Electrospun VDF-TeFE Scaffolds Modified by Copper and Titanium in Magnetron Plasma and Their Antibacterial Activity against MRSA
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of VDF-TeFE Scaffolds
2.2. Plasma Modification of VDF-TeFE Scaffolds
2.3. Characterization of VDF-TeFE Scaffolds
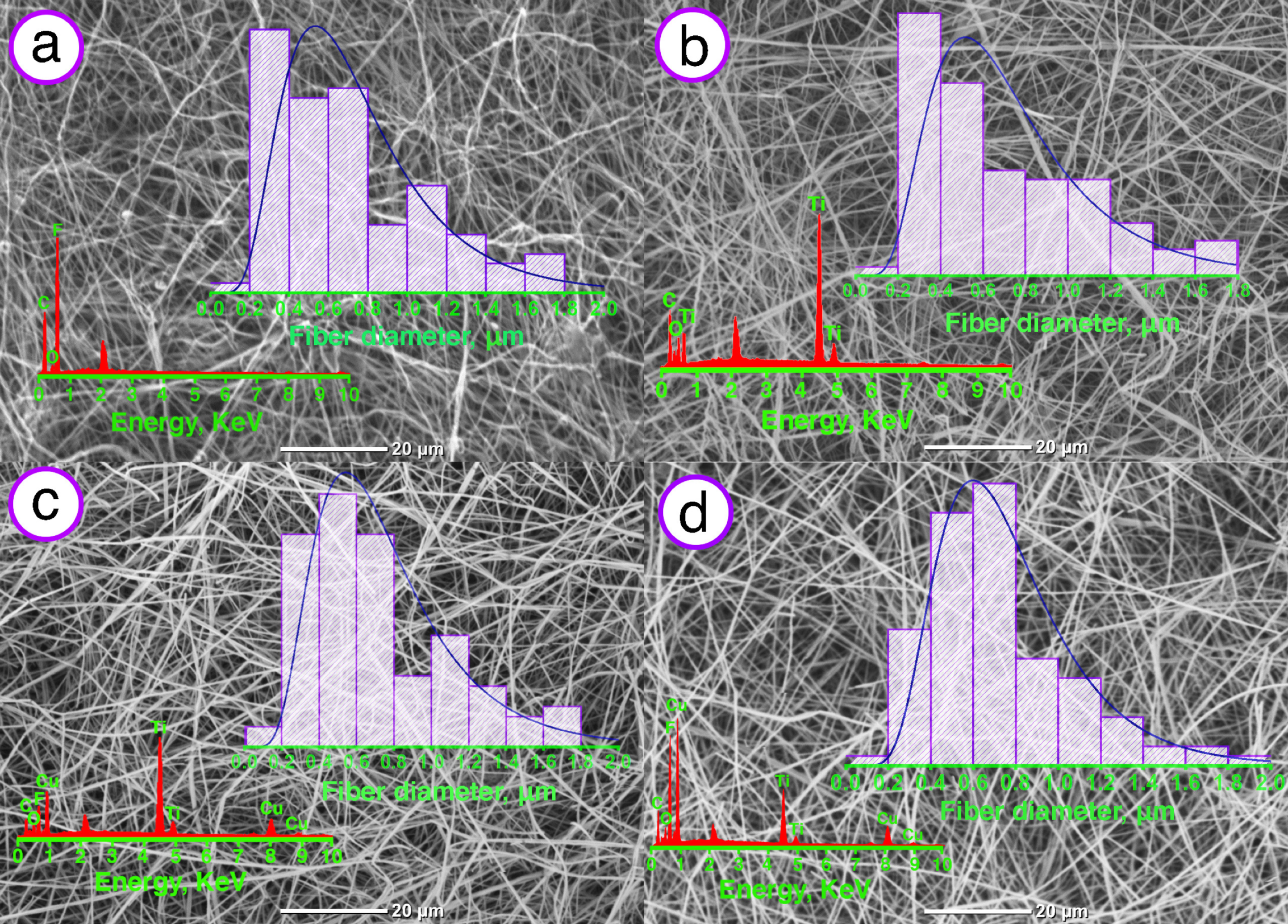
2.3.1. Scanning Electron Microscopy
2.3.2. Energy Dispersive X-ray Spectroscopy
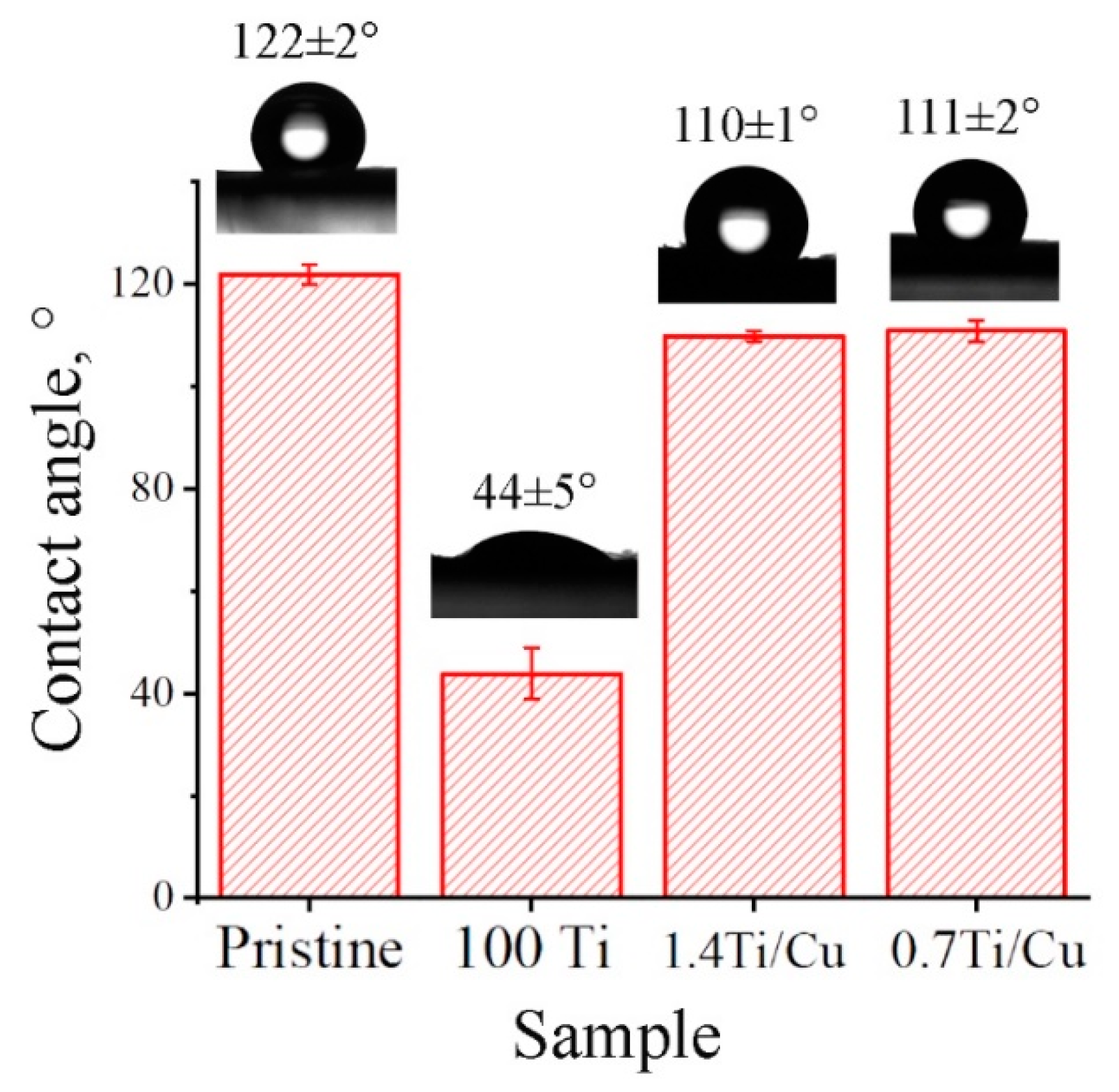
2.3.3. Wettability
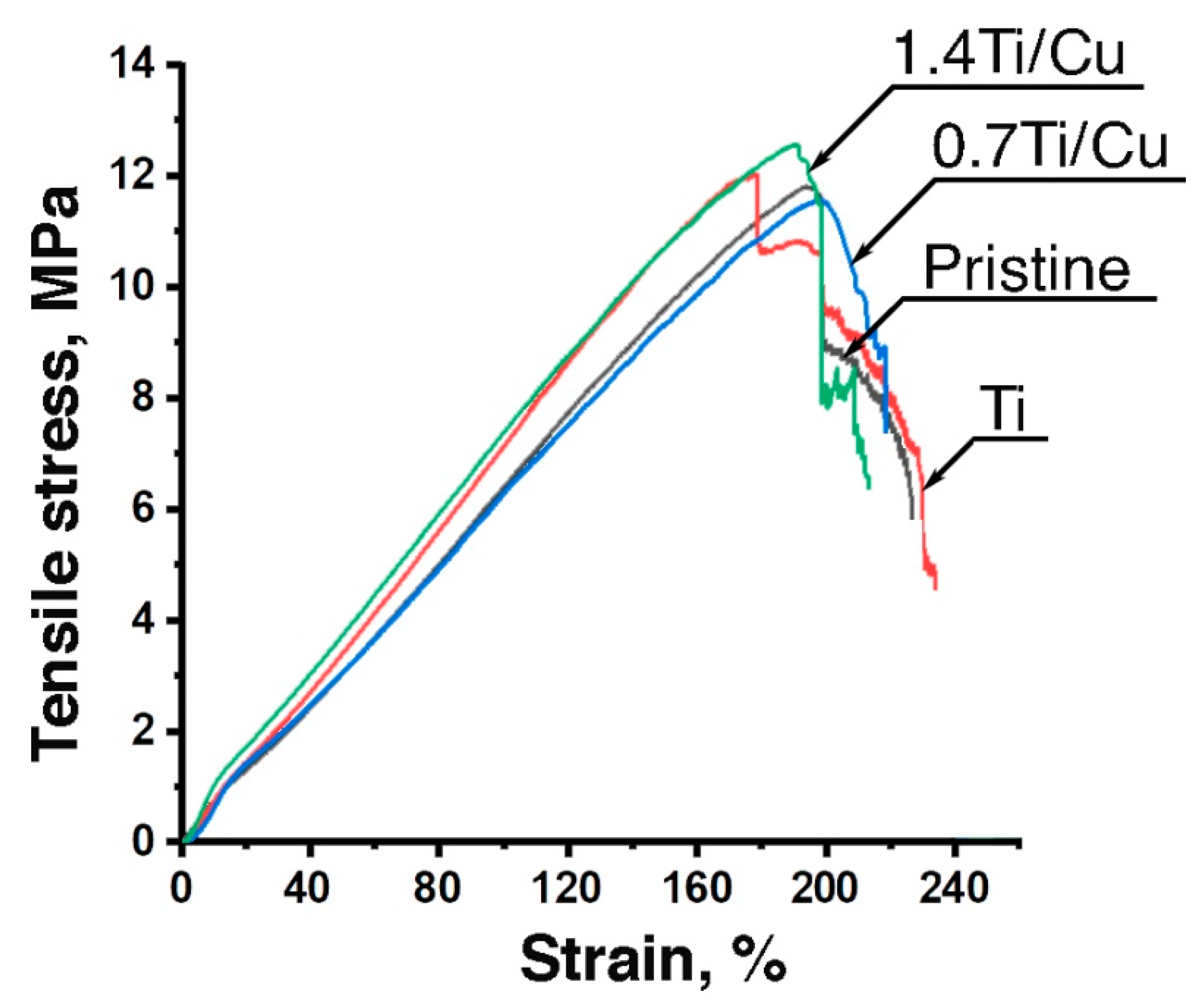
2.3.4. Mechanical Properties
2.3.5. Antibacterial Activity
2.4. Statistical Analysis
3. Results
3.1. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
3.2. Wettability
3.3. Mechanical Properties
3.4. Antibacterial Activity
4. Discussion
4.1. Influence of Plasma Modification by Ti/Cu on Morphology, Wettability and Mechanical Properties of VDF-TeFE Scaffolds
4.2. Influence of Plasma Modification by Ti/Cu on Elemental Composition of VDF-TeFE Scaffolds
4.3. Influence of Plasma Modification by Ti/Cu on Antibacterial Properties of VDF-TeFE Scaffolds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bolbasov, E.N.; Stankevich, K.S.; Sudarev, E.A.; Bouznik, V.M.; Kudryavtseva, V.L.; Antonova, L.V.; Matveeva, V.G.; Anissimov, Y.G.; Tverdokhlebov, S.I. The investigation of the production method influence on the structure and properties of the ferroelectric nonwoven materials based on vinylidene fluoride—Tetrafluoroethylene copolymer. Mater. Chem. Phys. 2016, 182, 338–346. [Google Scholar] [CrossRef]
