Hydrogel-Based Technologies for the Diagnosis of Skin Pathology
Abstract
1. Introduction
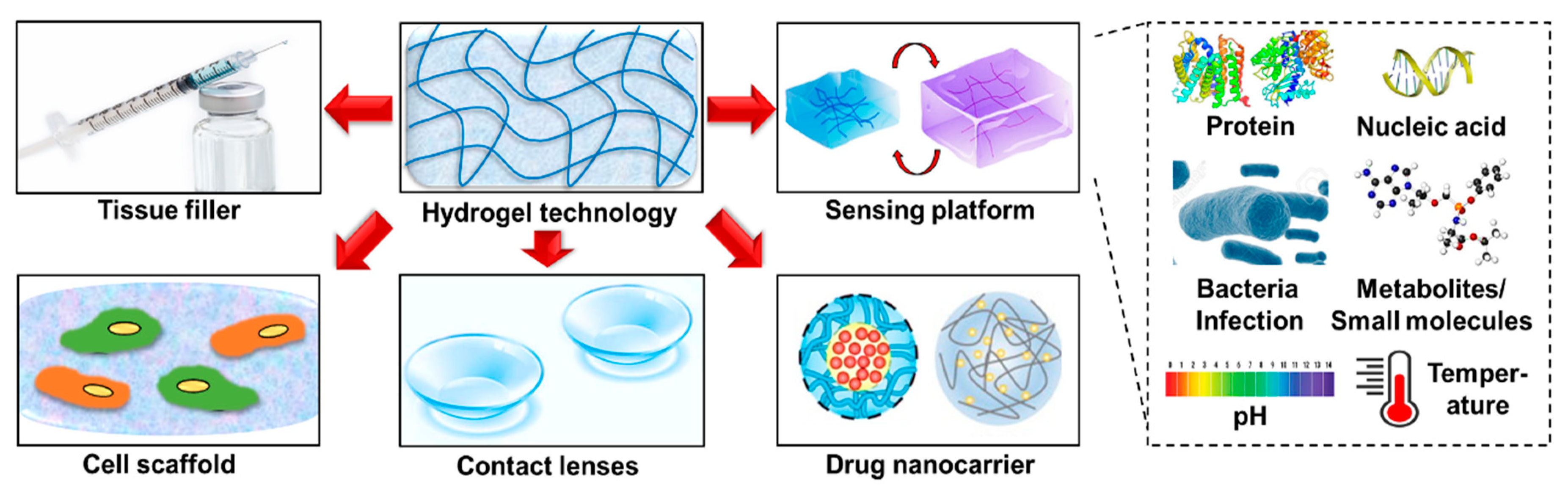
2. Synthesis and Preparation of Hydrogel Technologies
3. Hydrogel-Based Technologies for Point-of-Care (POC) Diagnosis/Monitoring of Skin Pathology
3.1. Importance of Skin Pathology Diagnosis and Monitoring
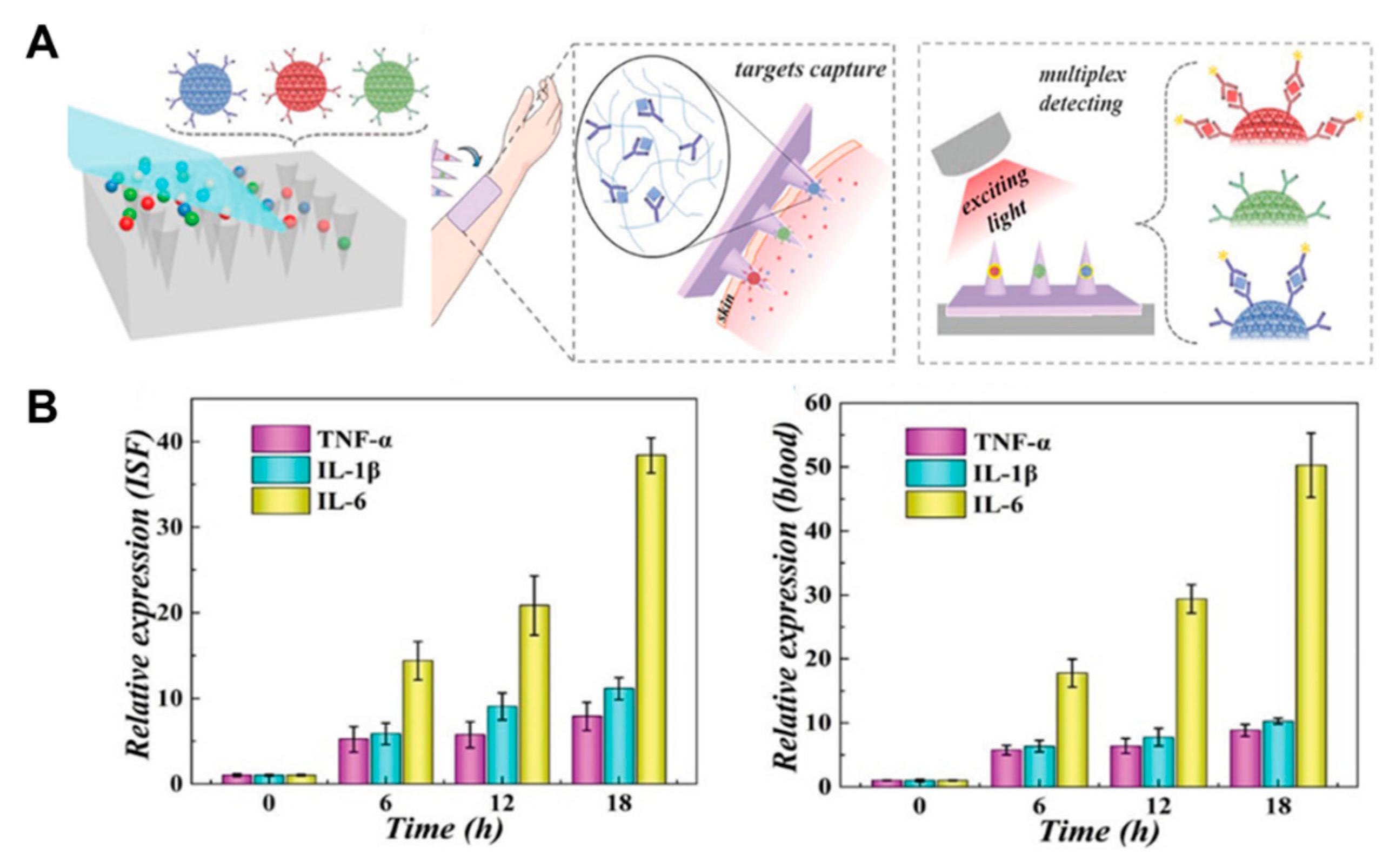
3.2. Proteins
3.3. Nucleic Acids
3.4. Metabolites and Reactive Species
3.5. Bacterial Infections
3.6. Other Markers
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Manafi, A.; Emami, A.-H.; Pooli, A.H.; Habibi, M.; Saidian, L. Unacceptable results with an accepted soft tissue filler: Polyacrylamide hydrogel. Aesthetic Plast. Surg. 2009, 34, 413–422. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Sheikhi, A.; Tutar, R.; Jahangiry, J.; Baidya, A.; Haghniaz, R.; Khademhosseini, A. Engineering tough, injectable, naturally derived, bioadhesive composite hydrogels. Adv. Health Mater. 2020, 9, e1901722. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Wang, C.; Cheng, Y.; Cheng, Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem. Commun. 2016, 52, 978–981. [Google Scholar] [CrossRef]
- Taylor, D.L.; Panhuis, M.I.H. Self-Healing hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Li, X.; Su, X.; Xiulan, S. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef]
- Varma, D.M.; Gold, G.T.; Taub, P.J.; Nicoll, S.B. Injectable carboxymethylcellulose hydrogels for soft tissue filler applications. Acta Biomater. 2014, 10, 4996–5004. [Google Scholar] [CrossRef]
- Magno, V.; Meinhardt, A.; Werner, C. Polymer hydrogels to guide organotypic and organoid cultures. Adv. Funct. Mater. 2020, 2000097. [Google Scholar] [CrossRef]
- Gu, Z.; Huang, K.; Luo, Y.; Zhang, L.; Mi, H.-Y.; Chen, Z.; Liao, G. Double network hydrogel for tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Bahia, M. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, G.; Irache, J.M. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug Deliv. Rev. 1998, 34, 191–219. [Google Scholar] [CrossRef]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core–shell microneedle gel for self-regulated insulin delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Wiraja, C.; Lio, D.C.S.; Xu, C. A double-layered microneedle platform fabricated through frozen spray-coating. Adv. Health Mater. 2020, 9, e2000147. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.W.T.; Shah, A.H.; Zheng, M.; Chang, H.; Wiraja, C.; Steele, T.W.J.; Xu, C. A self-adhesive microneedle patch with drug loading capability through swelling effect. Bioeng. Transl. Med. 2020, 5, e10157. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Eady, R.; Pope, F. Anatomy and organization of human skin. Rook’s Textb. Dermatol. 2004, 1, 3.2–3.80. [Google Scholar]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.C.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef]
- Schaefer, H.; Redelmeier, T. 1 Structure and dynamics of the skin barrier. In Skin Barrier; Karger Publishers: Basel, Switzerland, 1996; pp. 1–42. [Google Scholar]
- Mohammadi, M.H.; Araghi, B.H.; Beydaghi, V.; Geraili, A.; Moradi, F.; Jafari, P.; Janmaleki, M.; Valente, K.P.; Akbari, M.; Sanati-Nezhad, A. Skin diseases modeling using combined tissue engineering and microfluidic technologies. Adv. Health Mater. 2016, 5, 2459–2480. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Collins, M.; Williams, C.M.M.; Ma, H.-L. The pathology and immunology of atopic dermatitis. Inflamm. Allergy-Drug Targets 2011, 10, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wiraja, C.; Chew, S.W.T.; Xu, C. Nano-delivery systems for topical management of skin disorders. Mol. Pharm. 2020. [Google Scholar] [CrossRef]
- Palfreeman, A.; Mcnamee, K.E.; McCann, F.E. New developments in the management of psoriasis and psoriatic arthritis: A focus on apremilast. Drug Des. Dev. Ther. 2013, 7, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.L.; Dellavalle, R. Acne vulgaris. BMJ 2013, 346, f2634. [Google Scholar] [CrossRef]
- Abtin, A.; Jain, R.; Mitchell, A.; Roediger, B.; Brzoska, A.J.; Tikoo, S.; Cheng, Q.; Ng, L.G.; Cavanagh, L.L.; Von Andrian, U.H.; et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat. Immunol. 2013, 15, 45–53. [Google Scholar] [CrossRef]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin cancer: Epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef]
