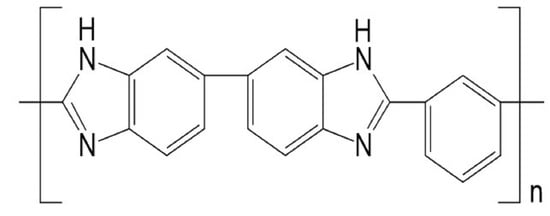
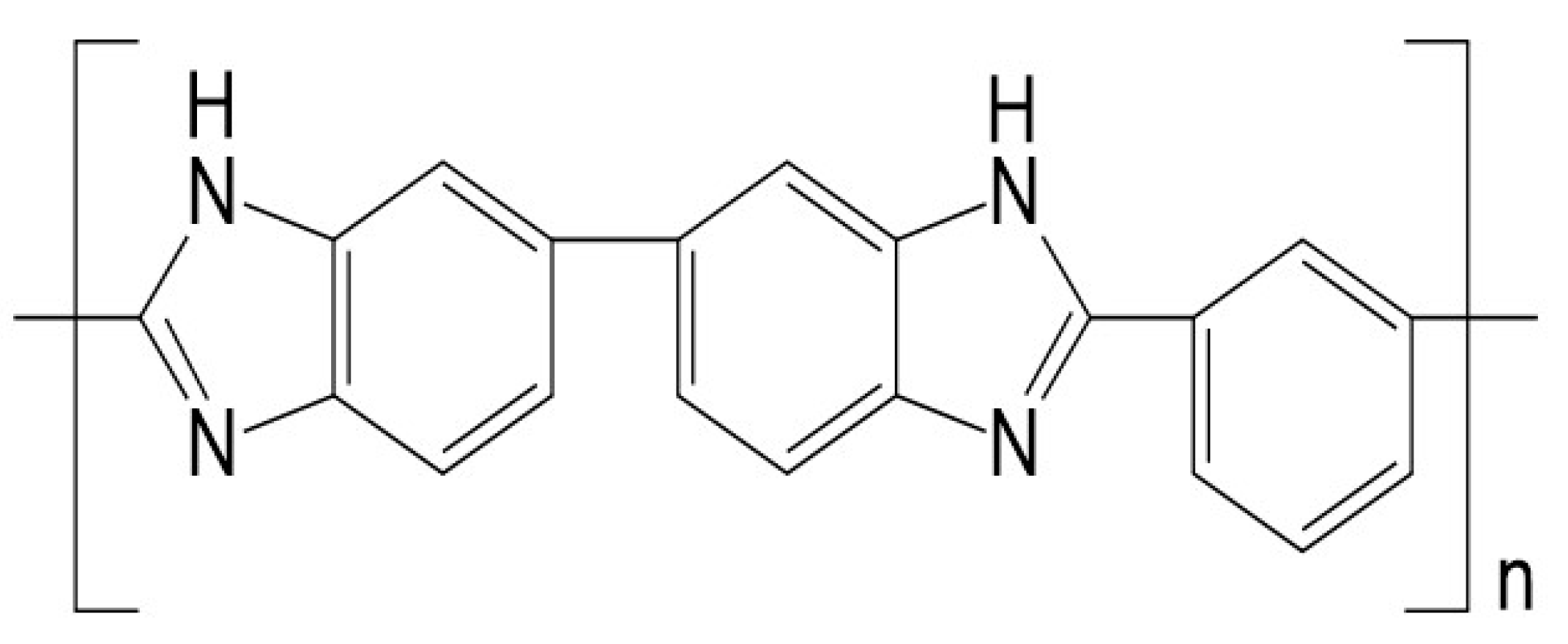
UV Photo-Oxidation of Polybenzimidazole (PBI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV-Visible Spectrophotometers
2.3. UV Photo-Oxidation
2.4. X-Ray Photoelectron Spectroscopy (XPS)
2.5. Contact Angle (CA) Goniometry
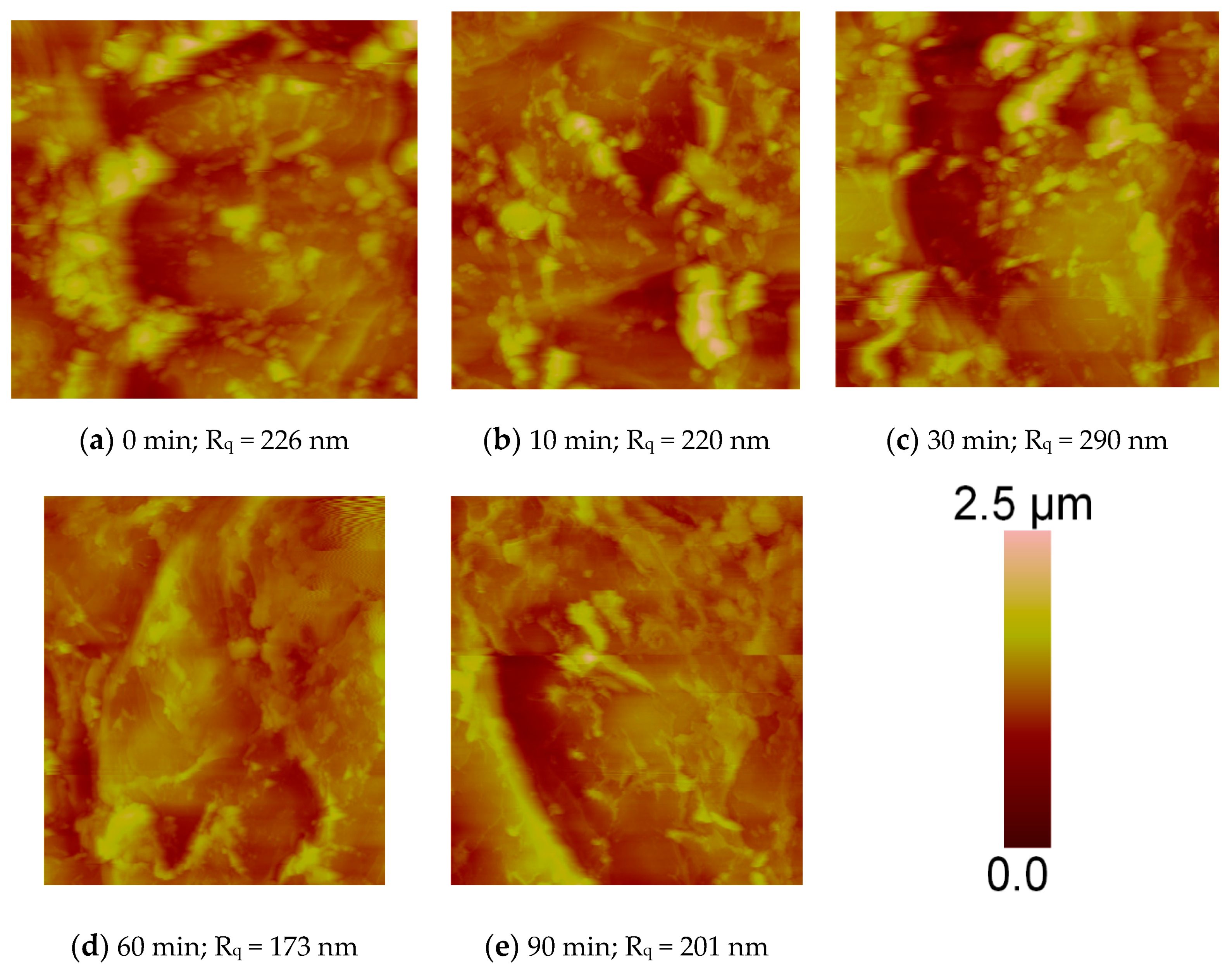
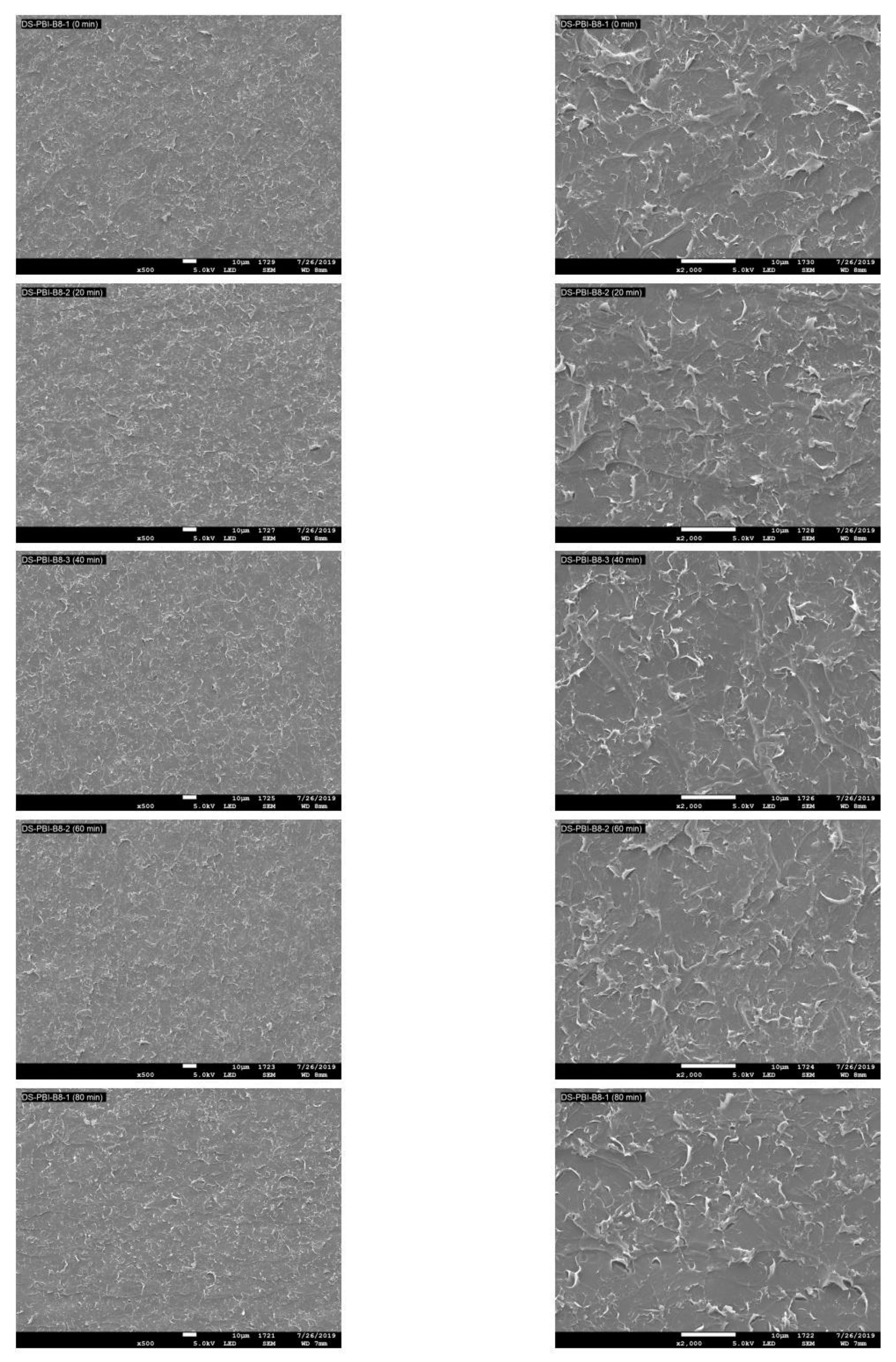
2.6. Surface Topography
3. Results
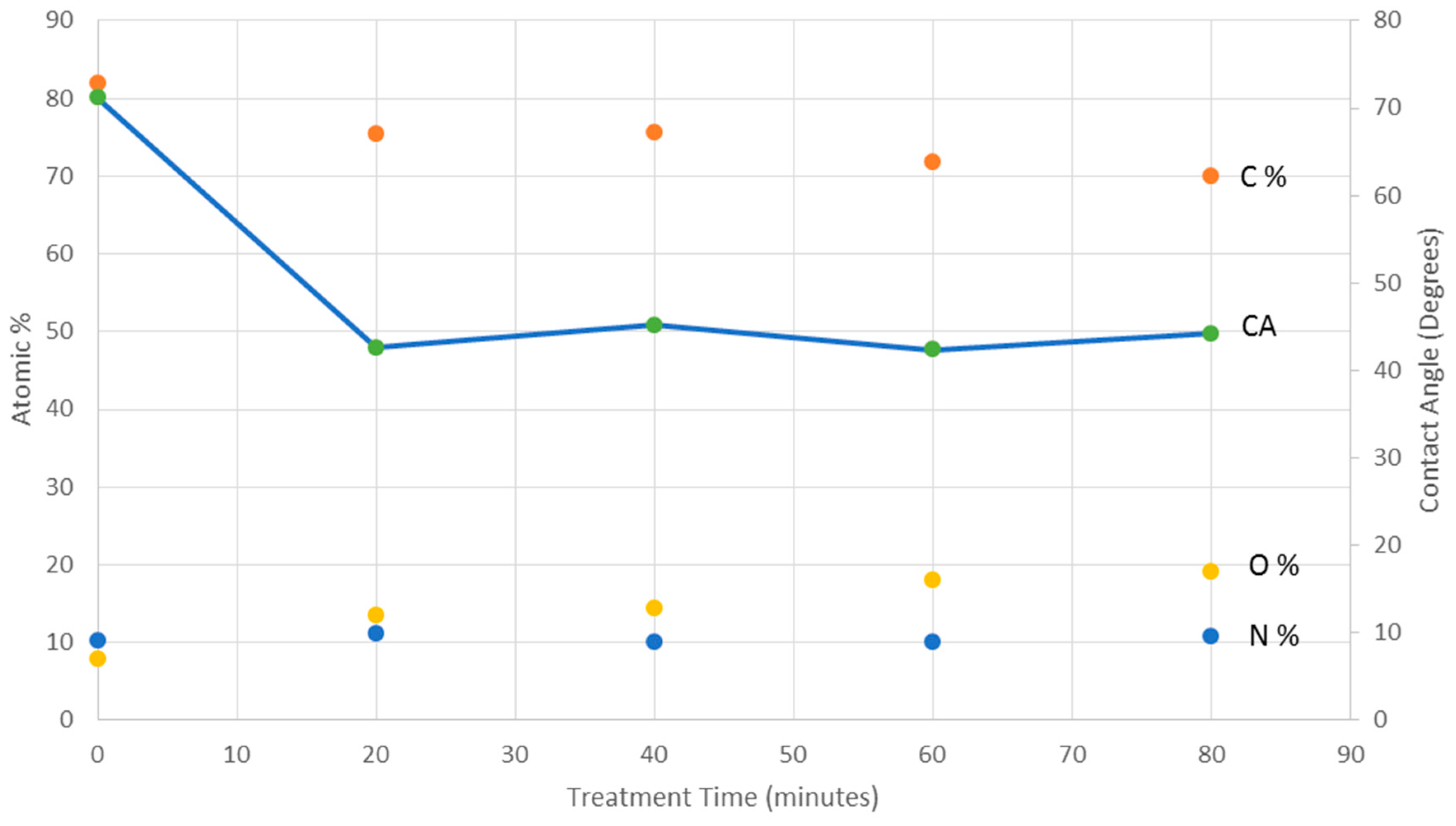
3.1. Quantitative XPS and Water Contact Angle
3.2. XPS Chemical State Analysis
3.3. Formatting of Mathematical Components
3.4. Surface Topography for PBI Treated with UV Photo-Oxidation
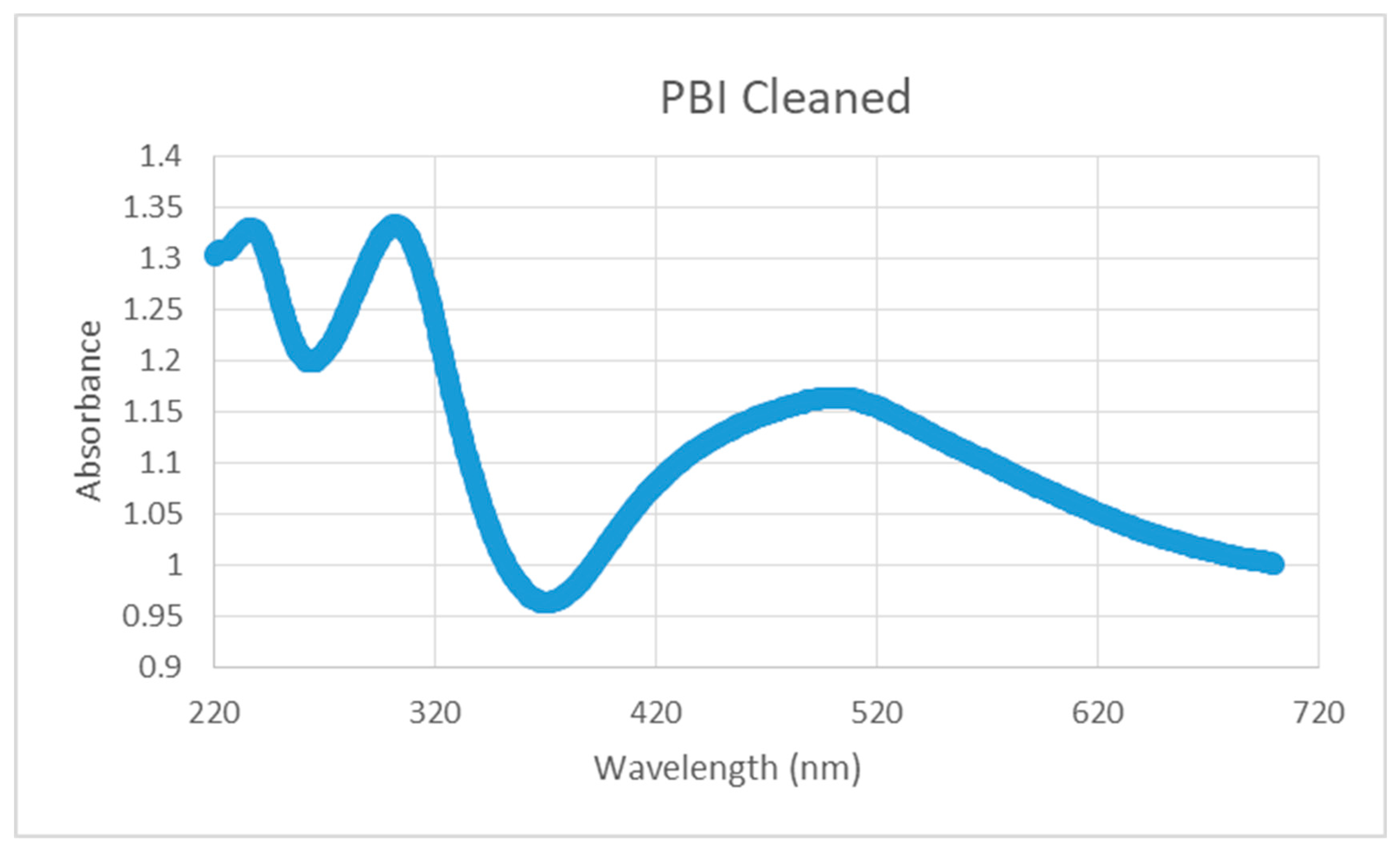
3.5. UV-Visible Photoabsorption Spectrum of PBI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.-T.; Weng, D.; Savinell, R.; Litt, M. Acid-Doped Polybenzimidazoles: A New Polymer Electrolyte. J. Electrochem. Soc. 1995, 142, L121. [Google Scholar] [CrossRef]