- Fang, X.-Q.; Liu, J.-X.; Gupta, V. Fundamental formulations and recent achievements in piezoelectric nano-structures: A review. Nanoscale 2013, 5, 1716. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liao, C.; Tjong, S.C. Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomaterials 2019, 9, 952. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Pereira, J.; Ribeiro, S.; Ribeiro, C.; Gombek, C.J.; Gama, F.M.; Gomes, A.C.; Patterson, D.A.; Lanceros-Méndez, S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polym. Test. 2015, 44, 234–241. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Badaraev, A.D.; Koniaeva, A.; Krikova, S.A.; Shesterikov, E.V.; Bolbasov, E.N.; Nemoykina, A.L.; Bouznik, V.M.; Stankevich, K.S.; Zhukov, Y.M.; Mishin, I.P.; et al. Piezoelectric polymer membranes with thin antibacterial coating for the regeneration of oral mucosa. Appl. Surf. Sci. 2020, 504, 144068. [Google Scholar] [CrossRef]
- Badaraev, A.D.; Nemoykina, A.L.; Bolbasov, E.N.; Tverdokhlebov, S.I. Magnetron plasma modification by co-sputtering copper target of electrospun fluoropolymer material to possess bacteriostatic properties. Mater. Today Proc. 2020, 22, 219–227. [Google Scholar] [CrossRef]
- Kelly, P.; Arnell, R. Magnetron co-sputtering: A review of recent developments and applications. Vacuum 2020, 56, 159–172. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Heun Koo, B.; Choi, D. Influence of complex SiF62−Ions on the PEO coatings formed on Mg–Al6–Zn1 alloy for enhanced wear and corrosion protection. Coatings 2020, 10, 94. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Heun Koo, B.; Jung, Y.-G.; Lee, J.H.; Choi, D. Effect of K2ZrF6 concentration on the two-step PEO Coating prepared on AZ91 Mg Alloy in Alkaline Silicate Solution. Materials 2020, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Ogwu, A.A.; Bouquerel, E.; Ademosu, O.; Moh, S.; Crossan, E.; Placido, F. An investigation of the surface energy and optical transmittance of copper oxide thin films prepared by reactive magnetron sputtering. Acta Mater. 2005, 53, 5151–5159. [Google Scholar] [CrossRef]
- Bolbasov, E.N.; Maryin, P.V.; Stankevich, K.S.; Kozelskaya, A.I.; Shesterikov, E.V.; Khodyrevskaya, Y.I.; Nasonova, M.V.; Shishkova, D.K.; Kudryavtseva, Y.A.; Anissimov, Y.G.; et al. Surface modification of electrospun poly-(l-lactic) acid scaffolds by reactive magnetron co-sputtering. Colloids Surf. B Biointerfaces 2018, 162, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, D.; Kaczmarek, D.; Antosiak, A.; Mazur, M.; Rybak, Z.; Rusak, A.; Osekowska, M.; Poniedzialek, A.; Gamian, A.; Szponar, B. Influence of Cu–Ti thin film surface properties on antimicrobial activity and viability of living cells. Mater. Sci. Eng. C 2015, 56, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sidelev, D.V.; Bleykher, G.A.; Bestetti, M.; Krivobokov, V.P.; Vicenzo, A.; Franz, S.; Brunella, M.F. A comparative study on the properties of chromium coatings deposited by magnetron co-sputtering with hot and cooled target. Vacuum 2017, 143, 479–485. [Google Scholar] [CrossRef]
