some warping (
Figure 6a–c). Immediately after placement of the drops (
Figure 6d), the liquid was transparent and the surface of the metal plates was clearly visible. The process of multicolored discoloration of the solution and the metal surface, respectively, was immediately visible to the students in the detailed image (
Figure 6d’). In this figure it can be seen, and it should be noted, that the inclined underground for the alignment to the camera was disadvantageous, because the typical ring structure of the reaction products was not formed. With the naked eye it could be seen already after a few minutes and particularly clearly after about 1 h that three areas marked in color appeared. On the one hand, there was an outer pink-violet area at the drop edge or where the drop was very flat, a ring-shaped reddish area, and a dark blue center. These three areas could be seen in varying degrees on all three metals (
Figure 6a’–d’). In particular, the blue region in the center was very evident on the gold-coated metal samples.
3.2.6. Experiment VI—Give Me Candy. Give Me Cake. Give Me Something Sour to Make!—Bacteria as Acid Manufacturers
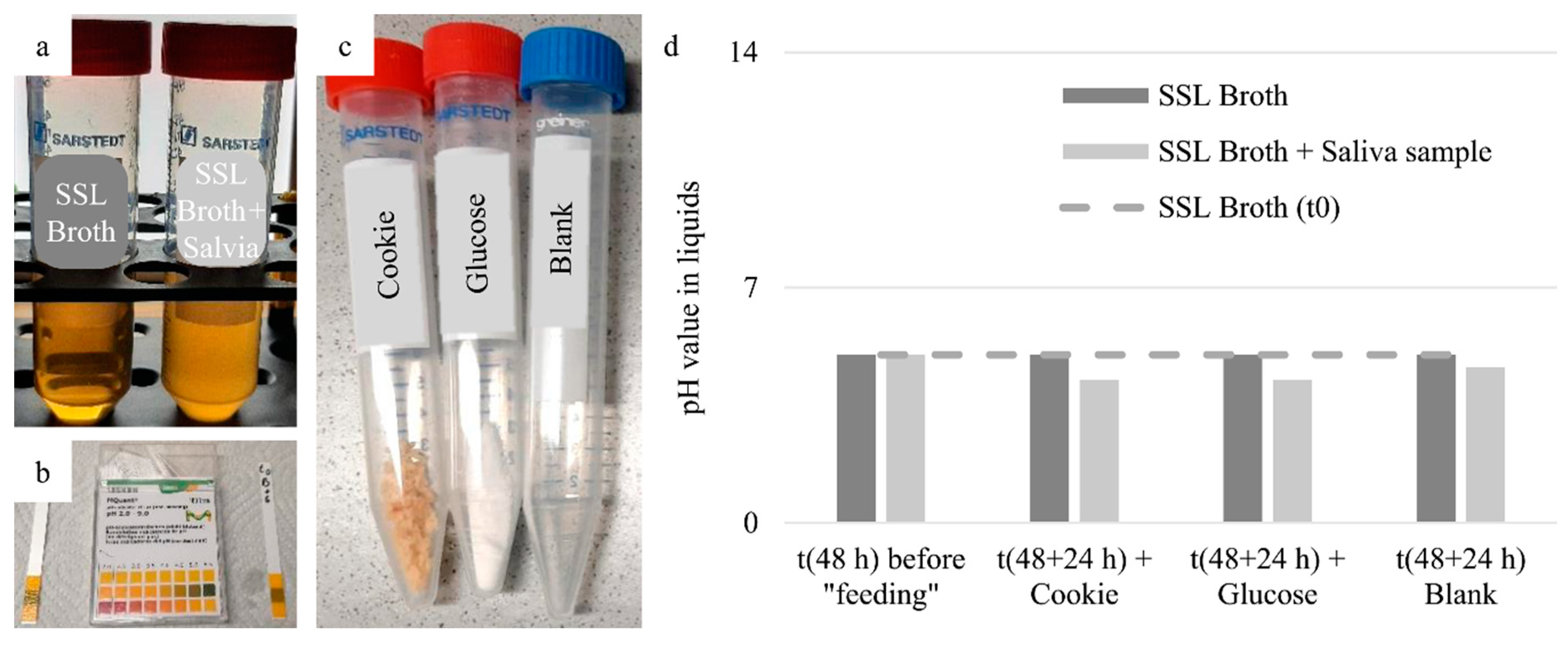
The broth was translucent at the beginning of the experiment and did not change this state during storage in the heating cabinet. By adding the saliva sample, a turbidity in the broth could be seen after about 48 h of storage (
Figure 7a). However, this turbidity was not accompanied by a change in pH (
Figure 7b,d). After dividing the two solutions into the three reaction vessels each (
Figure 7c) and additional storage for 24 h in the heating cabinet, a slight decrease in pH was seen in the case of the cultured saliva sample and a more pronounced decrease was seen in the case of the cultured saliva with glucose or crushed cookie in the broth (
Figure 7d). In all other cases, the pH value did not differ from the initial pH value.
