1. Introduction
The trend of increasing the firing temperature of ceramic products to achieve outstanding mechanical properties and extremely low porosity during sintering requires the use of thermally stable pigments. The main requirements for this class of pigments include high coloring capabilities, even if used in small amounts, and heat stability, accompanied by a resistance to dissolution, chemical agents, and solvent attack [
1]. Synthetic crystalline oxide compounds of two or more different metals are candidate materials for such an application, and they have been extensively used during the past few decades. The wide range of colors for these pigments is due to the presence of transition metals (V, Cr, Mn, Fe, Co, Cu) or rare earth metals (Pr, Nd) in oxidic crystalline phases known for their thermal stability [
2]. Past research has generally focused on two main directions: an economization and improvement of existing pigments or the discovery and implementation of new compounds with higher performing characteristics. However, due to limitations in available chemistry, very few novel compounds have been developed in the last few decades [
3,
4]. In the framework of the optimization of existing pigments, alternative synthetic routes can play an important role in accelerating the manufacturing and in reducing the cost of the process, as already demonstrated by some of the authors [
5,
6,
7,
8]. However, it must be taken into account that the value of pigments relies on their physical-optical properties, i.e., crystalline structure, particle size distribution, particle shape and agglomeration, and chemical purity and stability [
9]. From an environmental point of view, the possible absence of additives used to enhance the solid-state reactivity, known also as mineralizers, makes alternative synthetic routes preferable.
Due to their rapid, selective, and volumetric heating, microwaves have been widely used in the past to enhance solid-state reactions [
10,
11,
12], as well as the synthesis of ceramic pigments such as Al
2O
3/Cr
2O
3 and CeO
2:Pr pink pigment [
13,
14], and of several oxides of transition metals: Co–Fe, Ni–Fe, and Fe–Fe spinels [
15,
16].
Most of the existing literature work on the microwave synthesis of ceramic pigments involves hydrothermal synthesis or the solid-state reaction between precursors, using the 2.45 GHz ISM (Industrial, Scientific, and Medical) frequency and commercial or slightly modified multi-mode applicators. However, other ISM frequencies are available, as well as other microwave applicator designs, which could further enhance the microwave-assisted synthesis. As a matter of fact, microwave heating of the load is affected by the nature of the load itself (its dielectric, electric, and magnetic properties, as well as the volume and shape of the load) and by the nature and strength of the electromagnetic field inside the load volume. Frequency is determined by the microwave generator, but field strength is strongly affected by the load characteristics, position in the applicator, and microwave applicator geometry (Appendix A in [
10]). Hence, for a given load whose dielectric, electric, and magnetic properties are functions of frequency and temperature, it can be useful to investigate whether rationally changing the applicator geometry or microwave source frequency can be beneficial in terms of the energy efficiency or synthesis time.
The aim of this work is to present a case study on the preparation of blue CoAl2O4 pigment using different microwave applicators and generator frequencies, showing the advantages which can derive from a properly designed microwave reactor for the solid-state synthesis of such pigment. Attention has been also given to the temperature detection to avoid misinterpretation of real process temperature, as often occurs in the case of irradiated environments where common temperature sensors are difficult to use.
2. Materials and Methods
Industrial grade Co
3O
4 and Al(OH)
3 powders, supplied by Colorobbia Italia, were used as starting raw materials due to their capability of strongly coupling with microwaves at the typical 2.45 GHz frequency allocated for ISM uses. The stoichiometric ratio of powders, according to the balance:
was wet milled for 20 min using alumina balls as milling media, in a 1:1 weight ratio. The resulting slurry was dried at 120 °C and subsequently dry milled in an agate mortar with agate balls as milling media, in a 1:1 weight ratio. The obtained powders from the same batch were inserted into several porcelain crucibles and calcined at different temperatures, ranging from 1000 to 1200 °C, with holding time at the maximum temperature ranging from 15 to 120 min, followed by natural cooling inside the furnace. Different furnaces were used for calcination, namely:
Conventional heating furnace (muffle furnace, Nannetti mod PK/16, Italy).
Fixed power multi-mode microwave applicator, 2.45 GHz (CEM MAS 7000, 950 W).
Variable power multi-mode microwave applicator, 2.45 GHz (Radatherm VPS, maximum power 1800 W).
Variable power single mode applicator, 2.45 GHz (MKS Alter, maximum power 1200 W).
Variable power single mode applicator, 5.8 GHz (MKS Alter, maximum power 950 W).
The single mode applicators used for this study were based on the rectangular waveguide geometry (WR340 for the 2.45 GHz one and WR159 for the 5.8 GHz one), short circuited by a movable plunger. Microwave furnaces 3–5 were equipped with directional couplers, which allowed for the real-time measurement of the emitted and reflected power and hence, by difference, the power dissipated into the load (i.e., the sample plus the crucible and the refractory lining). The CEM MAS applicator could mount an optional SiC ring to achieve hybrid heating conditions, i.e., direct microwave absorption by the load and indirect heating of the load by heat transfer from the microwave-heated SiC ring [
10]. Temperature was monitored during the microwave processing using a sapphire fiber directly touching the upper free surface of the sample and connected to a signal conditioner (MIKRON M680 Infraducer, Mikron Infrared Inc., Santa Clara, CA, USA).
