Abstract
Cryogenic upgrading represents a promising route for the production of high-purity biomethane, aligning with current decarbonization goals and the increasing demand for renewable gases. This review provides a critical assessment of cryogenic technologies applied to biogas purification, focusing on process fundamentals, technological configurations, energy and separation performance, and their industrial integration potential. The analysis covers standalone cryogenic systems as well as hybrid configurations combining cryogenic separation with membrane or chemical pretreatment to enhance efficiency and reduce operating costs. A comparative evaluation of key performance indicators—including methane recovery, specific energy demand, product purity, and technology readiness level—is presented, along with a discussion of representative industrial applications. In addition, recent techno-economic studies are examined to contextualize cryogenic upgrading within the broader landscape of CO2 separation technologies. Environmental trade-offs, investment thresholds, and sensitivity to gas prices and CO2 taxation are also discussed. The review identifies existing technical and economic barriers, outlines research and innovation priorities, and highlights the relevance of process integration with natural gas networks. Overall, cryogenic upgrading is confirmed as a technically viable and environmentally competitive solution for biomethane production, particularly in contexts requiring liquefied biomethane or CO2 recovery. Strategic deployment and regulatory support will be key to accelerating its industrial adoption. The objectives of this review have been met by consolidating the current state of knowledge and identifying specific gaps that warrant further investigation. Future work is expected to address these gaps through targeted experimental studies and technology demonstrations.
1. Introduction
The global energy landscape is at a critical crossroads, driven by the urgent need to reconcile the ever-growing demand for energy with pressing environmental imperatives. Rising population and industrial expansion continue to fuel global energy consumption, with fossil fuels like crude oil and natural gas still dominating the mix. After the temporary downturn caused by the COVID-19 pandemic, global oil demand has continued its strong recovery. In 2024, it reached approximately 103.9 million barrels per day, and is projected to rise to 105 million b/d in 2025 [1]. Similarly, global natural gas demand grew by 2.7% in 2024, corresponding to an increase of around 115 billion cubic meters, and is expected to maintain a similar growth rate in 2025 [2]. Yet this reliance on fossil energy is increasingly unsustainable, given its major contribution to greenhouse gas emissions and climate change. This situation has catalyzed a fundamental transformation of the energy paradigm. Global attention has shifted toward clean, low-carbon energy sources, and within this context, biomethane has emerged as a renewable vector of particular promise. Derived from biogas—produced via anaerobic digestion of organic residues including sewage sludge, agricultural waste, and energy crops [3]—biomethane offers both environmental and energy security advantages. The anaerobic process not only diverts waste from landfills but simultaneously transforms it into usable energy, aligning with circular economy principles. The substrates for anaerobic digestion are diverse and locally available, including livestock manure, agro-industrial residues, source-separated organic municipal solid waste, and wastewater sludge [3]. This flexibility supports decentralized energy models and allows tailoring the process to local biomass availability.
Biomethane’s versatility further reinforces its role in decarbonization. It can serve diverse end-uses, such as industrial cogeneration, heating, domestic energy supply, and transportation fuel, effectively replacing natural gas [4]. Additionally, biomethane utilization directly contributes to the reduction of GHG emissions. The typical production pathway includes feedstock pretreatment, anaerobic digestion, and subsequent upgrading to produce pipeline-quality or liquefied biomethane, depending on the intended application. However, realizing this potential hinges on addressing the critical technical challenge of biogas purification. Biogas typically contains a mixture of methane (CH4), carbon dioxide (CO2), nitrogen, hydrogen sulfide, oxygen, and water vapor, which must be efficiently removed to obtain pipeline-quality biomethane. Depending on regional standards, biomethane for grid injection generally contains at least 90% CH4 (minimum threshold for pipeline-quality in the U.S.) and more commonly 96–98% CH4 to meet contractual and technical specifications in both U.S. and European networks, alongside strict limits for other components such as oxygen, hydrogen sulfide, and siloxanes [4,5].
Cryogenic upgrading technologies, based on extremely low-temperature processing, stand out for their ability to produce high-purity biomethane, often exceeding 99% CH4 [6]. These processes enable effective methane–CO2 separation and often facilitate CO2 recovery as a valuable byproduct [7].
Recent studies highlight the competitive advantages of cryogenic upgrading when integrated with liquefaction of natural gas (bio-LNG) routes. Compared to conventional methods such as membrane separation or chemical scrubbing, cryogenic systems can simultaneously deliver high methane purity and energy efficiency, while producing a liquefied product with increased energy density. For long-distance transport, bio-LNG (with ~21 MJ/L) is significantly more efficient than compressed biomethane [8]. Moreover, the potential for recovering liquid CO2 as a co-product enhances the environmental and economic performance of these systems [9,10].
The growing interest in biogas upgrading is also reflected in the diversification of technologies adopted across Europe. While membrane separation currently dominates the market, cryogenic methods are gaining momentum due to their integration potential and purity levels [11]. In parallel, favorable policies—such as the EU’s target to achieve 15% renewable fuels in transport by 2030—continue to push for biomethane adoption at scale [12]. In 2023, combined biogas and biomethane production in Europe reached 22 billion cubic meters (bcm), equivalent to approximately 234 TWh, representing about 7% of the EU’s natural gas consumption. Biomethane production alone grew to 4.9 bcm, marking a 21% year-on-year increase, and installed capacity rose to 6.4 bcm/year by Q1 2024, the highest annual increase recorded to date [13].
Beyond technical merit, cryogenic upgrading technologies are increasingly evaluated from a sustainability and techno-economic perspective. Recent assessments have compared the energy and exergy efficiencies of different cryogenic routes, such as distillation, anti-sublimation, and controlled freeze zone (CFZ) systems, in both standalone and integrated bio-LNG configurations. Reported efficiencies for optimized configurations exceed 95%, with payback periods under 2.5 years in favorable economic scenarios [14,15,16].
Against this backdrop, the present article aims to provide a critical and comprehensive review of cryogenic upgrading as a cornerstone technology in the biomethane value chain. The underlying principles, key configurations, and recent technological advances in cryogenic separation are analyzed in detail. Special attention is paid to comparing cryogenic options with conventional upgrading methods in terms of energy consumption, methane purity, carbon capture potential, and integration feasibility with downstream processes such as liquefaction. Furthermore, the review identifies knowledge gaps related to the comparative performance of emerging cryogenic techniques (e.g., CFZ vs. anti-sublimation) and their scalability. It also examines the challenges associated with high energy demands in refrigeration cycles, the behavior of contaminants under cryogenic conditions, and prospects for optimizing process efficiency through heat integration or hybridization. In summary, this review positions cryogenic upgrading as a key enabler in the large-scale deployment of biomethane, contributing not only to energy diversification but also to climate mitigation. It aims to support informed decision-making by policymakers, industry actors, and researchers seeking high-performance solutions in the transition toward a low-carbon energy system. Comparable reviews have been published in recent years, addressing cryogenic upgrading either as part of broader biogas purification overviews [5,6,11] or in focused assessments of specific configurations and integration strategies with bio-LNG production [10,17]. However, these works either cover cryogenic technologies only tangentially or emphasize thermodynamic modeling without a comprehensive cross-comparison of energy, economic, and environmental metrics. The present review builds on these contributions by offering a fully integrated techno-economic and environmental perspective, systematically contrasting multiple cryogenic routes under standardized performance indicators and highlighting their industrial deployment potential.
2. Principles and Configurations of Cryogenic Biogas Upgrading
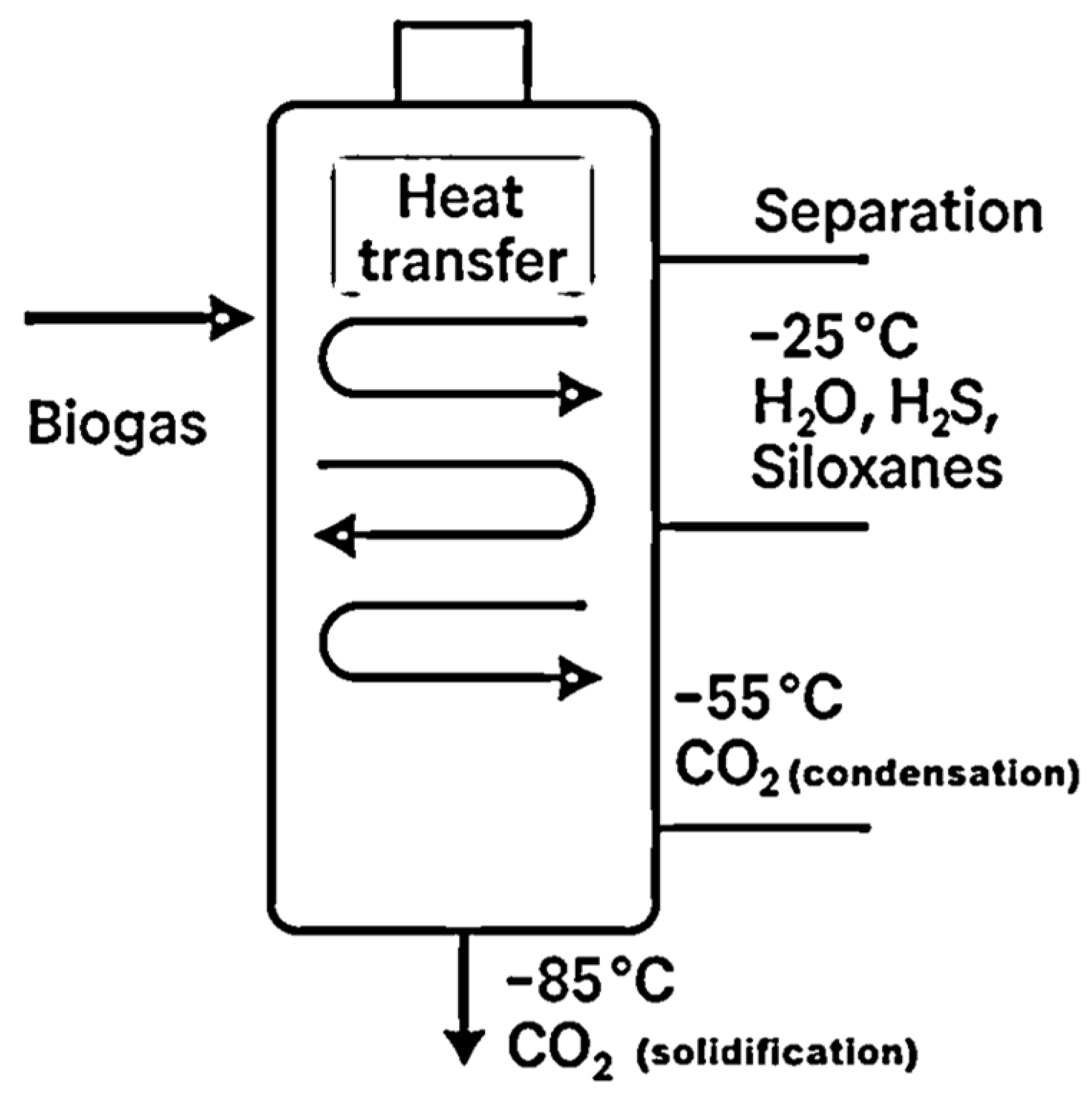
Cryogenic upgrading of biogas is founded on the principle of differential condensation behavior of its constituents under extremely low temperatures. Unlike conventional separation techniques that rely on chemical affinity or physical differences such as molecular size or permeability, cryogenic methods exploit the distinct phase-change thresholds of each compound present in biogas when progressively cooled. The aim is to isolate methane—biogas’s energy-rich component—by cooling the gas mixture to specific temperatures where impurities condense or solidify while methane remains in the vapor phase or is selectively liquefied [17]. The most common contaminants in biogas—such as carbon dioxide, water vapor, hydrogen sulfide, and siloxanes—exhibit varying condensation and solidification points. This variation enables a staged cooling strategy for their selective removal. For instance, water vapor tends to condense at relatively higher temperatures, allowing for early separation. Impurities like hydrogen sulfide and siloxanes can be extracted in subsequent steps as their respective liquefaction or freezing points are reached. Carbon dioxide, which is present in significant quantities (up to 50%), typically requires lower temperatures to undergo phase transition and is often the most technically challenging component to separate. The efficiency of the process depends critically on maintaining precise temperature gradients across multiple stages of compression and refrigeration [18]. Advanced temperature regulation ensures that each target compound transitions phase at its specific thermal threshold, thereby maximizing methane purity while reducing energy demand [19]. In standard configurations, the process begins at a working pressure of about 10 bar. Sequential cooling steps are then applied: at approximately −25 °C, most of the water vapor, hydrogen sulfide, and volatile siloxanes are removed; at around −55 °C, carbon dioxide begins to condense, and final CO2 purification is typically achieved at −85 °C when CO2 solidification is complete. The process is shown schematically in Figure 1. At this stage, methane remains in the gaseous phase, as its condensation temperature is significantly lower under these conditions [20]. The solid or liquid CO2 recovered through this process may be valorized as a commercial by-product, enhancing the economic feasibility of the system [21]. Alternatively, some systems employ a configuration involving initial gas drying and multi-stage compression up to 80 bar, which allows operation at moderately higher temperatures (−45 °C to −55 °C). However, this approach requires intermediate cooling stages during compression, which increases process complexity and energy consumption [17].
