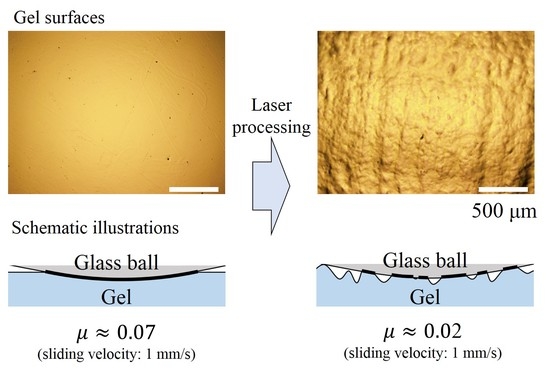
Enormously Low Frictional Surface on Tough Hydrogels Simply Created by Laser-Cutting Process
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of DN gel
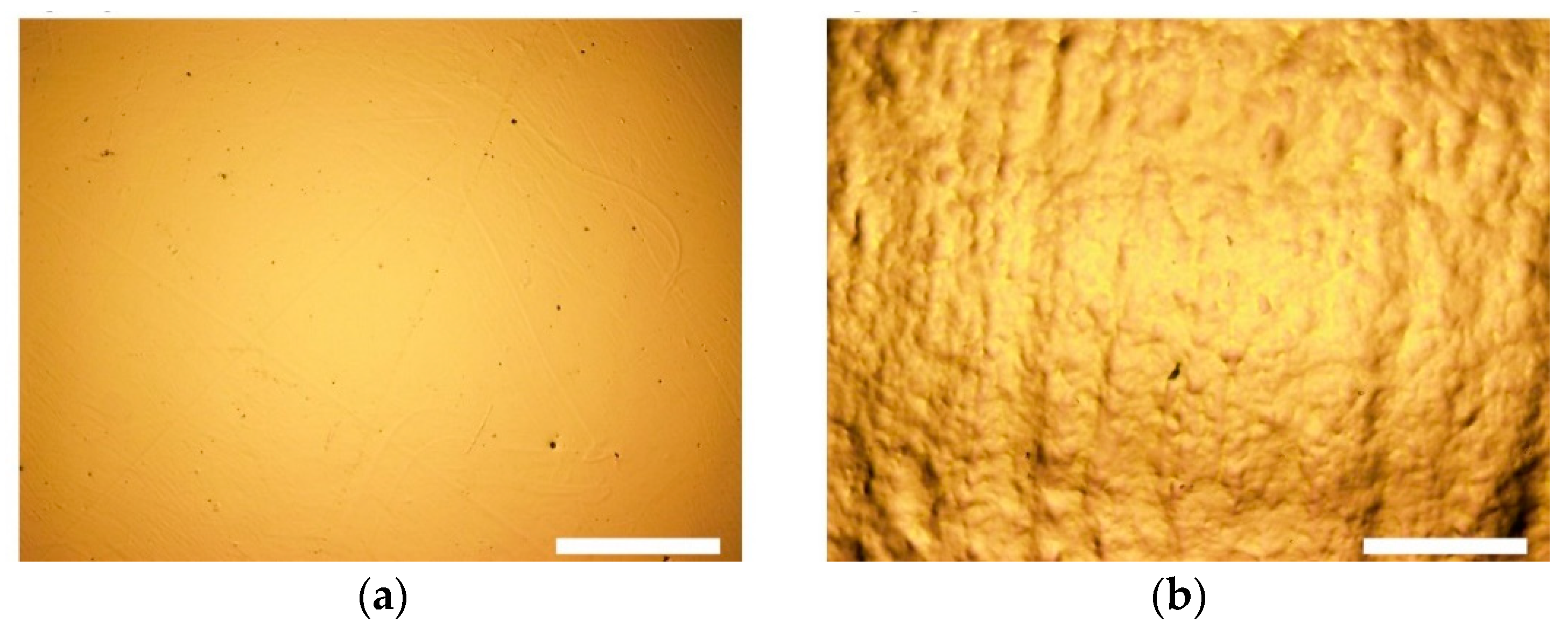
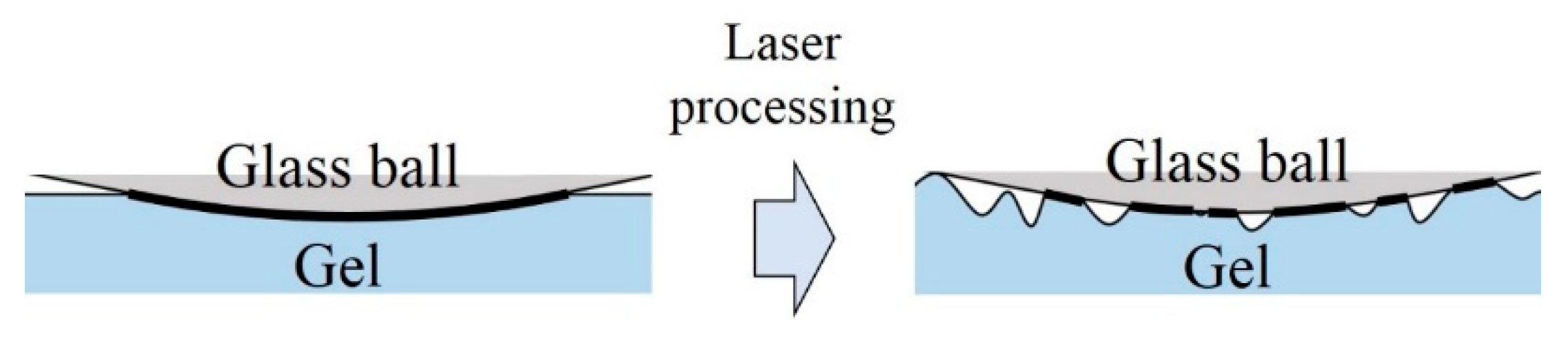
2.2. Laser Processing
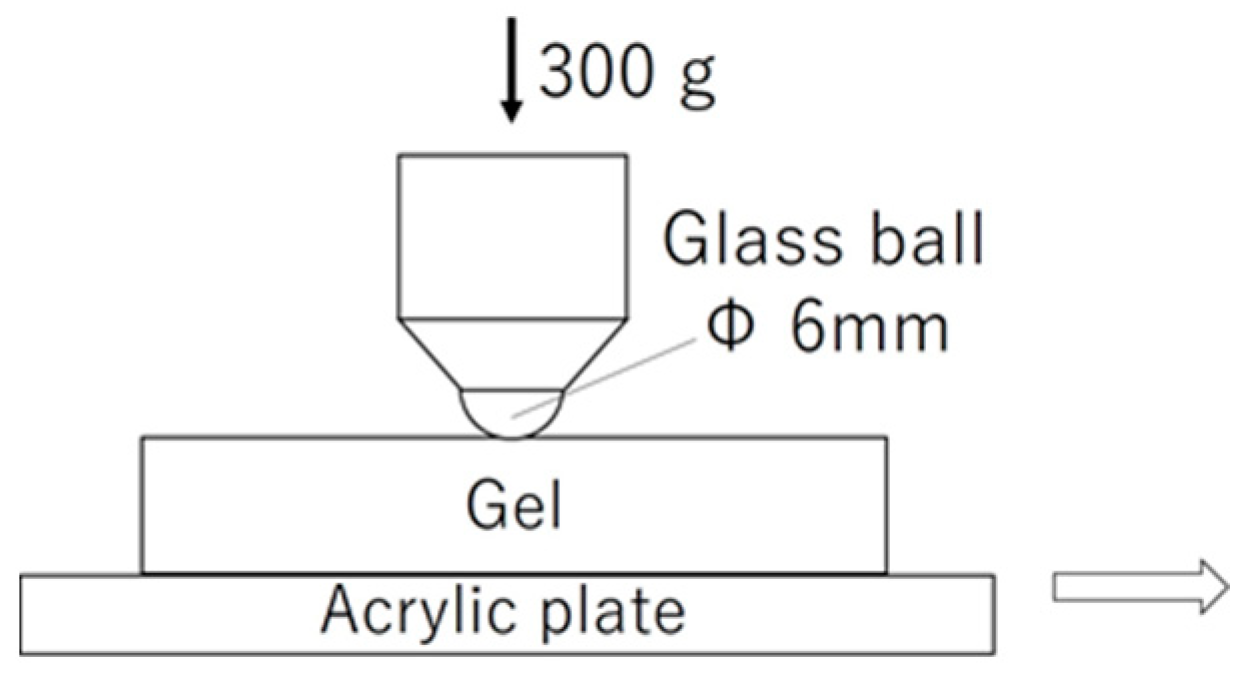
2.3. Friction Measurements
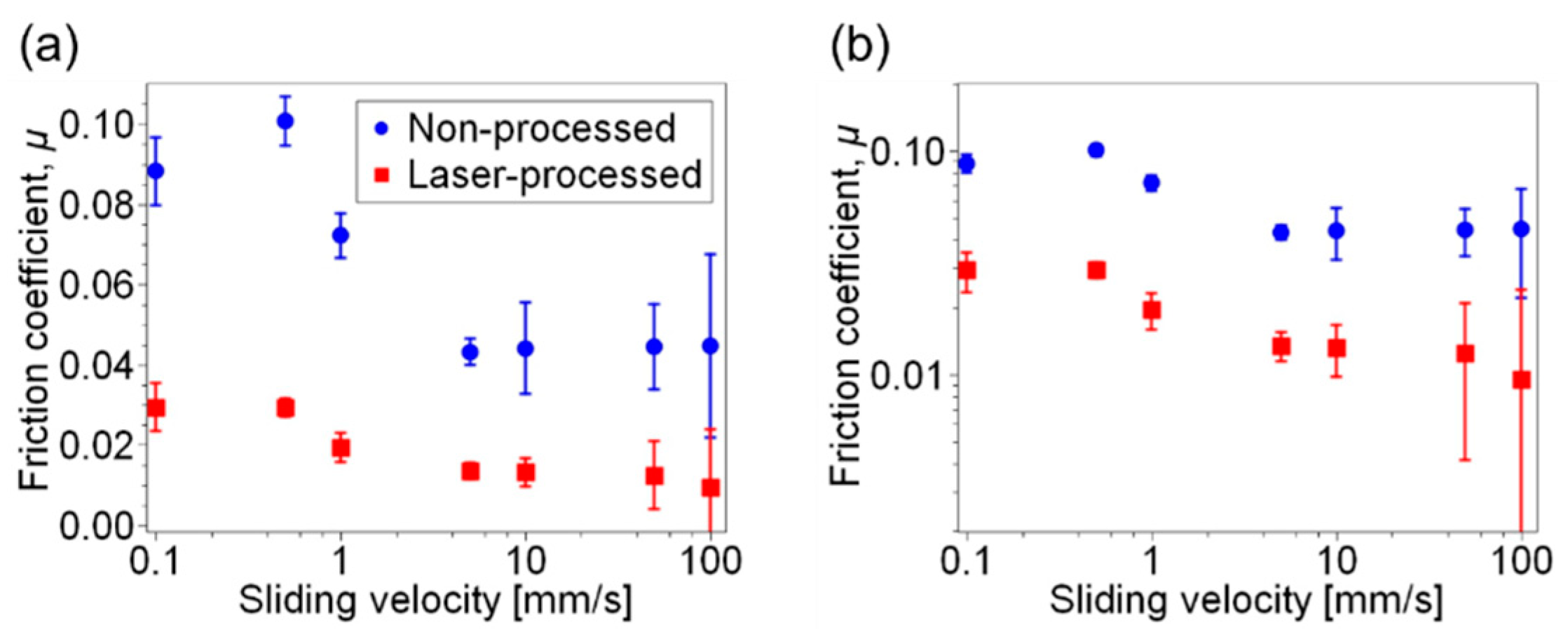
3. Results and Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Otsuki, M.; Matsukawa, H. Systematic Breakdown of Amontons’ Law of Friction for an Elastic Object Locally obeying Amontons’ Law. Sci. Rep. 2013, 3, 1586. [Google Scholar] [CrossRef] [PubMed]
- Katano, Y.; Nakano, K.; Otsuki, M.; Matsukawa, H. Novel Friction Law for the Static Friction Force based on Local Precursor Slipping. Sci. Rep. 2014, 4, 6324. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Asami, N.; Uragami, T. A Reversibly Antigen-Responsive Hydrogel. Nature 1999, 399, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Elisseeff, J.H. Mimicking Biological Functionality with Polymers for Biomedical Applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Collapse of Gels and the Critical Endpoint. Phys. Rev. Lett. 1978, 40, 820. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.; Sun, S.-T.; Nishio, I.; Swislow, G.; Shah, A. Phase Transitions in Ionic Gels. Phys. Rev. Lett. 1980, 45, 1636. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishio, I.; Sun, S.-T.; Ueno-Nishio, S. Collapse of Gels in an Electric Field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379. [Google Scholar] [CrossRef]