Calcining by microwave heating was performed partially in power- and in temperature-control mode: the set forward power was continuously applied until the maximum set temperature was reached (power-control); holding at the maximum temperature was performed by the control system of the furnace, turning ON/OFF the microwave generator (temperature-control). Hence, the overall time of exposure to microwave was certain only for the heating stage, while holding required different exposure times depending on the furnace type and its insulation. In the case of comparison with conventional heating processing, calcination was conducted entirely in the temperature-controlled mode, which makes the effective microwave exposure times more difficult to quantify.
Temperature monitoring by inserting the sapphire fiber on the precursors mixture was not able to appreciate the possible presence of hot spots, as it averages temperature on the fiber tip area (3 mm in diameter). Hence, the reported temperature should be regarded only as a possible variation of the global synthesis temperature and time, and not as an evidence of the reduction of the synthesis temperature.
Table 1 summarizes the synthesis procedure and parameters applied during this study.
The calcined powders were subjected to mineralogical analysis (Philips PW3710, Cu-Kα, Almelo, The Netherlands) and UV-VIS measurements (UV-Vis Perkin Elmer mod. Lambda 19, Waltham, MA, USA) to determine their position in the CIELab color space. Surface area analyses were performed on the as-synthesized powders by the Brunauer–Emmett–Teller (BET) method (Micromeritics Instrument, Gemini 2360, Norcross, GA, USA), using nitrogen as the adsorbing species. The obtained powders were also tested in simulated industrial cycle firing conditions for porcelanized stoneware tiles, by dispersion of 4 wt % of pigment powder into the ceramic body by wet milling for 60 min with alumina balls as milling media, fired at the maximum temperature of 1150 °C for 8 min.
An industrial grade cobalt aluminate pigment, supplied by Colorobbia Italia, was tested as well for comparison. Its declared synthetic route involves calcination at 1200 °C for 4 h in industrial kilns [
17].
3. Results and Discussion
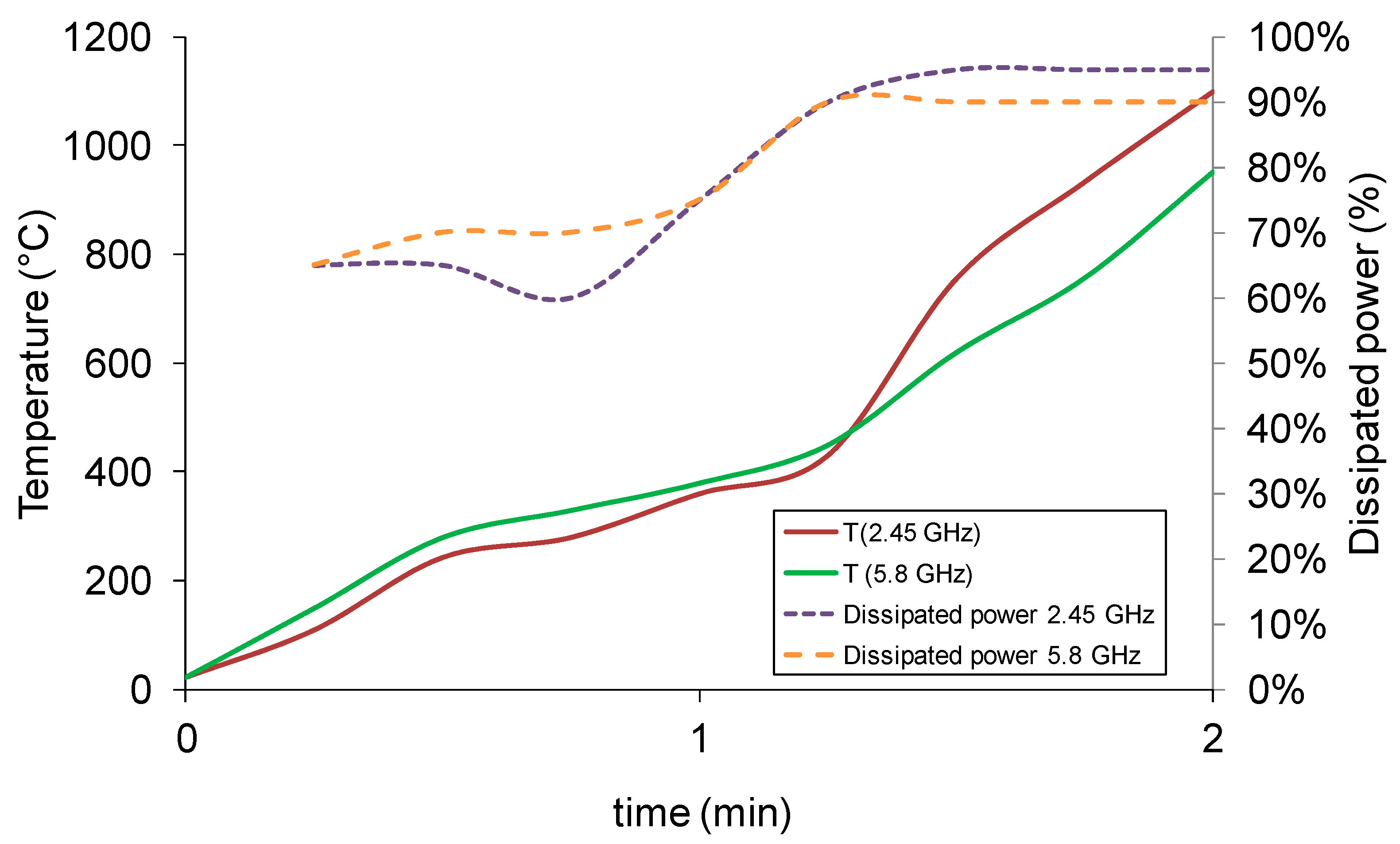
As expected, the microwave processing of the precursors mixture led to a rapid temperature increase of the load, as shown in
Figure 1, using single mode applicators.