- Kuypers, D.R. Skin problems in chronic kidney disease. Nat. Rev. Nephrol. 2009, 5, 157–170. [Google Scholar] [CrossRef]
- Demirseren, D.D.; Emre, S.; Akoglu, G.; Arpacı, D.; Arman, A.; Metin, A.; Cakır, B.; Arpaci, D.; Cakir, B. Relationship between skin diseases and extracutaneous complications of diabetes mellitus: Clinical analysis of 750 patients. Am. J. Clin. Dermatol. 2013, 15, 65–70. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-based strategies to advance therapies for chronic skin wounds. Annu. Rev. Biomed. Eng. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.P.; Spada, F. Hydrogels for atopic dermatitis and wound management: A superior drug delivery vehicle. Pharmarmaceutics 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wat, E.; Hui, P.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Fu, J.; Shao, K.; Wang, L.; Lan, X.; Shi, J. Biomimetic hydrogel for rapid and scar-free healing of skin wounds inspired by the healing process of oral mucosa. Acta Biomater. 2019, 100, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, N.; Mangır, N. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef]
- Zheng, X.; Ding, Z.; Cheng, W.; Lu, Q.; Kong, X.; Zhou, X.; Lu, G.; Kaplan, D.L. Microskin-inspired injectable msc-laden hydrogels for scarless wound healing with hair follicles. Adv. Health Mater. 2020, 9, e2000041. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: New York, NY, USA, 2007; Volume 153, pp. 37–65. [Google Scholar]
- Takigami, M.; Amada, H.; Nagasawa, N.; Yagi, T.; Kasahara, T.; Takigami, S.; Tamada, M. Preparation and properties of CMC gel. Trans. Mater. Res. Soc. Jpn. 2007, 32, 713. [Google Scholar]
- Chung, H.J.; Park, T.G. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today 2009, 4, 429–437. [Google Scholar] [CrossRef]
- Alupei, I.C.; Popa, M.; Hamcerencu, M.; Abadie, M. Superabsorbant hydrogels based on xanthan and poly(vinyl alcohol). Eur. Polym. J. 2002, 38, 2313–2320. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, E.P.; Lin, J.-D.; Tseng, T.-W.; Wang, Y.-S.; Urban, P.L. Hydrogel micropatches for sampling and profiling skin metabolites. Anal. Chem. 2014, 86, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.L.; Lin, Y.; Yang, C.; Manocchi, A.K.; Yuet, K.P.; Doyle, P.S.; Yi, H. Microfluidic fabrication of hydrogel microparticles containing functionalized viral nanotemplates. Langmuir 2010, 26, 13436–13441. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Brady, A.J.; Eltayib, E.; Meng, T.; Alonso-Vicente, A.; González-Vázquez, P.; Torrisi, B.M.; Vicente-Perez, E.M.; Mooney, K.; Jones, D.S.; et al. Hydrogel-forming microneedle arrays allow detection of drugs and glucose In Vivo: Potential for use in diagnosis and therapeutic drug monitoring. PLoS ONE 2015, 10, e0145644. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Choi, H.-J.; Jang, M.; Lee, H.; Lee, H.-S.; Lim, Y.-B.; Choi, H.-J.; Chae, Y. A CMOS VEGF sensor for cancer diagnosis using a peptide aptamer-based functionalized microneedle. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1288–1299. [Google Scholar] [CrossRef]
- Sivaharan, A.J.; Lau, W.M.; Ng, K.W. Gel-based, 3D visual and colorimetric detection of a skin cancer biomarker using immunodiagnostic microneedles. In Proceedings of the 10th APS International PharmSci Conference, London, UK, 11–13 September 2019. [Google Scholar]
- Griffiths, C.E.; van de Kerkhof, P.; Czarnecka-Operacz, M. Psoriasis and atopic dermatitis. Dermatol. Ther. 2017, 7, 31–41. [Google Scholar] [CrossRef]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef]
