Microwave-Assisted Industrial Scale Cannabis Extraction
Abstract
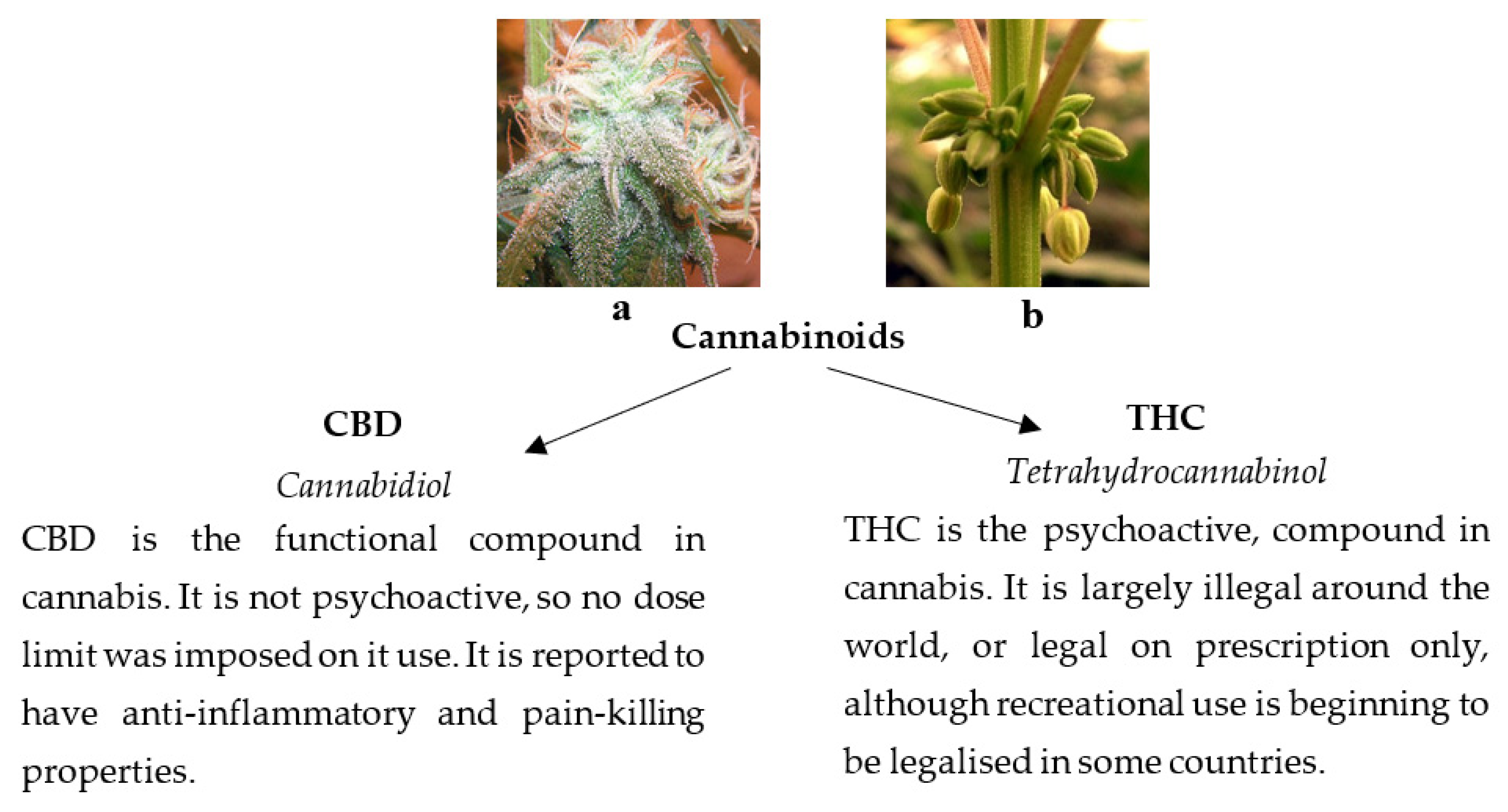
1. Introduction
2. Commercial Cannabis Extraction Methods
2.1. General Considerations
- Extraction efficiency, the percentage of bioactive compounds recovered through the entire extraction process;
- Extract quality and consistency, including the purity or “potency” of cannabinoids in the extract and also the relative amounts or “profile” of other potentially synergistic compounds such as terpenes;
- Throughput capacity and scalability, assessment of the extraction method and its efficient implementation at commercial scales vs. market demand;
- Environmental control, e.g., carbon footprint and safety, i.e., minimize risks to the consumers and worker safety.