- Davis, R.; Chin, J.; Lin, C.-C.; Petit, S. Accelerated weathering of polyaramid and polybenzimidazole firefighter protective clothing fabrics. Polym. Degrad. Stab. 2010, 95, 1642–1654. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Pyngrope, D.; Pradhan, R.; Jha, S.; Bhowmik, S.; Poulis, H.; Bui, V.T.; Bonin, H. Electron beam modification of space durable polymeric nano-adhesive bonding of ultra-high temperature resistant polymer. J. Polym. Eng. 2011, 31, 381–386. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Polymer fuel cells based on polybenzimidazole/H3PO4. Energy Environ. Sci. 2012, 5, 6436–6444. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Ye, R.; Henkensmeier, D.; Yoon, S.J.; Huang, Z.; Kim, D.K.; Chang, Z.; Kim, S.; Chen, R. Redox Flow Batteries for Energy Storage: A Technology Review. J. Electrochem. Energy Convers. Storage 2017, 15, 010801. [Google Scholar] [CrossRef]
- Bülbül, E.; Atanasov, V.; Mehlhorn, M.; Bürger, M.; Chromik, A.; Häring, T.; Kerres, J. Highly phosphonated polypentafluorostyrene blended with polybenzimidazole: Application in vanadium redox flow battery. J. Membr. Sci. 2019, 194–203. [Google Scholar] [CrossRef]