- Gottrup, F. A specialized wound-healing center concept: Importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am. J. Surg. 2004, 187, S38–S43. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2010, 17, 113–125. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Templeton, S.; Voelcker, N.H. A flexible and low power telemetric sensing and monitoring system for chronic wound diagnostics. Biomed. Eng. Online 2015, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.C.; Xu, C. Simplifying skin disease diagnosis with topical nanotechnology. SLAS Technol. Transl. Life Sci. Innov. 2018, 23, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.; Doherty, D.; Smithdale, R.; Franks, P. Clinical predictors of leg ulcer healing. Br. J. Dermatol. 2010, 162, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Chew, S.W.T.; Xu, C. Advances in the formulations of microneedles for manifold biomedical applications. Adv. Mater. Technol. 2020, 5. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. Adv. Health Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv. Mater. 2017, 29, 1702243. [Google Scholar] [CrossRef]
- Miller, P.R.; Taylor, R.M.; Tran, B.Q.; Boyd, G.; Glaros, T.; Chavez, V.H.; Krishnakumar, R.; Sinha, A.; Poorey, K.; Williams, K.P.; et al. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun. Boil. 2018, 1, 173. [Google Scholar] [CrossRef]
- Bond, M.; Richards-Kortum, R. Drop-to-drop variation in the cellular components of fingerprick blood. Am. J. Clin. Pathol. 2015, 144, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Romanyuk, A.V.; Zvezdin, V.N.; Samant, P.; Grenader, M.I.; Zemlianova, M.A.; Prausnitz, M.R. Collection of analytes from microneedle patches. Anal. Chem. 2014, 86, 10520–10523. [Google Scholar] [CrossRef] [PubMed]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-responsive hydrogels: Intelligent materials for biosensing and drug delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non melanoma skin cancer pathogenesis overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ding, J.; Shankowsky, H.A.; Tredget, E.E. The molecular mechanism of hypertrophic scar. J. Cell Commun. Signal. 2013, 7, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Neagu, M.; Constantin, C.; Tanase, C. Immune-related biomarkers for diagnosis/prognosis and therapy monitoring of cutaneous melanoma. Expert Rev. Mol. Diagn. 2010, 10, 897–919. [Google Scholar] [CrossRef] [PubMed]
- Ladwig, G.P.; Robson, M.C.; Liu, R.; Kuhn, M.A.; Muir, D.F.; Schultz, G. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002, 10, 26–37. [Google Scholar] [CrossRef]
- Yeo, D.C.; Chew, S.W.; Xu, C. Polymeric biomaterials for management of pathological scarring. ACS Appl. Polym. Mater. 2019, 1, 612–624. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Horev, L.; Rüffer, C.; Schlippe, G.; Voss, W.; Ma’Or, Z.; Oron, M.; Soroka, Y.; Frusic-Zlotkin, M.; Milner, Y.; et al. Non-invasive skin biomarkers quantification of psoriasis and atopic dermatitis: Cytokines, antioxidants and psoriatic skin auto-fluorescence. Biomed. Pharmacother. 2012, 66, 293–299. [Google Scholar] [CrossRef]
- Jiang, S.; Hinchliffe, T.E.; Wu, T. Biomarkers of an autoimmune skin disease-psoriasis. Genom. Proteom. Bioinform. 2015, 13, 224–233. [Google Scholar] [CrossRef]
- Lee, H.J.; Roh, Y.H.; Kim, H.U.; Kim, S.M.; Bong, K.W. Multiplexed immunoassay using post-synthesis functionalized hydrogel microparticles. Lab Chip 2019, 19, 111–119. [Google Scholar] [CrossRef]
- Battista, E.; Causa, F.; Netti, P. Bioengineering microgels and hydrogel microparticles for sensing biomolecular targets. Gels 2017, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, D.C.; Chapin, S.C.; Doyle, P.S. Multiplexed protein quantification with barcoded hydrogel microparticles. Anal. Chem. 2011, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, G.; Bian, F.; Cai, L.; Zhao, Y. Encoded microneedle arrays for detection of skin interstitial fluid biomarkers. Adv. Mater. 2019, 31, e1902825. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Cho, H.; Jeon, D.; Park, S.H.; Shin, D.-S.; Heo, C.Y. A matrix metalloproteinase sensing biosensor for the evaluation of chronic wounds. BioChip J. 2019, 13, 323–332. [Google Scholar] [CrossRef]
- Son, K.J.; Shin, D.-S.; Kwa, T.; Gao, Y.; Revzin, A. Micropatterned sensing hydrogels integrated with reconfigurable microfluidics for detecting protease release from cells. Anal. Chem. 2013, 85, 11893–11901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, D.S.; Tokuda, E.Y.; Leight, J.L.; Miksch, C.E.; Brown, T.E.; Anseth, K.S. Synthesis of microgel sensors for spatial and temporal monitoring of protease activity. ACS Biomater. Sci. Eng. 2017, 4, 378–387. [Google Scholar] [CrossRef]