- Decarboxylation, the process of converting non-active native acidic cannabinoids into their active, neutral forms via a thermal reaction;
- Winterization, the process of removing plant lipids and unwanted waxes by a secondary solvent, freezing and filtration;
- Decolorization, the process of removing chlorophyll and unwanted pigments;
- Secondary purification, the process of further purifying the extract to increase the potency or alter the composition of cannabinoids and other components, via various methods including distillation, chromatography, or crystallization.
2.2. Scale-Up Considerations
2.3. Available Methods Currently Used for Commercial Cannabis Extraction
- Supercritical CO2 (SC-CO2) extraction
- Pressurized gas (hydrocarbon) extraction
- Conventional organic solvent extraction
2.3.1. Supercritical CO2 (SC-CO2) Extraction
2.3.2. Pressurized Gas (Hydrocarbon) Extraction
2.3.3. Conventional Organic Solvent Extraction
3. Microwave-Assisted Extraction
3.1. General Considerations
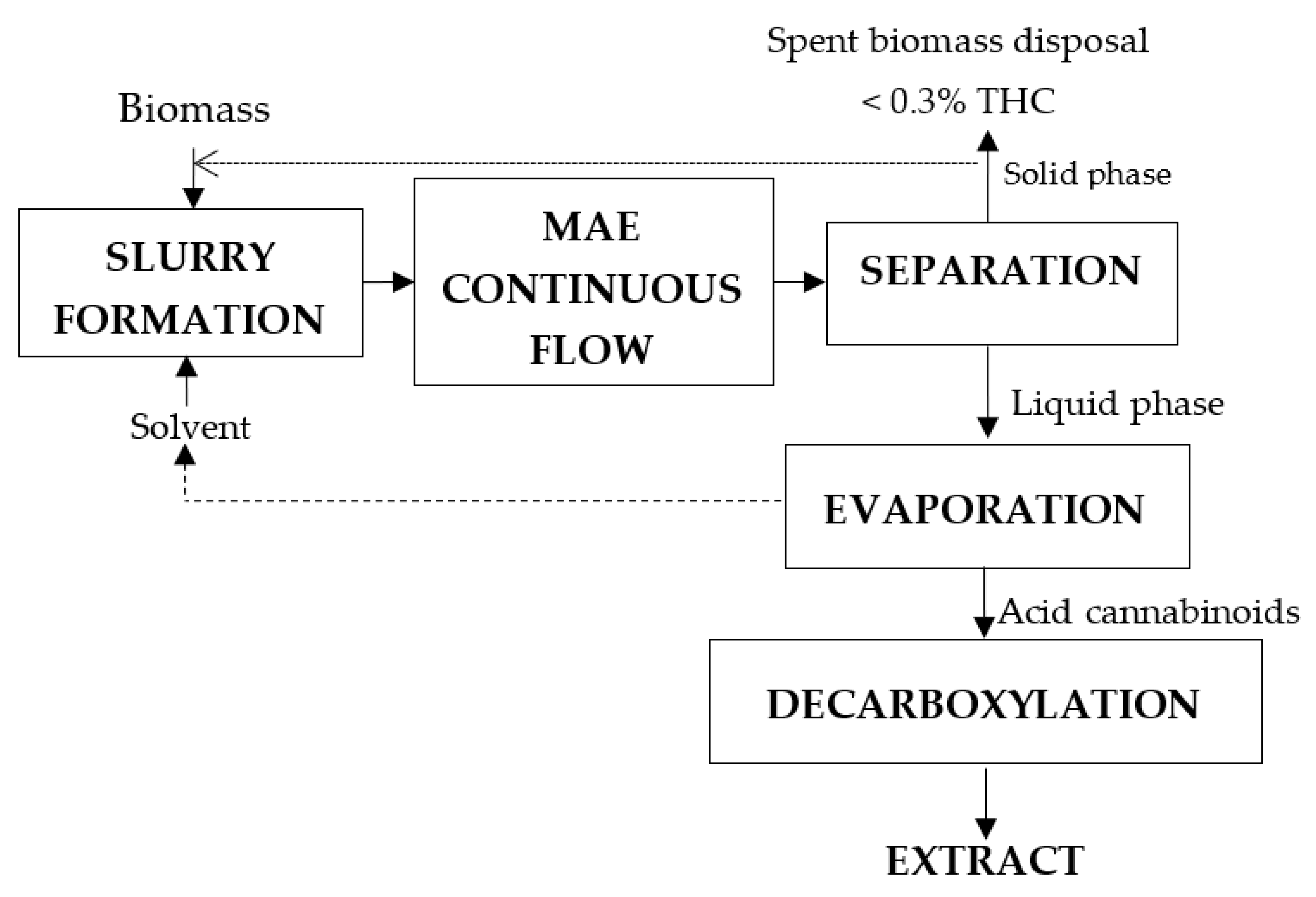
3.2. MAE of Cannabis via MAPTM
- Continuous-flow method at atmospheric pressure which allows for much higher volumes of cannabis biomass to be processed in much less time than existing extraction methods.
