Abstract
Reversible absorbents for safe storage of toxic hydrazine were developed. Various cross-linked polar polymers were examined as absorbents for hydrazine and its 35% aqueous solution, and structurally similar polymers were found to be suitable for effective absorption. Namely, cross-linked polyacrylamide (CPAM) was most effective among examined various hydrophilic polymers. CPAM absorbed 43- and 31-fold heavier amounts of absolute hydrazine and 35% aqueous solution, respectively, by simple soaking. Absorbed hydrazine could be quantitatively released either by N2 gas flow and compression, and the resulting absorbent reabsorbed hydrazine without loss of the absorption ability. The absorption ability was higher than conventional covalent storages, and the release protocol, without dissolution of the absorbent, are suitable for storage systems in hydrazine fuel cells.
1. Introduction
Hydrazine contains high energy and has been applied as a fuel. The most practical applications are fuels for reaction control systems of artificial satellites, auxiliary power units for spacecraft like the Space Shuttle, and propellants of rockets and spacecraft like the MESSENGER orbiting the planet Mercury [1,2,3]. The fighter jet F-16 Fighting Falcon also employs hydrazine as the fuel of the aircraft’s emergency power unit. The hypergolic property by mixing with N2O4, ammonium nitrate, and energetic ionic liquids realizes simple ignition systems suitable to space vehicles [1,2,3,4,5,6]. Another potential application is a fuel for basic fuel cells [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Hydrazine fuel cells using 10–15% aqueous solution have some advantages toward acidic typical fuel cells based on hydrogen or methanol. The output is as high as that of hydrogen fuel cells. The low corrosion properties of hydrazine solutions enable uses of metals such as Co, Ni, and carbon materials unable to be used in conventional fuel cells requiring Pt due to the harsh acidity. Liquid fuels are basically advantageous for consumers’ uses such as fuel stations for automobiles and domestic fuel cells by the safer methods of storage and refueling.
However, as other fuels, the high energy can cause unfortunate explosions originating from leakage of hydrazine-based fuels. The high toxicity of absolute or highly concentrated hydrazine used for rocket fuels is also a severe problem [9,21,22,23]. For example, inhalation exposures of hundreds ppm orders of hydrazine derivatives are reported to be lethal to various animals. This severe problem accentuates the importance of safe treatment protocols for hydrazine fuels.
For safe storage of hydrazine derivatives and its aqueous solution, immobilization of hydrazine derivatives has been examined to avoid leakage. Some polar polymers such as various polysaccharides serve as gellants for hydrazine and its derivatives [24,25,26,27,28,29,30]. The gels are thixotropic, and can be transformed to sols with fluidity suitable enough to be supplied to combustion engines of rockets through pipelines. However, the liquids containing the gellants may revert to gels again, which is responsible to clogging up the pipelines. Due to the drain of the gellants with the fuels, this system cannot be applied to rechargeable fuel systems, such as for cars and aircrafts. Accordingly, desirable immobilization systems are those with reversibility based on immobilizers remaining in fuel tanks. From this point of view, chemical immobilization of hydrazine in aqueous solution was attempted using cross-linked polymers bearing ketone moieties [10]. The reversible immobilization proceeds via the condensation of hydrazine with the ketone moieties to produce pendant hydrazone moieties, which can be hydrolyzed to release hydrazine via the treatment with hot water. However, the amount of storable hydrazine on the support is very low (below 5 wt%) due to the hydrophilic segment to give the affinity with aqueous hydrazine and the stoichiometric nature of the immobilization. With insufficient recyclability of approximately 80%, probably originating from the undesired Wolff–Kishner reduction consuming the ketone moieties, is also a problem.
We thought that a simple gelation system universally adoptable to various applications, namely rockets, aircrafts, and fuel cells, is more desirable. Materials focused on are absorbents based on insoluble polymers capable of absorbing high amounts of hydrazine to the weight of the absorbents without dissolution. Herein, we report absorbents for hydrazine enabling efficient and reversible gelation consisting of absorption by simple soaking and quantitative release by compression or N2 flow.