- Miao, Q.; Yeo, D.C.; Wiraja, C.; Zhang, J.; Ning, X.; Xu, C.; Pu, K. Near-infrared fluorescent molecular probe for sensitive imaging of keloid. Angew. Chem. Int. Ed. 2018, 57, 1256–1260. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef]
- Wiraja, C.; Yeo, D.C.; Lio, D.; Labanieh, L.; Lu, M.; Zhao, W.; Xu, C. Aptamer technology for tracking cells’ status & function. Mol. Cell. Ther. 2014, 2, 33. [Google Scholar] [CrossRef]
- Srinivas, R.L.; Chapin, S.C.; Doyle, P.S. Aptamer-functionalized microgel particles for protein detection. Anal. Chem. 2011, 83, 9138–9145. [Google Scholar] [CrossRef]
- Tejavibulya, N.; Colburn, D.A.; Marcogliese, F.A.; Yang, K.-A.; Guo, V.; Chowdhury, S.; Stojanovic, M.N.; Sia, S.K. Hydrogel microfilaments toward intradermal health monitoring. iScience 2019, 21, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Ohlendorf, J.; Chen, Y.; Taylor, U.D.; Rai, S.N.; Waigel, S.; Zacharias, W.; Hao, H.; McMasters, K.M. Identifying mRNA, MicroRNA and protein profiles of melanoma exosomes. PLoS ONE 2012, 7, e46874. [Google Scholar] [CrossRef] [PubMed]
- Affandi, A.J.; Radstake, T.R.; Marut, W. Update on biomarkers in systemic sclerosis: Tools for diagnosis and treatment. Semin. Immunopathol. 2015, 37, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Rinkevich, Y. Scars or Regeneration? Dermal fibroblasts as drivers of diverse skin wound responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.R.; Papenfuss, J.; Wong, R.; Motaal, A.; Tran, V.; Panko, J.; Krueger, G.G. An analysis of select pathogenic messages in lesional and non-lesional psoriatic skin using non-invasive tape harvesting. J. Investig. Dermatol. 2006, 126, 2234–2241. [Google Scholar] [CrossRef]
- Ra, S.H.; Li, X.; Binder, S.W. Molecular discrimination of cutaneous squamous cell carcinoma from actinic keratosis and normal skin. Mod. Pathol. 2011, 24, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.W.; Rehli, M.; Bosserhoff, A. miRNA expression profiling in melanocytes and melanoma cell lines reveals mirnas associated with formation and progression of malignant melanoma. J. Investig. Dermatol. 2009, 129, 1740–1751. [Google Scholar] [CrossRef]
- Lim, C.Z.J.; Zhang, L.; Zhang, Y.; Sundah, N.R.; Shao, H. New sensors for extracellular vesicles: Insights on constituent and associated biomarkers. ACS Sens. 2020, 5, 4–12. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Wong, R.; Tran, V.; Morhenn, V.; Hung, S.-P.; Andersen, B.; Ito, E.; Hatfield, G.W.; Benson, N.R. Use of RT-PCR and DNA microarrays to characterize RNA recovered by non-invasive tape harvesting of normal and inflamed skin. J. Investig. Dermatol. 2004, 123, 159–167. [Google Scholar] [CrossRef]
- Wiraja, C.; Yeo, D.C.; Lio, D.C.S.; Zheng, M.; Xu, C. Functional imaging with nucleic-acid-based sensors: Technology, application and future healthcare prospects. ChemBioChem 2018, 20, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wiraja, C.; Yeo, D.C.; Chang, H.; Lio, D.C.S.; Shi, W.; Pu, K.; Paller, A.S.; Xu, C. Oligonucleotide molecular sprinkler for intracellular detection and spontaneous regulation of mRNA for theranostics of scar fibroblasts. Small 2018, 14, 1802546. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wiraja, C.; Wee, M.; Yeo, D.; Hu, L.; Xu, C. Hairpin-structured probe conjugated nano-graphene oxide for the cellular detection of connective tissue growth factor mRNA. Anal. Chim. Acta 2018, 1038, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.C.; Wiraja, C.; Paller, A.S.; Mirkin, C.A.; Xu, C. Abnormal scar identification with spherical-nucleic-acid technology. Nat. Biomed. Eng. 2018, 2, 227–238. [Google Scholar] [CrossRef]
- Zhai, L.-Y.; Li, M.-X.; Pan, W.-L.; Chen, Y.; Pang, J.-X.; Zheng, L.; Chen, J.-X.; Duan, W.-J.; Li, M.-M. In Situ detection of plasma exosomal MicroRNA-1246 for breast cancer diagnostics by a Au nanoflare probe. ACS Appl. Mater. Interfaces 2018, 10, 39478–39486. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shapiro, S.J.; Chapin, S.C.; Doyle, P.S. Encoded hydrogel microparticles for sensitive and multiplex microRNA detection directly from raw cell lysates. Anal. Chem. 2016, 88, 3075–3081. [Google Scholar] [CrossRef]
- Si, Y.; Li, L.; Wang, N.; Zheng, J.; Yang, R.; Li, J. Oligonucleotide cross-linked hydrogel for recognition and quantitation of MicroRNAs based on a portable glucometer readout. ACS Appl. Mater. Interfaces 2019, 11, 7792–7799. [Google Scholar] [CrossRef]
