Abstract
Nanocrystallized C60 thin films of such as hexagonal, plate-like, and rod-like morphologies were recrystallized by poor solvent immersion, employing 1-propanol, 2-propanol, and butanol respectively. A C60 thin film fabricated by evaporation was immersed in the poor solvent, partially dissolving the surface C60. This was followed by the solvent rapidly reaching a supersaturated state, resulting in the induced recrystallization of the C60. C60 fine high-density crystals were successfully prepared using propanol, with crystal sizes varying between 84 and 141 nm by changing the immersion time. In addition, due to the 1-propanol recrystallizing solvated crystals which were formed through interactions between the solvent and the C60, uniform C60 fine crystals were obtained by the formation of a large number of nucleation sites.
Keywords:
C60; thin film; recrystallization; solvent immersion; morphology control; solvated crystal 1. Introduction
Organic semiconductor materials in the form of fullerenes have been intensively studied as attractive materials for various state-of-art devices such as field effect transistors [1], light emitting diodes [2], chemical sensors [3], photodetectors [4], and solar cells [5]. In several kinds of fullerenes, C60 shows promising optoelectronic properties [6,7,8] that are expected to be developed further. Crystallization of C60 and control of the morphology of their crystals is one of the approaches currently used in order to enhance these properties, and the morphology control of fine crystals has revealed novel optoelectronic properties that depend on crystal sizes and shapes [9,10,11]. In terms of the applications of C60 for various optoelectronic devices, morphology control of C60 fine crystals in thin film state enables control of nanostructures, which can improve device performances. In particular, approaches of morphology control through use of solutions are required for various optoelectronic devices that necessitate cost-effective process and higher device performance [12].
Several techniques have been developed to control the morphologies of C60 fine crystals without the demanding requirements of using templates, special equipment, high temperature, or high pressure. Using liquid-phase synthesis to obtain C60 fine crystals following the method of liquid-liquid interface precipitation [13] and reprecipitation [14] is one of the simplest techniques. These techniques can transform the morphologies of C60 fine crystals simply by changing the solvents used for recrystallizing the C60 molecules [15,16]; however, multiple steps are required to fabricate nanocrystallized C60 thin films and it is difficult to achieve precise control of nanostructures in a film state. To develop a method that overcomes these obstacles, several studies report using as-deposited C60 thin films to control the morphologies of C60 fine crystals. Kim et al. demonstrated C60 nanowires on a substrate, which were prepared by solvent vapor annealing (SVA) using good solvents to recrystallize C60 molecules, where their growth direction was controlled in vertical and lateral directions by adjusting Marangoni flows [17]. Nojiri et al. also fabricated a nanocrystallized C60 thin film prepared by SVA, using an as-deposited C60 thin film. This film improved charge transport ability of a C60 layer and power conversion efficiency from 1.8% to 2.1%, which was found to enhance solar cell performances [18]. These studies have shown that employing an as-deposited C60 thin film is a useful strategy for forming nanocrystallized C60 thin films via the recrystallization of C60.
In this work, we propose the poor solvent immersion to control the morphologies of nanocrystallized C60 thin films using as-deposited C60 thin film. These morphologies, such as hexagonal, plate-like, and rod-like, were successfully controlled by employing optimal poor solvents such as 1-propanol, 2-propanol, and butanol, respectively. In particular, 1-propanol formed uniform 84–141 nm size C60 fine crystals with high density through specific recrystallization process via solvation in this system. Poor solvent immersion simply formed nanocrystallized C60 thin films and it has potential application in versatile materials enabling them to control nanostructures.
2. Materials and Methods
2.1. Materials
C60 powder (99.9%) was purchased from Tokyo Chemical Industry (TCI) (Tokyo, Japan). Methanol (99.8%), ethanol (99.5%), 1-propanol (99.5%), 2-propanol (99.7%), butanol (99.0%), hexane (96.0%), cyclohexane (98.0%), and chloroform (99.0%) were used to recrystallize as-deposited C60 thin films and purchased from TCI (Tokyo, Japan).
2.2. Fabrication of Nanocrystallized C60 Thin Film
100 nm C60 thin films was thermally evaporated onto the ITO substrate at a pressure of 2 × 10−4 Pa. As-deposited C60 thin films were immersed into 15.0 mL various organic solvents in a plate. After immersion, nanocrystallized C60 thin films were dried for 12 h at room temperature (under ambient atmosphere).
2.3. Characterization of Nanocrystallized C60 Thin Film
X-ray diffraction (XRD) patterns of the samples were measured on a Smart Lab (Rigaku, Tokyo, Japan) (using Cu Kα radiation at 45 kV and 200 mA). The samples were observed by a JSM-6700F scanning electron microscope (SEM) (Japan Electron Optics Laboratory (JEOL), Tokyo, Japan) (accelerating voltage of 10 kV). Visible absorption spectra of the samples were obtained on a V-670 spectrophotometer (Japan Spectroscopic Corporation (JASCO), Tokyo, Japan) (detecting wavelength range of 200 to 800 nm).
3. Results and Discussion
3.1. Poor Solvent Immersion
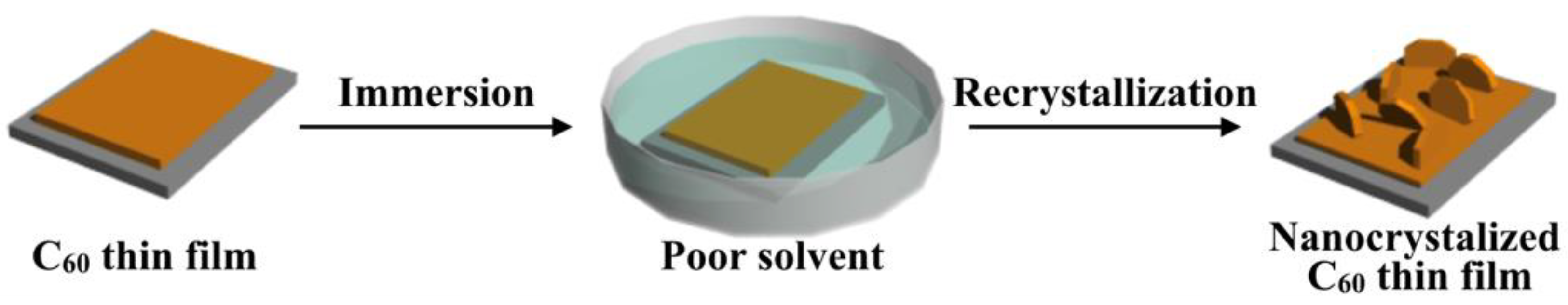
Figure 1 shows a schematic illustration of poor solvent immersion. In this method, a thermally evaporated C60 thin film is immersed in a poor solvent and the surface morphologies are controlled by the recrystallization of C60. When an as-deposited C60 thin film is immersed in a poor solvent, the C60 on the surface is partially dissolved. The solvent rapidly reaches a state of supersaturation because of its inability to dissolve large amounts of C60, and so recrystallization takes place at the surface of the C60 thin film followed by the formation of well-controlled C60 fine high-density crystals, which can induce a transformation in their morphologies in the thin film state.