2. Materials and Methods
2.1. Materials
Absolute hydrazine and poly(vinyl alcohol) (PVA) (degree of polymerization ≒ 2000, degree of saponification ≒ 80) were purchased from Tokyo Kasei Kogyo (Tokyo, Japan), and used without purification. Aqueous solution of hydrazine (35 wt%) and poly(N-vinyl pyrrolidone) (PVP) (Mw = 1,300,000) were purchased from Aldrich (Missouri, MO, USA), and used without purification. N-Isopropylacrylamide was purchased from Wako Chemical (Tokyo, Japan) and purified by recrystallization with a mixed solvent of hexane and diethyl ether. N,N-Dimethylacrylamide was purchased from Wako Chemical and purified by distillation under reduced pressure in the presence of CaH2. Acrylamide was purchased from Wako Chemical and purified by recrystallization with methanol. N-Hydroxymethylacrylamide and 2,2′-azobisisobutyronitrile (AIBN) were purchased from Tokyo Kasei Kogyo, and purified by recrystallization with methanol. Methylenebisacrylamide (MBAA) and formalin were purchased from Kanto Chemical (Tokyo, Japan), and used without purification. Bracken starch was purchased from Inoue Tengyokudo (Nara, Japan). Sulfuric acid (47% in H2O) was purchased from Wako Chemical, and used as 20% solution after dilution with water.
2.2. Methods
2.2.1. Synthesis of Polymers by Radical Polymerization (Typical Procedure)
Absorbents were prepared via a conventional free radical polymerization technique. A monomer, AIBN (1 mol%), and 1,4-dioxane (1 mL to 1 mmol of the monomer) were added in a glass tube under a nitrogen atmosphere. The tube was degassed, sealed, and heated at 60 °C for 4 h. The polymer was isolated by precipitation into diethyl ether and drying under reduced pressure. The molecular weight was estimated by size exclusion chromatography on a Tosoh (Tokyo, Japan) HLC-8220 GPC instrument equipped with four consecutive polystyrene gel columns [Tosoh TSK-gel (bead size and exclusion limit molecular weight); Super AW-H (9 μm and 4 × 107 g/mol), Super AW-5000 (7 μm and 4 × 106 g/mol), Super AW-4000 (6 μm and 4 × 105 g/mol), and Super AW-3000 (4 μm and 6 × 104 g/mol)] using N,N-dimethyl formamide (DMF) containing 10 mM lithium bromide as an eluent at 40 °C. Cross-linked polymers were prepared by radical polymerization using 0.500 g of monomer and 3 mol% amounts of MBAA initiated by potassium persulfate and tetramethylethylenediamine (10 wt% to monomer and 10 drops, respectively) in 10 mL of water, and purified by Soxhlet extraction with water.
Polyacrylamide (PAM): AM = 0.178 g, Yield = 0.152 g (74.0%), molecular weight was not measured due to the poor solubility in DMF.
Poly(N-hydroxymethylacrylamide) (PHMAM): HMAM = 0.253 g, Yield = 0.228 g (90.1%), molecular weight was not measured due to the poor solubility in DMF.
Poly(N-isopropyl acrylamide) (PNIPAM): NIPAM = 0.283 g, Yield = 0.233 g (82.0%), Mn (Mw/Mn) = 58,300 (2.60).
Poly(N,N-dimethylacrylamide) (PDMAM): DMAM = 0.249 g, Yield = 0.216 g (86.7%), Mn (Mw/Mn) = 35,000 (3.97).
Cross-linked PAM: (CPAM): AM = 0.500 g, Yield = 0.367 g (68.9%).
Cross-linked PHMAM (CPHMAM): HMAM = 0.500 g, Yield = 0.465 g (84.5%).
Cross-linked PDMAM (CPDMAM): DMAM = 0.500 g, Yield = 0.313 g (59.7%).
