The Effect of the Terminal Functional Groups on Fluoropolymer on Electrowetting Device Performance
Abstract
1. Introduction
2. Materials and Methods
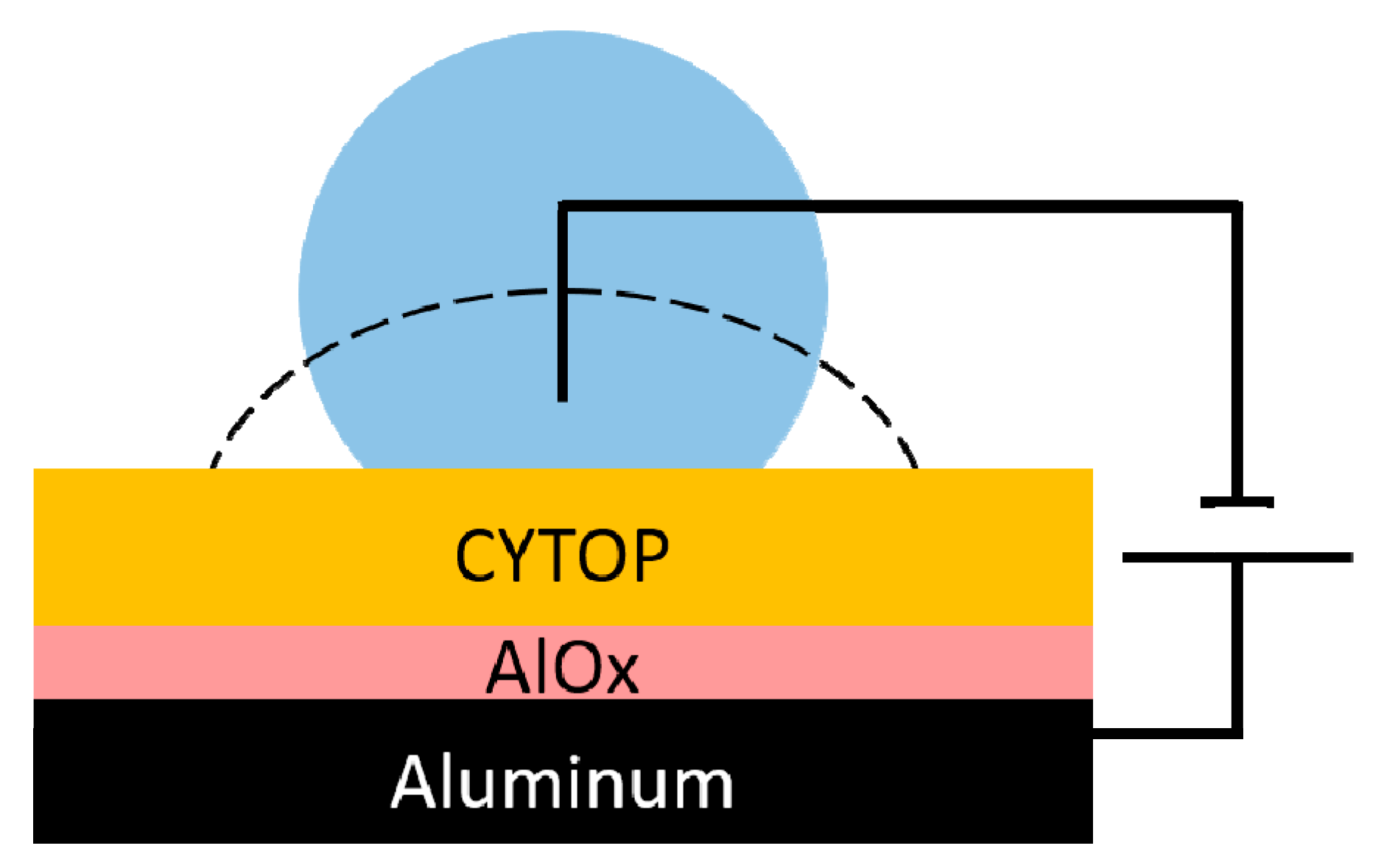
2.1. Fabrication Method for Basic EWOD Devices
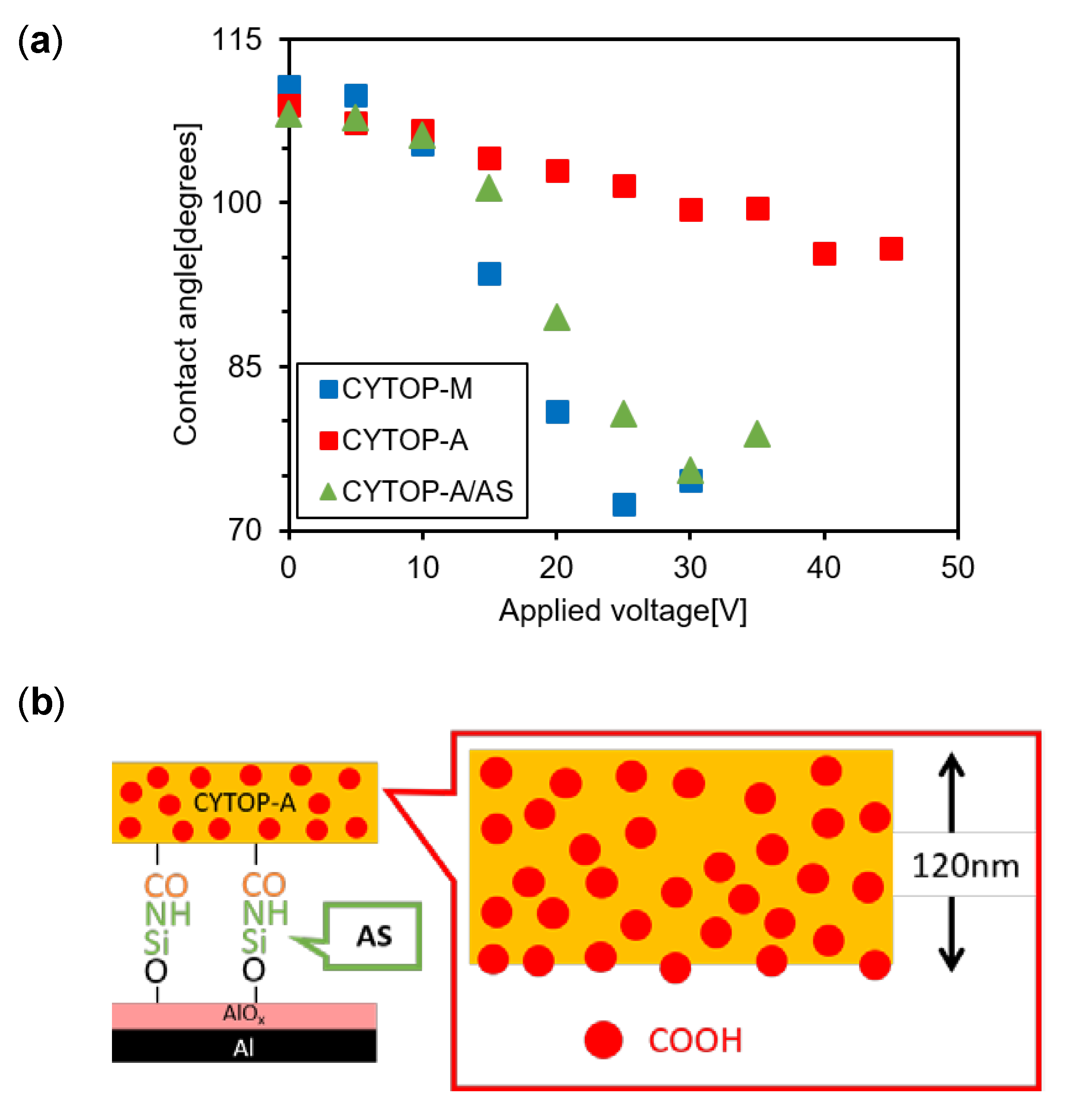
2.2. Silane Coupling Treatment for Formulation of an Amide Bond to the CYTOP-A/AlOx Interface
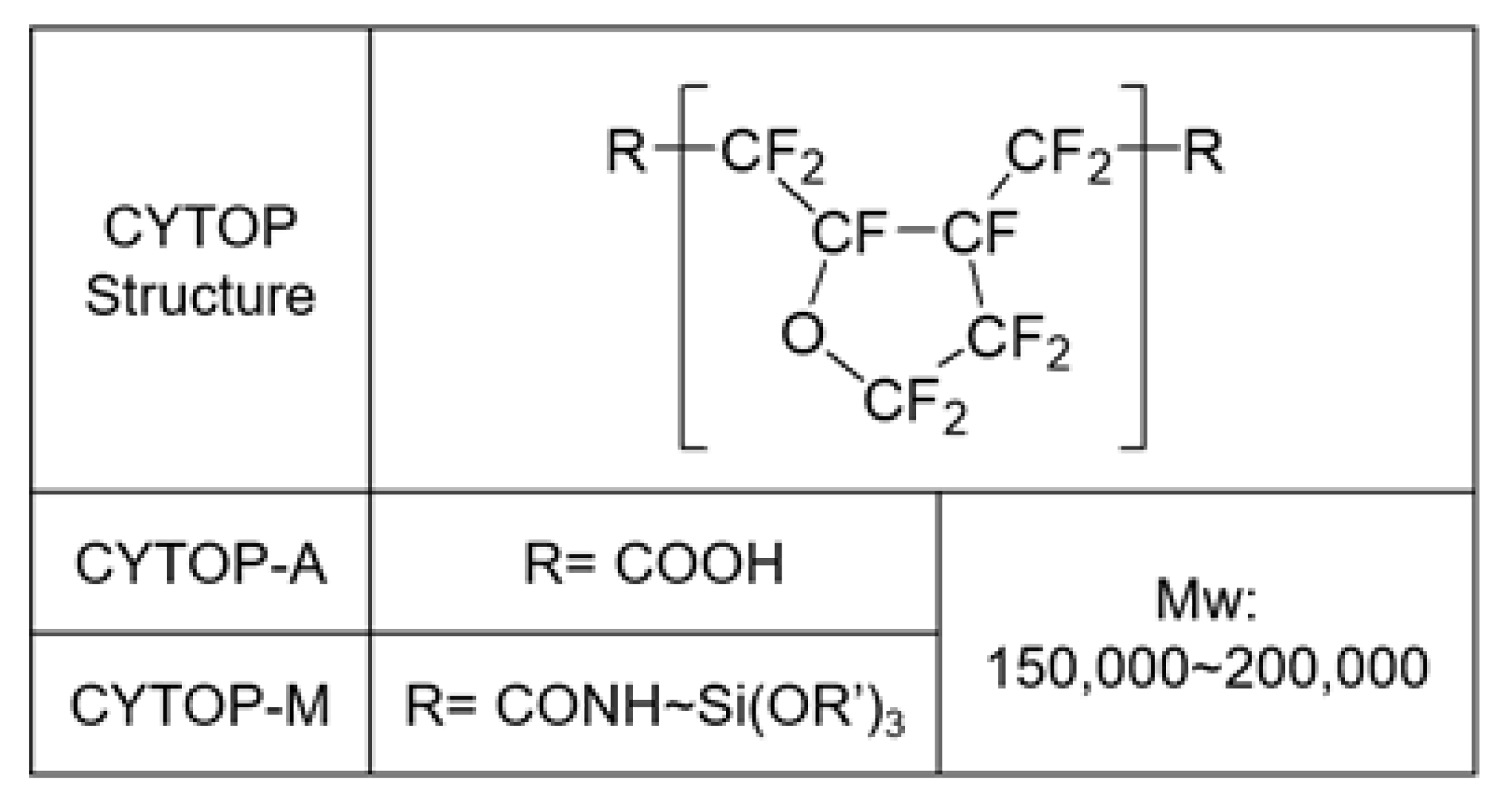
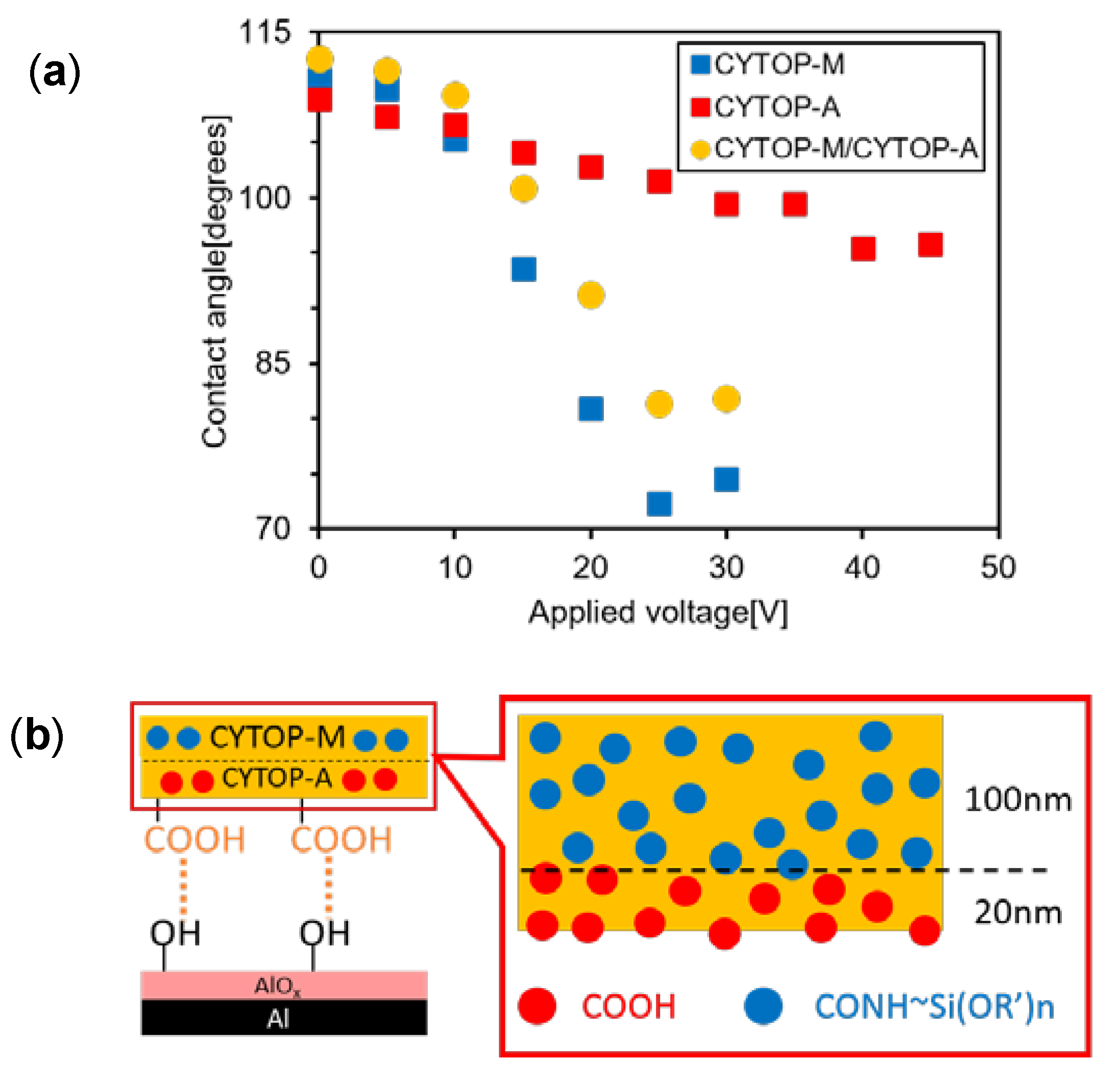
2.3. Double Layer Formation Using Two Types of CYTOP
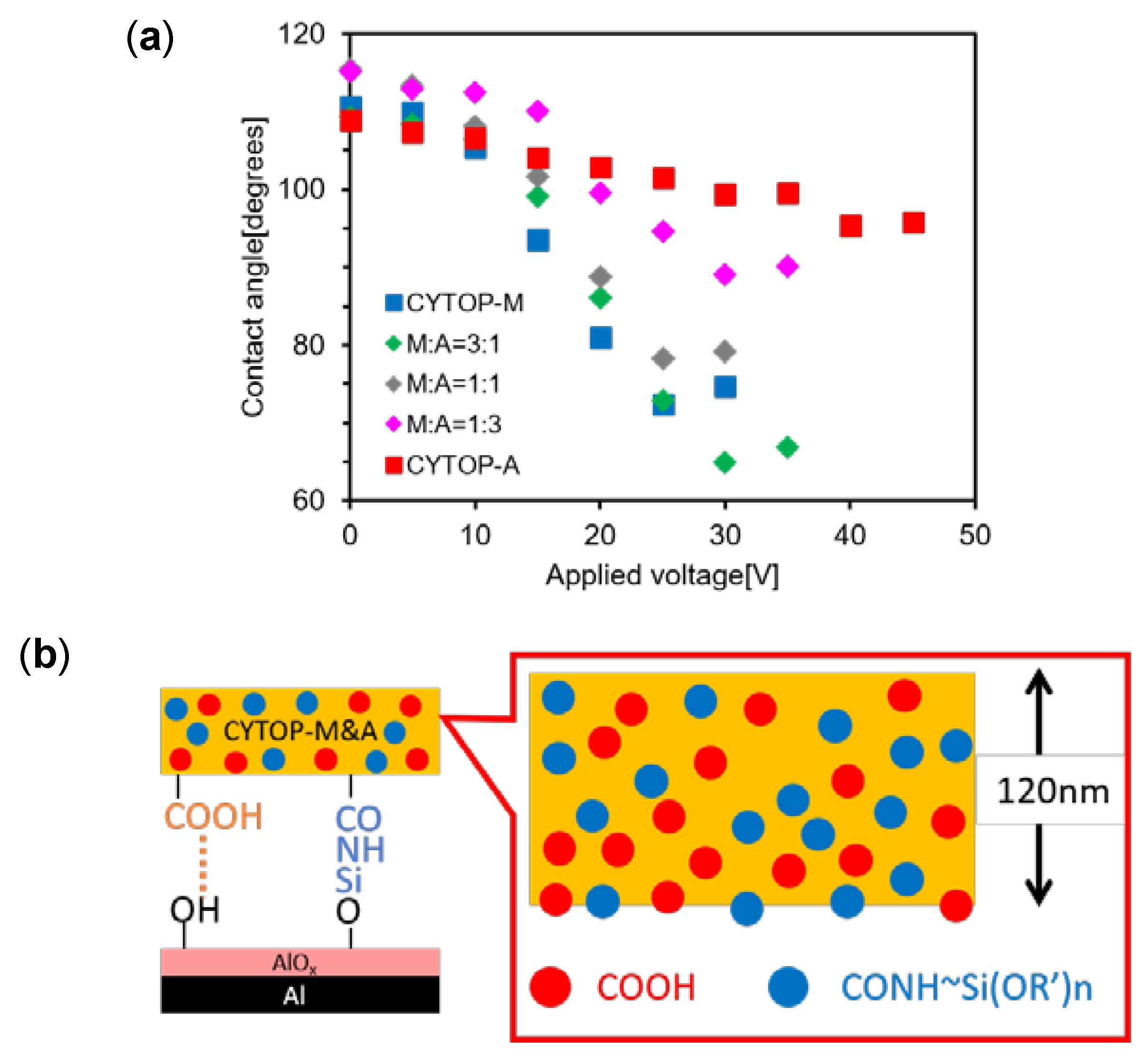