- Xiong, J.; Huo, P.; Ko, F.K. Introduction I Fabrication of ultrafine fibrous polytetrafluoroethylene porous membranes by electrospinning. J. Mater. Res. 2009, 24, 2755–2761. [Google Scholar] [CrossRef]
- Kolská, Z.; Řezníčková, A.; Hnatowicz, V.; Švorčík, V. PTFE surface modification by Ar plasma and its characterization. Vacuum 2012, 86, 643–647. [Google Scholar] [CrossRef]


| Modification Modes (Samples) | Discharge Power, W | Current, A | Modification Time, min | ||
|---|---|---|---|---|---|
| Cu | Ti | Cu | Ti | ||
| 100 Ti | - | 750 | - | 1.5 | 32.6 |
| Ti/Cu 1 | 65 | 750 | 0.15 | 1.5 | 26.6 |
| Ti/Cu 2 | 130 | 500 | 0.30 | 1.1 | 29.3 |
| Sample | Surface Porosity, % | Fiber Diameter, μm |
|---|---|---|
| Pristine | 58 ± 7 | 0.76 ± 0.37 |
| 100 Ti | 61 ± 6 | 0.75 ± 0.38 |
| Ti/Cu 1 | 62 ± 6 | 0.79 ± 0.37 |
| Ti/Cu 2 | 55 ± 9 | 0.78 ± 0.32 |
| Sample | Elemental Concentration, atom. % | Elemental Ratio | |||||
|---|---|---|---|---|---|---|---|
| C | O | F | Ti | Cu | C/O | Ti/Cu | |
| Pristine | 55.1 | 5.6 | 39.3 | - | - | 10.4 | - |
| Ti | 36.8 | 20.7 | 34.3 | 8.2 | - | 1.8 | - |
| Ti/Cu 1 | 37.4 | 15.8 | 29.2 | 10.3 | 7.3 | 2.4 | 1.4 |
| Ti/Cu 2 | 43.1 | 12.0 | 31.0 | 5.8 | 8.1 | 3.6 | 0.7 |
| Sample | Tensile Strength, MPa | Relative Elongation, % | Young’s Modulus, MPa |
|---|---|---|---|
| Pristine | 11.8 ± 0.8 | 226 ± 16 | 13.6 ± 0.9 |
| Ti | 12.0 ± 0.4 | 230 ± 18 | 15.3 ± 2.1 |
| Ti/Cu 1 | 11.6 ± 0.5 | 218 ± 24 | 13.2 ± 0.3 |