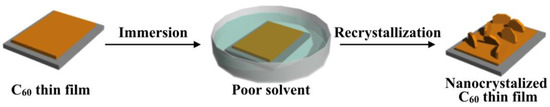
Figure 1.
Schematic illustration of morphology control of the nanocrystallized C60 thin films prepared by poor solvent immersion.
3.2. Optimization of Poor Solvent
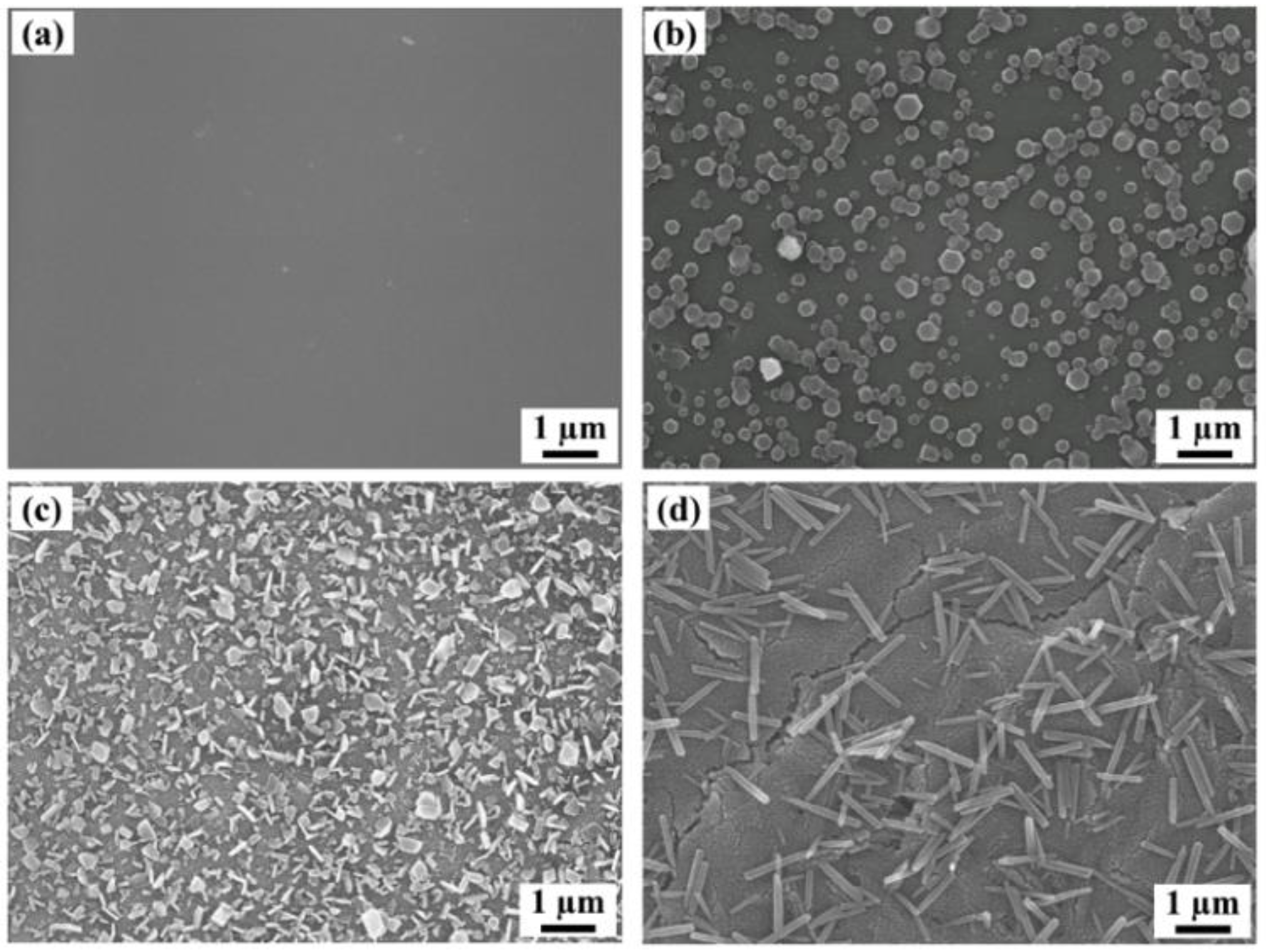
To determine the optimal solvent for recrystallizing C60 and controlling its morphology, various organic solvents such as methanol, ethanol, propanol, butanol, hexane, cyclohexane, and chloroform were tested. When the alcohol solvents (except for methanol and ethanol) were used, a large number of C60 fine crystals on thin films immersing for 60 min were observed in the scanning electron microscope (SEM) images shown in Figure 2. An extremely poor solvent such as methanol (0.000 mg/mL [19], Supplementary Materials Table S1) does not affect the surface morphology (Figure S1a) as significantly when compared to a C60 thin film fabricated through deposition by evaporation. Although ethanol is used as a treatment solvent in SVA and induces the recrystallization of C60 due to its low solubility (0.001 mg/mL, Table S1) [19], C60 fine crystals on thin film have lower density (Figure S1b) compared to the nanocrystallized C60 thin films recrystallized by propanol. In general, C60 is insoluble in polar solvents such as these, but C60 tends to increase solubility by increasing the number of carbon atoms in the solvents [19,20]. Butanol notably shows this tendency and forms 1 μm-sized rod-like C60 fine crystals due to increasing solubility and, as a result, the morphologies are drastically changed. However, recrystallized C60 fine crystals on the thin film are relatively low-density, and the SEM image (Figure 2d) shows cracks which severely damage the state of the thin film and represent an obstacle to application in various optoelectronic devices. Conversely, weak polar solvents at a certain level of solubility, such as hexane (0.043 mg/mL), cyclohexane (0.036 mg/mL), and chloroform (0.16 mg/mL), dissolve a large amount of C60 from thin films such that they do not induce recrystallization (Figure S1c–e, Table S1) [19]. According to experimental SEM observations, propanol recrystallized uniform C60 fine crystals on thin films with high density, so this was identified as the key solvent for controlling the morphologies of nanocrystallized C60 thin films. Interestingly, it is found that although 1-propanol and 2-propanol contain the same number of carbons, the resulting morphologies are significantly different. 1-propanol forms 300 nm sized C60 fine crystals with uniform size and shape, while 2-propanol, a structural isomer of 1-propanol, forms 500 nm–1 μm-sized C60 fine crystals.