- Choi, N.; Kim, J.; Chapin, S.C.; Duong, T.; Donohue, E.; Pandey, P.; Broom, W.; Hill, W.A.; Doyle, P.S. Multiplexed detection of mRNA using porosity-tuned hydrogel microparticles. Anal. Chem. 2012, 84, 9370–9378. [Google Scholar] [CrossRef]
- Roh, Y.H.; Sim, S.J.; Cho, I.-J.; Choi, N.; Bong, K.W. Vertically encoded tetragonal hydrogel microparticles for multiplexed detection of miRNAs associated with Alzheimer’s disease. Analyst 2016, 141, 4578–4586. [Google Scholar] [CrossRef]
- Roh, Y.H.; Lee, H.J.; Moon, H.J.; Kim, S.M.; Bong, K.W. Post-synthesis functionalized hydrogel microparticles for high performance microRNA detection. Anal. Chim. Acta 2019, 1076, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Rakszewska, A.; Stolper, R.J.; Kolasa, A.B.; Piruska, A.; Huck, W.T.S. Quantitative single-cell mRNA analysis in hydrogel beads. Angew. Chem. 2016, 128, 6810–6813. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Luan, C.; Zhao, Y.; Chen, B.; Liu, H.; Zhao, Y. Porous hydrogel encapsulated photonic barcodes for multiplex MicroRNA quantification. Adv. Funct. Mater. 2017, 28, 1704458. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, C.; Liu, H.; Zou, Y.; Zhang, X.; Kang, H.; Yang, C.J.; Tan, W. An aptamer cross-linked hydrogel as a colorimetric platform for visual detection. Angew. Chem. Int. Ed. 2010, 49, 1052–1056. [Google Scholar] [CrossRef]
- Si, Y.; Xu, L.; Wang, N.; Zheng, J.; Yang, R.; Li, J. Target MicroRNA-responsive DNA hydrogel-based surface-enhanced raman scattering sensor arrays for MicroRNA-marked cancer screening. Anal. Chem. 2020, 92, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Li, Y.; Jiang, C.; Chen, D.; Wen, Y.; Li, Z. Capillarity self-driven DNA hydrogel sensor for visual quantification of microRNA. Sens. Actuators B Chem. 2020, 313, 128036. [Google Scholar] [CrossRef]
- Al Sulaiman, D.; Chang, J.Y.H.; Bennett, N.R.; Topouzi, H.; Higgins, C.A.; Irvine, D.J.; Ladame, S. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano 2019, 13, 9620–9628. [Google Scholar] [CrossRef] [PubMed]
- Harchaoui, K.E.L.; Visser, M.; Kastelein, J.J.P.; Stroes, E.S.; Dallinga-Thie, G.M. Triglycerides and cardiovascular risk. Curr. Cardiol. Rev. 2009, 5, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Abaffy, T.; Möller, M.G.; Riemer, D.D.; Milikowski, C.; DeFazio, R.A. Comparative analysis of volatile metabolomics signals from melanoma and benign skin: A pilot study. Metabolomics 2013, 9, 998–1008. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Domenichiello, A.F.; Dey, A.K.; Yuan, Z.-X.; Goyal, A.; Rose, S.M.; Playford, M.P.; Ramsden, C.E.; Mehta, N.N. Bioactive lipid mediator profiles in human psoriasis skin and blood. J. Investig. Dermatol. 2018, 138, 1518–1528. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Wu, J.; Johnson, M.A.; Grapov, D.; Azizi, B.; Dhillon, J.; Fiehn, O. Metabolomics in psoriatic disease: Pilot study reveals metabolite differences in psoriasis and psoriatic arthritis. F1000Research 2014, 3, 248. [Google Scholar] [CrossRef]
- Dutkiewicz, E.P.; Hsieh, K.-T.; Wang, Y.-S.; Chiu, H.-Y.; Urban, P.L. Hydrogel micropatch and mass spectrometry–assisted screening for psoriasis-related skin metabolites. Clin. Chem. 2016, 62, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sarkar, R.; Nandi, S.; Porgador, A.; Jelinek, R. Detection of reactive oxygen species by a carbon-dot–ascorbic acid hydrogel. Anal. Chem. 2016, 89, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.P.; Reid, R.C.; Milton, R.D.; Minteer, S.D. A self-powered amperometric lactate biosensor based on lactate oxidase immobilized in dimethylferrocene-modified LPEI. Biosens. Bioelectron. 2016, 77, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramírez, J.; Wang, J.; Yardimci, C. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2014, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.J.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Srinivasan, G.; Chen, J.; Parisi, J.; Brückner, C.; Yao, X.; Lei, Y. An Injectable PEG-BSA-Coumarin-GOx hydrogel for fluorescence turn-on glucose detection. Appl. Biochem. Biotechnol. 2015, 177, 1115–1126. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; Carels, C.E.L.; Lundvig, D.M.S. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Afanas’Ev, I.B. Signaling by reactive oxygen and nitrogen species in skin diseases. Curr. Drug Metab. 2010, 11, 409–414. [Google Scholar] [CrossRef]