| Ti/Cu 2 | 12.6 ± 0.7 | 214 ± 22 | 15.1 ± 1.1 |
| Sample | Number of Bacteria, CFU/mL | R, % |
|---|---|---|
| Pristine | (6 ± 0.5) × 106 | - |
| Ti | (5.2 ± 0.9) × 106 | 13 |
| Ti/Cu 1 | (5.6 ± 0.6) × 105 | 91 |
| Ti/Cu 2 | (2.7 ± 0.8) × 105 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badaraev, A.D.; Lerner, M.I.; Sidelev, D.V.; Bolbasov, E.N.; Tverdokhlebov, S.I. Electrospun VDF-TeFE Scaffolds Modified by Copper and Titanium in Magnetron Plasma and Their Antibacterial Activity against MRSA. Technologies 2021, 9, 5. https://doi.org/10.3390/technologies9010005
Badaraev AD, Lerner MI, Sidelev DV, Bolbasov EN, Tverdokhlebov SI. Electrospun VDF-TeFE Scaffolds Modified by Copper and Titanium in Magnetron Plasma and Their Antibacterial Activity against MRSA. Technologies. 2021; 9(1):5. https://doi.org/10.3390/technologies9010005
Chicago/Turabian StyleBadaraev, Arsalan D., Marat I. Lerner, Dmitrii V. Sidelev, Evgeny N. Bolbasov, and Sergei I. Tverdokhlebov. 2021. "Electrospun VDF-TeFE Scaffolds Modified by Copper and Titanium in Magnetron Plasma and Their Antibacterial Activity against MRSA" Technologies 9, no. 1: 5. https://doi.org/10.3390/technologies9010005
APA StyleBadaraev, A. D., Lerner, M. I., Sidelev, D. V., Bolbasov, E. N., & Tverdokhlebov, S. I. (2021). Electrospun VDF-TeFE Scaffolds Modified by Copper and Titanium in Magnetron Plasma and Their Antibacterial Activity against MRSA. Technologies, 9(1), 5. https://doi.org/10.3390/technologies9010005