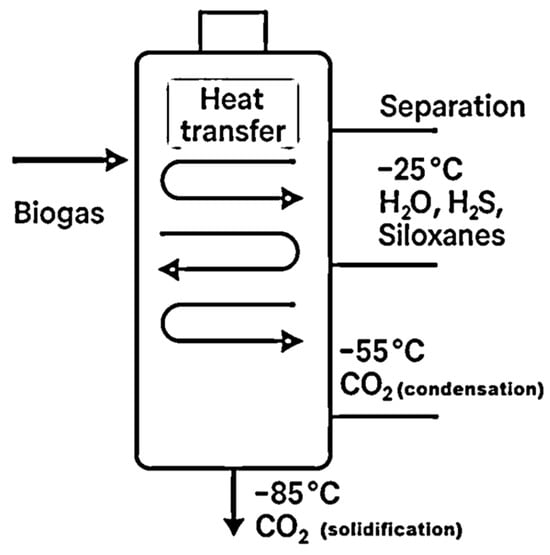
Figure 1.
Schematic representation of the cryogenic biogas upgrading process.
Among the multiple cryogenic technologies under development or commercial use, two broad categories dominate. Systems based on cryogenic distillation facilitate separation through differential boiling points, allowing methane and carbon dioxide to be isolated as liquid phases under well-controlled thermal conditions [22]. Meanwhile, anti-sublimation technologies rely on the direct solidification of CO2 onto cold surfaces, bypassing the liquid phase and offering potential benefits in compactness and reduced equipment footprint [23]. According to thermodynamic analyses, anti-sublimation configurations can operate efficiently under sub-atmospheric temperatures, offering CO2 capture with high selectivity and minimal methane loss [10]. These systems are especially attractive for distributed biomethane applications where on-site CO2 valorization is feasible, and transport of compressed gas is limited. Cryogenic packed beds have also been proposed as an alternative configuration. In these systems, the gas passes through a bed of cryogenically cooled material, where specific components are trapped via deposition [24]. Computational models have been developed to optimize key design parameters such as bed geometry, flow rate, and heat transfer conditions, aiming to maximize methane recovery while ensuring stable operation. Recent studies have shown that careful control of temperature gradients and packing structure is essential to avoid blockages caused by frost accumulation, and to improve the efficiency of contaminant capture [25]. Simulation tools also support the evaluation of system behavior during transient conditions, contributing to the development of robust and energy-efficient configurations [26].
3. Cryogenic Biogas Upgrading Technology
As described in Section 2, cryogenic biogas upgrading relies on the sequential cooling of the gas mixture to exploit the phase-change behavior of each constituent, enabling selective removal of CO2, H2O, H2S, and siloxanes while enriching methane to biomethane quality standards [27]. By progressively lowering the temperature under defined pressure conditions, impurities such as CO2, H2O, H2S, and siloxanes can be effectively removed, resulting in a methane-enriched stream that meets biomethane quality standards.
A widely implemented operational regime maintains the system at approximately 10 bar, under which cooling stages are configured to selectively condense and separate unwanted compounds at their characteristic temperature thresholds [28]. Water vapor, hydrogen sulfide, and siloxanes are typically eliminated at around −25 °C, while carbon dioxide requires lower temperatures—liquefying near −55 °C and ultimately solidifying at −85 °C to achieve complete separation [29]. As an alternative, some configurations apply initial drying followed by multi-stage compression up to 80 bar. This allows operation at moderately higher cryogenic temperatures, between −45 °C and −55 °C, but requires intermediate cooling during compression steps, increasing system complexity and energy demands [30]. To improve process flexibility and economic performance, numerous modifications and design alternatives have been proposed in recent years, ranging from novel cycle architectures to hybrid and intensified systems [17,31,32]. These technological developments are examined in detail throughout the following subsections.
The main cryogenic technologies currently applied or under development for biogas upgrading include the following: cryogenic distillation, dual-pressure cryogenic distillation, anti-sublimation, controlled freeze zone (CFZ) technology, the Ryan–Holmes process, and various hybrid cryogenic processes (e.g., membrane–cryogenic, adsorption–cryogenic, hydrate-based, and ammonia absorption–cryogenic configurations). A comparative overview of their main advantages and disadvantages is provided in Table 1. These technological developments are examined in detail throughout the following subsections.

Table 1.
Comparative advantages and disadvantages of cryogenic biogas upgrading technologies.
3.1. Cryogenic Distillation Process
Cryogenic distillation is a thermally-driven technology used to separate carbon dioxide from biogas by exploiting the specific phase-change behavior of CO2 at low temperatures and elevated pressures. The process enables CO2 to be recovered in liquid form while producing biomethane of high purity. Traditionally, this method is associated with high energy demands, which can negatively impact its economic competitiveness compared to other upgrading techniques [33]. To address these limitations, recent developments have focused on integrating process intensification techniques and hybrid systems that significantly improve energy performance. Reported results indicate that, when properly designed, hybrid cryogenic distillation schemes can achieve up to 70% energy savings compared to baseline configurations [34].
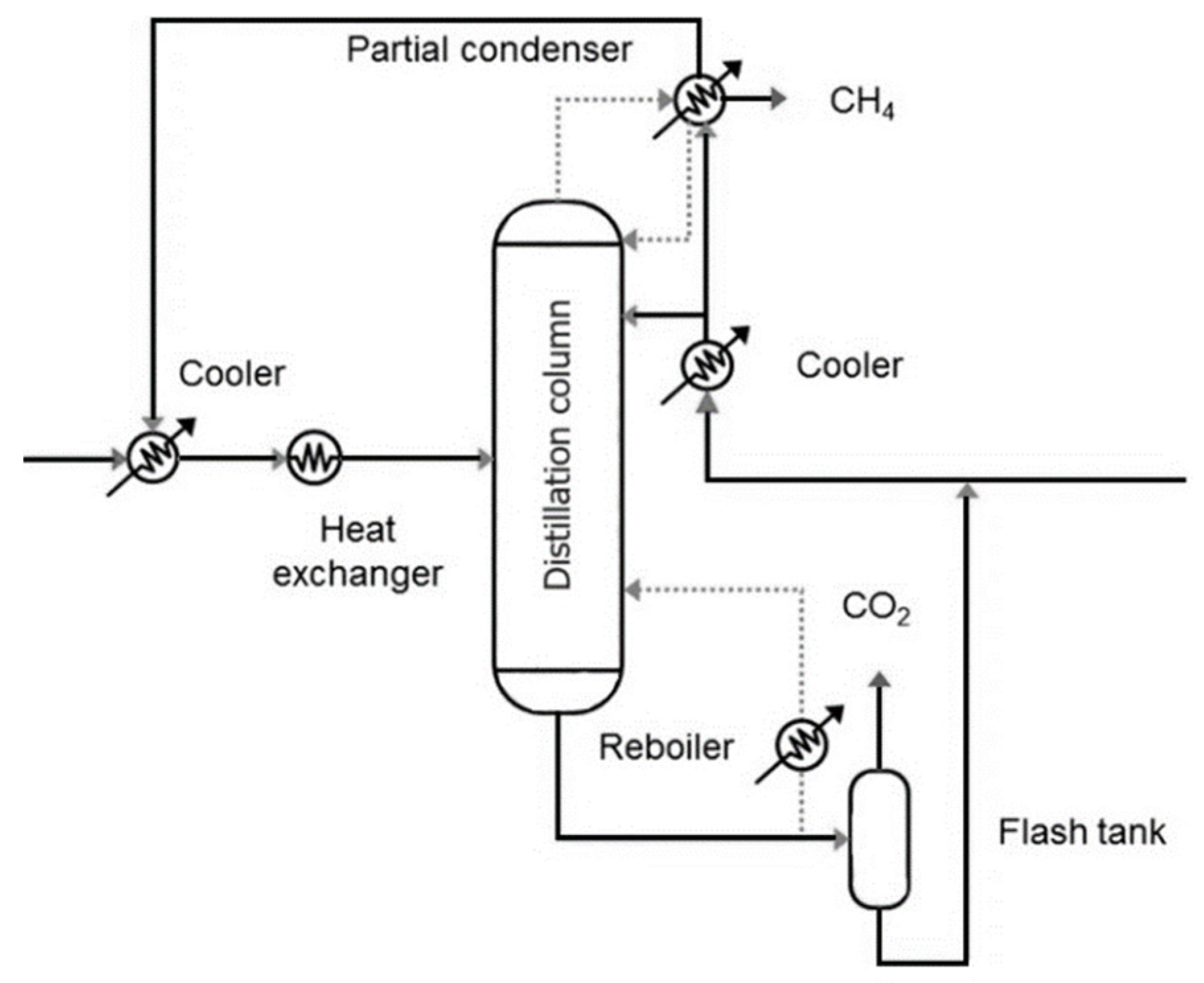
Although most cryogenic distillation systems have been validated under natural gas purification scenarios, several authors have outlined their potential applicability to biogas upgrading [35,36]. Figure 2 shows a representative setup in which the gas stream is pretreated to remove trace contaminants and then cooled in two stages before entering a distillation column. Inside the column, separation is based on boiling point differences: methane, as the lightest compound, is collected at the top via a partial condenser, while CO2 is recovered as a bottom product in liquid form. A fraction of the CO2 stream is recycled to assist in the reboiler duty of the pre-vaporization unit, whereas the rest is extracted for commercial use, thereby enhancing the system’s economic viability.
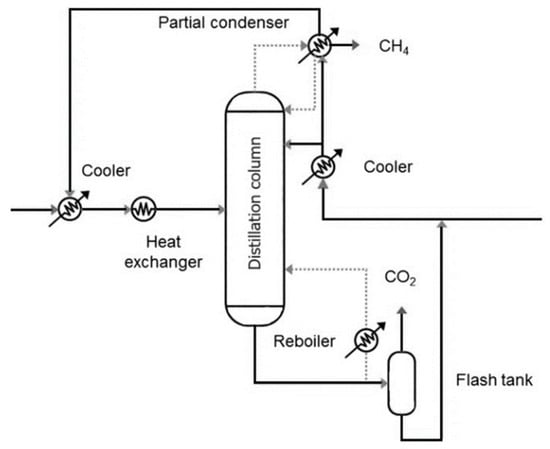
Figure 2.
Schematic representation of a cryogenic distillation system for biogas upgrading. Precooled biogas enters the distillation column, where high-purity methane is recovered overhead and liquid CO2 is collected at the bottom. Part of the CO2 is recycled to support reboiler vaporization.
In the work by Yousef et al. [37], cryogenic distillation was optimized to avoid CO2 freezing within the column, a common issue that may disrupt process performance. They investigated the influence of feed pressure, CO2 concentration, temperature, reflux ratio, and feed location within the column. Simulation results demonstrated that a CO2 purity of 99.5 mol% could be attained at a specific energy consumption of 0.31 kWh/Nm3 of raw biogas, while methane purity reached up to 97 mol% without CO2 solidification. Notably, the tendency for CO2 freezing was found to remain constant across a wide range of inlet CO2 concentrations, suggesting robustness in process operation. In a complementary study [38], a novel design combining a four-stage compression train, a distillation column, and an integrated flash separator was proposed to optimize energy recovery and separation efficiency. This configuration yielded a record CH4 purity of 97.2 mol%, outperforming previous single-column systems. At the same time, a valuable CO2 co-product of more than 99% purity was generated. The associated energy penalty was just 0.38 kWh/Nm3 of clean gas, positioning the system among the most efficient reported to date in terms of methane recovery, CO2 valorization, and overall process economics.