- Cheng, P.; Zhang, J.; Huang, J.; Miao, Q.; Xu, C.; Pu, K. Near-infrared fluorescence probes to detect reactive oxygen species for keloid diagnosis. Chem. Sci. 2018, 9, 6340–6347. [Google Scholar] [CrossRef]
- Chen, X.; Tian, X.; Shin, I.; Yoon, J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2011, 40, 4783. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.K.; Scharff-Poulsen, A.M.; Olsen, L.F. Horseradish peroxidase embedded in polyacrylamide nanoparticles enables optical detection of reactive oxygen species. Anal. Biochem. 2007, 366, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.-M.; Sedano, S.; Jiang, Q.; Duan, Y.; Shen, W.; Jiang, J.-H.; Zhong, W. Encapsulation of ionic nanoparticles produces reactive oxygen species (ROS)-responsive microgel useful for molecular detection. Chem. Commun. 2018, 54, 4329–4332. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, S. Electrochemical quantification of reactive oxygen and nitrogen: Challenges and opportunities. Anal. Bioanal. Chem. 2009, 394, 95–105. [Google Scholar] [CrossRef]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Grice, E.A. The skin microbiome: A focus on pathogens and their association with skin disease. PLoS Pathog. 2014, 10, e1004436. [Google Scholar] [CrossRef]
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Wei, X.; Wu, Q.; Zhang, J.; Zhang, Y.; Guo, W.; Chen, M.; Gu, Q.; Cai, Z.; Lu, M. Synthesis of precipitating chromogenic/fluorogenic β-glucosidase/β-galactosidase substrates by a new method and their application in the visual detection of foodborne pathogenic bacteria. Chem. Commun. 2017, 53, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.-M.S.; Laabei, M.; Jenkins, T.; Schönherr, H. Autonomously sensing hydrogels for the rapid and selective detection of pathogenic bacteria. Macromol. Rapid Commun. 2015, 36, 2123–2128. [Google Scholar] [CrossRef]
- Ebrahimi, M.-M.S.; Voss, Y.; Schönherr, H. Rapid detection of Escherichia colivia enzymatically triggered reactions in self-reporting chitosan hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 20190–20199. [Google Scholar] [CrossRef]
- Thet, N.T.; Alves, D.R.; Bean, J.E.; Booth, S.; Nzakizwanayo, J.; Young, A.E.R.; Jones, B.V.; Jenkins, T. Prototype development of the intelligent hydrogel wound dressing and its efficacy in the detection of model pathogenic wound biofilms. ACS Appl. Mater. Interfaces 2015, 8, 14909–14919. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Nandi, S.; Jelinek, R. Carbon-dot–hydrogel for enzyme-mediated bacterial detection. RSC Adv. 2017, 7, 588–594. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Luan, C.; Liu, Y.; Chen, B.; Zhao, Y. Aptamer-based hydrogel barcodes for the capture and detection of multiple types of pathogenic bacteria. Biosens. Bioelectron. 2018, 100, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Massad-Ivanir, N.; Shtenberg, G.; Zeidman, T.; Segal, E. Construction and characterization of porous SiO2/hydrogel hybrids as optical biosensors for rapid detection of bacteria. Adv. Funct. Mater. 2010, 20, 2269–2277. [Google Scholar] [CrossRef]
- Zepon, K.M.; Martins, M.M.; Marques, M.S.; Heckler, J.M.; Morisso, F.D.P.; Moreira, M.G.; Ziulkoski, A.L.; Kanis, L.A. Smart wound dressing based on κ-carrageenan/locust bean gum/cranberry extract for monitoring bacterial infections. Carbohydr. Polym. 2019, 206, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bennison, L.; Miller, C.; Summers, R.; Minnis, A.; Sussman, G.; McGuiness, W. The pH of wounds during healing and infection: A descriptive literature review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 63. [Google Scholar]
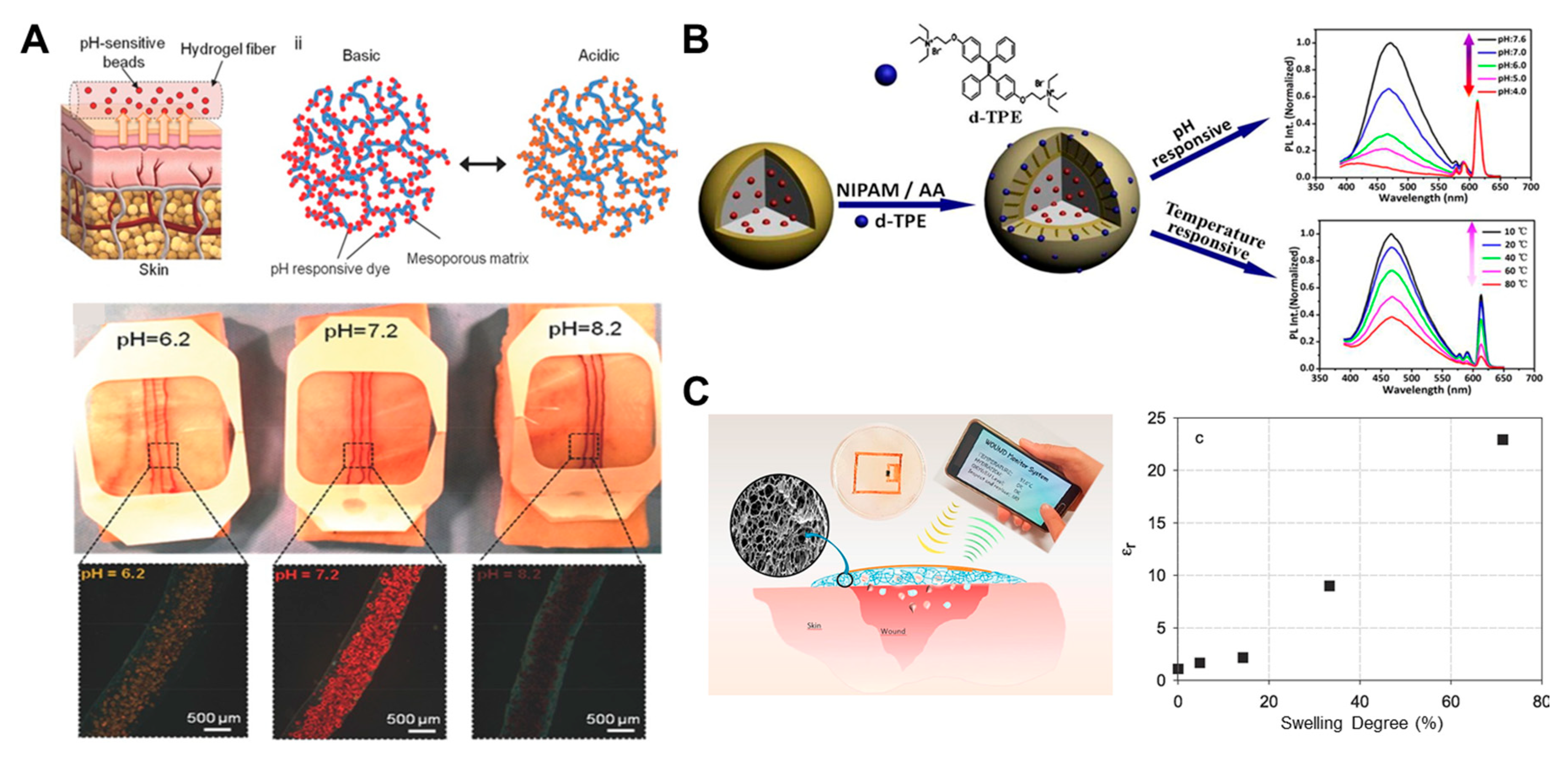