Figure 1 shows also that, independent from the microwave frequency applied, there is a steep increase of the dissipated power (i.e., the power absorbed by the load) near 400 °C. Cobalt oxide has been classified as a microwave active material [
18] and more recently the dielectric and magnetic properties of cobalt oxide-based mixtures have been measured [
19], showing that the good microwave absorption is due also to magnetic losses, which, depending on the particle size and shape, can occur at different frequencies. The microwave absorption of cobalt oxide near the 2.45 GHz frequency is an increasing function of temperature. Therefore, due to the relatively low thermal conductivity of the powders, thermal runaway is likely to occur [
20]. Literature data for cobalt oxide also show that in the 2–14 GHz range a decrease in the dielectric and magnetic losses as a function of frequency is expected [
21], leading to possible differences in the microwave heating rate depending on the microwave generator operating frequency. On the other hand, the use of aluminum hydroxide in the gibbsite form is known to improve the microwave absorption of materials below 300 °C [
22], due to the formation of an amorphous component containing a variable amount of weakly bound molecular water [
23]. Hence, the observed heating behavior reported in
Figure 1 is in agreement with the dielectric behavior of cobalt oxide. On the other hand, the initial heating of the load proceeds more slowly, and is characterized by lower dissipated power, despite the presence of the (OH) groups associated with the gibbsite. However, it must be taken into account that, for simply ground gibbsite, at about 300 °C most of the OH