- Achieved higher rates of consistency and quality because the process does not require stopping and restarting material flows.
- Scale-up to industrial scale without the need to purchase an endless supply of new machinery and without the use of pressurised batch vessels.
- Eliminates additional steps required in most extraction methods, such as winterisation.
- Ability to achieve high extraction efficiency at industrial scale. Typical recovery of active compounds via MAE is up to 95%.
- The contact time between the biomass and solvent before, during and after microwave treatment can be adjusted much more easily.
- It is possible to precisely control biomass residence time in the microwave zone and—if desired—separate the biomass from the solvent very quickly after treatment, or continue contact for any length of time at any temperature, depending on the desired outcome.
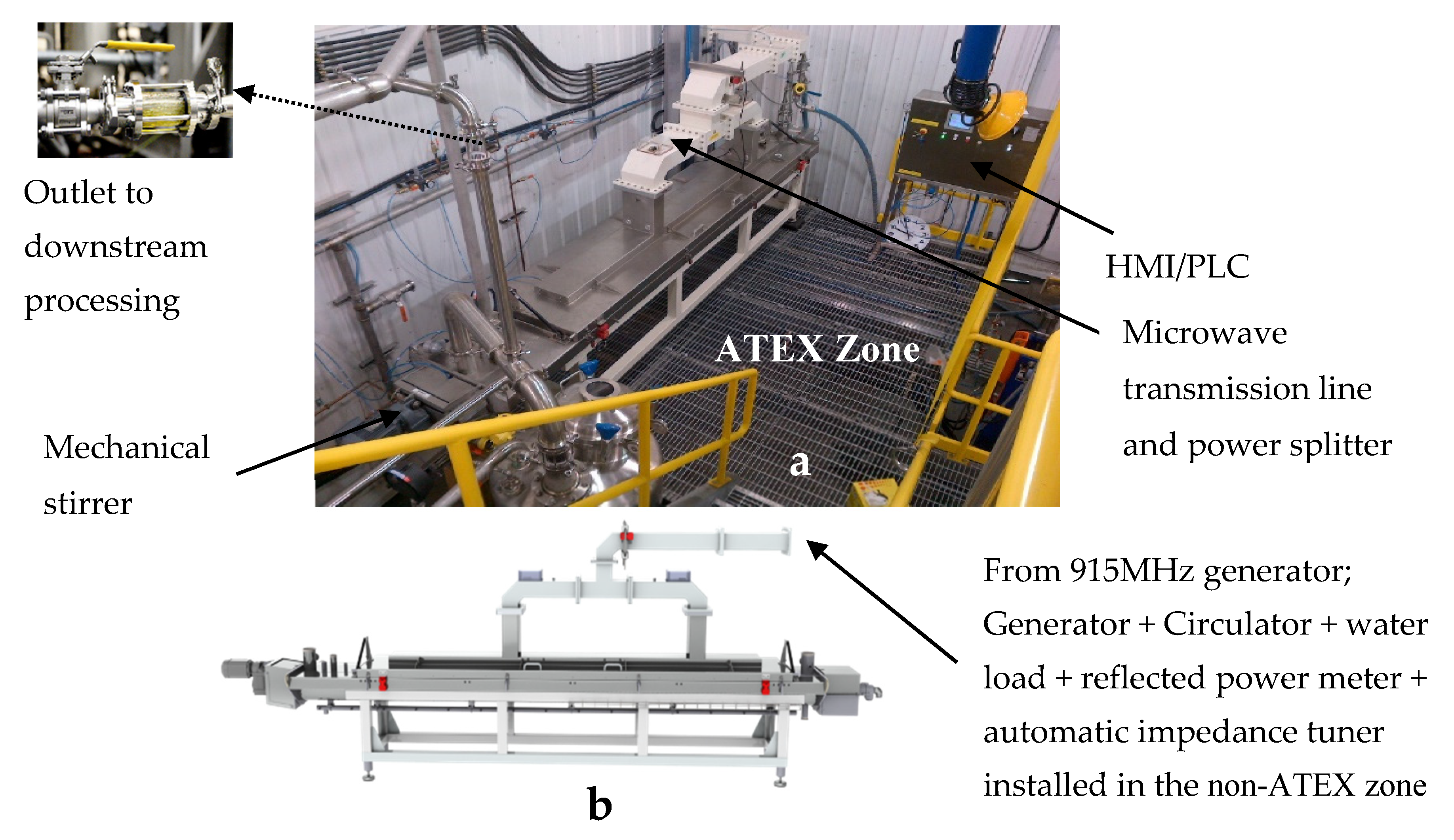
- The use of multiple microwave field deposition points through the use of a split waveguide and a “ridge wave deposition” allowing for non-uniform dispersal of the wave from the inlet to the outlet to account for changing dielectric properties as the material is treated.
- It has an automatic impedance matching unit that allows for constant, automatic adjustment of the field strength and microwave energy absorption maximization.
- It has a built-in mechanical agitator with variable speed control to randomize movement of biomass thus making the field uniform for the materials at all times.
- It is fully automated (operators simply input desired MW parameters on an HMI and it runs itself while connected to the plant PLC systems).
- It is fully ATEX or “hazardous zone” classified, meaning it can be used with any flammable liquid and be completely safe.
3.3. Economics of MAE
- Badly designed reactors (geometry & chosen microwave frequency) vs. quantity & type of reaction mixture.
