Abstract
The development of alternative energies has become a concern for all countries to ensure domestic energy supply and provide environmental friendliness. One of the providential alternative energies is biodiesel. Biodiesel, commonly stated as fatty acid alkyl ester (FAAE), is a liquid fuel intended to substitute petroleum diesel. Nevertheless, implementation of pure biodiesel is not recommended for conventional diesel engines. It holds poor values of cold flow properties, as the effect of high saturated FAAE content contributes to this constraint. Several processes have been proposed to enhance cold flow properties of biodiesel, but this work focuses on the skeletal isomerization process. This process rearranges the skeletal carbon chain of straight-chain FAAE into branched isomeric products to lower the melting point, related to the good cold flow behavior. This method specifically requires an acid catalyst to elevate the isomerization reaction rate. And then, sulfated tin(IV) oxide emerged as a solid superacid catalyst due to its superiority in acidity. The results of biodiesel isomerization over this catalyst and its modification with iron had not satisfied the expectation of high isomerization yield and significant CFP improvement. However, they emphasized that the skeletal isomers demonstrated minimum impact on biodiesel oxidation stability. They also affirmed the role of an acid catalyst in the reaction mechanism in terms of protonation, isomerization, and deprotonation. Furthermore, the metal promotion was theoretically necessary to boost the catalytic activity of this material. It initiated the dehydrogenation of linear hydrocarbon before protonation and terminated the isomerization by hydrogenating the branched carbon chain after deprotonation. Finally, the overall findings indicated promising prospects for further enhancement of catalyst performance and reusability.
1. Introduction
Energy is critical for humans to carry out all their activities. Consequently, the rapid growth of global human residents over the past decade has forced the rising of energy demands [1]. Additionally, the prompt technological evolution in this era has changed world lifestyle and economic development, yielding a significant multiplication in energy requirement [2]. Nevertheless, the positive elevation in energy demand is not matched by its supply. The presence of existing energy sources, especially fossils, experiences a declining trend every year [3]. This situation strongly causes energy scarcity and unaffordability [4]. Moreover, the application of fossil fuels must be controlled to prevent negative impacts on humans and the environment because of its harmful emissions [5,6]. To respond to those conditions, the most adopted strategy is optimizing the utilization of fossil energy as well as developing alternative energies to ensure energy security and sovereignty. One of the providential alternative fuels is biodiesel.
Biodiesel, commonly stated as fatty acid esters, is synthesized from various fatty acid-rich feedstocks and short-chain alcohols by an esterification or transesterification process [7,8]. The fatty acid-rich feedstocks are increasing in variety and can be classified into five groups: safe-to-eat oils, inedible oils, animal fats, wastes, and microalgae-microorganisms [9,10,11,12,13]. Globally, total biodiesel production (Figure 1) has been recorded as increasing in the period of 2019–2024. Compared to 2019, the accumulated production in 2023 expanded more than 10 billion liters. Further, the European Union (EU), the United States (US), Indonesia, and Brazil were the four largest biodiesel producers in the world in the last five years [14]. In the future, they are predicted to still dominate the production and will gradually intensify the replacement of vegetable oils with low-cost feedstocks [15].
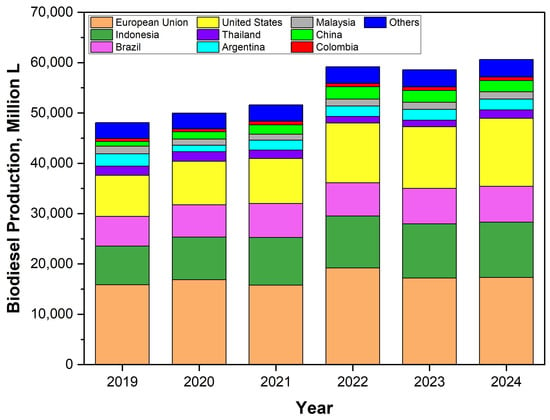
Figure 1.
Quantity and distribution of worldwide biodiesel production in the period of 2019–2024 (data were obtained from OECD and FAO [14]).
In application, this cleaner-combustion diesel is intended to reduce the dependence on petro-diesel [16]. Generally, biodiesel is mixed with petro-diesel at a certain volumetric percentage. Implementation of pure biodiesel or mixed fuel with high biodiesel concentration is not recommended for conventional diesel engines. Some of the considered reasons are (1) low oxidation stability [17]; (2) poor cold flow properties [18]; and (3) high rubber-dissolving properties [19]. These three points have significant negative impacts on engine performance. Based on the revealed problems, this paper is devoted to further discussing the obstacle(s) in cold flow properties (CFPs).