Moreover, recent numerical studies have explored the feasibility of process intensification in cryogenic distillation by adopting column internals that improve mass and heat transfer performance. For instance, the use of structured packing and enhanced heat integration has been shown to reduce condenser and reboiler loads, especially when CO2-rich feeds are treated at optimized pressure levels [39]. Other experiments have demonstrated that cascading refrigeration stages and adjusting the CO2 reflux ratio can further suppress freezing risk while maintaining operational efficiency [40]. These innovations underscore the ongoing evolution of cryogenic distillation from an energy-intensive method to a viable and scalable solution for biogas upgrading.
3.2. Dual-Pressure Cryogenic Distillation Process
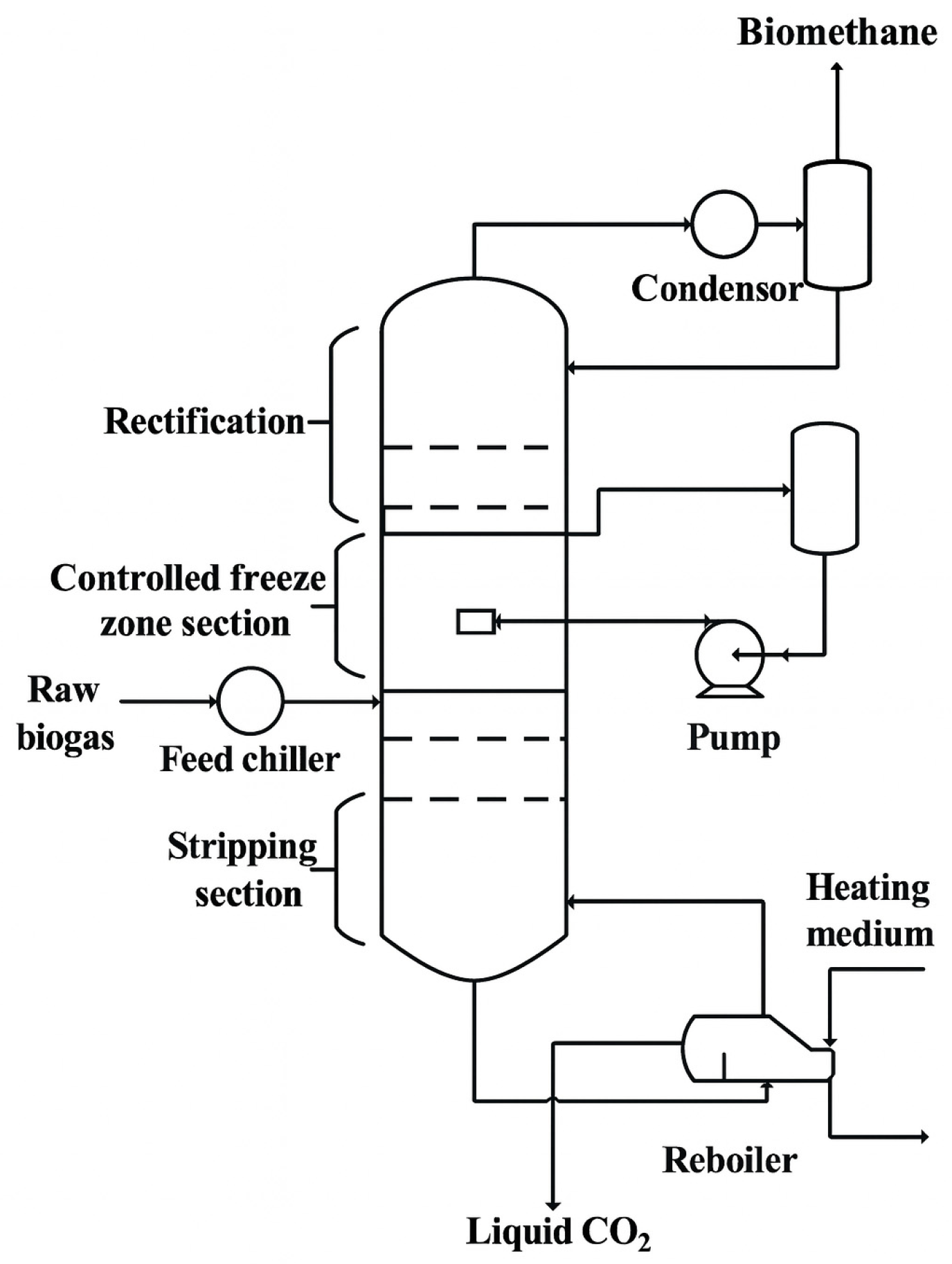
The process of removing carbon dioxide from gas through a dual-pressure low-temperature (or cryogenic) distillation process represents a significant advancement in gas purification, especially in fields containing acid gas, for example, natural gas that is typically laden with acidic compounds like CO2 and H2S. These fields, accounting for about 40% of the globe’s natural gas reserves, span continents from Europe and America to Asia. Yet, to harness these resources effectively, eliminating acidic components is paramount [36]. Such components, including CO2 and H2S, can cause severe corrosion in pipelines and processing equipment, reduce heating value, and pose safety and environmental hazards if not removed. The process proposed by Pellegrini et al. [41] tackles these hurdles with an innovative approach, employing two distillation stages at varying pressures alongside an intermediary heating phase (Figure 3). Initially, at high pressure, the feed mixture undergoes distillation, facilitating CO2 separation in liquid form. Subsequently, at lower pressure, further methane purification is achieved. In this configuration, acidic components such as CO2 and H2S are removed predominantly in the high-pressure column, where they condense or remain in the liquid phase and exit as a bottom product. This liquid stream is then withdrawn from the system for valorization or disposal, ensuring that only methane-rich gas proceeds to the low-pressure column.
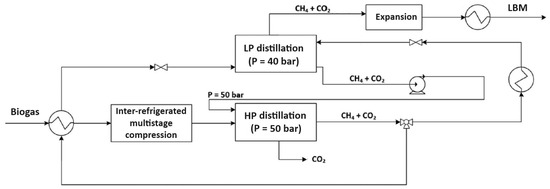
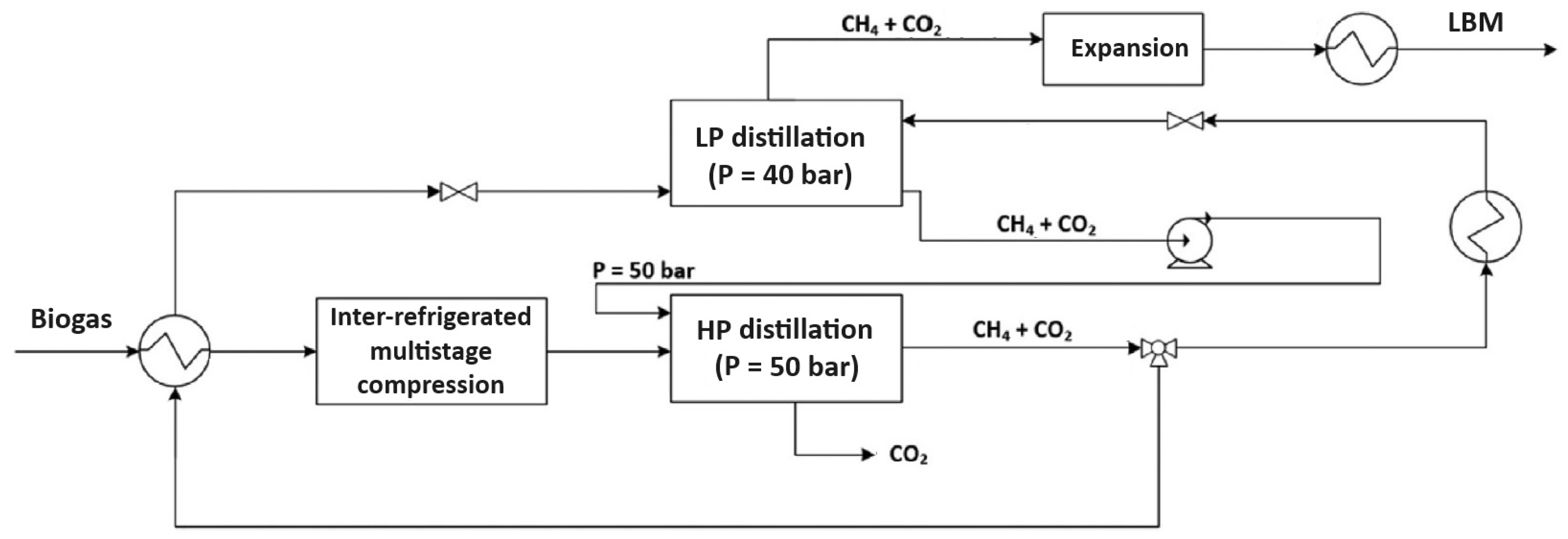
Figure 3.
Schematic representation of a dual-pressure cryogenic distillation process for biogas upgrading. The system integrates high- and low-pressure distillation columns with intermediate heat exchange and expansion to produce liquid biomethane (LBM) and recover high-purity CO2.
More specifically, purification occurs within two distillation units: the first operates at high pressure (50 bar), while the second operates below methane’s critical pressure (40 bar). The high-pressure section functions akin to the stripping section of a conventional distillation column, where liquid reflux is facilitated by recycling the flow from the low-pressure section’s bottom. Similarly, the low-pressure section acts as the enrichment section: it features a partial condenser at the top, and the feed gas flow originates from the high-pressure section’s output. The gas stream exiting the top of the low-pressure section meets the required methane purity specifications. According to the Pellegrini patent [41], methane purity in the low-pressure top product can reach up to 100 mol%, while CO2 purity in the high-pressure bottom stream exceeds 99.9 mol%, demonstrating the high selectivity of the process. Liquid biomethane (LBM) is subsequently generated via an appropriate liquefaction process. The high-pressure distillation section’s bottom product comprises highly pure carbon dioxide. The incoming biogas feed is precooled in a primary heat exchanger utilizing the available cooling capacity from an intermediate process stream, necessitating preheating before entering the low-pressure section. After precooling, the biogas is compressed to 50 bar and further cooled to its dew point at that pressure before entering the high-pressure distillation section. Compression follows precooling to reduce the compression power by lowering the feed stream’s temperature.
The high-pressure section effectively eliminates input CO2; the bottom stream is high-pressure liquid CO2, while the top product stream is methane-rich (approximately 6.5 mol% CO2) [41]. Since the high-pressure section operates above methane’s critical pressure, obtaining pure methane through distillation in a single 50 bar unit is infeasible. Hence, final purification occurs in the low-pressure section at 40 bar. Outputs from the low-pressure section include an upper methane gas flow and a lower methane-rich liquid flow pumped back to the high-pressure section. The feed flow enters the high-pressure section on the fourth tray from the top, while liquid reflux from the low-pressure section’s bottom is pumped and introduced into the first tray from the top. The upper gas flow from the high-pressure section undergoes splitting into two streams. Before entering the low-pressure section’s bottom, a portion of the high-pressure section’s upper product flow is heated and expanded to the low-pressure section’s operating pressure, ensuring a temperature higher than its dew point. This prevents solid phase formation during expansion, with heat sourced from the input raw biogas stream precooled prior to compression. The remaining upper product flow from the high-pressure section is cooled to 50 bar (below CO2s’ solubility limit) and expanded to the low-pressure section’s operating pressure, yielding a liquid flow at its bubble point, fed into the low-pressure section one theoretical plate above the gas feed flow [42].
A pivotal advantage of this method lies in its adeptness at handling sour gas, even with substantial CO2 and other impurities like higher hydrocarbons and hydrogen sulfide. This adaptability stems from meticulously tailored pressure and temperature conditions at each distillation phase, preventing solid CO2 formation and ensuring efficient acid component separation. This is achieved by exploiting the different phase-change temperatures of CH4, CO2, and H2S under tailored pressure–temperature conditions: CO2 and H2S condense or solidify at higher temperatures than methane, allowing their selective removal as liquid or solid phases in the bottom streams of each distillation section. Moreover, the process exhibits versatility, proving equally effective for both conventional natural gas and biogas derived from organic waste. This adaptability renders it a multipurpose solution for gas purification across diverse industrial and environmental settings [41]. Recent literature supports the industrial relevance and scalability of this approach. According to Pellegrini et al. [19], dual-pressure cryogenic distillation demonstrates strong operational efficiency: it utilizes only 14% of the produced biomethane as internal energy—significantly lower than anti-sublimation (~22%) and MEA (monoethanolamine) scrubbing (~29%) schemes. Furthermore, most of this energy (61%) is used for cooling, with no requirement for high-temperature reboiler heat. In terms of process stages, about 38% of energy is consumed in compression, ~32% in upgrading, and ~30% in liquefaction, while CO2 pressurization is effectively zero, since CO2 is handled as a pressurized liquid.