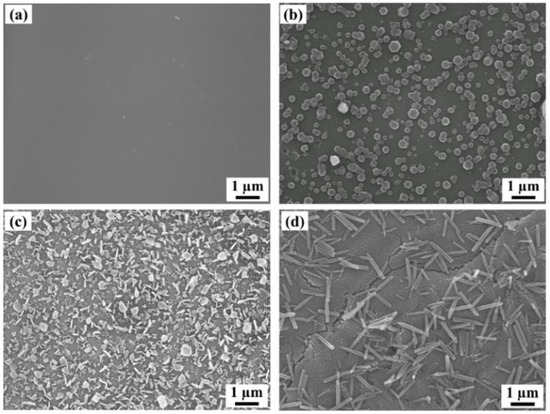
Figure 2.
SEM images of (a) a thermally evaporated C60 thin film and (b) hexagonal, (c) plate-like, and (d) rod-like C60 fine crystals on the films prepared by immersion in 1-propanol, 2-propanol, and butanol as a poor solvent for 60 min, respectively.
3.3. Crystal Structure
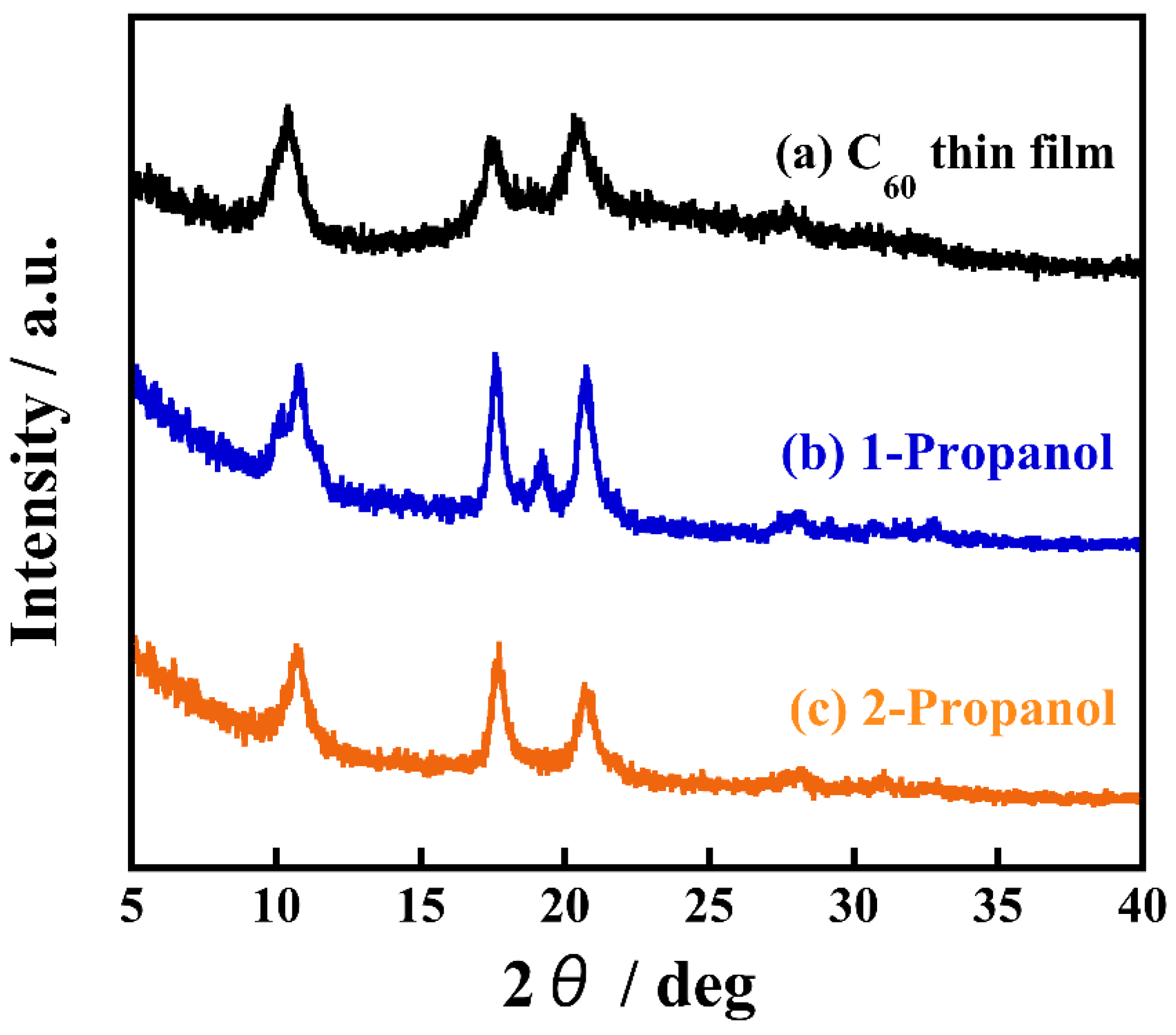
To explore the most significant factors that contribute to structural differences, we measured the crystal structures of the nanocrystallized C60 thin films prepared with 1-propanol and 2-propanol using X-ray diffraction (XRD) analysis, as shown in Figure 3. A nanocrystallized C60 thin film recrystallized by 2-propanol shows diffraction peaks at 2θ = 10.4, 17.6, 20.7, which is consistent with an XRD pattern of as-deposited C60 thin film and corresponds to a typical face-centered cubic (fcc) C60 crystal structure [21]. The other film, recrystallized by 1-propanol instead of 2-propanol, clearly exhibits a diffraction peak at 2θ = 19.2, which is not detected for the as-deposited C60 film. This suggests 1-propanol forms a different crystal structure to the FCC structure of a nanocrystallized C60 thin film prepared by 2-propanol. Our previous study on C60 fine crystals prepared by the reprecipitation method found that C60 interacts and cocrystallizes with various solvents, resulting in the formation of solvated crystals that incorporate the solvent [21]. Moreover, studying the phase transformation of C60 fine crystals and the associated change of their shapes induced by the exchanging of incorporated solvents revealed that