2.2.2. Synthesis of Cross-Linked PVA (CPVA)
PVA (2.00 g) was dissolved in distilled water (10 mL) at 75 °C in a round-bottom flask. The flask was cooled to 40 °C, and an aqueous dispersion of bracken starch (1.00 g in 10 mL of water) was added. Then, the mixture was stirred at 80 °C until the mixture became a paste. The paste was cooled to 40 °C, and 20% sulfuric acid (12.0 mL) and 37% formalin (5.0 mL) were added. The mixture was stirred at 60 °C for 5 h. The precipitate was collected by filtration and washed with water for 30 min (3 times). A white solid was obtained after drying under reduced pressure (1.79 g, yield = 76.0%).
2.2.3. Gelation of Hydrazine with Absorbents
An absorbent (100 mg) was added in a 5-mL glass vial. Then, hydrazine or its aqueous solution was slowly added drop-wise until the added liquids were not absorbed. The excess amounts of hydrazine were removed with cotton swabs. The amounts of the absorbed hydrazine were determined gravimetrically.
2.2.4. Releasing of Hydrazine from Hydrazine gels by N2 Gas Flow (Typical Procedure)
Hydrazine gel (100 mg, CPAM/hydrazine = 1/29 (w/w)) was placed in a cylindrical glass tube (length = 3.0 cm, diameter = 0.5 cm) equipped with a N2 gas inlet and a hydrazine outlet leading to a cold trap cooled with liquid N2. N2 gas was flowed at a flow rate of 100 mL/min. The amount of released hydrazine was determined by the gravimetric change of the glass tube containing gel measured at every one hour after wiping off the hydrazine droplets emitted from the gel.
2.2.5. Releasing of Hydrazine from Hydrazine Gels by Mechanical Compression
Hydrazine gel (1.255 g, CPAM/hydrazine = 1/23 (w/w)) was filled in a 10-mL plastic syringe equipped with cotton at the outlet. The plunger directly attached with an IMADA (Toyohashi, Japan) DS2-500N force meter was pushed at a pressure of 85 N/cm2. The syringe containing gel was weighed, and the amount of released hydrazine was determined by the gravimetric decrease (0.554 g, 46 wt% to absorbed hydrazine).
3. Results and Discussion
3.1. Solubility of Polymers in Anhydrous and Aqueous Hydrazine
First, the solubility of water-soluble polymers was examined for anhydrous hydrazine and 35 wt% aqueous hydrazine solution (Table 1). The examined polymers are PNIPAM, PDMAM, PVP, PA, PHMAM, and PVA (Figure 1). Protic polymers without hydrophobic moieties, namely PAM, PHMAM, and PVA, had higher affinity with hydrazine. A plausible reason is the multiple and intermolecular hydrogen bonds between the slightly acidic protons and the basic nitrogen atoms in hydrazine, and the Lewis basic structures such as carbonyl groups and the active protons in the polymers, respectively. The high polarity of hydrazine (dielectric constant = 52) presumably resulted in the lower affinity with the aprotic polymers. This solubility correlates with the Hildebrand solubility parameters (δ) [31]. The δ values may be calculated by various methods, and the calculated values for some polymers differ with the methods. Specifically, the reported δ values for PNIPAM range widely from 19.2 to 28.0, and a typical and average value is indicated in Table 1. The protic polymers with small substituents have higher δ than the aprotic polymers except PVP, and exhibited high affinity with hydrazine. PAM bearing the most analogous structure with hydrazine and having the highest δ value was soluble in both anhydrous hydrazine and 35 wt% hydrazine aq. The polymers having moderately high δ values, namely PHMAM, PVA, and PVP, showed relatively lower affinity with hydrazine and the aqueous solution. PVA was dissolved in hydrazine at room temperature without any treatment, but swelled in 35 wt% hydrazine aq. This result indicates that PVA have higher affinity toward hydrazine than water requiring heating for dissolving of PVA. The insolubility of PNIPAM having an active hydrogen atom in absolute hydrazine would have originated from the high hydrophobicity of the isopropyl group, which cannot be overcome by the affinity based on the hydrogen bonds. The lower affinity of PVP toward the high δ value is the absence of active hydrogen capable of interacting with the basic nitrogen atom in hydrazine. As a result of the hydrophobic isopropyl group, PNIPAM was soluble in 35 wt% hydrazine aq. below 0 °C, showing lower critical solution temperature behavior.