2.4. Thin Layer Preparation Using a Solution Blended with Two Types of CYTOP
2.5. Characterization and Electrowetting Test
3. Results and Discussion
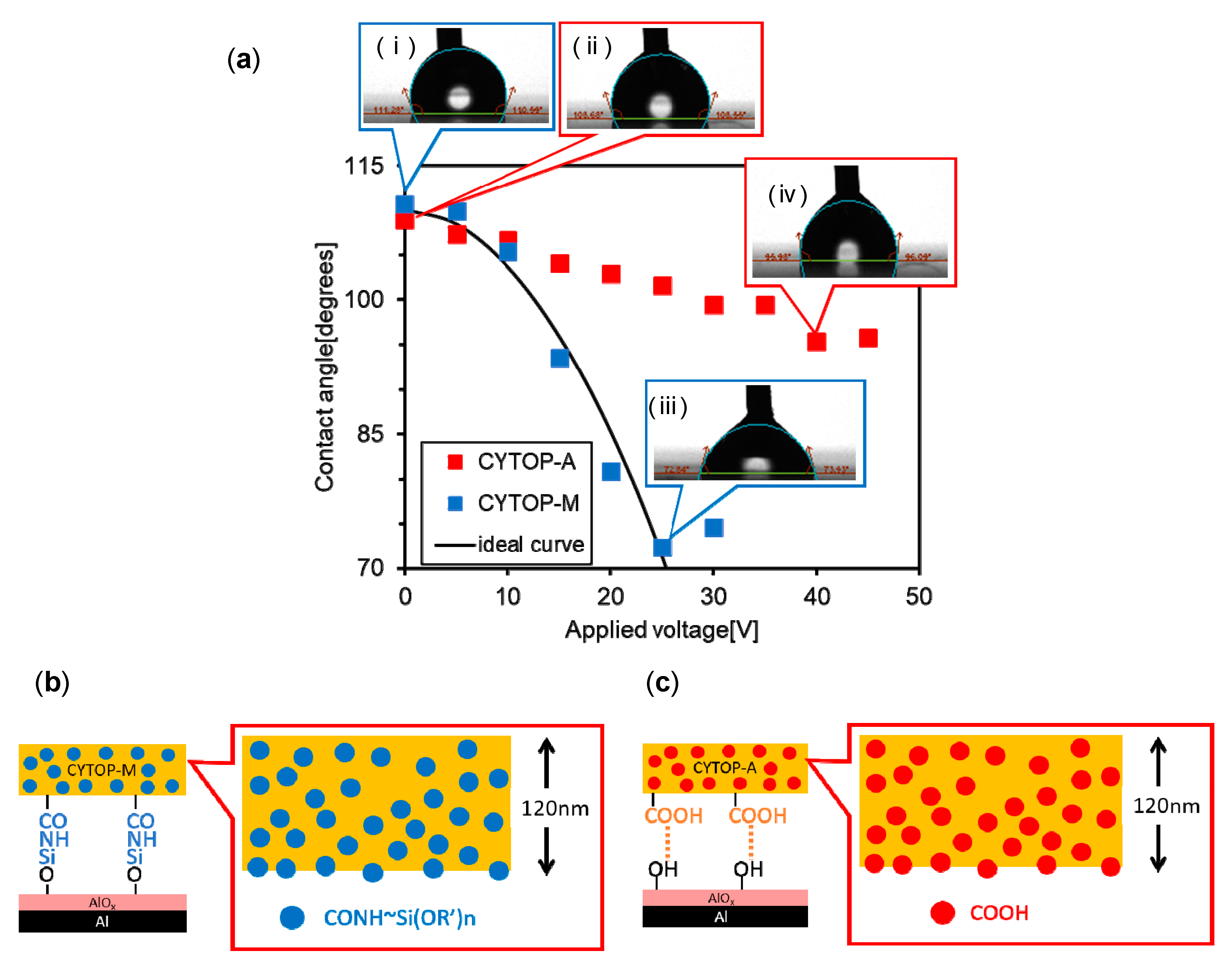
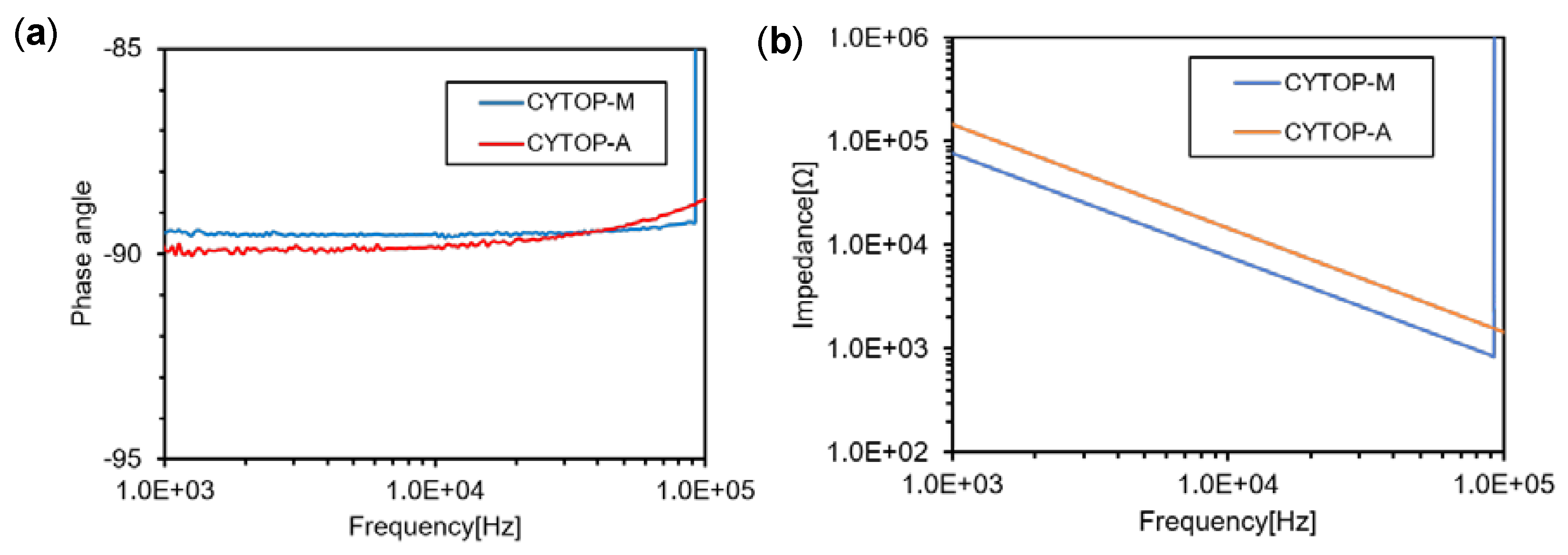
3.1. Comparison between CYTOP-M and CYTOP-A
3.2. Effects of Amino Silane Coupling Treatment on the CYTOP-A/AlOx Interface
3.3. Effect of the Double Layer Structure of CYTOP-A and CYTOP-M
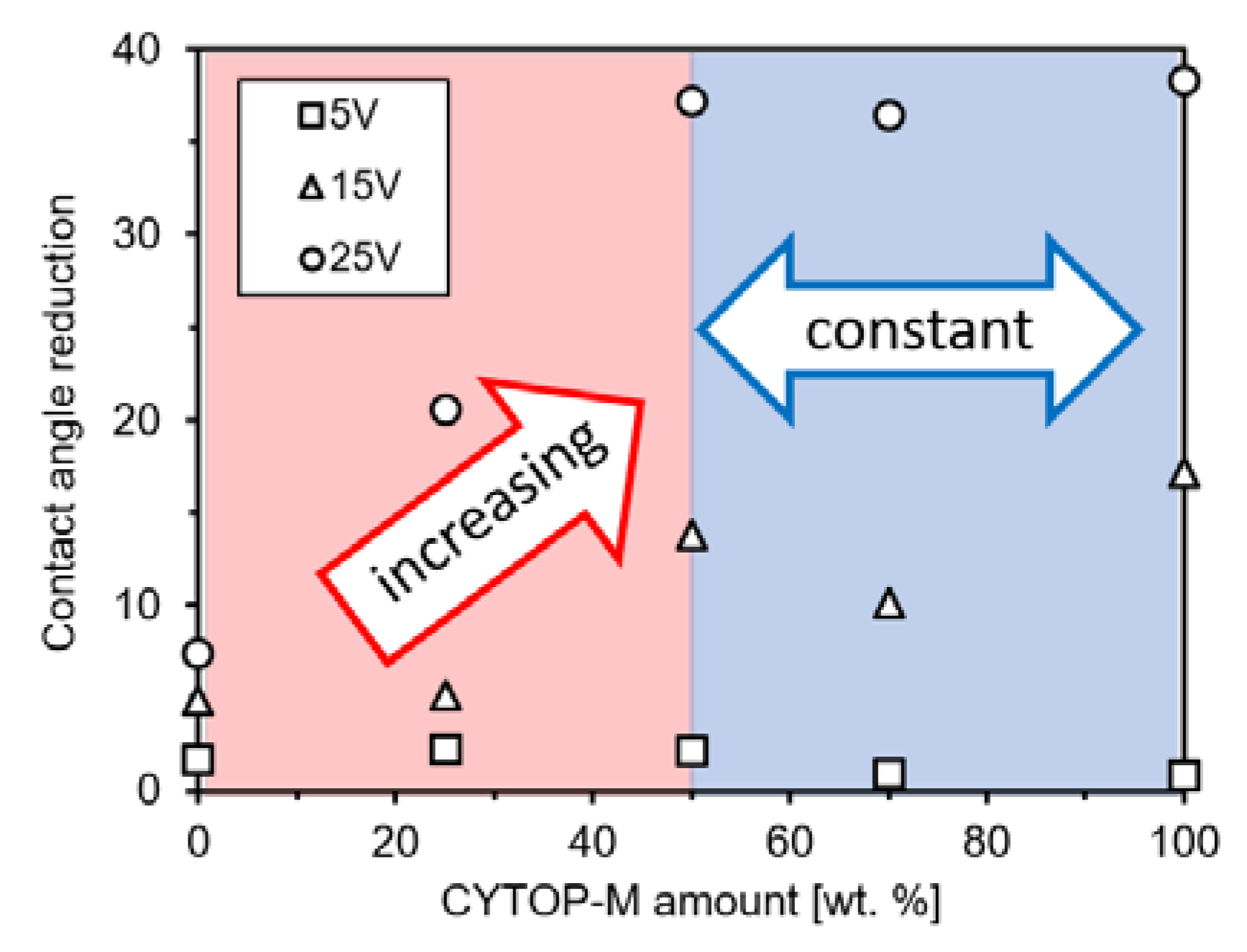
3.4. Correlation of Amide Groups (High Polar) and Carboxy Groups (Low