- Jung, M.; Lee, W.; Noh, C.; Konovalova, A.; Yi, G.S.; Kim, S.; Kwon, Y.; Henkensmeier, D. Blending polybenzimidazole with an anion exchange polymer increases the efficiency of vanadium redox flow batteries. J. Membr. Sci. 2019, 580, 110–116. [Google Scholar] [CrossRef]
- Gubler, L.; Scherer, G.G. A Proton-Conducting Polymer Membrane as Solid Electrolyte—Function and Required Properties. Fuel Cells I 2008, 215, 1–14. [Google Scholar]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Calvert, J.G.; Pitts, J.N. Photochemistry; John Wiley & Sons: New York, NY, USA, 1966; pp. 205–209. [Google Scholar]
- Okabe, H. Photochemistry of Small Molecules; John Wiley & Sons: New York, NY, USA, 1978; p. 179. [Google Scholar]
- Hess, G. Ozone Conflict Rages. Chem. Eng. News Arch. 2014, 92, 24–25. [Google Scholar]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers; John Wiley & Sons: Chichester, UK, 1991. [Google Scholar]
- Losito, I.; Malitesta, C.; De Bari, I.; Calvano, C.D. X-ray photoelectron spectroscopy characterization of poly(2,3-diaminophenazine) films electrosynthesised on platinum. Thin Solid Films 2005, 473, 104–113. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Tsai, M.-H.; Wu, C.; Bai, S. Electronic properties of heterocyclic aromatic hydroxyl rigid-rod polymers. Polymer 2004, 45, 459–465. [Google Scholar] [CrossRef]
- Kushwaha, O.S.; Avadhani, C.V.; Singh, R.P. Effect of UV Rays on Degradation and Stability of High Performance Polymer Membranes. Adv. Mater. Lett. 2014, 5, 272–279. [Google Scholar] [CrossRef]
- Weir, N.A. Photo and photooxidative reactions of polystyrene and of ring substituted polystyrenes. Dev. Polym. Degrad. 1982, 4, 143–188. [Google Scholar]
- Geuskens, G.; Baeyens-Volant, D.; Delaunois, G.; Lu-Vinh, Q.; Piret, W.; David, C. Photooxidation of polymers I. A quantitative study of the chemical reactions resulting from irradiation of polystyrene at 253.7 nm in the presence of oxygen. Euro. Polym. J. 1978, 14, 291–297. [Google Scholar] [CrossRef]