Table 1.
Solubility of water-soluble polymers in hydrazine and 35 wt% hydrazine aq.
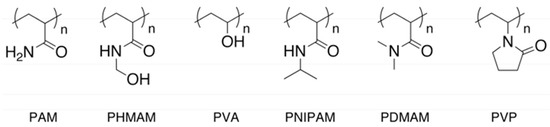
Figure 1.
Structures of examined polymers.
3.2. Gelation of Hydrazine with Cross-Linked Polymers
We prepared insoluble absorbents for practical applications. CPAM, CPHMAM, and CPVA were selected by their high affinity. CPDMAM was also examined as a reference. CPAM, CPHMAM, and CPDMAM were obtained via cross-linking copolymerization with MBAA (3 mol% to monomers). CPVA was prepared by acetalization of PVA with formalin. The absorption of absolute hydrazine and 35 wt% hydrazine aq. was examined at room temperature (Table 2). The amounts of gelation were defined as the amounts required for losing fluidity (Figure 2).

Table 2.
Saturated amounts of anhydrous hydrazine and 35 wt% hydrazine aq. absorbed by cross-linked absorbents (cross-linker contents = 3 mol%).
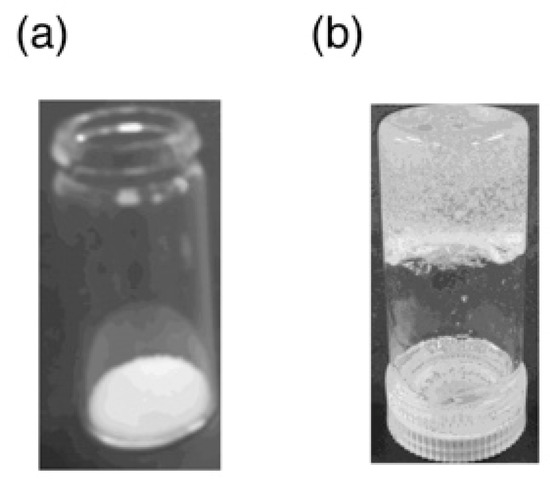
Figure 2.
Photo images of (a) CPAM (100 mg) and (b) gel of CPAM absorbing the saturated amount of anhydrous hydrazine.
CPAM was the best absorbent for both anhydrous and aqueous solution of hydrazine, as can be expected from the solubility. The absence of active hydrogen atoms in CPDMAM resulted in lower absorption ability in spite of the presence of the carbonyl groups accepting the active hydrogen atoms of hydrazine to improve the absorption ability. The low absorption ability of CPVA can be attributed to the strong hydrogen bonding between the hydroxyl groups and the lower affinity between the OH and the protic amide groups.
In order to develop more effective absorbent, the effect of the cross-linker content of CPAM was examined for absorption of anhydrous hydrazine (Figure 3). The maximum absorption of 58-fold in weight was attained using 1 mol% amount of MBAA. The amounts of absorbed hydrazine are comparable to gelation with polysaccharides typically used in 1–10 wt% to hydrazine [24,25,26,27,28,29,30]. The lower cross-linker content resulted in low cross-linking efficiency leading to lower ability to keep hydrazine in the gel. As the increase of the cross-linker content, the absorption efficiency decreased gradually. This decrease may be ascribed to the increase in the cross-linking density preventing swell by hydrazine.