- Changes in the microwave absorbance of the reaction mixture due to modifications of its temperature, chemical composition, and phase when applicable (e.g., evaporation). This results in a gradual or rapid shift in the power absorbed by the reaction mixture and therefore, for a fixed Pf, an increase in Pr, which is a common problem in obtaining good quality products with good energy efficiency.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Cannabis sativa and Hemp. In Nutraceuticals: Efficacity, Safety and Toxicity; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 735–754. [Google Scholar] [CrossRef]
- Joy, J.E.; Watson, S.J., Jr.; Benson, J.A., Jr. The Medical Value of Marijuana and Related Substances. In Marijuana and Medicine: Assessing the Science Base. Consensus Study Report; National Academies Press: Washington, DC, USA, 1999; Available online: https://www.nap.edu/catalog/6376/marijuana-and-medicine-assessing-the-science-base (accessed on 29 June 2020). [CrossRef]
- EMCDDA. Medical Use of Cannabis and Cannabinoids, Questions and Answers for Policymaking; EMCDDA: Lisbon, Portugal, 2018; Available online: http://www.emcdda.europa.eu (accessed on 10 May 2020). [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press (US): Washington, DC, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK423845/ (accessed on 27 June 2020). [CrossRef]
- EMCDDA. Insights, An Overview of Cannabis Potency in Europe. 2004. Available online: http://www.emcdda.europa.eu (accessed on 10 May 2020).
- Hanus, L.O.; Meyer, S.M.; Munoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Bindesri, S.D.; Jebailey, R.; Albarghouthi, N.; Pye, C.C.; Brosseau, C.L. Spectroelectrochemical and computational studies of tetrahydrocannabinol (THC) and carboxy-tetrahydrocannabinol (THC-COOH). Analyst 2020, 145, 1849–1857. [Google Scholar] [CrossRef]
- Hazekamp, A.; Fischedick, J.T.; Dıez, M.L.; Lubbe, A.; Ruhaak, R.L. Chemistry of Cannabis. In Comprehensive Natural Products II. Chemistry and Biology; Mander, L., Liu, H.-W., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2010; pp. 1033–1084. [Google Scholar]
- Gazendam, A.; Nucci, N.; Gouveia, K.; Khalik, H.A.; Rubinger, L.; Johal, H. Cannabinoids in the Management of Acute Pain: A Systematic Review and Meta-analysis. Cannabis and Cannabinoid Research. Ahead of print. Available online: https://www.liebertpub.com/doi/10.1089/can.2019.0079 (accessed on 9 April 2020).