- Tamayol, A.; Akbari, M.; Zilberman, Y.; Comotto, M.; Lesha, E.; Serex, L.; Bagherifard, S.; Chen, Y.; Fu, G.; Ameri, S.K.; et al. Flexible pH-sensing hydrogel fibers for epidermal applications. Adv. Health Mater. 2016, 5, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Guenther, M.; Sorber, J.; Suchaneck, G.; Arndt, K.-F.; Richter, A. Chemical and pH sensors based on the swelling behavior of hydrogels. Sens. Actuators B Chem. 2005, 111, 555–561. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart bandage for monitoring and treatment of chronic wounds. Small 2018, 14, 1703509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, C.; Yang, X.; Shen, B.; Sun, Y.; Chen, Y.; Xu, X.; Sun, H.; Yu, K.; Yang, B.; et al. pH and temperature-sensitive hydrogel nanoparticles with dual photoluminescence for bioprobes. ACS Nano 2016, 10, 5856–5863. [Google Scholar] [CrossRef] [PubMed]
- Ajovalasit, A.; Caccami, M.C.; Amendola, S.; Sabatino, M.A.; Alotta, G.; Zingales, M.; Giacomazza, D.; Occhiuzzi, C.; Marrocco, G.; Dispenza, C. Development and characterization of xyloglucan-poly (vinyl alcohol) hydrogel membrane for Wireless Smart wound dressings. Eur. Polym. J. 2018, 106, 214–222. [Google Scholar] [CrossRef]
- Nakagami, G.; Sanada, H.; Iizaka, S.; Kadono, T.; Higashino, T.; Koyanagi, H.; Haga, N. Predicting delayed pressure ulcer healing using thermography: A prospective cohort study. J. Wound Care 2010, 19, 465–472. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart bandages: The future of wound care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef]
- Maruyama, H.; Masuda, T.; Honda, A.; Arai, F. Local temperature measurement using specrta shift of quantum-dot hydrogel sensor. In Proceedings of the 2011 11th IEEE International Conference on Nanotechnology, Portland, OR, USA, 15–19 August 2011; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2011; pp. 319–322. [Google Scholar]
- Ge, G.; Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Muscle-inspired self-healing hydrogels for strain and temperature sensor. ACS Nano 2019, 14, 218–228. [Google Scholar] [CrossRef]
- Augustin, M.; Kirsten, N.; Körber, A.; Wilsmann-Theis, D.; Itschert, G.; Staubach-Renz, P.; Maul, J.-T.; Zander, N. Prevalence, predictors and comorbidity of dry skin in the general population. J. Eur. Acad. Dermatol. Venereol. 2018, 33, 147–150. [Google Scholar] [CrossRef]
- Harding, K. Diagnostics and Wounds, a Consensus Document; World Union of Wound Healing Societies (WUWHS): Turin, Italy, 2007. [Google Scholar]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8, S4–S9. [Google Scholar] [CrossRef]
- Occhiuzzi, C.; Ajovalasit, A.; Sabatino, M.A.; Dispenza, C.; Marrocco, G. RFID epidermal sensor including hydrogel membranes for wound monitoring and healing. In Proceedings of the 2015 IEEE International Conference on RFID (RFID), San Diego, CA, USA, 15–17 April 2015; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2015; pp. 182–188. [Google Scholar]
- Moore, Z.; Webster, J. Dressings and topical agents for preventing pressure ulcers. Cochrane Database Syst. Rev. 2018, 12, CD009362. [Google Scholar] [CrossRef]
- Boyko, T.V.; Longaker, M.T.; Yang, G.P. Review of the current management of pressure ulcers. Adv. Wound Care 2018, 7, 57–67. [Google Scholar] [CrossRef]
- Liao, M.; Liao, H.; Ye, J.; Wan, P.; Zhang, L. Polyvinyl alcohol-stabilized liquid metal hydrogel for wearable transient epidermal sensors. ACS Appl. Mater. Interfaces 2019, 11, 47358–47364. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A Bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar]
- Samant, P.; Prausnitz, M.R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci. USA 2018, 115, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.-W. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Health Mater. 2018, 8, 1801019. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.J.; Jüngel, A.; Rimann, M.; Wuertz-Kozak, K. Advances in the biofabrication of 3D skin In Vitro: Healthy and pathological models. Front. Bioeng. Biotechnol. 2018, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Michael, S.; Bharti, K.; Ferrer, M.; Song, M.J. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 2020, 12, 035002. [Google Scholar] [CrossRef]
- He, X.-P.; Hu, X.-L.; James, T.D.; Yoon, J.; Tian, H. Multiplexed photoluminescent sensors: Towards improved disease diagnostics. Chem. Soc. Rev. 2017, 46, 6687–6696. [Google Scholar] [CrossRef]