3.3. Anti-Sublimation Process
The production of liquefied biomethane via the anti-sublimation process involves the utilization of heat exchange surfaces to refine biogas, operating within the solid–vapor equilibrium region at atmospheric pressure [43]. In this process, CO2 freezes directly within the gas stream, resulting in methane enrichment. This innovative approach, patented by Clodic et al. [44], is based on a specially designed cold chamber where CO2 solidifies upon contact with cryogenic refrigerants. Its modular configuration allows for the decoupling of vaporization and solidification stages in separate chambers, thus enhancing operational flexibility. The process configuration is illustrated in Figure 4.
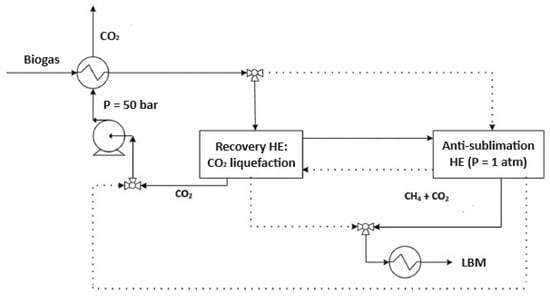
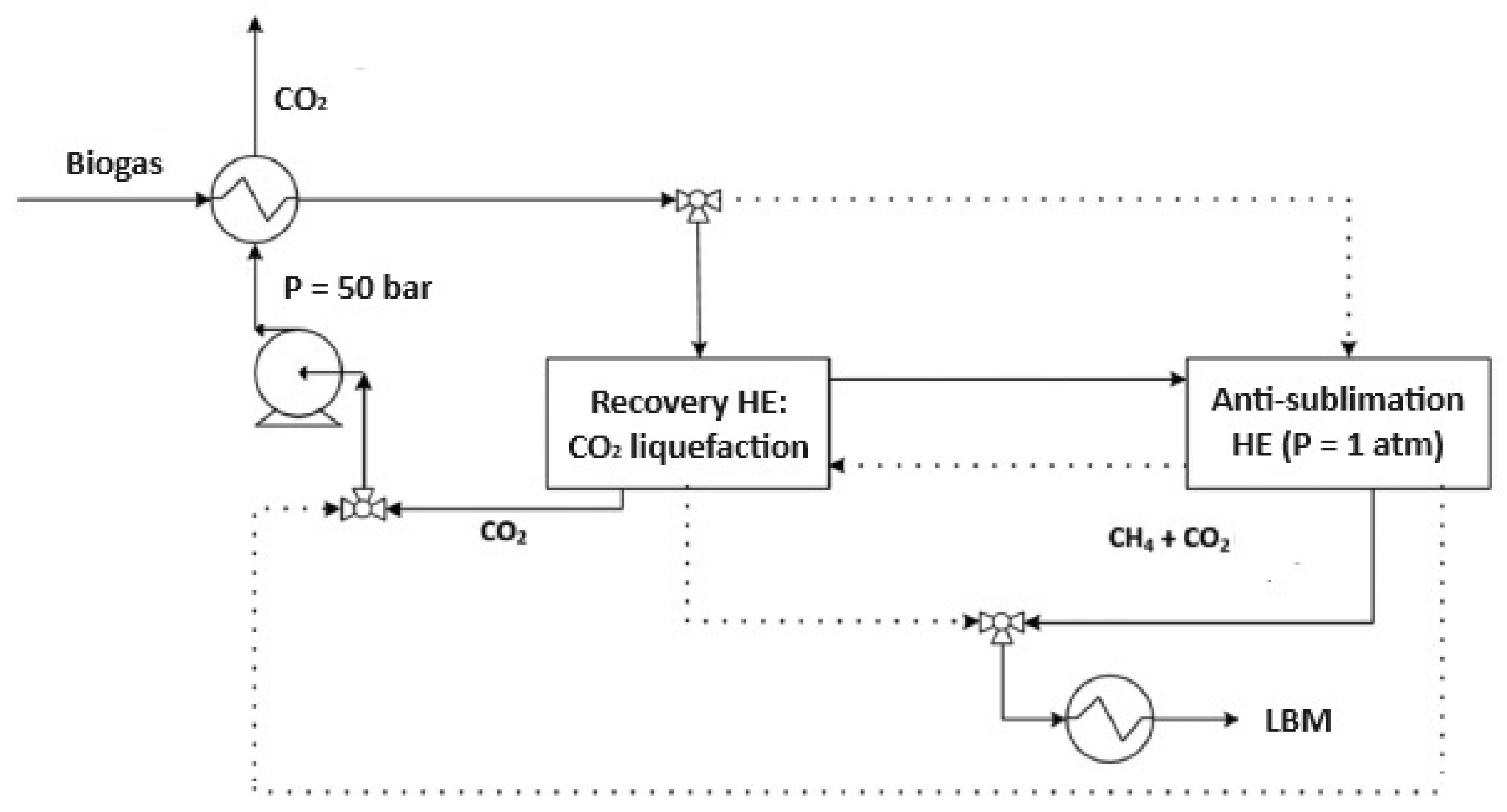
Figure 4.
Schematic representation of biogas purification via the anti-sublimation process. The system integrates a CO2 liquefaction stage (Recovery Heat Exchanger, HE) and an anti-sublimation unit operated at atmospheric pressure, enabling CH4 enrichment and Liquid Biomethane (LBM) production through sequential cooling and solid CO2 separation.
In the anti-sublimation process, purification is achieved through dry ice formation within a sealed cryogenic system. Two dynamic heat exchangers (HE in Figure 4) alternate between purification and regeneration phases, enabling continuous operation. Figure 4 depicts solid lines for the operational phase and dashed lines for the regeneration cycle. The incoming raw biogas, typically at ambient temperature and pressure, is first routed through a precooling exchanger that recovers cold from the liquid CO2 stream in the recovery loop. This precooled gas then passes through the recovery exchanger, where residual dry ice from the previous cycle contributes additional cooling. By melting this layer of CO2, temperatures as low as −51 °C are attained, and the resulting CO2 is collected in liquid form. The liquid CO2 is then pressurized to 50 bar and reheated in the recovery exchanger. To reach the anti-sublimation threshold, further cooling is required to bring the gas temperature down to approximately −160 °C, enabling the direct solidification of CO2 from the gas phase. This is achieved with an external refrigeration system where nitrogen acts as the final cooling agent [16]. Inside the anti-sublimation exchanger, a stable layer of solid CO2 accumulates, reducing CO2 concentration in the methane-rich stream to below 50 ppm at atmospheric pressure. The purified gas is subsequently liquefied in a dedicated cryogenic condenser for downstream storage and use.
The anti-sublimation process requires precise control of pressure and temperature, guided by the CH4–CO2 phase diagram, to ensure efficient CO2 solidification. Simulation studies conducted in Aspen Plus indicate that by reducing feed gas temperature to the CO2 sublimation point, extraction purities up to 90% can be achieved [45]. Energy consumption ranges from 0.286 to 1.3 kWh/kgCO2 removed, depending on operational conditions and the degree of thermal integration. Under optimal configurations, the lowest consumption case also corresponds to a specific cost of approximately 0.1 €/kg of CO2 [10,16,45], estimated as a levelized cost of process including both capital expenditure (CAPEX) and fixed and variable operating expenditure (OPEX), for a plant capacity of 1000 m3/h, a 30-year lifetime, and a 10% discount rate. CAPEX was calculated using Guthrie’s bare module method for major process equipment, with fixed OPEX set at 2% of CAPEX and variable OPEX covering electricity, low-pressure steam, and refrigerant.
Additional insights from Naquash et al. [10] further support the practical implementation of this process. In comparative analyses, anti-sublimation exhibited the highest refrigeration demand among cryogenic systems, with over 99% of total energy consumption allocated to cooling duties, largely due to the necessity of reaching extreme cryogenic temperatures. Despite this, it enables CH4 recoveries of over 98% and achieves CO2 concentrations well below 100 ppm, making it suitable for high-purity biomethane production. However, the lack of process flexibility under variable flow rates and the need for robust frost management strategies were identified as key limitations. The design’s integrated heat exchange between incoming gas and recovered CO2 stream plays a pivotal role in energy efficiency, particularly in reducing the refrigeration load of the final purification stage. By leveraging the cold potential of sublimated or liquefied CO2, the system reduces external cooling demands during startup and regeneration cycles. This integrated thermodynamic behavior illustrates the technological ingenuity of anti-sublimation and underscores its relevance for both bio-LNG production and high-value CO2 recovery.
3.4. Controlled Freeze Zone Process
Controlled Freeze Zone, or CFZ technology, is a cryogenic one-step distillation process developed by Exxon Mobil over four decades ago to efficiently separate CO2 and other acid gases from natural gas streams [46]. Originally demonstrated in a pilot unit with a processing capacity of 0.02 MNm3/day and CO2 concentrations ranging from 15% to 65%, this technology achieved outlet gas CO2 levels as low as 100 ppm. Following initial success, it was scaled up to accommodate natural gas flows up to 30 MNm3/day while maintaining output specifications. The key innovation of CFZ lies in its integration of vapor–liquid–solid equilibrium within a single distillation column. The column is structured into three main zones: an upper rectifying section (packed bed), a central CFZ chamber, and a lower stripping section. The central freezing zone contains a spray subzone for distributing liquid CO2, a freezing zone where solid CO2 forms, and a melting zone where the solids are recovered as liquid. Pretreated and dehydrated natural gas enters the upper section. As methane rises and vaporizes, a CO2-enriched liquid is sprayed into the CFZ chamber, where it solidifies under cryogenic conditions. The solid CO2 accumulates, melts, and is withdrawn as a high-purity liquid at the bottom, while purified CH4 exits at the top. The process is illustrated in Figure 5, which depicts the structural layout of the integrated CFZ column.
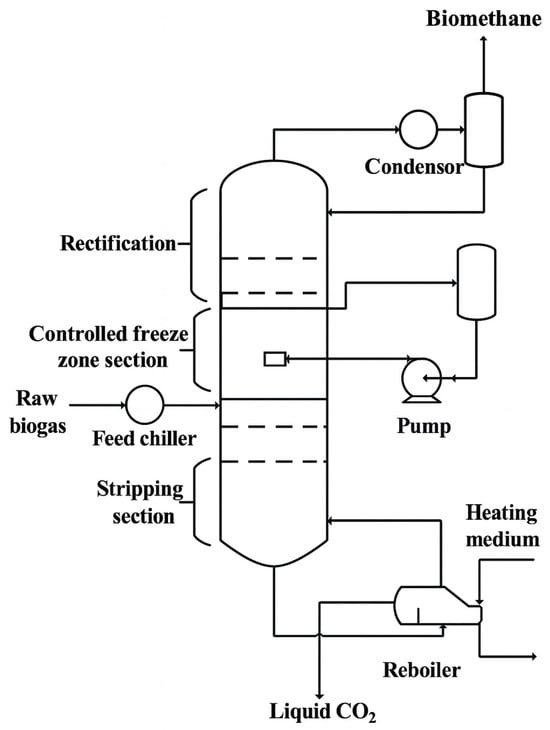
Figure 5.
Purification of biogas through controlled freeze zone technology. The CFZ system integrates rectifying and stripping packed sections with a central chamber where CO2 is frozen and melted in sequence. Liquid CO2 is sprayed into the freezing zone, forming dry ice that is later melted and removed as a pure liquid stream, while high-purity methane exits from the top of the column.
According to Exxon Mobil, this configuration reduces capital costs by 10–30% compared to conventional absorption systems [47], with added operational benefits such as simplified process flow and reduced refrigerant demand. However, its performance diminishes in gas mixtures with high ethane or heavier hydrocarbons, which tend to co-solidify and hinder selectivity, thereby increasing separation complexity and cost. A comparative techno-economic and performance analysis between CFZ and dual-pressure cryogenic distillation (Pellegrini process) was conducted on the Natuna and Bojonegoro gas fields in Indonesia [21]. At Natuna, CFZ achieved a CH4 purity of 95.62% and CO2 recovery of 99.04%, outperforming Pellegrini’s scheme in CO2 separation but slightly lower in methane purity (91.98% CH4, 97.86% CO2 recovery). At Bojonegoro, however, Pellegrini’s approach surpassed CFZ in both parameters, reaching 99.91% CH4 purity and 99.99% CO2 recovery, compared to CFZ’s 97.99% and 98.45%, respectively. Despite these mixed results in purity, CFZ demonstrated a lower CO2 capture cost, at 7.9 USD/tonne, compared to 10.26 USD/tonne for Pellegrini distillation. This cost advantage is attributed to the integration of freezing and melting within a single column, reduced reboiler duty, and minimized refrigerant inventory. According to additional insights from Spitoni et al. [31], CFZ technology exhibits superior thermodynamic integration and a compact footprint, which enhances modularity and scalability—particularly in offshore or decentralized applications. Moreover, the direct collection of liquid CO2 facilitates its downstream valorization for industrial use.