Cold flow properties (CFPs) are classified as physical properties measuring the fluidity characteristics of fuel in cold conditions [20]. The measurable parameters of biodiesel CFPs are cloud point, cold filter plugging point, and pour point. Cloud point (CP) is interpreted as the temperature at which the adhered solidified fuel particles during cooling begin to exhibit a cloudy emersion in the liquid bulk [20]. Cold filter plugging point (CFPP) is provided as the CFP parameter to measure the temperature at which biodiesel cannot flow properly by way of a specific filtration apparatus (4.5 × 10−5 m) for a finite time (max. 60 s) [21]. Furthermore, temperatures below the CFPP lead to massive solidification, causing loss of fluidity [22]. In that condition, the measured temperature is expressed as pour point (PP).
Basically, cold flow properties of biodiesel are specifically affected by saturation degree of fatty esters [23]. Saturated fatty ester (SFE) is regularly proven to have higher oxidation stability, but poorer cold flow properties than unsaturated fatty ester (UFE) with the same number of carbon atoms [24]. Moreover, the increasing quantity of double bonds in the carbon chain of fatty ester has the effect of decreasing oxidative stability and elevating cold fluidity characteristics [25]. As shown in Figure 2, the melting points of C-18 fatty acid methyl esters are going lower in line with alleviating saturation degree. In other words, the order of CFP from good to poor is methyl linolenate (C18:3), methyl linoleate (C18:2), methyl oleate (C18:1-cis), and methyl stearate (C18:0). Nevertheless, the order of oxidation stability from good to poor is inversely proportional to cold flow properties.
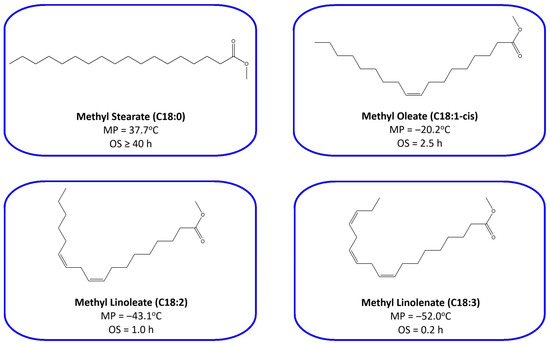
Figure 2.
Melting point (MP) and oxidation stability (OS) characteristics of C18-fatty acid methyl esters (data were obtained from Anwar and Garforth [25], Knothe and Dunn [26], Moser [27]).
Based on these characteristics, several processes have been promoted to improve the biodiesel CFPs, including chemical insertion, fractionation, alkyl modification, isomerization, and cracking. However, this article focuses on isomerization (specifically the branching process) because this process has minimal impact on oxidation stability. Based on Figure 3, the principal target is to modify straight-chain saturated fatty esters which have higher melting points into branched forms which have better CFPs [3]. At the same time, the oxidative stability of branched-chain saturated fatty esters does not change significantly. Moreover, they have only a slight effect on cetane number and viscosity issues [25,28].
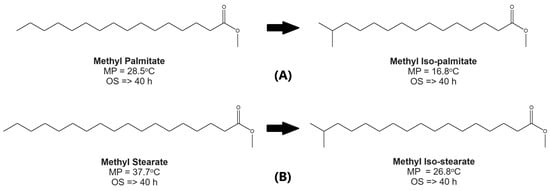
Figure 3.
General reaction formula, melting point (MP), and oxidation stability (OS) of components for (A) isomerization of methyl palmitate; (B) isomerization of methyl stearate (data were obtained from Anwar and Garforth [25], Knothe and Dunn [26], Moser [27]).
Generally, hydrocarbon branching isomerization requires an acid catalyst to accelerate the reaction [29]. To date, the isomerization catalysts were preferred in solid form. This was because they have the advantage of having a larger surface area, are easier to separate, and are more able to be reactivated than the homogeneous ones [21,25]. Based on the acidity characteristic, the solid catalysts are divided into two categories: (i) solid acid catalyst; and (ii) solid superacid catalyst. According to the basis of catalyst compound, the solid acid catalysts can be classified into zeolite, clay, and alumina. The previous isomerization results using these catalysts for short- and long-chain hydrocarbons are tabulated in Table 1. According to Table 1, the skeletal isomerization was mostly carried out under high pressure at a temperature range of 160–310 °C. The longer the carbon chain required, the higher the reaction temperature to branch the carbon chain. For fatty acid or ester raw materials, the pressurized reactions were run at a temperature of 250–285 °C and catalyst loading of 0.5–5 wt% for up to 24 h. In this condition, the isomeric product yields were reported to vary up to 85.7%. The higher isomerization temperature promoted the hydrocarbon cracking process [30].