the solvated crystals had a hexagonal crystal structure [22]. As a nanocrystallized C60 thin film recrystallized by 1-propanol shows a diffraction peak at 2θ = 19.2, this exhibits that 1-propanol interacts with C60 and forms solvated crystals. It is well known that C60 can be occupied with versatile chemical species such as alkali metal atoms, halogen molecules, and small hydrocarbon molecules, as well as solvent molecules. The intercalation of solvent into the lattice of C60 minimizes the lattice energy and forms various crystal shapes [23]. It assumes that solvated crystals are formed because of 1-propanol has lower steric hindrance than 2-propanol. However, this may need further study. It can therefore be considered that the solvation is a significant factor for obtaining uniformly sized C60 fine crystals on thin films.
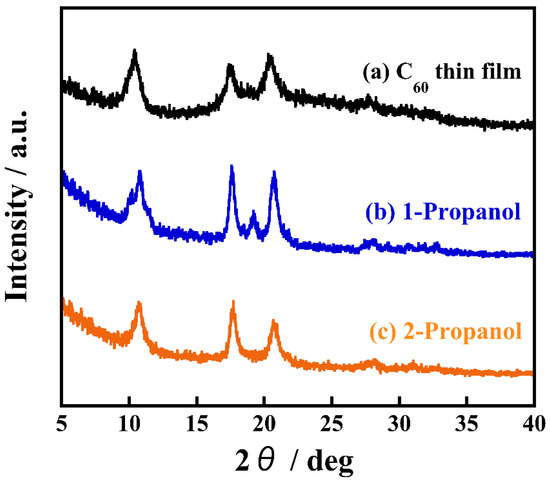
Figure 3.
XRD patterns of (a) a thermally evaporated C60 thin film and (b) hexagonal, (c) plate-like nanocrystallized C60 thin films prepared by using 1-propanol and 2-propanol as a poor solvent, respectively.
3.4. Recrystallization Using 1-Propanol
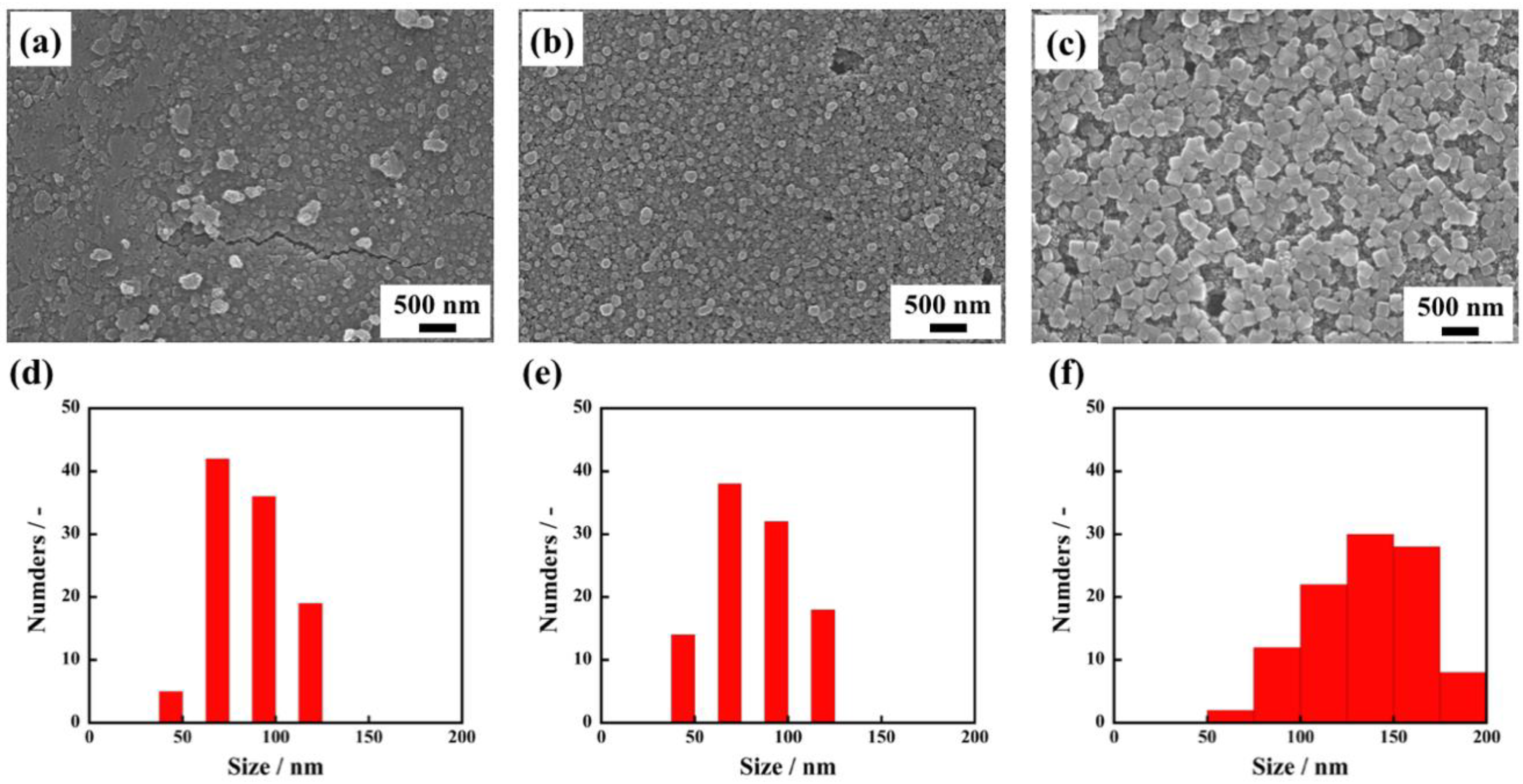
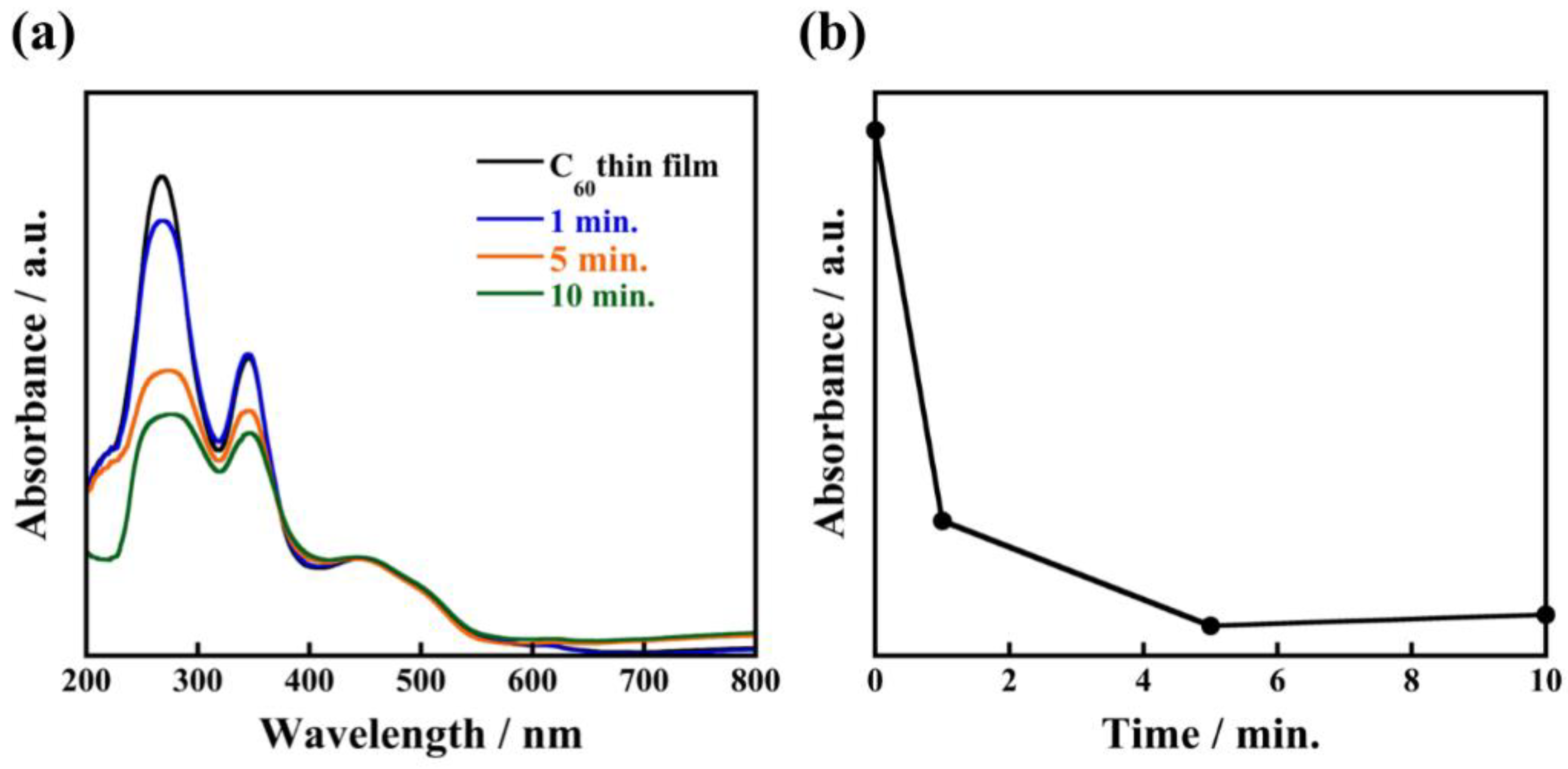
To investigate the effect of solvation by 1-propanol on the formation of uniform C60 fine crystals, we also recorded time-resolved observation of the recrystallization process of C60 fine crystals during the immersion. Figure 4 shows SEM images of nanocrystallized C60 thin films and their crystal size distributions when immersed for 1 min, 5 min, and 30 min. From SEM observation, it