CFZ remains a promising option for high-volume natural gas upgrading, especially where cryogenic utilities are already present or CO2 recovery for reuse is a priority. Nevertheless, its implementation in biogas treatment remains limited, primarily due to the lower scale and compositional variability of typical biogas streams. However, its technical underpinnings continue to inform the development of hybrid cryogenic–adsorption systems being considered in recent research for biomethane production [48].
3.5. Ryan–Holmes Process
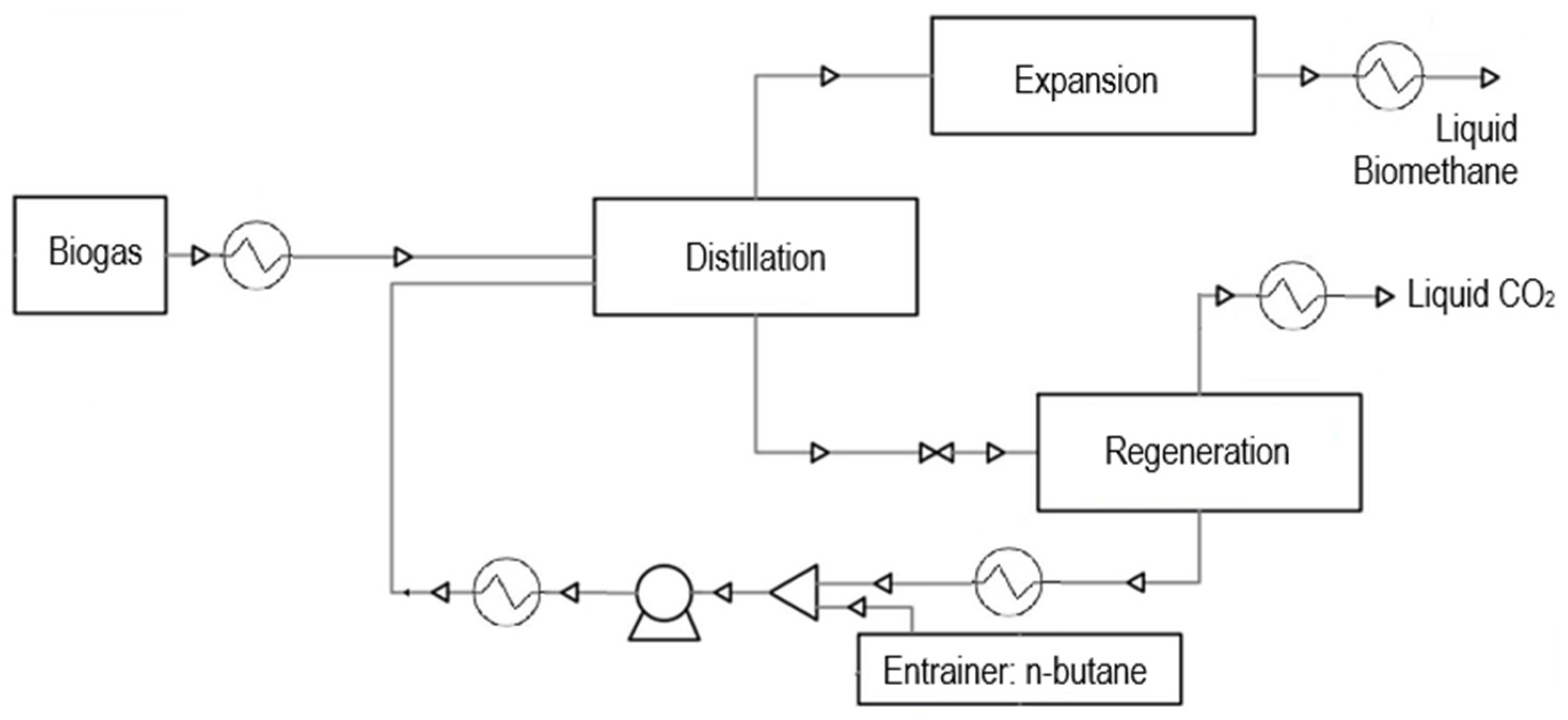
The Ryan–Holmes process [49] (Figure 6) represents a cryogenic biogas upgrading route based on extractive distillation, which facilitates carbon dioxide removal by modifying the phase behavior of the CH4–CO2 mixture. This is achieved by incorporating an entrainer that elevates the critical locus and displaces the CO2 freezing boundary toward lower pressures and temperatures, thus minimizing the risk of CO2 solidification [19]. The process typically employs heavier hydrocarbons, such as n-butane, as entraining agents. These components interact with CO2 to depress its freezing point and promote efficient separation from methane. In optimized designs, typical entrainer losses are below 1% molar of the circulating flow, although values up to 2–3% may occur in less efficient recovery configurations. The overall system comprises five principal units: (i) biogas compression, (ii) extractive distillation, (iii) entrainer recovery and regeneration, (iv) biomethane liquefaction, and (v) CO2 condensation and pumping.

Figure 6.
Purification of biogas through the Ryan–Holmes process. Cryogenic upgrading by extractive distillation using n-butane as entrainer, which shifts the CO2 freezing boundary to prevent solid formation and enables efficient separation of methane, CO2, and heavier hydrocarbons.
Initially, the raw biogas feed is compressed to approximately 40 bar to prepare it for extractive distillation. Within the distillation column, n-butane is injected as a solvent. Its presence shifts the critical region of the gas mixture, enabling lower-temperature operation without encountering the risk of CO2 freeze-out. The biogas feed enters the column at an upper tray, optimizing the contact with the descending entrainer and maximizing separation efficiency. The overhead product from this column is primarily composed of methane and undergoes further cooling and depressurization via a liquid expander and condensation stage, ultimately yielding liquefied biomethane at near-atmospheric pressure. Conversely, the bottom stream from the extractive distillation unit—rich in CO2 and n-butane—is first depressurized to 30 bar and then introduced into a regeneration column. In the regeneration section, thermal input enables the selective desorption and recovery of CO2 in liquid form. The n-butane, now separated, is condensed, cooled, and recycled back to the extractive distillation stage, thereby closing the entrainer loop and minimizing solvent losses. The extractive distillation column itself operates under a temperature gradient that decreases from bottom to top—heated at the reboiler and cooled at the condenser—allowing stable phase separation throughout the column height. In contrast, the regeneration column maintains relatively higher temperature levels to ensure effective desorption and recovery of the entrainer. The Ryan–Holmes process is distinguished by its relatively high energy consumption, largely attributable to the reboiler duty required for entrainer regeneration and the condenser duty associated with methane product recovery. Nevertheless, its ability to circumvent CO2 solidification without operating at extreme pressures or employing anti-sublimation steps makes it a promising alternative for biogas upgrading.
Originally developed for sour gas treatment in enhanced oil recovery operations, the Ryan–Holmes process was specifically designed to address two critical technical challenges, the formation of a CO2–C2H6 azeotrope and the high freezing point of CO2, both of which compromise the performance of conventional cryogenic distillation. By incorporating an extractive recycle loop, this process suppresses CO2 solid formation and breaks the azeotropic barrier, enabling efficient separation of methane, CO2, and light hydrocarbons [50].
Numerous large-scale natural gas processing facilities have implemented this technology successfully, including the Seminole Unit operated by Amerada Hess, the Willard Unit by ARCO, the GMK South Field by Mobil Oil Corporation, and the Wasson Denver Unit by Shell Oil. These applications confirm the maturity and industrial viability of the process under high CO2 concentration conditions. In comparison with amine scrubbing systems, the Ryan–Holmes process presents multiple advantages: lower dehydration requirements, elimination of corrosive solvents, the use of natural gas liquids as solvents, and the production of high-pressure CO2 streams that are readily suitable for geological reinjection. Additionally, the process achieves high recovery rates of heavier hydrocarbons—such as ethane and propane—providing an important revenue stream, particularly in scenarios where methane content in the feed gas is reduced [50,51].
Beyond its traditional configuration, the Ryan–Holmes process has also been explored in hybrid schemes combined with membrane technologies to improve cost-effectiveness and operational flexibility. Membrane units may be installed upstream for bulk CO2 removal prior to cryogenic distillation, reducing the load on the distillation system and optimizing overall energy use. Alternatively, membranes can be integrated downstream of the CO2 stripper, particularly in the overhead stream rich in CO2 and ethane, to further purify the CO2 product and break azeotropic mixtures. This hybrid approach has been demonstrated to lower utility costs by up to 20% and capital expenditure by approximately 25% compared to stand-alone cryogenic systems [50,52].
3.6. Hybrid Cryogenic Processes
Building upon the foundational concepts discussed in the Ryan–Holmes process and its membrane-integrated variants, hybrid cryogenic systems have emerged as a promising direction to overcome some of the limitations associated with conventional cryogenic separation. These integrated configurations aim to improve energy efficiency, process flexibility, and cost-effectiveness by combining cryogenic methods with other separation technologies, such as membranes, adsorption, or absorption, to create synergistic effects. Cryogenic technologies alone may face several barriers to effective application, including high energy consumption, production costs, and the availability of cooling energy sources [53]. In this context, hybrid cryogenic processes, such as those integrating cryogenic adsorption with membrane separation, have been proposed to mitigate these drawbacks and enable more sustainable biogas upgrading or CO2 capture routes [54]. For instance, an experimental study assessed the energy-saving opportunities of a cryogenic–membrane hybrid process designed for biogas refining applications [55]. In this system, membrane modules were evaluated under a range of operating temperatures, revealing excellent energy performance—0.8 MJ per kilogram of CH4—and high product purity, reaching 98% for methane and 99.8% for CO2, when operated at temperatures between −30 and 40 °C. In another work based on simulation, a hybrid system combining vacuum cryogenic adsorption with subsequent CO2 liquefaction was examined [56]. The results showed that a total energy input of 1.4 GJ per tonne of CO2 was required to achieve a product stream with 98.2% CO2 purity, indicating the potential of such configurations under moderate thermal demand. From an economic perspective, a techno-economic assessment evaluated the cost implications of a hybrid membrane–cryogenic process for CO2 capture, reporting that the avoided CO2 cost associated with an 85% capture ratio was approximately 9% higher than that of a traditional amine absorption process [57]. Although this figure reflects a relative disadvantage in terms of direct cost, the hybrid system demonstrated favorable performance when factors such as solvent regeneration and environmental compatibility were considered. A comparative study further analyzed cryogenic hybrid processes for biogas upgrading, showing that while conventional membrane-integrated systems achieved lower overall costs, the cryogenic hybrid alternatives offered notable advantages in terms of plant-level benefits and system robustness under variable feed conditions [58]. These findings underline the potential of hybridization as a design principle in advanced CO2 separation systems.
Beyond membrane integration, cryogenic hydrate-based processes also present a viable option for high-purity CO2 recovery. These systems rely on hydrate formation at low temperatures and high pressures, following a preliminary cryogenic separation stage that reduces the CO2 content of the feed stream [59]. The sequential operation allows efficient impurity removal and CO2 capture through solid hydrate formation. Similarly, cryogenic absorption processes using ammonia (NH3) have gained attention as an alternative for biogas upgrading. NH3-based absorption offers several advantages, such as low regeneration energy requirements, compatibility with low-temperature operation, and the commercial availability of NH3 as a working fluid [60]. However, the practical implementation of these emerging hybrid processes still requires further optimization to enhance selectivity, scalability, and cost competitiveness under real operating conditions.
4. Energy Performance of Cryogenic Biomethane Upgrading Technologies
An integral aspect of cryogenic upgrading for biomethane production involves conducting a thorough energy analysis [61]. This analysis is essential for assessing the efficiency and viability of the process, focusing on various facets of energy utilization. Central to the energy analysis is the examination of energy consumption across the biomethane production process. This encompasses evaluating the energy inputs required for cryogenic cooling, compression, and liquefaction, as well as any ancillary processes involved in pretreatment and post-treatment stages [62]. Understanding energy consumption profiles facilitates the identification of optimization opportunities to minimize energy wastage and enhance overall process efficiency.