− groups are eliminated in a single endothermic event, and the dehydration reaction is completed at 700 °C [
24].
Rather interestingly,
Figure 1 also indicates the existence of frequency-related effects: despite starting at the same dissipated power level, the sample heated at 2.45 GHz frequency presents a slightly higher average absorption of microwaves, accompanied by a higher temperature raise at the end of the 2-min processing. Moreover, above 400 °C the heating rate of the 2.45 GHz-processed load is much higher than its 5.8 GHz counterpart, indicating that the higher loss factor of the cobalt oxide precursor still plays a role, which is progressively lost as the synthesis takes place (the heating rate after 1.45 min is almost identical).
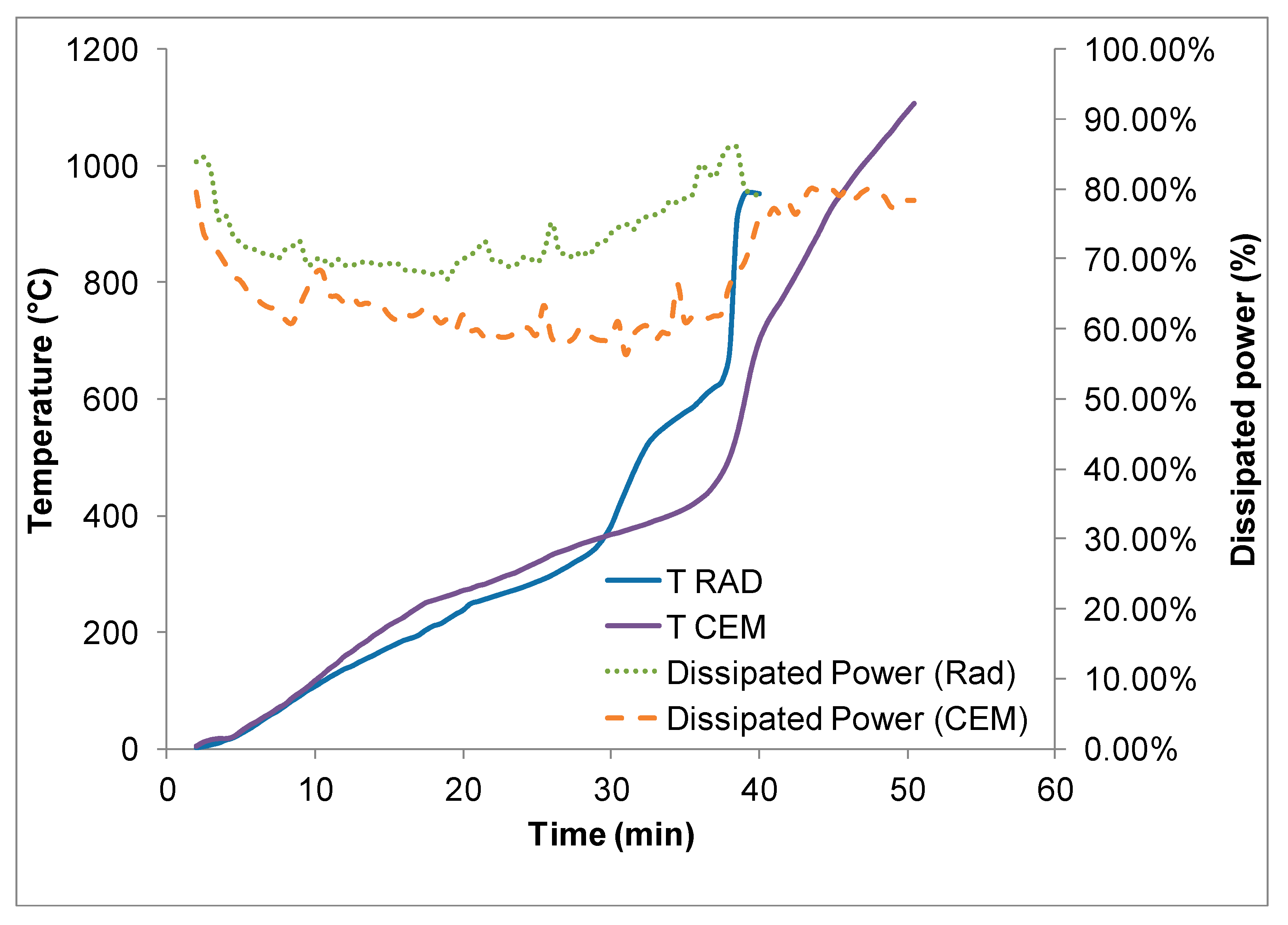
Microwave processing in multi-mode applicators, both operating at 2.45 GHz, led to a much lower heating rate, despite the use of power densities similar to the single mode applicator ones: 600 W power was applied to a 10 g sample, but it required much longer time to reach 1000 °C, which cannot be explained when only considering the lower average dissipated power, as shown in
Figure 2.
This lack of efficiency is partially due to the much lower electric field strength in the multi-mode type of applicators, compared to the single mode ones, as well as to the lack of proper impedance matching systems, which are installed on the single mode applicators. Moreover, the measurement of the dissipated power is the