is clearly seen that average crystal sizes increased to 84 ± 35 nm, 100 ± 19 nm, and 141 ± 39 nm with increasing immersion times. Interestingly, even with an immersion time as short as 1 min (corresponding to Figure 5a and Figure S2a), a large number of small-sized C60 fine crystals are observed. Based on these observations of real-time crystal growth, it appears that when C60 thin film fabricated through an evaporation method is immersed in 1-propanol, the solution immediately reaches a supersaturation state because 1-propanol forms solvated crystals with the C60 molecules, which results in the immediate formation of a large number of nucleation sites in the thin film. Consequently, a large number of nuclei grow in this proposed process forming uniform solvated C60 fine crystals with high-density in thin film. In addition, time-resolved ultraviolet and visible absorption spectra of the nanocrystallized C60 thin films revealed that the absorbance at 268 nm decreased with increasing immersion times, as plotted in Figure 5b. The absorption peak at 268 nm, which corresponds to electron transfer from gg to t2u [24], rapidly decreased after immersion for 1 min. It can be deduced that 1-propanol accelerates the recrystallization of C60 due to solvation, resulting in a decrease of the absorbance originating from an individual C60 molecule. These results give evidence for the proposed recrystallization process taking place through the formation of a large number of nuclei and solvation, which is a significant step forward for recrystallizing C60 fine crystals with uniform size.