- Arai, K.D.; Saito, A.; Ito, K.; Uematsu, Y.; Ueno, T.; Fujii, Y.; Nishio, I. Isobars, the Coexistence Curve, and the Critical Exponent β of N-Isopropylacrylamide Gels Obtained Using A Simple Experimental Method. Phys. Rev. E 2013, 87, 022603. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kimura, J.; Fujii, Y.; Nishio, I. Volume Phase Transition of N-Isopropylacrylamide Gels Crosslinked by a Crosslinker with Six Hands. Phys. Rev. E 2013, 88, 062601. [Google Scholar] [CrossRef] [PubMed]
- Osada, Y.; Matsuda, A. Shape Memory in Hydrogels. Nature 1995, 376, 219. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Takahashi, T.; Yamaguchi, T.; Ichijo, H. Self-Oscillating Gel. J. Am. Chem. Soc. 1996, 118, 5134–5135. [Google Scholar] [CrossRef]
- Yoshida, R.; Takahashi, T.; Yamaguchi, T.; Ichijo, H. Self-Oscillating Gels. Adv. Mater. 1997, 9, 175–178. [Google Scholar] [CrossRef]
- Gong, J.P.; Higa, M.; Iwasaki, Y.; Katsuyama, Y.; Osada, Y. Friction of Gels. J. Phys. Chem. B 1997, 101, 5487–5489. [Google Scholar] [CrossRef]
- Gong, J.P.; Osada, Y. Gel Friction: A Model Based on Surface Repulsion and Adsorption. J. Chem. Phys. 1998, 109, 8062. [Google Scholar] [CrossRef]
- Gong, J.P.; Iwasaki, Y.; Osada, Y.; Kurihara, K.; Hamai, Y. Friction of Gels. 3. Friction on Solid Surfaces. J. Phys. Chem. B 1999, 103, 6001–6006. [Google Scholar] [CrossRef]
- Gong, J.P. Friction and Lubrication of Hydrogels—Its Richness and Complexity. Soft Matter 2006, 2, 544–552. [Google Scholar] [CrossRef]
- Kosukegawa, H.; Fridrici, V.; Kapsa, P.; Sutou, Y.; Adachi, K.; Ohta, M. Friction Properties of Medical Metallic Alloys on Soft Tissue-Mimicking Poly(Vinyl Alcohol) Hydrogel Biomodel. Tribol. Lett. 2013, 51, 311–321. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Mizukami, M.; Tanabe, T.; Furukawa, H.; Kurihara, K. Friction of Polymer Hydrogels Studied by Resonance Shear Measurements. Soft Matter 2015, 11, 6192–6200. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Sato, H.; Miyakoshi, T.; Kitano, T.; Kanebako, H.; Aadachi, K. Friction Control of Mechanical Seals in a Ventricular Assist Device. Biosurf. Biotribol. 2015, 1, 135–143. [Google Scholar] [CrossRef][Green Version]