The principal metrics employed to evaluate cryogenic upgrading performance include the Specific Energy Consumption (SEC) and the overall Energy Efficiency (η). The SEC is defined as
where E total is the total energy consumed by the process (kWh), and is the mass flow rate of biomethane produced (kg). The Energy Efficiency (η) is defined as
where E useful is the energy content of the biomethane product (e.g., lower heating value basis), and E input is the total energy supplied to the upgrading process.
Moreover, the energy analysis entails exploring energy efficiency metrics to assess the effectiveness of energy utilization within the cryogenic upgrading system [63]. Quantifying SEC and η provides a direct basis for comparing different process configurations and identifying areas for improvement. This involves evaluating the ratio of useful output energy (biomethane production) to input energy (electricity, refrigeration, etc.). Quantifying energy efficiency metrics enables the identification of areas for improvement and the implementation of strategies to enhance overall system performance. Additionally, energy recovery mechanisms play a crucial role in the energy analysis of cryogenic biomethane upgrading. Strategies such as heat integration, waste heat recovery, and the utilization of renewable energy sources can significantly reduce the net energy demand of the process [64]. By maximizing energy utilization and harnessing energy from various sources, the overall sustainability and economic viability of cryogenic biomethane production can be enhanced.
A comparative analysis of energy consumption among three prominent cryogenic biogas upgrading technologies, dual-pressure low-temperature distillation, Ryan–Holmes process, and anti-sublimation process [17], revealed significant insights. In the dual-pressure low-temperature distillation process, compression energy accounts for 38% of the total energy consumed, primarily due to the necessity of high pressures (40–50 bar). Additionally, approximately 62% of the total energy is attributed to the cryogenic duty essential for low-temperature distillation, which is crucial for producing liquefied biomethane. Contrastingly, the Ryan–Holmes process, featuring extractive distillation with a regenerative step, allocates 32% of the total energy to upgrading and 12% to heating load. In the anti-sublimation process, the upgrading step emerges as the most energy-intensive, consuming about 74% of the total energy. This intensity arises from the solid-to-vapor phase change involved in removing CO2 from CH4 during upgrading, consuming nearly all the energy for cooling duty. Moreover, a comparison of upgrading technologies concerning biomethane purity versus energy consumption highlights the challenges faced by cryogenic distillation in achieving high biomethane purity due to CO2 freezing, particularly at low temperatures within the distillation column. Dual-pressure low-temperature distillation configurations demonstrate enhanced purity levels, reaching up to 99%. Conversely, single-column distillation processes offer slightly lower purity but are preferable when CO2 purity is not a constraint, presenting an energy-efficient option. The anti-sublimation process, which leverages CO2 freezing, has been evaluated against conventional methods, showing promising results in terms of biomethane purity, albeit with higher specific energy consumption due to the energy-intensive solidification of CO21 and compression requirements. These findings underscore ongoing efforts to optimize energy utilization in cryogenic biogas upgrading processes.
Additional insights from the comprehensive assessment by Naquash et al. [10] reinforce the differences in energy profiles and reveal new benchmarks for evaluating process performance. Table 2 summarizes the comparative results reported in this study. The dynamic simulations carried out by these authors show that dual-pressure cryogenic distillation can achieve specific energy consumption values as low as 0.30 kWh/kgCH4, with refrigeration and compression duties accounting for 61% and 39% of total energy use, respectively. Notably, the elimination of reboiler requirements through full cryogenic operation contributes to lowering overall utility demand. The Ryan–Holmes process, although advantageous for some gas compositions, generally presents higher energy intensities, ranging between 0.44 and 0.55 kWh/kgCH4, primarily due to the energy needs for both the refrigerant loop and chemical solvent regeneration. In contrast, the anti-sublimation system displays the highest energy demand, exceeding 0.75 kWh/kgCH4 in most configurations, with more than 99% of energy allocated to cooling to reach solid–gas equilibrium temperatures. Despite this, its superior separation selectivity allows CH4 recoveries above 98% and CO2 concentrations below 100 ppm. Naquash et al. [10] also identify that operating pressure strongly influences refrigeration needs: increasing pressure to 50 bar can cut condenser duty by up to 50%, though it may raise compression or even melting zone loads if thermal recovery is not implemented. Importantly, they demonstrate that heat integration strategies—particularly pinch-based exchanger network design combined with internal Joule–Thomson expansion of CO2-rich side streams—can lower net cooling energy demand by up to 47% in dual-pressure schemes. Such internal cold recovery is not always feasible in anti-sublimation setups, where temperature control and frost management limit integration potential.

Table 2.
Comparative energy indicators for cryogenic biogas upgrading technologies [10,17].
These numerical insights stress that while anti-sublimation excels in separation purity, it does so at the cost of higher energy intensity. Dual-pressure distillation offers a better balance between purity and energy efficiency, and Ryan–Holmes provides operational flexibility but with moderate energy penalties. The choice of configuration ultimately depends on the specific boundary conditions of each case—such as feed gas composition, biomethane purity requirements, availability of cryogenic utilities, and integration with liquefaction chains or CO2 valorization routes. In any scenario, improving system-wide energy efficiency remains the most consistent strategy to enhance the techno-economic feasibility of cryogenic upgrading.
5. Techno-Economic Assessment of Cryogenic Biomethane Upgrading
Economic analysis also plays a pivotal role in determining the feasibility and economic viability of implementing cryogenic upgrading processes for biomethane production. This section focuses on evaluating various economic factors crucial for assessing the profitability and competitiveness of cryogenic biomethane production technologies. The economic analysis encompasses a thorough examination of capital investment requirements, operational expenditures, and potential revenue streams associated with cryogenic biomethane production facilities [68]. Initial capital investment considerations include equipment procurement, infrastructure development, and installation costs essential for establishing cryogenic upgrading plants. Operational costs constitute a significant aspect of the economic analysis, encompassing ongoing expenses such as energy consumption, maintenance, and labor costs associated with the operation of cryogenic biomethane production facilities. Understanding these operational expenses is essential for estimating the overall cost of biomethane production and determining its economic viability. In addition to costs, revenue generation opportunities are evaluated, primarily through the sale of biomethane and potential by-products. Revenue streams may include income from biomethane sales, carbon credit trading, or other renewable energy incentives available in the market [69]. Assessing market demand, pricing dynamics, and regulatory frameworks is crucial for accurately forecasting revenue and assessing the economic feasibility of cryogenic biomethane production projects.
Cost–benefit analysis and financial modeling techniques are employed to evaluate the financial performance metrics of cryogenic biomethane production projects. Parameters such as net present value (NPV), return on investment (ROI), and internal rate of return (IRR) are analyzed to gauge the project’s economic attractiveness and profitability over its lifecycle [70]. Furthermore, sensitivity analysis is conducted to assess the impact of key variables, such as feedstock costs, energy prices, and market conditions, on the financial performance of cryogenic biomethane production projects. Identifying sensitivities and potential risks enables stakeholders to develop mitigation strategies and make informed decisions regarding investment in cryogenic biomethane production technologies [71].
A recent study on the economic evaluation of cryogenic technologies [17] yields significant insights into investment and production costs. Firstly, comparing biogas upgrading through single-column and dual-column cryogenic distillation processes at different capacities reveals that higher capacity leads to decreased investment costs while maintaining constant feed composition and operating conditions. Additionally, transitioning from a single-column to a dual-column distillation process results in a slight increase in investment costs, with percentages varying depending on plant capacities, at 1.30% for 1400 m3/h plants and 2.94% for 600 m3/h plants. Furthermore, the cost analysis between cryogenic distillation and anti-sublimation technologies demonstrates that cryogenic distillation incurs higher production costs due to the need for elevated pressures for biogas, consequently increasing the cost of LBM production. In contrast, the desublimation process operates below the CO2 triple point, avoiding the need for high pressures, resulting in lower production costs (0.2 EUR/kg) compared to cryogenic distillation (0.45 EUR/kg). According to the authors, the economic trade-offs associated with cryogenic technologies for biogas upgrading highlight the importance of considering both investment and production costs in technology selection and process optimization.
Additional insights [14] complement this economic outlook by providing comparative cost indicators under standardized performance assumptions. Their techno-economic simulations indicate that dual-pressure cryogenic distillation (DPCD) has a lower methane loss and specific energy consumption per kg of CH4 recovered than both single-column cryogenic distillation and anti-sublimation systems, which directly affects production cost. In terms of capital investment, DPCD configurations exhibit marginally higher equipment costs due to the inclusion of a second distillation column and advanced exchanger networks, but this is offset by improved energy efficiency and lower operational costs. In contrast, anti-sublimation systems, while simpler in design, show higher total cost of ownership due to the large cooling demands and the need for precision frost management infrastructure. Naquash et al. [10] report specific production costs for cryogenic systems ranging between 0.36 and 0.44 EUR/kg of CH4 depending on feed composition and heat integration level, while anti-sublimation systems, despite achieving high product purity, often surpass 0.50 EUR/kg CH4 due to elevated refrigeration requirements and limited thermal recovery. Their analysis further emphasizes that economies of scale and integration with bio-LNG value chains are decisive for improving cost-effectiveness, especially in decentralized or modular applications.
To facilitate a direct comparison of the main cryogenic biomethane upgrading configurations discussed above, Table 3 summarizes the key techno-economic indicators, including capital and operating cost trends, specific energy consumption, methane losses, and production costs under standardized performance assumptions.

Table 3.
Summary of techno-economic indicators for cryogenic biomethane upgrading technologies [10,17].
6. Overcoming Bottlenecks and Emerging Pathways for Cryogenic Biomethane Production
Cryogenic technologies for biomethane upgrading are emerging as high-purity, low-emission alternatives to conventional methods. However, their broader implementation is still constrained by a complex mix of technical, economic, and systemic challenges. Among the most critical is the significant energy demand associated with cooling, phase change, and separation at very low temperatures. Maintaining cryogenic conditions requires robust refrigeration systems and multi-stage compression, which together can represent up to 90% of the total energy input in some configurations. Reducing these demands through energy integration, internal cold recovery, and advanced thermal management is essential for enhancing sustainability and lifecycle efficiency [74].
Equally important are the CAPEX and OPEX, which are often higher than those of chemical or membrane-based systems. Cryogenic upgrading requires specialized components—such as cryogenic heat exchangers, distillation columns, and vacuum-insulated pipelines—that significantly increase equipment and installation costs. Operational expenses, particularly electricity for refrigeration and compression, represent a recurring burden. Strategies such as heat pump coupling, use of renewable electricity, and optimization of pressure and temperature levels are being explored to reduce the overall cost per unit of biomethane produced [75].
Scaling cryogenic systems poses another hurdle. While pilot-scale and small demonstration plants (typically <1000 m3/h) have shown technical feasibility, there is a lack of standardized engineering solutions for medium- and large-scale units that can operate continuously and reliably under fluctuating feedstock and ambient conditions. Modular design principles and dynamic simulation tools are being developed to guide the scale-up process, ensuring process stability, control precision, and rapid start-up and shut-down capabilities under load variation [76]. The variability in biogas composition, both in terms of CH4/CO2 ratio and presence of trace components (e.g., NH3, H2S, siloxanes), further complicates process control. These variations affect condensation temperatures, freezing risks in distillation columns, and the selectivity of sublimation mechanisms. Advanced process analytical technologies (PATs) and real-time gas monitoring tools are being proposed to support adaptive process control, enabling cryogenic systems to self-regulate according to the fluctuating properties of the biogas stream [77].
In terms of future trends, innovation in core cryogenic components is key. Notable developments include magnetocaloric refrigeration systems, mixed refrigerant cycles tailored to biogas upgrading, and compact multi-stream plate-fin heat exchangers that enhance thermal performance while minimizing pressure drop. In parallel, digital twins and AI-driven process optimization are being introduced to monitor performance, detect anomalies, and optimize energy and product recovery across multi-criteria targets [78]. The integration of cryogenic upgrading with renewable energy systems (solar PV, wind, hydro) is gaining traction, particularly in remote or off-grid installations. When co-located with renewable electricity generation, these systems can operate flexibly during surplus power periods, turning electricity into storable, high-purity methane. This concept aligns with the EU’s “smart sector integration” strategy and supports grid balancing through Power-to-Gas-to-Power loops [79].