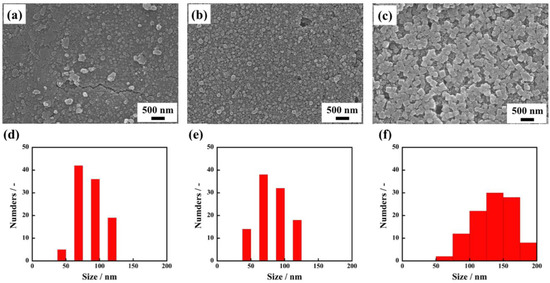
Figure 4.
SEM images of nanocrystallized C60 thin films immersed in 1-propanol for 1 min. (a), 5 min. (b), 30 min (c), and the size distributions of C60 fine crystals recrystallized for 1min (d), 5 min (e), 30 min (f).
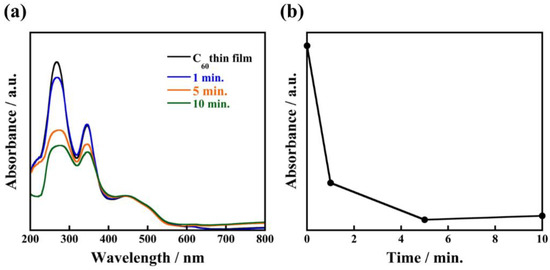
Figure 5.
Absorption spectra of nanocrystallized C60 thin films immersed in 1-propanol for 1 min, 5 min, 10 min (a) and the relationship between absorbance at wavelength of 268 nm and immersion times (b).
3.5. Recrystallization Using 2-Propanol
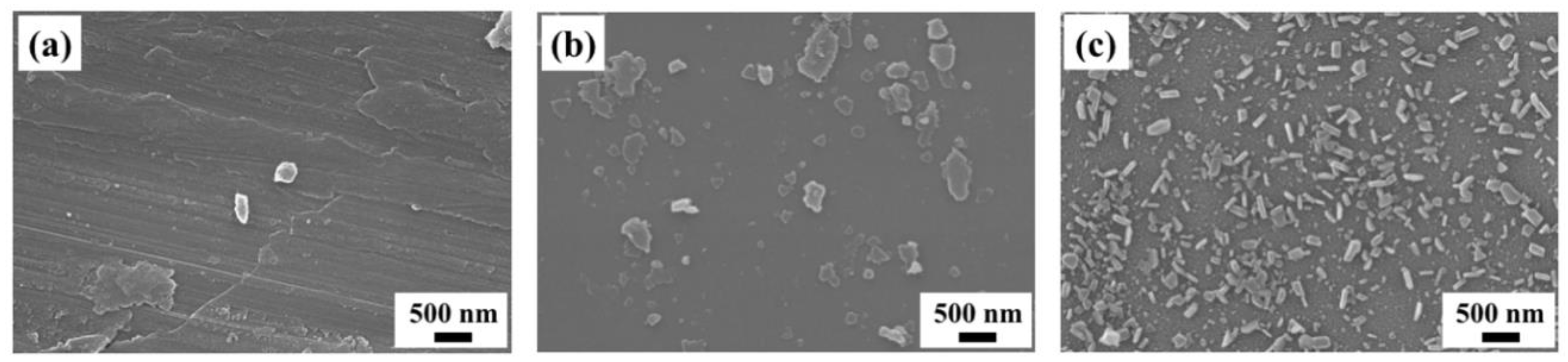
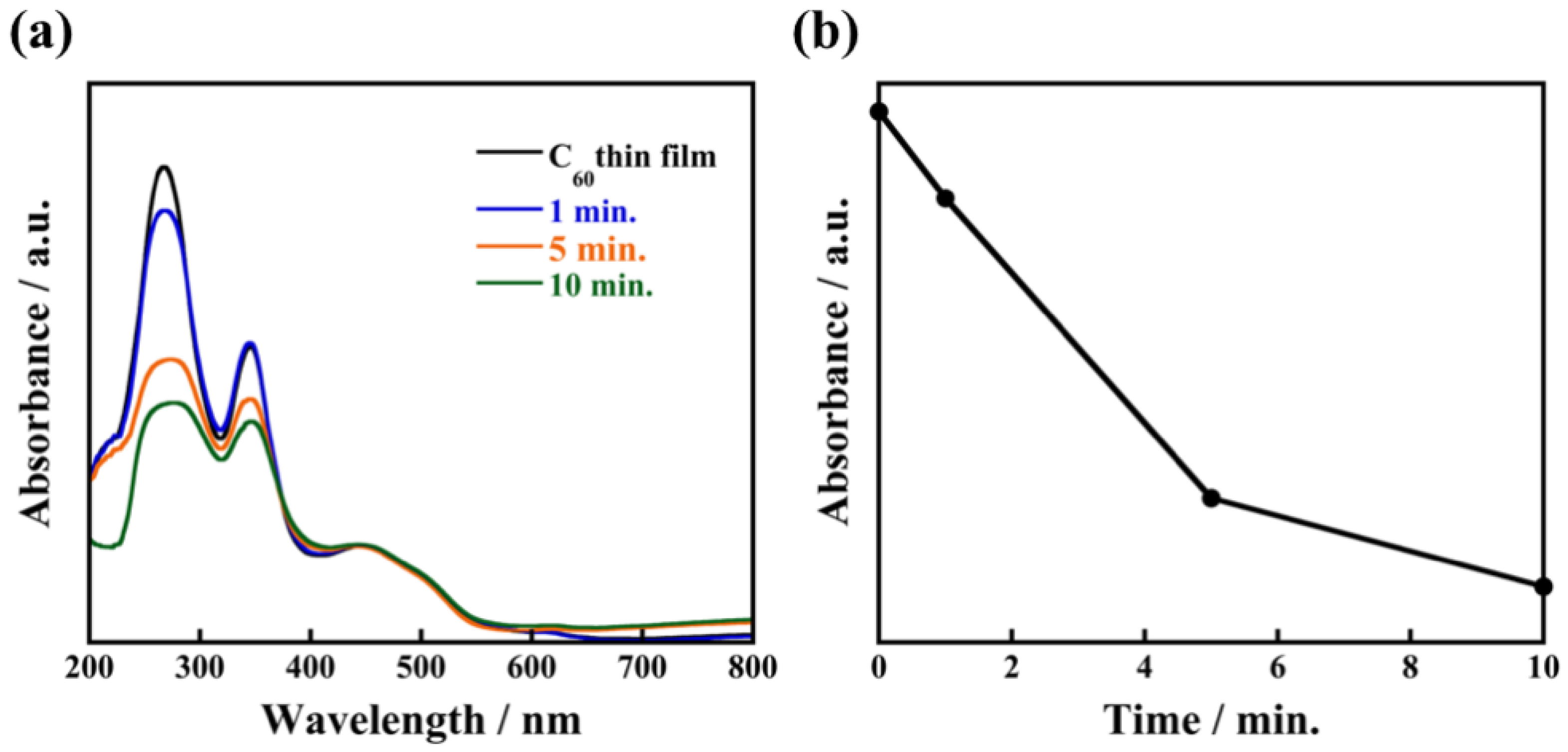
In contrast, when an as-deposited C60 film is immersed in 2-propanol for 1 min, C60 fine crystals are not observed in Figure 6a. After immersion for 5 min, a small number of C60 fine crystals are formed, which grow to a size in the range of 500 nm–1 μm with plate-like morphology after being immersed for 30 min. From such non-uniformity of crystal size and the small number of crystals compared to the case of 1-propanol, the recrystallization process using 2-propanol is clearly different. Considering that 2-propanol does not cocrystallize with C60, we propose the following model of the recrystallization process: When an as-deposited C60 thin film is immersed in 2-propanol, dissolution of C60 from the surface is dominant. After sufficient dissolution, 2-propanol slowly reaches the supersaturation state ((slower than 1-propanol) so only a small number of nuclei are formed. As a result, the C60 fine crystals are recrystallized with non-uniform size. XRD patterns of the nanocrystallized C60 thin film (Figure S3) show that the diffraction peaks almost disappear after immersion for 1 min, which suggests dissolution of C60 from the thin film is dominant during this time. After immersion for 5 min, distinct XRD peaks reappear due to the recrystallization of C60. The absorption peak at 268 nm rapidly decreases after immersion for 5 min as shown in Figure 7, which indicates that the dissolved C60 is supplied for recrystallization on the surface of the nanocrystallized C60 thin film. These results support our proposed model of the recrystallization process in 2-propanol which, due to the dissolution process taking a finite amount of times, takes longer to reach supersaturation state than 1-propanol, resulting in the formation of non-uniform C60 fine crystals with non-uniform size.