- Kanda, K.; Adachi, K. Shear Strength of Protein Film Formed by Friction of SiC/SiC Sliding Pair in Plasma Environment. Biotribology 2017, 10, 26–34. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and Characterization of pH- and Temperature-Responsive Nanocomposite Double Network Hydrogels. Mater. Sci. Eng. C 2013, 33, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Hidema, R.; Gong, J.; Furukawa, H. Development of Optical 3D Gel Printer for Fabricating Free-Form Soft & Wet Industrial Materials and Evaluation of Printed Double-Network Gels. J. Solid Mech. Mater. Eng. 2013, 7, 163–168. [Google Scholar]
- Wada, M.; Hidema, R.; Chiba, T.; Yamada, K.; Yamada, N.; Gong, J.; Furukawa, H. Surface and Bulk Mechanical Properties of Soft and Wet Materials. J. Solid Mech. Mater. Eng. 2013, 7, 228–234. [Google Scholar] [CrossRef]
- Wada, M.; Kameyama, T.; Arai, M.; Yamada, K.; Makino, M.; Kawakami, M.; Furukawa, H. Friction Measurement of Functional Gel Mechanical Materials. Microsyst. Technol. 2016, 22, 77–81. [Google Scholar] [CrossRef]
- Wada, M.; Yamada, K.; Kameyama, T.; Yamada, N.; Yoshida, K.; Saito, A.; Makino, M.; Khosla, A.; Kawakami, M.; Furukawa, H. Electric Control of Friction on Surface of High-Strength Hydrogels. Microsyst. Technol. 2018, 24, 639–646. [Google Scholar] [CrossRef]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast Laser Processing of Materials: from Science to Industry. Light: Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef]
- Jin, Z.; Dowson, D. Bio-friction. Friction 2013, 1, 100–113. [Google Scholar] [CrossRef]