- Teh, S.-S.; Birch, E. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- Truta, E.; Gille, E.; Toth, E.; Maniu, M. Biochemical Differences in Cannabis Sativa, L. Depending on Sexual Phenotype. J. Appl. Genet. 2002, 43, 451–462. [Google Scholar] [PubMed]
- Prapatsorn, T.; Surapol, N.; Pipop, C.; Sirot, C. Characteristics of cannabinoids composition of Cannabis plants grown in Northern Thailand and its forensic application. Forensic Sci. Int. 2013, 115, 164–170. [Google Scholar] [CrossRef]
- Nahler, G.; Jones, T.M. Pure cannabidiol versus cannabidiol-containing extracts: Distinctly different multi-target modulators. J. Altern. Complement. Integr. Med. 2018, 4, 262–271. [Google Scholar] [CrossRef]
- Smith, D.; Bloor, R.; George, C.; Pysanenko, A.; Spanel, P. Release of toxic ammonia and volatile organic compounds by heated cannabis and their relation to tetrahydrocannabinol content. Anal. Methods 2015, 7, 4104–4110. [Google Scholar] [CrossRef]
- Romano, L.; Hazekamp, A. An overview of galenic preparation methods for medicinal cannabis. Curr. Bioact. Compd. 2019, 15, 174–195. [Google Scholar] [CrossRef]
- Spindle, T.R.; O’Bonn-Miller, M.; Vandrey, R. Changing landscape of cannabis: Novel products, formulations, and methods of administration. Curr. Opin. Psychol. 2019, 30, 98–102. [Google Scholar] [CrossRef]
- Villena, K. Cannabis in Beauty and Personal Care: Prospects, Opportunities and Challenges; Passport, Euromonitor International: London, UK, November 2019. [Google Scholar]
- Wang, M.; Wang, Y.-H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; El Sohly, M.A.; Khan, I.A. Decarboxylation study of acidic cannabinoids. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Perino-Issartier, S.; Maingonnat, J.-F.; Chemat, F. Microwave Food Processing. In RSC Green Chemistry No. 10. Alternatives to Conventional Food Processing; Proctor, A., Ed.; The Royal Society of Chemistry: London, UK, 2011; pp. 415–458. [Google Scholar] [CrossRef]
- Zhou, L.; Lie, Y.; Briers, H.; Fan, J.; Remon, J.; Nystrom, J.; Budarin, V.; Macquarrie, D.; McElroy, C.R. Natural Product Recovery from Bilberry (Vaccinium myrtillus L.) Presscake via Microwave Hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3676–3685. [Google Scholar] [CrossRef]
- Agarwal, C.; Mathe, K.; Hofmann, T.; Csoka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis Sativa L. Optimized by Response Surface Methodology. Food Eng. Mater. Sci. Nanotechnol. 2018, 83, 701–710. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Cannabinoids: Extraction Methods, Analysis, and Physicochemical Characterization. Stud. Nat. Prod. Chem. 2018, 61, 143–163. [Google Scholar]