difference between the emitted microwave power and reflected microwave power, hence it includes not only the heat dissipated in the load, but also possible heat losses of the applicator walls, of the lining, etc. Nevertheless, both multi-mode applicators presented the same increase of heating rate occurring above 400 °C and an increasing trend of the dissipated power as a function of temperature, similar to the single mode applicator results. The higher microwave absorption (higher dissipated power) recorded at low temperatures is probably ascribable to some residual moisture of the load.
Increasing the microwave incident power to achieve electric field strengths similar to the ones used in single mode applicator led to localized overheating of the load, with subsequent thermal runaway, as shown in
Figure 3.
In order to investigate possible improvements related to microwave synthesis, the maximum calcination temperature and treatment duration were varied as described in the Materials and Methods section. The same precursors mixture was calcined both in conventional as well as in microwave furnaces, using a multi-mode CEM applicator, as its built-in mode stirrer can achieve a better temperature distribution homogeneity, albeit at the cost of energy efficiency (discussed later).
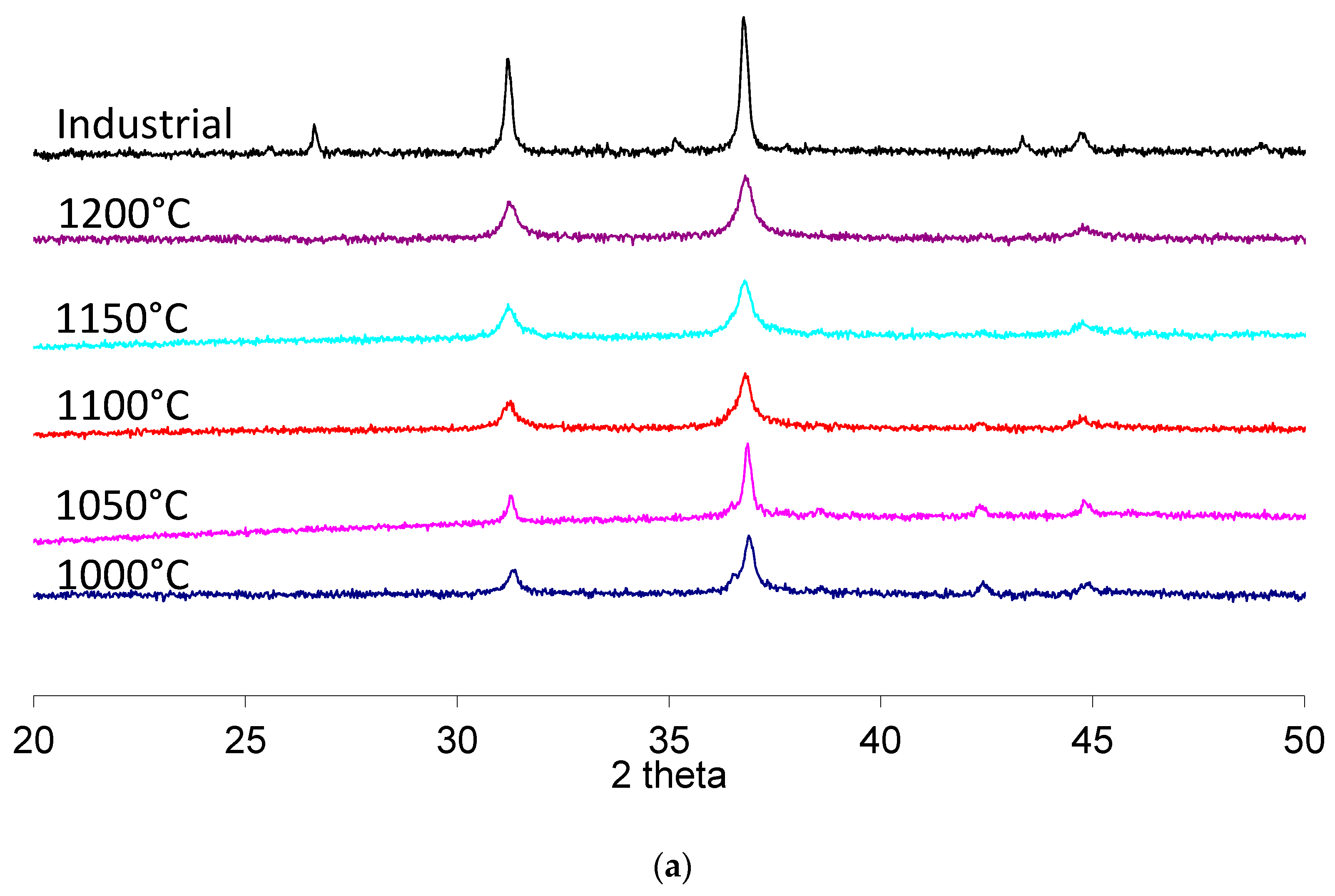
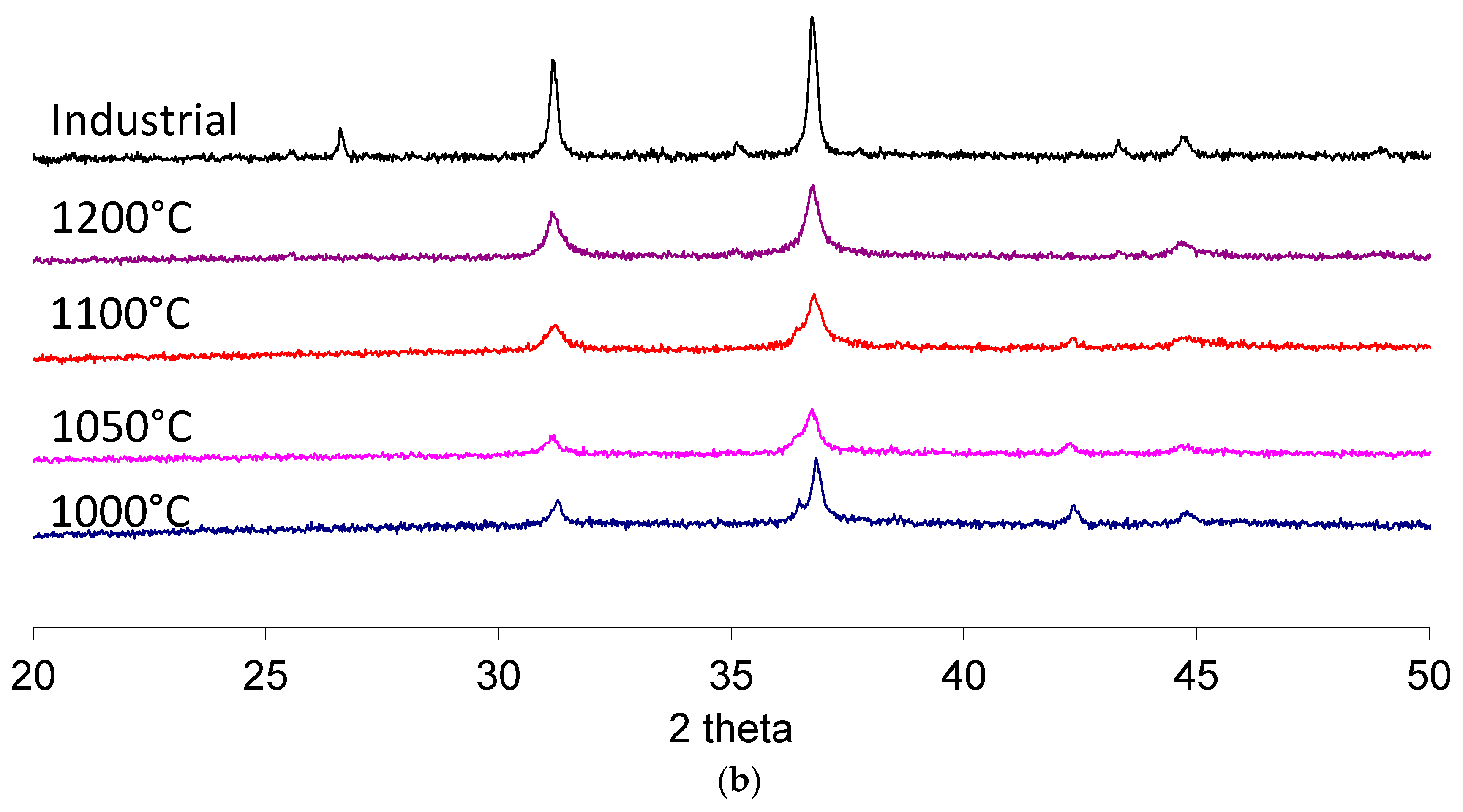
Figure 4 shows the comparison between calcinations conducted at different temperatures using conventional (a) or microwave (b) heating in the temperature-controlled mode, 10 °C/min up to the maximum temperature, with no holding time.