- Baumgart, T.; Hess, S.T.; Webb, W.W. Imaging Coexisting Fluid Domains in Biomembrane Models Coupling Curvature and Line Tension. Nature 2003, 425, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Sugimoto, R.; Vestergaard, M.C.; Nagasaki, T.; Takagi, M. Membrane Disc and Sphere: Controllable Mesoscopic Structures for The Capture and Release of a Targeted Object. J. Am. Chem. Soc. 2010, 132, 10528–10532. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Morita, M.; Miyakawa, M.; Sugimoto, R.; Hatanaka, A.; Vestergaard, M.C.; Takagi, M. Size-Dependent Partitioning of Nano/Micro-Particles Mediated by Membrane Lateral Heterogeneity. J. Am. Chem. Soc. 2012, 134, 13990–13996. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Hamada, T.; Vestergaard, M.C.; Takagi, M. Endo- and Exocytic Budding Transformation of Slow-Diffusing Membrane Domains Induced by Alzheimer’s Amyloid Beta. Phys. Chem. Chem. Phys. 2014, 16, 8773–8777. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Fujii, Y.; Nishio, I. Deformation of Lipid Membranes Containing Photoresponsive Molecules in Response to Ultraviolet Light. J. Phys. Chem. B 2014, 118, 4115–4121. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Horii, K.; Fujii, Y.; Nishio, I. Real-Time Observation of Liposome Bursting Induced by Acetonitrile. ChemPhysChem 2014, 15, 2609–2912. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Takashima, A.; Nishio, I. Effect of Dibucaine Hydrochloride on Raft-Like Lipid Domains in Model Membrane Systems. MedChemComm 2015, 6, 1444–1451. [Google Scholar] [CrossRef]
- Yoshida, K.; Mitsumori, R.; Horii, K.; Takashima, A.; Nishio, I. Acetonitrile-Induced Destabilization in Liposomes. Colloids Interfaces 2018, 2, 6. [Google Scholar] [CrossRef]
- Ota, T.; Yoshida, K.; Tase, T.; Sato, K.; Tanaka, M.; Saito, A.; Takamatsu, K.; Kawakami, M.; Furukawa, H. Influence of 3D-Printing Conditions on Physical Properties of Hydrogel Objects. Mech. Eng. J. 2018, 5, 17-00538. [Google Scholar] [CrossRef]