- Gačanin, J.; Synatschke, C.V.; Weil, T. Biomedical applications of DNA-based hydrogels. Adv. Funct. Mater. 2019, 30, 1906253. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel actuators and sensors for biomedical soft robots: Brief overview with impending challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Tran, K.T.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- De France, K.; Xu, F.; Hoare, T. Macroporous hydrogels: Structured macroporous hydrogels: Progress, challenges, and opportunities (Adv. Healthcare Mater. 1/2018). Adv. Health Mater. 2018, 7, 1870006. [Google Scholar] [CrossRef]
- Rafael, D.F.; Andrade, F.; Martinez-Trucharte, F.; Basas, J.; Seras-Franzoso, J.; Palau, M.; Gomis, X.; Burgos, M.P.; Blanco, A.; López-Fernández, A.; et al. Sterilization procedure for temperature-sensitive hydrogels loaded with silver nanoparticles for clinical applications. Nanomaterials 2019, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Haridas, N.; Rosemary, M. Effect of steam sterilization and biocompatibility studies of hyaluronic acid hydrogel for viscosupplementation. Polym. Degrad. Stab. 2019, 163, 220–227. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Sartore, L. Effects of gamma sterilization on the physicomechanical and thermal properties of gelatin-based novel hydrogels. Polym. Eng. Sci. 2019, 59, 2533–2540. [Google Scholar] [CrossRef]
- Soliman, S.M.; Malak, N.S.A.; El-Gazayerly, O.N.; Rehim, A.A. Formulation of microemulsion gel systems for transdermal delivery of celecoxib: In Vitro permeation, anti-inflammatory activity and skin irritation tests. Drug Discov. Ther. 2010, 4, 459–471. [Google Scholar]
- Paliwal, S.; Hwang, B.H.; Tsai, K.Y.; Mitragotri, S. Diagnostic opportunities based on skin biomarkers. Eur. J. Pharm. Sci. 2013, 50, 546–556. [Google Scholar] [CrossRef]
- Wisniewski, N.A.; Nichols, S.P.; Gamsey, S.J.; Pullins, S.; Au-Yeung, K.Y.; Klitzman, B.; Helton, K.L. Tissue-integrating oxygen sensors: Continuous tracking of tissue hypoxia. In Single Molecule and Single Cell Sequencing; Springer: New York, NY, USA, 2017; Volume 977, pp. 377–383. [Google Scholar]
- Milo, S.; Thet, N.T.; Liu, D.; Nzakizwanayo, J.; Jones, B.V.; Jenkins, T. An In-Situ infection detection sensor coating for urinary catheters. Biosens. Bioelectron. 2016, 81, 166–172. [Google Scholar] [CrossRef]
- Wang, C.; Javadi, A.; Ghaffari, M.; Gong, S. A pH-sensitive molecularly imprinted nanospheres/hydrogel composite as a coating for implantable biosensors. Biomaterials 2010, 31, 4944–4951. [Google Scholar] [CrossRef]




| Challenges | Descriptions | Potential Solutions |
|---|---|---|
| Sensor sensitivity and consistency | Isolation of sufficient samples: ISF, exudate Complexity with human subjects: complicating diseases and behaviors | Adjusting hydrogel patch/MN, coupling of external technology (e.g., suction force) Multiplexing markers with internal references |
| Scaling-up fabrication of hydrogel technology | Batch-to-batch variation Cost of production Sterilization procedures Prolonging shelf-life | Improvement of fabrication techniques (e.g., soft/photo lithography, 3D printing) Standardized good manufacturing practice Post-synthesis steam sterilization and gamma irradiation Controlled cold and humid storage conditions |
| Proper and safe clinical adaptation | Proper interpretation Risks of inflammation (i.e., erythema and swelling) | Thorough assessment and validation (especially during early stage) Optimization of the application procedures (pre-treatment, timing, frequency, and removal/cleaning) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiraja, C.; Ning, X.; Cui, M.; Xu, C. Hydrogel-Based Technologies for the Diagnosis of Skin Pathology. Technologies 2020, 8, 47. https://doi.org/10.3390/technologies8030047
Wiraja C, Ning X, Cui M, Xu C. Hydrogel-Based Technologies for the Diagnosis of Skin Pathology. Technologies. 2020; 8(3):47. https://doi.org/10.3390/technologies8030047
Chicago/Turabian StyleWiraja, Christian, Xiaoyu Ning, Mingyue Cui, and Chenjie Xu. 2020. "Hydrogel-Based Technologies for the Diagnosis of Skin Pathology" Technologies 8, no. 3: 47. https://doi.org/10.3390/technologies8030047
APA StyleWiraja, C., Ning, X., Cui, M., & Xu, C. (2020). Hydrogel-Based Technologies for the Diagnosis of Skin Pathology. Technologies, 8(3), 47. https://doi.org/10.3390/technologies8030047