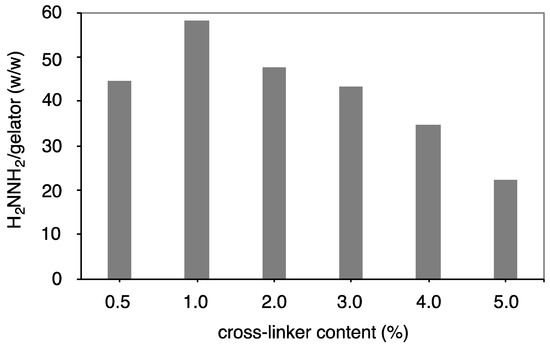
Figure 3.
Effect of cross-linker content on saturated amount of anhydrous hydrazine absorbed by CPAM.
3.3. Release of Absorbed Hydrazine
We tried two methods, N2 gas flow and compression, for releasing of absorbed hydrazine from the hydrazine gels. First, the releasing by N2 gas flow is described. Hydrazine gel prepared from CPAM and anhydrous hydrazine [1/29 (w/w)] was placed in a glass tube, and hydrazine was released by N2 flow. CPAM used was prepared using 4 mol% amount of MBAA. The amount of released hydrazine was measured gravimetrically (Figure 4). Higher flow rate of N2 resulted in higher releasing rate, and 97% of hydrazine was released after 6 h by 100 mL/min of N2 flow. All the releasing rates are almost constant during the releasing experiments, suggesting good mobility of hydrazine in the gels. The remained dried absorbent could absorb identical amount of hydrazine again, and absorbed hydrazine was released almost quantitatively by the same treatment (Table 3). This result demonstrates the reversibility of this system.
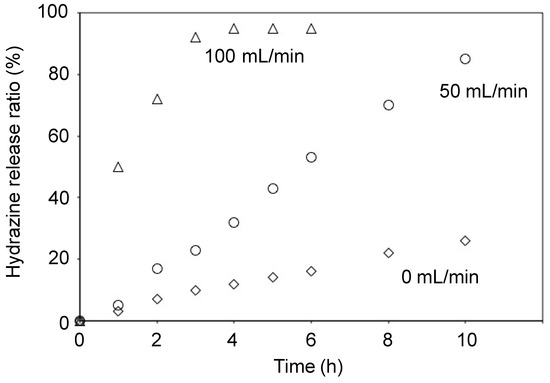
Figure 4.
Releasing of hydrazine from CPAM-based hydrazine gel (CPAM/hydrazine = 1/29 (w/w)) by N2 flow.

Table 3.
Absorption of hydrazine with CPAM and releasing of hydrazine from CPAM-based hydrazine gel by N2 gas flow.
The second method is the releasing by compression. The hydrazine gel prepared from hydrazine and CPAM (w/w = 23/1, 5% cross-linked) was filled in a plastic syringe equipped with a cotton filter, and the releasing was conducted by pushing the plunger at 85 N/m2. As a result, 46% amount of hydrazine was released. The resulting hydrazine gel absorbed hydrazine by immersion into hydrazine, and the reabsorbed amount was identical to the first absorption (23-fold weight to the absorbent). Although the release was not quantitative under this compression condition, this release method by compression is suitable to be built in fuel cell systems.
5. Conclusions
A reversible gelation system for hydrazine was developed for applications to safe storage-release of hydrazine fuels for hydrazine-based basic fuel cells. Various cross-linked polymers absorbed hydrazine by simple soaking without dissolution. CPAM was the best absorbent among various hydrophilic cross-linked polymers examined, and absorbed 43- and 31-fold heavier amounts of absolute hydrazine and 35% aqueous solution, respectively, by simple soaking. Absorbed hydrazine could be quantitatively released either by N2 gas flow and compression. The resulting absorbent after the release process remained without dissolution, and reabsorbed hydrazine without loss of the absorption ability. These simple methods of release and the recyclability are suitable for installing into fuel cell systems. Accordingly, the developed reversible gelation system will provide hydrazine fuel cells safety in charge and storage of the hydrazine fuels.