- Ion, J. Laser Processing of Engineering Materials: Principles, Procedure and Industrial Application; Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Pirani, F.; Sharma, N.; Moreno-Cencerrado, A.; Fossati, S.; Petri, C.; Descrovi, E.; Toca-Herrera, J.L.; Jonas, U.; Dostalek, J. Optical Waveguide-Enhanced Diffraction for Observation of Responsive Hydrogel Nanostructures. Macromol. Chem. Phys. 2017, 218, 1600400. [Google Scholar] [CrossRef]
- Nykanen, A.; Nuopponen, M.; Hiekkataipale, P.; Hirvonen, S.-P.; Soininen, A.; Tenhu, H.; Ikkala, O.; Mezzenga, R.; Ruokolainen, J. Direct Imaging of Nanoscopic Plastic Deformation below Bulk Tg and Chain Stretching in Temperature-Responsive Block Copolymer Hydrogels by Cryo-TEM. Macromolecules 2008, 41, 3243–3249. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Yahagi, H.; Wada, M.; Kameyama, T.; Kawakami, M.; Furukawa, H.; Adachi, K. Enormously Low Frictional Surface on Tough Hydrogels Simply Created by Laser-Cutting Process. Technologies 2018, 6, 82. https://doi.org/10.3390/technologies6030082
Yoshida K, Yahagi H, Wada M, Kameyama T, Kawakami M, Furukawa H, Adachi K. Enormously Low Frictional Surface on Tough Hydrogels Simply Created by Laser-Cutting Process. Technologies. 2018; 6(3):82. https://doi.org/10.3390/technologies6030082
Chicago/Turabian StyleYoshida, Kazunari, Hikaru Yahagi, Masato Wada, Toshiki Kameyama, Masaru Kawakami, Hidemitsu Furukawa, and Koshi Adachi. 2018. "Enormously Low Frictional Surface on Tough Hydrogels Simply Created by Laser-Cutting Process" Technologies 6, no. 3: 82. https://doi.org/10.3390/technologies6030082
APA StyleYoshida, K., Yahagi, H., Wada, M., Kameyama, T., Kawakami, M., Furukawa, H., & Adachi, K. (2018). Enormously Low Frictional Surface on Tough Hydrogels Simply Created by Laser-Cutting Process. Technologies, 6(3), 82. https://doi.org/10.3390/technologies6030082