- Ternelli, M.; Brighenti, V.; Anceschi, L.; Poto, M.; Bertelli, D.; Licatac, M.; Pellati, F. Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes. J. Pharm. Biomed. 2020, 186, 113296. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Rezet Jambrak, A.; Munetaka, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. Available online: www.rsc.com (accessed on 2 April 2020). [CrossRef]
- King, J.K.; Srinivas, K.; Zhang, D. Advances in Critical Fluid Processing. In RSC Green Chemistry No. 10. Alternatives to Conventional Food Processing; Proctor, A., Ed.; The Royal Society of Chemistry: London, UK, 2011; pp. 93–144. [Google Scholar] [CrossRef]
- Ribeiro Grijó, D.; Vieitez Osorio, I.A.; Cardozo-Filho, L. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers. J. CO2 Util. 2018, 28, 174–180. [Google Scholar] [CrossRef]
- Attard, T.M.; Hunt, A.J. Supercritical Carbon Dioxide Extraction of Lipophilic Molecules. In Green Chemistry Series No. 57, Supercritical and Other High-Pressure Solvent Systems: For Extraction, Reaction and Material Processing; Hunt, A.J., Attard, T.M., Eds.; The Royal Society of Chemistry: London, UK, 2018; pp. 40–76. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Moreno, T.; Montanes, F.; Tallon, S.J.; Fenton, T.; King, J.W. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: An overview of different processing options. J. Supercrit. Fluids 2020, 161, 104850. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Martínez Ramírez, J.A.; Rivera Monroy, Z.J.; King, J.W.; Parada-Alfonso, F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Angela, M.; Meireles, A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds. Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: New York, NY, USA; Heildelberg, Germany; Dordrech, The Netherlands; London, UK, 2013; pp. 15–52. [Google Scholar]
- Chemat, F.; Abert Vian, M.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Patrascu, M.; Radoiu, M. Rose Essential Oil Extraction from Fresh Petals Using Synergetic Microwave & Ultrasound Energy: Chemical Composition and Antioxidant Activity Assessment. J. Chem. Eng. 2016, 10, 136–142. [Google Scholar] [CrossRef]
- Radoiu, M.; Splinter, S.; Popek, T. Continuous industrial-scale microwave-assisted extraction of high-value ingredients from natural biomass. In Proceedings of the 53rd IMPI’s Microwave Power Symposium, Las Vegas, NV, USA, 18–20 June 2019. [Google Scholar]
- Drinić, Z.; Vladic, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Kiprovski, B.; Vidovic, S. Microwave-assisted extraction of cannabinoids and antioxidants from Cannabis sativa aerial parts and process modeling. J. Chem. Technol. Biotechnol. 2020, 95, 831–839. [Google Scholar] [CrossRef]
- Radoiu, M. Industrial Microwave Reactors: Components and set-up, In Microwave Chemistry; Cravotto, G., Carnaroglio, D., Eds.; Walter De Gruyter GmbH: Berlin, Germany, 2017; pp. 65–90. ISBN 978-3-11-047992-8. [Google Scholar]