- Partridge, R.H. Vacuum-ultraviolet absorption spectrum of poly-styrene. J. Chem. Phys. 1967, 47, 4223–4227. [Google Scholar] [CrossRef]
- Khot, A.; Bailey, A.; Debies, T.; Takacs, G. XPS studies of poly(acrylic acid) grafted onto UV photo-oxidized polystyrene surfaces. J. Adhes. Sci. Technol. 2012, 26, 2627–2639. [Google Scholar] [CrossRef]
- Wells, R.K.; Royston, A.; Badyal, J.P.S. Direct Evidence for the Generation of Phenyl Radicals and Crosslinking during the Photolysis of a Polystyrene Film. Macromolecules 1994, 27, 7465–7468. [Google Scholar] [CrossRef]
- Omar, O.; Ha, B.; Vega, K.; Fleischer, A.; Moon, H.; Shertok, J.; Bailey, A.; Mehan, M.; Gupta, S.K.; Takacs, G.A. Reaction of ozone with polybenzimidazole (PBI). Ozone Sci. Eng. 2018, 40, 392–398. [Google Scholar] [CrossRef]


| Binding | Species | Treatment Time (min) | ||||
|---|---|---|---|---|---|---|
| Energy (eV) | 0 | 20 | 40 | 60 | 80 | |
| 284.6 | C-C sp2 | 53.6 | 48.4 | 47.5 | 46.3 | 41.8 |
| 285.1 | C-C sp3 | 16.3 | 13.3 | 13.3 | 13.2 | 13.7 |
| 285.7 | C-N= | 9.0 | 6.8 | 9.4 | 8.2 | 8.1 |
| 286.0 | 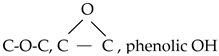 | 11.7 | 11.4 | 11.2 | 11.8 | 11.4 |
| 287.0 | C=O, aldehyde | 4.3 | 6.8 | 6.2 | 6.0 | 6.0 |
| 287.9 | N-C=O | 1.0 | 4.7 | 3.8 | 4.7 | 6.4 |
| 288.6 | O-C=O, carboxylic acid | 2.5 | 5.6 | 5.9 | 7.0 | 9.8 |
| 289.8 | O=C-O-C=O, O-(C=O)-O | 1.3 | 2.1 | 2.1 | 2.3 | 3.0 |
| 292.0 | Energy Loss | 0.5 | 0.9 | 0.6 | 0.7 | 0.8 |
| Binding | Species | Treatment Time (min) | ||||
|---|---|---|---|---|---|---|
| Energy (eV) | 0 | 20 | 40 | 60 | 80 | |
| 398.4 | C=N | 39.7 | 32.6 | 32.3 | 27.2 | 23.9 |
| 400.2 | C-N | 53.9 | 60.0 | 60.2 | 65.1 | 68.4 |
| 402.0 | N-O | 6.4 | 7.4 | 7.4 | 7.7 | 7.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shedden, D.; Atkinson, K.M.; Cisse, I.; Lutondo, S.; Roundtree, T.; Teixeira, M.; Shertok, J.; Mehan, M.; Thompson, G.K.; Gupta, S.K.; et al. UV Photo-Oxidation of Polybenzimidazole (PBI). Technologies 2020, 8, 52. https://doi.org/10.3390/technologies8040052
Shedden D, Atkinson KM, Cisse I, Lutondo S, Roundtree T, Teixeira M, Shertok J, Mehan M, Thompson GK, Gupta SK, et al. UV Photo-Oxidation of Polybenzimidazole (PBI). Technologies. 2020; 8(4):52. https://doi.org/10.3390/technologies8040052
Chicago/Turabian StyleShedden, Devon, Kristen M. Atkinson, Ibrahim Cisse, Shin Lutondo, Tyshawn Roundtree, Michilena Teixeira, Joel Shertok, Michael Mehan, Gregory K. Thompson, Surendra K. Gupta, and et al. 2020. "UV Photo-Oxidation of Polybenzimidazole (PBI)" Technologies 8, no. 4: 52. https://doi.org/10.3390/technologies8040052
APA StyleShedden, D., Atkinson, K. M., Cisse, I., Lutondo, S., Roundtree, T., Teixeira, M., Shertok, J., Mehan, M., Thompson, G. K., Gupta, S. K., & Takacs, G. A. (2020). UV Photo-Oxidation of Polybenzimidazole (PBI). Technologies, 8(4), 52. https://doi.org/10.3390/technologies8040052