Further works are ongoing for practical applications in fuel cells for vehicles. The challenges remained involve further optimization of materials, examination on tolerance to temperature and repeated gelation-release cycles, and designs of systems for delivery of fuels into cells. We will report the update soon.
Author Contributions
Conceptualization, B.O.; Methodology, B.O. and Y.S.; Validation, B.O. and Y.S.; Formal Analysis, Y.S.; Investigation, Y.S.; Resources, Y.S.; Data Curation, Y.S.; Writing, B.O.; Visualization, B.O.; Supervision, B.O.; Project Administration, B.O.; Funding Acquisition, B.O.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McNutt, R.L., Jr.; Solomon, S.C.; Gold, R.E.; Leary, J.C. The MESSENGER Team, The MESSENGER mission to Mercury: Development history and early mission status. Adv. Space Res. 2006, 38, 564–571. [Google Scholar] [CrossRef]
- Leary, J.C.; Conde, R.F.; Dakermanji, G.; Engelbrecht, C.S.; Ercol, C.J.; Fielhauer, K.B.; Grant, D.G.; Hartka, T.J.; Hill, T.A.; Jaskulek, S.E.; et al. The MESSENGER spacecraft. Space Sci. Rev. 2007, 131, 187–217. [Google Scholar] [CrossRef]
- Jain, S.R. Self-igniting Fuel-oxidizer Systems and Hybrid Rockets. J. Sci. Ind. Res. 2003, 62, 293–310. [Google Scholar]
- Lai, K.Y.; Zhu, R.S.; Lin, M.C. Why mixtures of hydrazine and dinitrogen tetroxide are hypergolic? Chem. Phys. Lett. 2012, 537, 33–37. [Google Scholar] [CrossRef]
- Solomon, Y.; DeFini, S.J.; Pourpoint, T.L.; Anderson, W.E. Gelled Monomethyl Hydrazine Hypergolic Droplet Investigation. J. Propuls. Power 2013, 29, 79–86. [Google Scholar] [CrossRef]
- Davis, S.M.; Yilmaz, N. Advances in Hypergolic Propellants: Ignition, Hydrazine, and Hydrogen Peroxide Research. Adv. Aerosp. Eng. 2014, 2014, 729313. [Google Scholar] [CrossRef]
- Evans, G.E.; Kordesch, K.V. Hydrazine-air fuel cells. Hydrazine-air fuel cells emerge from the laboratory. Science 1967, 158, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Meibuhr, S.G. Surface-Catalyzed Anodes for Hydrazine Fuel Cells I. Preparation of the Substrate. J. Electrochem. Soc. 1974, 121, 1264–1270. [Google Scholar] [CrossRef]
- Demirci, U.B. Direct liquid-feed fuel cells: Thermodynamic and environmental concerns. J. Power Sources 2007, 169, 239–246. [Google Scholar] [CrossRef]
- Asazawa, K.; Yamada, K.; Tanaka, H.; Oka, A.; Taniguchi, M.; Kobayashi, T. A platinum-free zero-carbon-emission easy fuelling direct hydrazine fuel cell for vehicles. Angew. Chem. Int. Ed. 2007, 46, 8024–8027. [Google Scholar] [CrossRef] [PubMed]
- Asazawa, K.; Sakamoto, T.; Yamaguchi, S.; Yamada, K.; Fujikawa, H.; Tanaka, H.; Oguro, K. Study of anode catalysts and fuel concentration on direct hydrazine alkaline anion-exchange membrane fuel cells. J. Electrochem. Soc. 2009, 156, B509–B512. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Direct hydrazine fuel cells: A review. Appl. Catal. B Environ. 2010, 98, 1–9. [Google Scholar] [CrossRef]