5. Conclusions
Transferring an experimental lecture into the digital space requires, first of all, conscientious planning and consideration of the learning objectives of the experiments. Subsequently, the technical boundary conditions for the experiments, the broadcast of the lecture, and suitable audience response systems can be determined. In the present case, the challenge was to enable the participation of the students, even though the experiments were exclusively demonstration experiments conducted during a synchronous lecture. In the following, the three essential aspects of technology, subject content, and didactics are summarized.
The technical aspect can be concluded briefly, as with the current state of the art (laptop, second monitor, camera, microphones) and the available tools (web communication with video, audio and chat, student learning platforms, joint pin boards, and voting platforms) there is an easily accessible and flexible basis for the broadcast of synchronous lectures. There may even be a benefit to broadcasting an experiment from a real lab by having better technical equipment available and implicitly teaching laboratory skills and behavior. This may be perceived as an increase in value compared to experiments that should otherwise have been performed in a scaled-down manner in a lecture hall by students. Video broadcasting also offers the advantage that all students are equally close, which is sometimes not feasible with larger practical groups in face-to-face courses.
The technical aspects of the experiments performed can be summarized as follows. It is a great challenge to select experiments that can be completed entirely within the scheduled time. In addition, the experiments should be logically linked with each other (as in the case shown, the types of corrosion of metals and the attack of tooth substances) and idle phases should no longer occur. For this purpose, sections of explanation of experiments or discussions with students are suitable to fill these phases. To a certain extent, long-term experiments are also suitable, and their evaluation then takes place in the experimental lecture. Here, a prelude (e.g., in the form of a video of the experiment start) with asynchronous queries can provide a timely divided and thus repeated occupation with the topic.
The didactic evaluation of the experimental lecture should be based primarily on the possibilities of student activation, since this is one of the essential components of a practically conducted experiment in contrast to a seminar or lecture. Putting the focus on preparatory aspects, the questioning of expectations and the joint discussion of results makes it possible to compensate for the missing hands-on aspect to a large extent. The actual scientific learning objectives can be fulfilled by digital audience response systems if the experiments are designed accordingly. In this way, the students’ interest (according to their own assessment) can be maintained for the duration of the “only observation elements” of the experiments. The feedback indicates that students perceived the digital experiments as adequate experimentation, if a certain student influence and animating approach to the audience is integrated. This was realized using an asynchronous as well as a synchronous communication platform, whose technical implementation was rated as very good and good. The joint discussion of experimental outcomes is especially vital if assumptions on results are requested in advance and can be discussed subsequently. The selection of the experiments should be closely oriented to the content of the lecture so that the didactic role of the event is not lost by a drift in the context of the content. For a positive perception, an evaluation of the course by the students and its assessment together with the students was considered to be of great value.