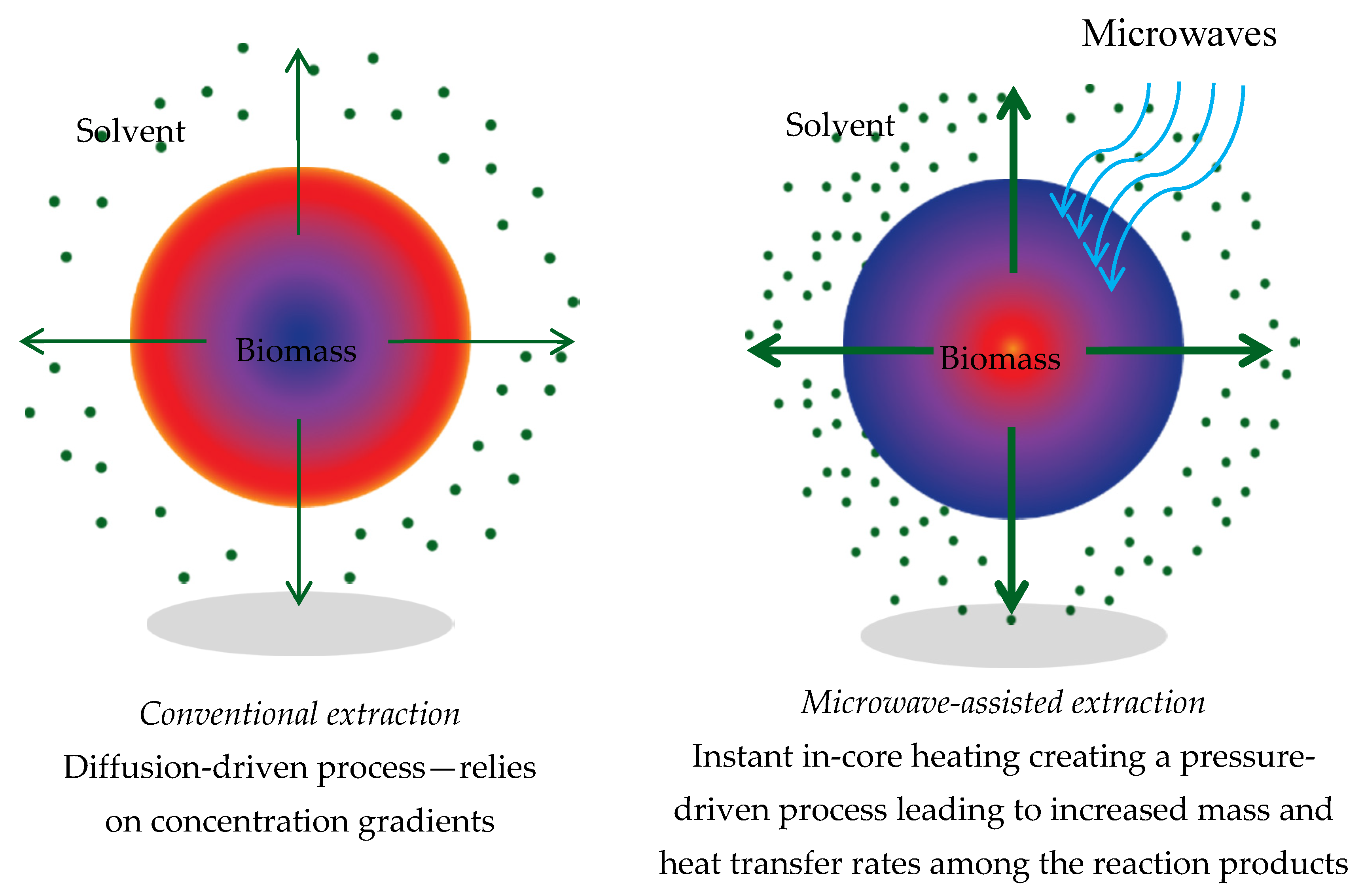
| Conventional Extraction | Microwave Assisted Extraction |
|---|---|
| Mechanism via diffusion | Pressure-enhanced mass transfer |
| Concentration gradient of actives between the biomass and the solvent is the driving force | Microwave energy is selectively absorbed by the residual water present in the biomass cells |
| Diffusion is slow, particularly as the actives become more concentrated in the solvent | Results in rapid pressure buildup within cells leading to a pressure-driven mass transfer of actives (pop-corn effect) |
| Eventually reaches a saturation point | Extraction is very fast and not limited by an equilibrium state—transfer continues as long as energy is applied |
| Requires high solvent ratios and multiple extraction stages to achieve reasonable recovery of actives | Results in short extraction times, reduced solvent requirements and fewer extraction stages |
| Run | Mass of Biomass kg | Purity of Cannabis Extract THC % | THC Recovery in the Extract % |
|---|---|---|---|
| 1 | 100 | 61.4 ± 0.04 | 92.6 |
| 2 | 100 | 55.1 ± 0.4 | 93.4 |
| Frequency | 915 MHz a | 2450 MHz a |
|---|---|---|
| Number of generators to deliver 75 kW | 1 (×72 kW) | 12 (×6 kW) |
| Generator price b Total price for microwave generators | 90 k$ 1 × 90 k$ = 90 k$ | 10 k$ 12 × 10 k$ = 120 k$ |
| Microwave transmission line (waveguides, impedance tuners, and other waveguide components required to transmit the microwave power from the generator to the reactor) | 1 × 15 c k$ = 15 k$ | 12 × 5d k$ = 60 k$ |
| CAPEX (microwave generators and microwave transmission line) | 105 k$ | 180 k$ |
| Main consumable, magnetron | ||
| Operation lifetime e Price/unit Total/operation lifetime | 6000 h 8 k$ = 8 k$ | 7000 h 2.5 k$ = 30 k$ |
| Mains electricity consumption f,g | ~85 kW | ~100 kW |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radoiu, M.; Kaur, H.; Bakowska-Barczak, A.; Splinter, S. Microwave-Assisted Industrial Scale Cannabis Extraction. Technologies 2020, 8, 45. https://doi.org/10.3390/technologies8030045
Radoiu M, Kaur H, Bakowska-Barczak A, Splinter S. Microwave-Assisted Industrial Scale Cannabis Extraction. Technologies. 2020; 8(3):45. https://doi.org/10.3390/technologies8030045
Chicago/Turabian StyleRadoiu, Marilena, Harmandeep Kaur, Anna Bakowska-Barczak, and Steven Splinter. 2020. "Microwave-Assisted Industrial Scale Cannabis Extraction" Technologies 8, no. 3: 45. https://doi.org/10.3390/technologies8030045
APA StyleRadoiu, M., Kaur, H., Bakowska-Barczak, A., & Splinter, S. (2020). Microwave-Assisted Industrial Scale Cannabis Extraction. Technologies, 8(3), 45. https://doi.org/10.3390/technologies8030045