Polar) Quantities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Satoh, W.; Hosono, H.; Suzuki, H. On-Chip Microfluidic Transport and Mixing Using Electrowetting and Incorporation of sensing Functions. Anal. Chem. 2015, 77, 6857–6863. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A.; Feenstra, B.J. Video-speed electronic paper based on electrowetting. Lett. Nat. 2003, 425, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Krupenkin, T.; Taylor, J.A. Reverse electrowetting as a new approach to high-power energy harvesting. Nat. Commun. 2011, 2, 448. [Google Scholar] [CrossRef] [PubMed]
- Sohail, S.; Mistri, E.A.; Khan, A.; Banerjee, S.; Biswas, K. Fabrication and performance study of BST/Teflon nanocomposite thin film for low voltage electrowetting devices. Sens. Actuators A 2016, 238, 122–132. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Hong, S.-M.; Park, S.-S.; Hong, Y.-P. The Movement of Micro Droplet with the Effects of Dielectric Layer and Hydrophobic Surface Treatment with R. F. Atmospheric Plasma in EWOD Structure. J. Phys. Conf. Ser. 2006, 34, 650–655. [Google Scholar] [CrossRef]
- Narasimhan, V.; Park, S.Y. An Ion Gel as a Low-Cost, Spin-Coatable, High-Capacitance Dielectric for Electrowetting-on-Dielectric (EWOD). Langmuir 2015, 31, 8512–8518. [Google Scholar] [CrossRef] [PubMed]
- Sawane, Y.B.; Ogale, S.B.; Banpurkar, A.G. Low Voltage Electrowetting on Ferroelectric PVDF-HFP Insulator with Highly Tunable Contact Angle Range. Appl. Mater. Interfaces 2016, 8, 24049–24056. [Google Scholar] [CrossRef] [PubMed]
- Roques-Carmes, T.; Aldeek, F.; Balan, L.; Corbel, S.; Schneider, R. Aqueous Dispersions of core/shell CdSe quantum dots as nanofluids for electrowetting. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 269–277. [Google Scholar] [CrossRef]
- Mibus, M.; Hu, X.; Knospe, C.; Reed, M.L.; Zangari, G. Failure Modes during Low-Voltage Electrowetting. Appl. Mater. Interfaces 2016, 8, 15767–15777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Parkes, W.; Haworth, L.I.; Ross, A.W.S.; Stevenson, J.T.M.; Walton, A.J. Room-Temperature Fabrication of Anodic Tantalum Pentoxide for Low-Voltage Electrowetting on Dielectric (EWOD). J. Microelectromechanical Syst. 2008, 17, 1481–1488. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oishi, E.; Araki, N.; Goto, T.; Awano, H.; Takahashi, T. The Effect of the Terminal Functional Groups on Fluoropolymer on Electrowetting Device Performance. Technologies 2019, 7, 52. https://doi.org/10.3390/technologies7030052
Oishi E, Araki N, Goto T, Awano H, Takahashi T. The Effect of the Terminal Functional Groups on Fluoropolymer on Electrowetting Device Performance. Technologies. 2019; 7(3):52. https://doi.org/10.3390/technologies7030052
Chicago/Turabian StyleOishi, Eri, Noritoshi Araki, Teruya Goto, Hiroshi Awano, and Tatsuhiro Takahashi. 2019. "The Effect of the Terminal Functional Groups on Fluoropolymer on Electrowetting Device Performance" Technologies 7, no. 3: 52. https://doi.org/10.3390/technologies7030052
APA StyleOishi, E., Araki, N., Goto, T., Awano, H., & Takahashi, T. (2019). The Effect of the Terminal Functional Groups on Fluoropolymer on Electrowetting Device Performance. Technologies, 7(3), 52. https://doi.org/10.3390/technologies7030052