- Rees, N.; Compton, R. Carbon-free energy: A review of ammonia- and hydrazine-based electrochemical fuel cells. Energy Environ. Sci. 2011, 4, 1255–1260. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukasawa, K.; Nishino, E.; Yamaguchi, S.; Yamada, K.; Tanaka, H.; Bae, B.; Miyatake, K.; Watanabe, M. Anion conductive block poly(arylene ether)s: Synthesis, properties, and application in alkaline fuel cells. J. Am. Chem. Soc. 2011, 133, 10646–10654. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Chinchilla, J.; Asazawa, K.; Sakamoto, T.; Yamada, K.; Tanaka, H.; Strasser, P. Noble metal-free hydrazine fuel cell catalysts: EPOC effect in competing chemical and electrochemical reaction pathways. J. Am. Chem. Soc. 2011, 133, 5425–5431. [Google Scholar] [CrossRef] [PubMed]
- Granot, E.; Filanovsky, B.; Presman, I.; Kuras, I.; Patolsky, F. Hydrazine/air direct-liquid fuel cell based on nanostructured copper anodes. J. Power Sources 2012, 204, 116–121. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrog. Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Yan, X.; Meng, F.; Xie, Y.; Liu, J.; Ding, Y. Direct N2H4/H2O2 fuel cells powered by nanoporous gold leaves. Sci. Rep. 2012, 2, 941. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhong, X.; Dong, Z.; Wang, J.; Jin, J.; Ma, J. A highly active hydrazine fuel cell catalyst consisting of a Ni-Fe nanoparticle alloy plated on carbon materials by pulse reversal. RSC Adv. 2012, 2, 5038–5040. [Google Scholar] [CrossRef]
- Akbar, K.; Kim, J.; Lee, Z.; Kim, M.; Yi, Y.; Chun, S. Superaerophobic graphene nano-hills for direct hydrazine fuel cells. NPG Asia Mater. 2017, 9, e378. [Google Scholar] [CrossRef]
- Choudhary, G.; Hansen, H. Human health perspective on environmental exposure to hydrazines: A review. Chemosphere 1998, 37, 801–843. [Google Scholar] [CrossRef]
- Ritz, B.; Zhao, Y.; Krishnadasan, A.; Kennedy, N.; Morgenstern, H. Estimated effects of hydrazine exposure on cancer incidence and mortality in aerospace workers. Epidemiology 2006, 17, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Chong, C.H.; Ng, W.T.; Lim, D. Hydrazine inhalation hepatotoxicity. Occup. Med. 2007, 57, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.; Gupta, B.L.; Pandey, M. Formulation & storage studies on hydrazine-based gelled propellants. Def. Sci. J. 1996, 46, 435–442. [Google Scholar] [CrossRef]
- Kubota, N.; Fujiyoshi, H.; Ayabe, T. Gelling propellant and manufacture therefor. JP. Patent Application No. 3,876,469, 10 November 2006. (In Japanese). [Google Scholar]
- Rahimi, S.; Hasan, D.; Perets, A. Development of laboratory-scale gel-propulsion technology. J. Propul. Power 2004, 20, 93–100. [Google Scholar] [CrossRef]
- Rahimi, S.; Perets, A.; Natan, B. On share rheology of gel propellants. Propellants Explos. Pyrotech. 2007, 32, 165–174. [Google Scholar] [CrossRef]
- Rahimi, S.; Perets, A.; Natan, B. Rheological Matching of Gel Propellants. J. Propul. Power 2010, 26, 376–379. [Google Scholar] [CrossRef]
- Rahimi, S.; Weihs, D. Gelled fuel simulant droplet impact onto a solid surface. Propellants Explos. Pyrotech. 2011, 36, 273–281. [Google Scholar] [CrossRef]
- Su, X.; Kimura, S.; Wada, M.; Kim, U.-J.; Kuga, S. Complexation of hydrazine with native cellulose in water and toluene. Cellulose 2013, 20, 1023–1029. [Google Scholar] [CrossRef]
- Barton, A.F.M. Handbook of Polymer-Liquid Interaction Parameters and Solubility Parameters; CRC Press: Boca Raton, FL, USA, 1990; ISBN 0-8493-3544-2. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).