Moreover, policy support and financial instruments play a crucial role in fostering adoption. Feed-in tariffs, renewable gas guarantees of origin, carbon pricing mechanisms, and inclusion of liquefied biomethane in national energy mixes all contribute to improving project bankability. The current revision of the Renewable Energy Directive III (RED III) directive and the introduction of CO2 performance standards for heavy-duty vehicles are expected to stimulate demand for high-purity biomethane produced via cryogenic pathways. From a circular economy perspective, cryogenic upgrading enables valorization of industrial off-gases (e.g., from pulp and paper, breweries, wastewater treatment) by integrating biogas upgrading with CO2 purification and liquefaction for reuse. The high pressure and purity of recovered CO2 from cryogenic systems make it suitable for food-grade chemical synthesis and carbon capture and utilization (CCU) applications. Notably, cryogenic separation technologies offer several distinct advantages that make them highly attractive for advanced biogas upgrading applications. They enable the production of methane (CH4) with purity levels exceeding 99%, suitable for direct injection into natural gas distribution grids or for conversion into bio-LNG, meeting stringent quality standards for both uses. Furthermore, these systems facilitate the co-production of high-quality carbon dioxide (CO2) at pipeline pressure, which reduces or eliminates the need for energy-intensive post-treatment and compression stages. An additional benefit lies in the minimal use of chemical absorbents or solvents, which not only decreases operational complexity and cost but also avoids the generation of hazardous secondary waste streams, aligning with environmental regulations. Moreover, cryogenic upgrading is naturally compatible with bio-LNG value chains, enabling direct liquefaction of the upgraded methane and thus enhancing energy efficiency and system integration. These features position cryogenic technologies as a strategic component within circular economy frameworks and decarbonized energy systems, as emphasized in recent market analyses forecasting their growing role in renewable gas infrastructure [80].
Nevertheless, some systemic challenges remain unresolved. For instance, anti-sublimation technologies, while highly selective, demand precise frost management and pose scale-up difficulties due to the formation and accumulation of solid CO2 [43,81]. Likewise, cryogenic distillation systems must carefully avoid CO2 freeze-out in condenser zones, which may require reboiler adjustments or external heat pulses—complicating thermal balance and increasing CAPEX [28,82]. Furthermore, controlled freeze zone and Ryan–Holmes processes, although promising, are not yet fully commercialized for biogas. Their integration into modular, decentralized plants requires tailored designs, robust automation, and hybridization with upstream pretreatment and downstream liquefaction units. These systems can benefit from ongoing innovations in cryogenic insulation materials, vacuum super-jacketing, and magnetic valve control for ultra-low temperature operations.
To overcome these technical constraints, recent studies recommend integrating cryogenic upgrading directly with biomethane liquefaction, creating compact plants capable of producing bio-LNG for off-grid transport or marine use. This co-location allows cold recovery, improving energy efficiency by 15–25% depending on feed composition and operating pressure. Complementary integration with LNG regasification terminals is also being explored, allowing cascaded cold energy reuse and minimizing thermal losses during conversion stages. Advanced modeling tools—such as multi-objective genetic algorithms, pinch analysis, and exergy-based cost allocation—are being used to fine-tune process parameters and identify optimal trade-offs between energy efficiency, CAPEX, CH4 recovery, and CO2 quality. These models are particularly relevant when designing hybrid systems that combine cryogenic distillation with membrane or pressure swing adsorption (PSA) pre-separation steps. In parallel, life cycle assessment (LCA) and techno-economic analysis (TEA) studies are indispensable to quantify the environmental and economic impacts of each configuration under real-world constraints. Parameters such as global warming potential (GWP), energy return on investment (EROI), and levelized cost of methane (LCOM) are used to benchmark cryogenic technologies against alternatives [83]. Finally, from a policy perspective, targeted incentives for small-scale and modular systems, as well as recognition of liquefied biomethane in national fuel mandates, can significantly accelerate the deployment of cryogenic upgrading technologies. Pilot programs, preferential grid access, and exemptions from fossil gas taxation are potential mechanisms to foster innovation and market entry.
Author Contributions
Conceptualization and writing—original draft preparation, D.H.; writing—review and editing, J.M.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge support for this work from the ECLOSION project (Project No. MIG-20211071), funded by the Centre for the Development of Industrial Technology (CDTI) under the 2021 call of the “Science and Innovation Missions” Programme, within the Recovery, Transformation and Resilience Plan. This project is supported by the Ministry of Science and Innovation and co-funded by the European Union through the Next Generation EU fund.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| bcm | billion cubic meters |
| CAPEX | Capital Expenditure |
| CFZ | Controlled Freeze Zone |
| DPCD | Dual-Pressure Cryogenic Distillation |
| EROI | Energy Return on Investment |
| GHG | Greenhouse Gases |
| GWP | Global Warming Potential |
| HE | Heat Exchanger |
| LCA | Life Cycle Assessment |
| LBM | Liquid Biomethane |
| LCOM | Levelized Cost of Methane |
| LNG | Liquefied Natural Gas |
| OPEX | Operational Expenditure |
| PSA | Pressure Swing Adsorption |
| SEC | Specific Energy Consumption |
| TEA | Techno-Economic Analysis |
References
- International Energy Agency. Oil Market Report—December 2024; International Energy Agency: Paris, France, 2024; Available online: https://www.iea.org/reports/oil-market-report-december-2024 (accessed on 15 July 2025).
- International Energy Agency. Global Energy Review 2025: Natural Gas Section; International Energy Agency: Paris, France, 2025; Available online: https://www.iea.org/reports/global-energy-review-2025/natural-gas (accessed on 15 July 2025).
- Archana, K.; Visckram, A.S.; Kumar, P.S.; Manikandan, S.; Saravanan, A.; Natrayan, L. A review on recent technological breakthroughs in anaerobic digestion of organic biowaste for biogas generation: Challenges towards sustainable development goals. Fuel 2024, 358, 130298. [Google Scholar] [CrossRef]
- Bakkaloglu, S.; Hawkes, A. A comparative study of biogas and biomethane with natural gas and hydrogen alternatives. Energy Environ. Sci. 2024, 17, 1482–1496. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Wongwises, S.; Shadloo, M.S. A review of recent progress in biogas upgrading: With emphasis on carbon capture. Biomass Bioenergy 2022, 160, 106422. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Tarannum, K.; Chowdhury, A.T.; Rafa, N.; Nuzhat, S.; Kumar, P.S.; Vo, D.V.N.; Lichtfouse, E.; Mahlia, T.M.I. Biogas upgrading, economy and utilization: A review. Environ. Chem. Lett. 2021, 19, 4137–4164. [Google Scholar] [CrossRef]
- Werkneh, A.A. Biogas impurities: Environmental and health implications, removal technologies and future perspectives. Heliyon 2022, 8, e10864. [Google Scholar] [CrossRef] [PubMed]
- Arteconi, A.; Polonara, F. LNG as vehicle fuel and the problem of supply: The Italian case study. Energy Policy 2013, 62, 503–512. [Google Scholar] [CrossRef]
- Ghorbani, B.; Ebrahimi, A.; Ziabasharhagh, M. Thermodynamic and economic evaluation of biomethane and carbon dioxide liquefaction process in a hybridized system of biogas upgrading process and mixed fluid cascade liquefaction cycle. Process Saf. Environ. Prot. 2021, 151, 222–243. [Google Scholar] [CrossRef]
- Naquash, A.; Agarwal, N.; Nizami, M.; Nga, N.N.; Aziz, M.; Lee, M. Unlocking the potential of cryogenic biogas upgrading technologies integrated with bio-LNG production: A comparative assessment. Appl. Energy 2024, 371, 123720. [Google Scholar] [CrossRef]
- Galloni, M.; Di Marcoberardino, G. Biogas upgrading technology: Conventional processes and emerging solutions analysis. Energies 2024, 17, 2907. [Google Scholar] [CrossRef]
- International Energy Agency. Use of Renewable Energy for Transport in Europe. Available online: https://www.eea.europa.eu/en/analysis/indicators/use-of-renewable-energy-for (accessed on 16 March 2024).
- European Biogas Association. EBA Statistical Report 2024.; European Biogas Association: Brussels, Belgium, 2024; Available online: https://www.europeanbiogas.eu/publications/eba-statistical-report-2024/ (accessed on 15 July 2025).
- Yousef, A.M.I.; Eldrainy, Y.A.; El-Maghlany, W.M.; Attia, A. Upgrading biogas by a low-temperature CO2 removal technique. Alex. Eng. J. 2016, 55, 1143–1150. [Google Scholar] [CrossRef]
- Suri, S.U.K.; Khan, J.; Majeed, K.; Ahmed, F. Efficient carbon capture for enhanced biomethane purity via cryogenic distillation. J. Agric. Value Addit. 2025, 8, 44–73. [Google Scholar] [CrossRef]
- Spitoni, M.; Pierantozzi, M.; Comodi, G.; Polonara, F.; Arteconi, A. Theoretical evaluation and optimization of a cryogenic technology for carbon dioxide separation and methane liquefaction from biogas. J. Nat. Gas Sci. Eng. 2019, 62, 132–143. [Google Scholar] [CrossRef]
- Naquash, A.; Qyyum, M.A.; Haider, J.; Bokhari, A.; Lim, H.; Lee, M. State-of-the-art assessment of cryogenic technologies for biogas upgrading: Energy, economic, and environmental perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111826. [Google Scholar] [CrossRef]
- Asgharian, H.; Iov, F.; Araya, S.S.; Pedersen, T.H.; Nielsen, M.P.; Baniasadi, E.; Liso, V. A review on process modeling and simulation of cryogenic carbon capture for post-combustion treatment. Energies 2023, 16, 1855. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Langé, S. Biogas to liquefied biomethane via cryogenic upgrading technologies. Renew. Energy 2018, 124, 75–83. [Google Scholar] [CrossRef]
- Aena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Vilches, L.F.; Navarrete, B.; Zhang, Z. Biogas upgrading by cryogenic techniques. Environ. Chem. Lett. 2019, 17, 1251–1261. [Google Scholar] [CrossRef]
- Anugraha, R.P.; Pratiwi, V.D.; Renanto, R.; Juwari, J.; Islami, A.N.; Bakhtiar, M.Y. Techno-economic analysis of CO2 cryogenic distillation from high CO2 content gas field: A case study in Indonesia. Chem. Eng. Res. Des. 2024, 202, 226–234. [Google Scholar] [CrossRef]
- Qiao, S.; Xu, M.; Lv, X.; Zhao, H. Analysis and Optimization of Cryogenic Distillation Systems: For Reducing Distillation Energy Consumption. Chem. Eng. Technol. 2025, 48, e202400296. [Google Scholar] [CrossRef]
- Luberti, M.; Ballini, E.; Capocelli, M. Unveiling the Potential of Cryogenic Post-Combustion Carbon Capture: From Fundamentals to Innovative Processes. Energies 2024, 17, 2673. [Google Scholar] [CrossRef]
- Surmi, A.; Shariff, A.M.; Lock, S.S.M. Techno-Economic Assessment of Cryogenic Rotating Packed Beds for Nitrogen Removal from Natural Gas. Results Eng. 2025, 26, 104918. [Google Scholar] [CrossRef]
- Nizami, M.; Purwanto, W.W. Techno-Economic Analysis of Natural Gas High CO2 Content for Dimethyl Ether Production with Different CO2 Separation Using CFZ and Membrane Technologies. IOP Conf. Ser. Earth Environ. Sci. 2021, 673, 012015. [Google Scholar] [CrossRef]
- Hashemi, S.E.; Sarker, S.; Lien, K.M.; Schnell, S.K.; Austbø, B. Cryogenic vs. Absorption Biogas Upgrading in Liquefied Biomethane Production—An Energy Efficiency Analysis. Fuel 2019, 245, 294–304. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Ababneh, H.; Al-Muhtaseb, S.A. A review on the solid–liquid–vapor phase equilibria of acid gases in methane. Greenh. Gases Sci. Technol. 2022, 12, 566–579. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valor. 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ghorbani, B.; Manizadeh, A. Cryogenic biogas upgrading process using solar energy (process integration, development, and energy analysis). Energy 2020, 203, 117834. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Gallego, L.M.; Vega, F.; Navarrete, B. Cryogenic techniques: An innovative approach for biogas upgrading. In Emerging Technologies and Biological Systems for Biogas Upgrading; Kumar, G., Nguyen, D.D., Dhar, B.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 159–186. [Google Scholar] [CrossRef]
- Langè, S.; Pellegrini, L.A.; Vergani, P.; Lo Savio, M. Energy and economic analysis of a new low-temperature distillation process for the upgrading of high-CO2 content natural gas streams. Ind. Eng. Chem. Res. 2015, 54, 9770–9782. [Google Scholar] [CrossRef]
- Maqsood, K.; Ali, A.; Shariff, A.B.; Ganguly, S. Process intensification using mixed sequential and integrated hybrid cryogenic distillation network for purification of high CO2 natural gas. Chem. Eng. Res. Des. 2017, 117, 414–438. [Google Scholar] [CrossRef]
- Shen, M.; Tong, L.; Yin, S.; Liu, C.; Wang, L.; Feng, W.; Ding, Y. Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review. Sep. Purif. Technol. 2022, 299, 121734. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Ingrosso, S. Thermodynamic framework for cryogenic carbon capture. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; Volume 48, pp. 475–480. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. Low-temperature distillation process for CO2/CH4 separation: A study for avoiding CO2 freeze-out. J. Heat Transf. 2018, 140, 042001. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. Upgrading biogas to biomethane and liquid CO2: A novel cryogenic process. Fuel 2019, 251, 611–628. [Google Scholar] [CrossRef]
- Alam, S.; Begum, S.; Anupoju, G.R.; Yarasi, S.; Nakka, L.; Chenna, S. Modeling for upgrading biogas to syngas. In Biogas and Biomethane; Elsevier: Amsterdam, The Netherlands, 2024; pp. 165–177. [Google Scholar] [CrossRef]
- Wibawa, G.; Nafi, F.A.M.; Permatasari, A.; Mustain, A. Application of Peng–Robinson equation of state for calculating solid–vapor and solid–liquid equilibrium of CH4–CO2 system. Mod. Appl. Sci. 2015, 9, 177. [Google Scholar] [CrossRef]
- Pellegrini, L.A. Process for the Removal of CO2 from Acid Gas. WO Patent WO2014/125398A1, 2014. Available online: https://patents.google.com/patent/EP2917666A2 (accessed on 25 March 2024).