Figure 6.
SEM images of nanocrystallized C60 thin films immersed in 1-propanol for (a) 1 min, (b) 5 min, (c) 30 min.
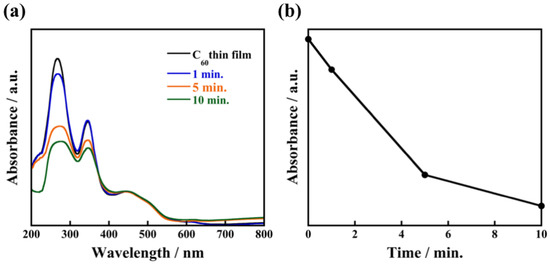
Figure 7.
Absorption spectra of nanocrystallized C60 thin films immersed in 2-propanol for 1 min, 5 min, 10 min (a) and the relationship between absorbance at wavelength of 268 nm and immersion times (b).
4. Conclusions
Nanocrystallized C60 thin films with hexagonal, plate-like, and rod-like morphologies were successfully prepared by poor solvent immersion. Uniform average crystal sizes could be controlled between 84 and 141 nm by changing immersion times and using 1-propanol as the poor solvent. Due to the 1-propanol interacting with C60 molecules, uniform C60 fine crystals were recrystallized in a thin film via a proposed mechanism associated with the formation of solvated crystals. Size- and shape-controlled synthesis of C60 fine crystals on thin film state by poor solvent immersion can therefore provide an appropriate synthetic route to producing optoelectronic materials.
Supplementary Materials
The following are available online at http://www.mdpi.com/2227-7080/6/2/51/s1, Figure S1: SEM images of the C60 thin film immersed using (a) methanol, (b) ethanol, (c) hexane, (d) cyclohexane, and (e) chloroform for 60 min, respectively; Figure S2: High magnification SEM images of nanocrystallized C60 thin films immersed in 1-propanol for 1 min (a), 5 min (b), (c) 30 min, and C60 thin films immersed in 2-propanol for (d) 1 min, (e) 5 min, (f) 30 min; Figure S3: XRD patterns of (a) a thermally evaporated C60 thin film and nanocrystallized C60 thin film immersed in 1-propanol for (b) 1 min, (c) 5 min, (d) 10 min, (e) 30 min, respectively; Table S1: Solubility of C60 in various solvents.
Author Contributions
Conceptualization, A.M.; Methodology, A.M.; K.U.; M.T.; Y.T.; B.L.; Formal Analysis, M.T.; M.D.; Writing Original Draft Preparation, K.U.; Writing Review and Editing, K.U.; A.M.; Supervision, A.M.; Funding Acquisition, A.M.; All authors have read and approved the final manuscript.
Funding
This work was partially supported by a research grant from The Cosmetology Research Foundation, Japan Society for the Promotion of Science KAKENHI Grant Number JP 18K04805.