All peaks of the higher temperature synthesis are referred to the spinel CoAl2O4 phase, except for the small peak at 26.6° in 2 theta for the industrial pigment, which could indicate the use of mineralizers to favor the high temperature synthesis at the solid state or the presence of unreacted alumina.
The two peaks corresponding to 2 theta angle values close to 37° and to 45°, both ascribable to the cobalt aluminate spinel planes (311) and (400), respectively, were used to evaluate the advancement of the solid-state reaction. The peak at 37° is actually made of two superimposed peaks, as is clearly visible in the 1000 °C samples, where the α-Al
2O
3, (110) planes reflections are present. At higher temperatures, alumina progressively reacts with the cobalt-based precursor, resulting in the cobalt aluminate spinel and the single peak. This single peak is also present in the case of the industrial standard pigment. Hence, the progressive transition toward the single peak configuration, also evident in the higher temperature processed samples, can be taken as a measurement of the effective formation of the desired compound. A further peak at 43° is also indicative of unreacted precursors (α-Al
2O
3, (113) planes), and it progressively disappears when the complete formation of the blue pigment is achieved. These trends are in agreement with recent literature involving the solid-state synthesis of cobalt aluminate [
25,
26,
27].
The results indicate that, in the case of conventional heating or microwave heating, temperatures higher than 1100 °C are required. Noticeably, the 37° peak for the highest calcination temperature is broader than its industrial counterpart, indicating that a possible incomplete conversion of the precursors occurred. However, precursors processed by microwave heating present much sharper peaks, indicating that a possible higher degree of reaction was achieved. Hence, a further series of treatments at different isothermal holding times was performed to investigate possible microwave processing-related effects and to achieve results similar to the industrial standard.
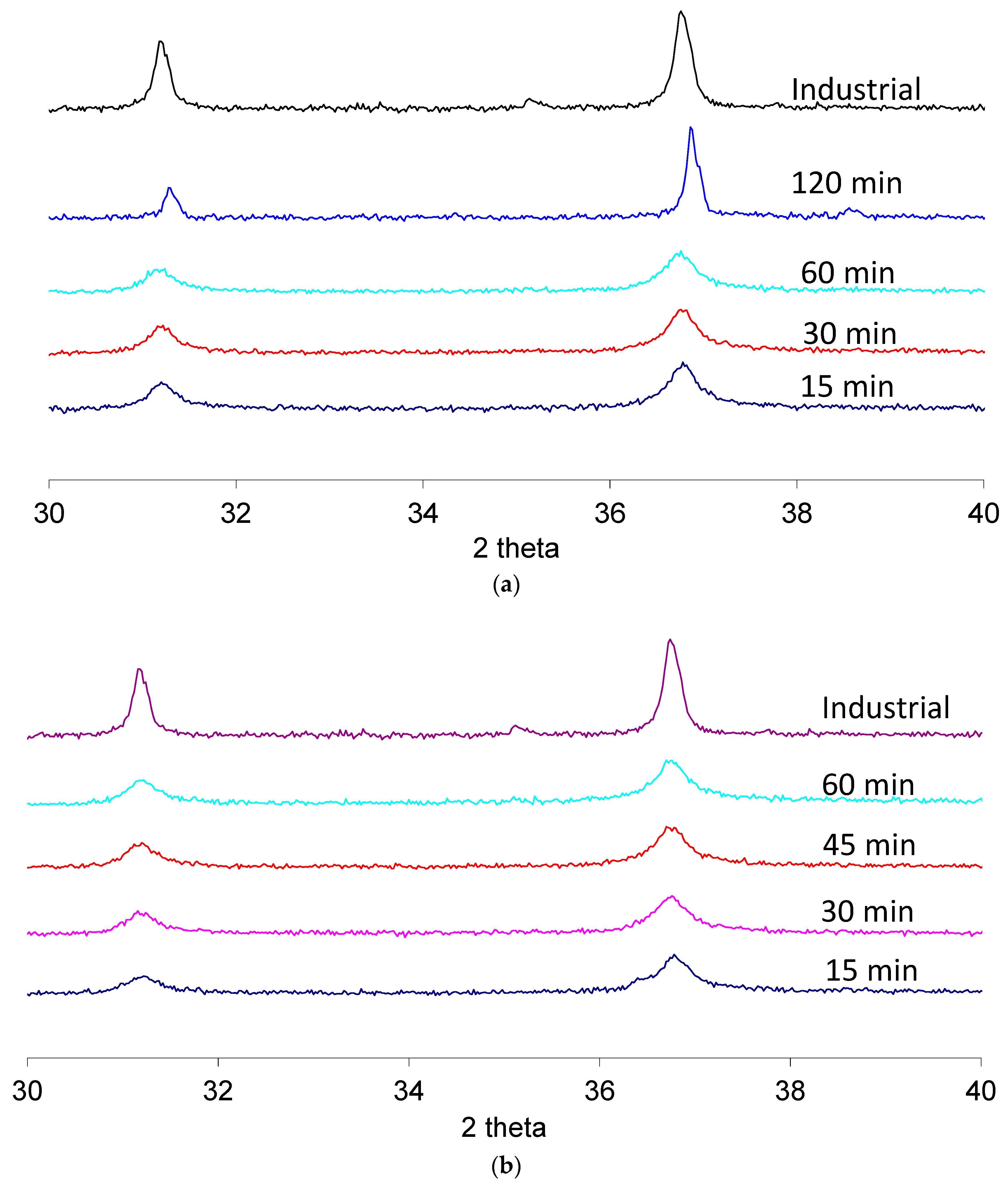
Figure 5 shows the results achieved varying the holding time at 1200 °C, in the case of conventional heating (a), or at 1100 °C, in the case of microwave heating (b), where a faster kinetic is expected based on the previous results. XRD patterns are magnified in order to investigate the 37° peak.
In the case of conventional processing at 1200 °C, the peak at 37° is completely formed starting from 30 min holding time, as it occurs in the case of microwave processing, despite the lower calcination temperature (1100 °C). In the case of prolonged processing at 1200 °C (i.e., for 2 h), the results are comparable with the ones achieved in the industrial practice.