- Fedele, M. The Dual Pressure Low-Temperature Distillation Process: First Pilot Plant Experimental Campaign and Comparison with a Hybrid Natural Gas Purification Technology. Ph.D. Thesis, Politecnico di Milano, Milan, Italy, 2016. Available online: https://www.politesi.polimi.it/handle/10589/137526 (accessed on 16 July 2025).
- Suri, S.U.K.; Majeed, M.K.; Ahmad, M.S. Simulation analysis of novel integrated LNG regasification-Organic Rankine Cycle and anti-sublimation process to generate clean energy. Energies 2023, 16, 2824. [Google Scholar] [CrossRef]
- Clodic, D.; Younes, M. Method and System for Extracting Carbon Dioxide by Antisublimation for Storage Thereof. US Patent US 7,073,348 B2, 2016. Available online: https://patents.google.com/patent/US7073348B2/en (accessed on 26 March 2024).
- Schach, M.O.; Oyarzún, B.; Schramm, H.; Schneider, R.; Repke, J.U. Feasibility study of CO2 capture by anti-sublimation. Energy Procedia 2011, 4, 1403–1410. [Google Scholar] [CrossRef]
- Tian, H.; Kang, K.; Shi, L.; Sun, R. Parameter analysis of CO2 capture with anti-sublimation process. Energy Eng. 2020, 117, 267. [Google Scholar] [CrossRef]
- Mart, C.J.C. Controlled Freeze Zone™ Technology—An Integrated Solution for Processing Sour Natural Gas. In Proceedings of the Global Climate and Energy Project Research Symposium, Stanford, CA, USA, 7–9 October 2009. [Google Scholar]
- D’Adamo, I.; Ribichini, M.; Tsagarakis, K.P. Biomethane as an energy resource for achieving sustainable production: Economic assessments and policy implications. Sustain. Prod. Consum. 2023, 35, 13–27. [Google Scholar] [CrossRef]
- Ryan, J.M.; Schaffert, F.W. CO2 recovery by the Ryan/Holmes process. Chem. Eng. Prog. 1984, 80, 53–56. [Google Scholar]
- Lastari, F. Ryan-Holmes and Modified Ryan-Holmes Processes for LNG Production. Ph.D. Thesis, Curtin University of Technology, Perth, Australia, December 2009. Available online: https://core.ac.uk/download/pdf/195631893.pdf (accessed on 17 July 2025).
- Tomlinson, T.R.; Limb, D.I. Propane Recovery, Ryan/Holmes Process. In Encyclopedia of Chemical Processing and Design; McKetta, J.J., Ed.; Marcel Dekker: New York, NY, USA, 2017; Volume 45, pp. 59–78. [Google Scholar]
- Bi, Y.; Ju, Y. Review on cryogenic technologies for CO2 removal from natural gas. Front. Energy 2022, 16, 793–811. [Google Scholar] [CrossRef]
- Gkotsis, P.; Kougias, P.; Mitrakas, M.; Zouboulis, A. Biogas upgrading technologies–Recent advances in membrane-based processes. Int. J. Hydrogen Energy 2023, 48, 3965–3993. [Google Scholar] [CrossRef]
- Vakili, S.S.; Kargari, A.; Sanaeepur, H. Cryogenic-membrane gas separation hybrid processes. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 349–368. [Google Scholar]
- Song, C.; Fan, Z.; Li, R.; Liu, Q.; Kitamura, Y. Efficient biogas upgrading by a novel membrane-cryogenic hybrid process: Experiment and simulation study. J. Membr. Sci. 2018, 565, 194–202. [Google Scholar] [CrossRef]
- Fong, J.C.L.Y.; Anderson, C.J.; Xiao, G.; Webley, P.A.; Hoadley, A.F. Multi-objective optimisation of a hybrid vacuum swing adsorption and low-temperature post-combustion CO2 capture. J. Clean. Prod. 2016, 111, 193–203. [Google Scholar] [CrossRef]
- Anantharaman, R.; Berstad, D.; Roussanaly, S. Techno-economic performance of a hybrid membrane–liquefaction process for post-combustion CO2 capture. Energy Procedia 2014, 61, 1244–1247. [Google Scholar] [CrossRef]
- Oliveira, L.G.; Cremonez, P.A.; Machado, B.; da Silva, E.S.; Silva, F.E.B.; Corrêa, G.C.G.; Alves, H.J. Updates on biogas enichment and purification methods: A review. Can. J. Chem. Eng. 2023, 101, 2361–2390. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.; Park, J.H.; Kim, S.C.; Won, S.O.; Kang, Y.T. Study on CO2 hydrate formation characteristics with promoters for CO2 capture and cold thermal energy transportation. J. Clean. Prod. 2021, 295, 126392. [Google Scholar] [CrossRef]
- Atzori, F.; Barzagli, F.; Dai, S.; Concas, A.; Cao, G. A new model-aided approach for the design of packed columns for CO2 absorption in aqueous NH3 solutions. Chem. Eng. Sci. 2024, 288, 119780. [Google Scholar] [CrossRef]
- Rogala, Z.; Stanclik, M.; Łuszkiewicz, D.; Malecha, Z. Perspectives for the Use of Biogas and Biomethane in the Context of the Green Energy Transformation on the Example of an EU Country. Energies 2023, 16, 1911. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, X.; Zhang, Y.; Gao, W.; Chen, W.; Peng, H. Energy, conventional exergy and advanced exergy analysis of cryogenic recuperative organic rankine cycle. Energy 2023, 268, 126648. [Google Scholar] [CrossRef]
- Wen, N.; Tan, H.; Qin, X. Simulation and analysis of a peak regulation gas power plant with advanced energy storage and cryogenic CO2 capture. Energy Storage Sav. 2023, 2, 479–486. [Google Scholar] [CrossRef]
- Tayyebi, M.; Derakhshani Molayousefi, M. A review on the effect of various rolling regimes (cryo, cold, warm, hot) and post-annealing on high-entropy alloys: Microstructure evolution, deformation mechanisms, and mechanical properties. Arch. Civ. Mech. Eng. 2023, 24, 16. [Google Scholar] [CrossRef]
- Bauer, F.; Persson, T.; Hulteberg, C.; Tamm, D. Biogas upgrading–technology overview, comparison and perspectives for the future. Biofuels Bioprod. Biorefin. 2013, 7, 499–511. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology—A review of biogas cleaning, upgrading and utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Vilardi, G.; Bassano, C.; Deiana, P.; Verdone, N. Exergy and energy analysis of three biogas upgrading processes. Energy Convers. Manag. 2020, 224, 113323. [Google Scholar] [CrossRef]
- Wivell, A.S. Biogas and Biomethane Production from Anaerobic Digesters and the Influences of Policies and Carbon Credit Systems on the Biogas Markets in the United States and the European Union. Ph.D. Thesis, Politecnico di Milano, Milan, Italy, 2022. Available online: https://www.politesi.polimi.it/retrieve/1652019f-44f8-4b3a-b298-7ab7dc874cc7/Thesis%20Report%20Austin%20Scott%20Wivell.pdf (accessed on 19 July 2025).
- Sganzerla, W.G.; da Rosa, R.G.; Barroso, T.L.C.T.; Castro, L.E.N.; Forster-Carneiro, T. Techno-economic assessment of on-site production of biomethane, bioenergy, and fertilizer from small-scale anaerobic digestion of jabuticaba by-product. Methane 2023, 2, 113–128. [Google Scholar] [CrossRef]
- Grahn, M.; Malmgren, E.; Korberg, A.D.; Taljegard, M.; Anderson, J.E.; Brynolf, S.; Wallington, T.J. Review of electrofuel feasibility—Cost and environmental impact. Prog. Energy 2022, 4, 032010. [Google Scholar] [CrossRef]
- Barrio, M.D.H.; Marroquín, J.M.M.; Corona, F. Transformación de biogás en biometano: Revisión de las tecnologías disponibles (Biogas upgrading: Review of available technologies). DYNA Energ. Sosten. 2017, 6, 12. [Google Scholar] [CrossRef]
- Miltner, M.; Makaruk, A. Review on available biogas upgrading technologies and innovations towards advanced solutions. J. Clean. Prod. 2017, 161, 1329–1337. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Haider, J.; Qadeer, K.; Valentina, V.; Khan, A.; Yasin, M.; Lee, M. Biogas to liquefied biomethane: Assessment of 3P’s–Production, processing, and prospects. Renew. Sustain. Energy Rev. 2020, 119, 109561. [Google Scholar] [CrossRef]
- Sulewski, P.; Ignaciuk, W.; Szymańska, M.; Wąs, A. Development of the biomethane market in Europe. Energies 2023, 16, 2001. [Google Scholar] [CrossRef]
- Tamburrano, P.; Distaso, E.; Salvatori, M.; Fedele, M.; Meschia, M.; Amirante, R. A novel small-scale biomethane liquefaction process: Assessment through a detailed theoretical analysis. Appl. Therm. Eng. 2023, 233, 121145. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Helwani, Z.; Kim, J. An overview of biogas upgrading via pressure swing adsorption: Navigating through bibliometric insights towards a conceptual framework and future research pathways. Energy Convers. Manag. 2024, 306, 118268. [Google Scholar] [CrossRef]
- Gan, H.; Zhang, H.; Fang, S.; Iqbal, Q.; Yao, Y.; Xu, Z.; Wang, K. Comparative study of waste heat recovery systems based on thermally driven refrigeration cycles in cryogenic air separation units. Int. J. Refrig. 2023, 156, 133–149. [Google Scholar] [CrossRef]
- Calise, F.; Cappiello, F.L.; Cimmino, L.; d’Accadia, M.D.; Vicidomini, M. Integration of photovoltaic panels and solar collectors into a plant producing biomethane for the transport sector: Dynamic simulation and case study. Heliyon 2023, 9, e14613. [Google Scholar] [CrossRef]
- Mark Wide Research. Biogas Upgrading Market Analysis 2025–2034; Mark Wide Research: USA, 2025; Available online: https://markwideresearch.com/biogas-upgrading-market (accessed on 15 August 2025).
- Tengku Hassan, T.N.A.; Shariff, A.M.; Mohd Pauzi, M.M.I.; Khidzir, M.S.; Surmi, A. Insights on cryogenic distillation technology for simultaneous CO2 and H2S removal for sour gas fields. Molecules 2022, 27, 1424. [Google Scholar] [CrossRef]
- Ababneh, H.; AlNouss, A.; Al-Muhtaseb, S.A. Carbon capture from post-combustion flue gas using a state-of-the-art, anti-sublimation, solid–vapor separation unit. Processes 2022, 10, 2406. [Google Scholar] [CrossRef]
- Hiloidhari, M.; Kumari, S. Biogas upgrading and life cycle assessment of different biogas upgrading technologies. In Emerging Technologies and Biological Systems for Biogas Upgrading, 1st ed.; Academic Press: London, UK, 2021; pp. 413–445. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).