Acknowledgments
The authors are very grateful to Atsushi Ito for supporting and valuable discussion on this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ohashi, H.; Tanigaki, K.; Kumashiro, R.; Sugihara, S.; Hiroshiba, S.; Kimura, S.; Kato, K.; Takata, M. Low-glancing-angle X-ray diffraction study on the relationship between crystallinity and properties of C60 field effect transistor. Appl. Phys. Lett. 2004, 84, 520–522. [Google Scholar] [CrossRef]
- Lee, J.Y. Efficient hole injection in organic light-emitting diodes using C60 as a buffer layer for Al reflective anodes. Appl. Phys. Lett. 2006, 88, 073512. [Google Scholar] [CrossRef]
- Lin, H.-B.; Shih, J.-S. Fullerene C60-cryptand coated surface acoustic wave quartz crystal sensor for organic vapors. Sens. Actuators B Chem. 2003, 92, 243–254. [Google Scholar] [CrossRef]
- Tsai, W.-W.; Chao, Y.-C.; Chen, E.-C.; Zan, H.-W.; Meng, H.-F.; Hsu, C.-S. Increasing organic vertical carrier mobility for the application of high speed bilayered organic photodetector. Appl. Phys. Lett. 2009, 95, 213308. [Google Scholar] [CrossRef]
- Sariciftci, N.-S.; Smilowitz, L.; Heeger, A.-J.; Wudl, F. Photoinduced Electron Transfer from a Conducting Polymer to Buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Akada, M.; Hirai, T.; Takeuchi, J.; Yamamoto, T.; Kumashiro, R.; Tanigaki, K. Superconducting phase sequence in Rx C60 fullerides (R=Smand Yb). Phys. Rev. B 2006, 73, 632–636. [Google Scholar] [CrossRef]
- Guldi, D.M.; Prato, M. Excited-State Properties of C60 Fullerene Derivatives. Acc. Chem. Res. 2000, 33, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Peumans, P.; Uchida, S.; Forrest, S.-R. Efficient bulk heterojunction photovoltaic cells using small- molecular-weight organic thin films. Nature 2003, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Kuwasaki, Y.; Hamamoto, K.; Nagata, S.; Obayashi, A.; Kuwabara, M. Structural characterization of C60nanowhiskers formed by the liquid/liquid interfacial precipitation method. Surf. Interface Anal. 2003, 35, 117–120. [Google Scholar] [CrossRef]
- Kizuki, T. Chapter 8 in Situ Transmission Electron Microscopy of Fullerene Nanowhiskers and Related Carbon Nanomaterials. In Fullerene Nanowhiskers, 1st ed.; Miyazawa, K., Tachibana, M., Mashino, T., Kizuka, T., Ochiai, Y., Eds.; Pan Stanford Publishing Pte., Ltd.: Singapore, 2011; pp. 103–116. [Google Scholar]
- Matsuishi, K. Chapter 11 Optical Properties of Fullerene Nanowhiskers. In Fullerene Nanowhiskers, 1st ed.; Miyazawa, K., Tachibana, M., Mashino, T., Kizuka, T., Ochiai, Y., Eds.; Pan Stanford Publishing Pte., Ltd.: Singapore, 2011; pp. 147–162. [Google Scholar]
- Thomas, M.; Worfolk, B.J.; Rider, D.A.; Taschuk, M.T.; Buriak, J.M.; Brett, M.J. C60 Fullerene Nanocolumns-Polythiophene Heterojunctions for Inverted Organic Photovoltaic Cells. ACS Appl. Mater. Interfaces 2011, 3, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.; Miyazawa, K. Size-Tunable Hexagonal Fullerene (C60) Nanosheets at the Liquid-Liquid Interface. J. Am. Chem. Soc. 2007, 129, 13816–13817. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Nalwa, H.S.; Oikawa, H.; Okada, S.; Matsuda, H.; Minami, N.; Kakuta, A.; Ono, K.; Mukoh, A.; Nakanishi, H. Novel Preparation Method of Organic Microcrystals. Jpn. J. Appl. Phys. 1992, 31, L1132–L1134. [Google Scholar] [CrossRef]
- Miyazawa, K. Chapter 1 Introduction of Fullerene Nanowhiskers. In Fullerene Nanowhiskers, 1st ed.; Miyazawa, K., Tachibana, M., Mashino, T., Kizuka, T., Ochiai, Y., Eds.; Pan Stanford Publishing Pte., Ltd.: Singapore, 2011; pp. 1–23. [Google Scholar]
- Masuhara, A.; Tan, Z.; Kasai, H.; Nakanishi, H.; Okikawa, H. Chapter 7 Fabrication and Characterization of C60 Fine Crystals and Their Hybridization. In Fullerene Nanowhiskers, 1st ed.; Miyazawa, K., Tachibana, M., Mashino, T., Kizuka, T., Ochiai, Y., Eds.; Pan Stanford Publishing Pte., Ltd.: Singapore, 2011; pp. 89–101. [Google Scholar]
- Kim, J.; Park, C.; Park, J.E.; Chu, K.; Choi, H.C. Vertical Crystallization of C60 Nanowires by Solvent Vapor Annealing Process. ACS Nano 2013, 7, 9122–9128. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, K.; Shahiduzzaman, M.; Yamamoto, K.; Kuwabara, T.; Takahashi, K.; Taima, T. Interpenetrating heterojunction photovoltaic cells based on C60 nano-crystallized thin films. Org. Electron. 2016, 38, 107–114. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Tse, D.S.; Malhotra, R.; Lorents, D.C. Solubility of fullerene (C60) in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar] [CrossRef]
- Hansen, C.M.; Smith, A.L. Using Hansen solubility parameters to correlate solubility of C60 fullerene in organic solvents and in polymers. Carbon 2004, 42, 1591–1597. [Google Scholar] [CrossRef]
- Masuhara, A.; Tan, Z.; Ikeshima, M.; Sato, T.; Kasai, H.; Oikawa, H.; Nakanishi, H. Cyclic transformation in shape and crystal structure of C60 microcrystals. CrystEngComm 2012, 14, 7787–7791. [Google Scholar] [CrossRef]
- Tan, Z.; Masuhara, A.; Kasai, H.; Nakanishi, H.; Oikawa, H. Thermal-induced shape transformation of solvated C60 microcrystals. Carbon 2013, 64, 370–376. [Google Scholar] [CrossRef]
- Park, C.; Park, J.E.; Choi, H.C. Crystallization-Induced Properties from Morphology-Controlled Organic Crystals. Acc. Chem. Res. 2014, 47, 2353–2364. [Google Scholar] [CrossRef] [PubMed]
- Graja, A.; Farges, J.-P. Optical spectra of C60 and C70 complexes: Their similarities and differences. Adv. Mater. Opt. Electron. 1998, 8, 215–228. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).