However, it must be taken into account, when comparing conventional and microwave heating, that microwave heating (when conducted in a heating rate (10 °C/min) controlled mode) leads to prolonged off times of the microwave generator, especially during the holding step. Hence, the processing time indicated does not correspond to the effective microwave irradiation time, but only to the time of exposure at a given temperature [
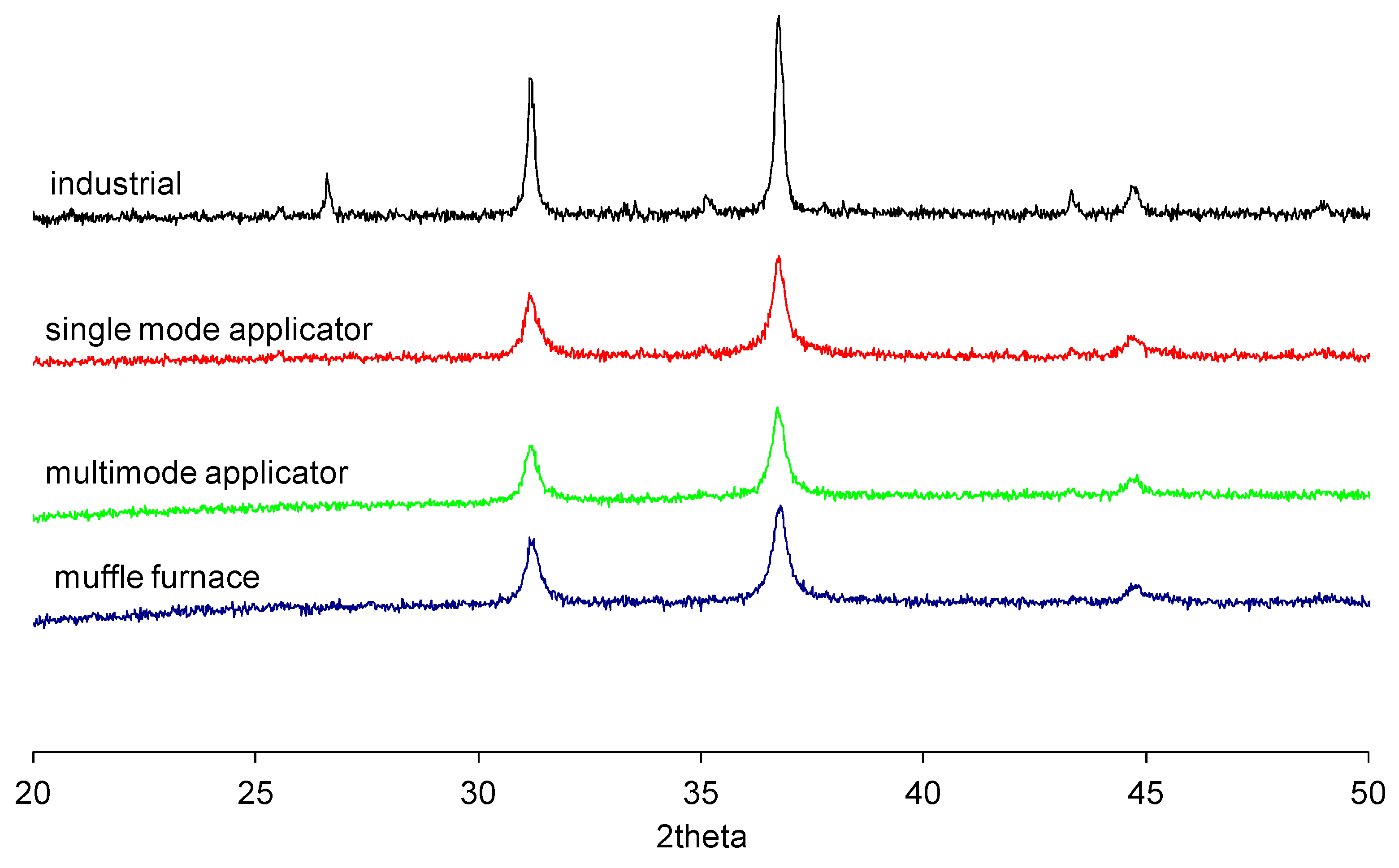
28]. For this reason, a second set of experiments was performed, setting only the maximum temperature (power-controlled mode) of 1200 °C, 60 min of holding time (temperature-controlled mode), and using different microwave applicators, which are expected to lead to different electric field strengths in the load, and hence an expected different tendency towards localized overheating and hot spots formation. The results, again in comparison with the industrial standard, are shown in
Figure 6, where, due to the similarity of the results obtained in different applicators of the same kind, only one pattern for a single mode and one for a multi-mode applicator are presented. Independent from the synthetic route used, the mineralogical analysis confirmed that blue CoAl
2O
4 pigment was obtained, with the single peak at 37°.
Surface area measurements were performed on the as-synthesized particles, processed at 1200 °C by different techniques, and mildly milled to reduce agglomeration, and finally were compared to the industrial standard (2.4 m
2/g). In the case of microwave processing, independent from the type of furnace and frequency used, the average surface area was 1.8 m
2/g, a value slightly lower than the one obtained in the case of conventional heating in a muffle furnace (3.2 m
2/g). It is well known from literature [
29,
30,
31] that, for a given set of starting raw materials, surface area is affected by the calcination temperature, decreasing with increasing calcining temperature. The obtained results seem to indicate that, in the case of microwave heating, despite an average set temperature of 1200 °C, localized overheating could have led to further sintering of the powders, thus lowering the surface area. Similarly, the values measured in the case of the conventionally heated pigment are higher than those obtained in the case of the industrial pigment, where mineralizers and possibly higher temperatures have been used during calcination.
UV-Vis measurements were used to calculate the Hunter CIELab parameters of the as-calcined powders and after inserting 4 wt % of powders in a porcelanized stoneware ceramic body.
Table 2 shows the results before and after firing, comparing the conventionally and microwave-heated samples (2.45 GHz, single mode applicator) with the industrial standard.
The results are extremely similar to those obtained in the case of fired products, independent of the technique used, while a small difference (lower absolute value of b *, indicating a less intense blue hue) exists between the industrial powders and the laboratory-prepared powders. However, a large difference was encountered when considering the specific energy consumption to achieve a unit mass of the desired pigment.
Table 3 summarizes the results obtained by integrating the electrical power absorbed by the line over time, divided by the amount of pigment synthesized. The data collected in this study allows for a comparison of the different techniques (conventional and microwave heating at different frequencies and in different applicators), including a comparison of the efficiency of the generators in the balance, which is a known drawback of dielectric heating applications. For comparison’s sake, the results achieved by microwave hybrid heating are included as well [
32]: in this case, heating occurs in the presence of an auxiliary microwave absorber, namely a SiC ring positioned around the precursors. This is a well-known set up often used to heat loads which present a low microwave absorption at room temperature, that increases as a function of temperature.
The data in
Table 3 show that conventional heating is the less advantageous technique, but it must be taken into account that the figures refer to the heat required for bringing the whole furnace to calcination temperature starting from room temperature, while in the industrial environment such a furnace would be already at high temperatures. Hence, the real data on conventional heating could be corrected to an order of magnitude smaller. The same conclusion applies to the heating process in the microwave furnaces, but in this case the selectivity of microwaves leads to the preferential heating of the load/pigment powder alone, while the furnace is almost unaffected, at least not as far as heat transfer from the load is concerned. In the case of the use of auxiliary SiC, the specific energy consumption results are similar to those calculated for the conventional furnace, as expected, since the entire furnace (including the SiC ring) is heated to the processing temperature starting from room temperature. It is also evident that the use of properly designed applicators, such as the single mode applicator, equipped with impedance matching devices, is the best solution, independent from the frequency of the generator, even if the use of 2.45 GHz gives the most favorable results for CoAl
2O
4 pigment. This fact becomes even more evident when comparing the theoretical specific energy consumption, i.e., the ratio between the time integral of the dissipated power curve and the mass of pigment obtained. In this case, the figure is deprived of reflections and of the applicator end generator efficiency, which leads to a promising average value of 118 kJ/g for the multi-mode applicator operating at 2.45 GHz and of 12.5 kJ/g in the case of single mode applicator, 2.45 GHz.