For the second catalyst type, the solid superacid catalyst is an evolution of the solid acid catalyst, which has very strong acidity and proper stability against thermal exposures [31]. The solid catalysts are classified as superacid when their acidity is stronger than pure sulfuric acid. In other word, the Hammet indicator (Ho) of a solid superacid catalyst must be less than or equal to −12 [32]. Arata et al. [33] has comprehensively studied the acid strength of various anion-modified metal oxides, such as sulfated zirconium oxide (SO4/ZrO2), tin oxide (SO4/SnO2), titanium oxide (SO4/TiO2), aluminum oxide (SO4/Al2O3), and hafnium oxide (SO4/HfO2). The highest acid strength was shown by sulfated tin oxide (calcined at 550 °C) with an Ho value of −18 [33]. Then, the performance of metal oxide-based superacid catalysts for branching isomerization is summarized in Table 2. According to data in Table 2, this catalyst type successfully isomerized hydrocarbons, varying from short to long carbon chains. Generally, superacid catalysts with sulfate anions required lower reaction temperatures than tungstated catalysts. The sulfated catalyst tended to accelerate the cracking process when the reaction was conducted at a higher temperature [34]. Later, isomerization using this superacid catalyst could be carried out at lower temperature and atmospheric pressure.
Specific to sulfated tin oxide (STO), previous studies in Table 2 reported that this superacid catalyst demonstrated catalytic activity in isomerizing several short-chain hydrocarbons. Nevertheless, only a few studies have further explored the role of this catalyst to isomerize long-chain hydrocarbon, especially biodiesel. Therefore, this research aimed to synthesize sulfated tin oxide and examine it in a catalytic isomerization reaction for improvement of biodiesel cold flow properties. This reaction was atmospherically conducted at a temperature of 200 °C to prevent evaporation of fatty esters at higher temperatures. This reason was supported by previous research presented in Table 1 and Table 2 involving long-chain hydrocarbon feeds that temperatures higher than 200 °C were mostly applied for high-pressure isomerization reactions. Meanwhile, lower temperatures tended to decline catalyst activity [34]. Additionally, temperatures higher than 235 °C potentially led to hydrocarbon cracking [35]. Moreover, the metal modification, such as iron, in STO was also carried out to investigate the effect of metal in the reaction.

Table 1.
Previous studies of solid acid catalysts for hydrocarbon skeletal isomerization.
Table 1.
Previous studies of solid acid catalysts for hydrocarbon skeletal isomerization.
| Basis of Catalyst Compound | Catalyst | Feed | Product | Catalyst Loading (wt%) | Temp. (°C) | Pressure (bar) | Time (h) | Yield (wt%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Zeolite | Ferrierite | Oleic and linoleic acid | Iso-stearic acid | 5.0 | 260 | 9.3 | 8 | 77.3 | [36] |
| Ferrierite | Oleic and linoleic acid | Iso-stearic acid | 5.0 | 260 | 9.3 | 24 | 83.1 | [37] | |
| Ferrierite | Oleic and linoleic acid | Iso-stearic acid | 5.0 | 260 | 5.5 | 4 | 85.7 | [38] | |
| Mordenite | Oleic and linoleic acid | Iso-stearic acid | 5.0 | 250 | ~13.8 | 6 | 70.2 | [39] | |
| β-zeolite | Oleic and linoleic acid | Iso-stearic acid | 5.0 | 260 | 9.3 | 24 | 70.5 | [37] | |
| Pt/β-zeolite | Methyl palmitate | Methyl Iso-palmitate | 0.5 | 285 | 40 | 16 | ±16.0 | [40] | |
| ZSM-5 | Oleic and linoleic acid | Iso-stearic acid | 5.0 | 260 | 9.3 | 24 | 81.8 | [37] | |
| Clay | Clay | Oleic acid | Iso-stearic acid | 4.3 | 250 | ~1.4 | 2.5 | 30.8 | [41] |
| Montmorillonite | Oleic acid | Iso-stearic acid | 5.0 | 250 | 5.0 | 4 | 46.0 | [42] | |
| Bentonite | Methyl oleate | Methyl iso-stearate | 5.0 | 250 | ~13.8 | 24 | 29.6 | [43] | |
| Alumina (Al2O3) | Pt/γ-Al2O3 | n-hexane | 2-methyl pentane 3-methyl pentane | n.a. | 180 | 20 | 2 | 89.7 | [44] |
| Pt/γ-Al2O3 | n-heptane | 2,2-dimethyl pentane 2,3-dimethyl pentane 3-ethyl pentane | n.a. | 180 | 20 | 2 | 58.2 | [44] | |
| Pt/γ-Al2O3 | n-octane | 2,3-dimethyl hexane 3,3-dimethyl hexane 3-ethyl hexane 3-methyl heptane | n.a. | 180 | 20 | 2 | 41.5 | [44] | |
| Pt/Cl/γ-Al2O3 | n-hexane | 2-methyl pentane 3-methyl pentane 2,3-dimethyl butane 2,2-dimethyl butane | WHSV = 1.8 h−1 | 160 | 30 | 0.56 | 66.2 | [45] | |
| Pd/SiO2-Al2O3 | n-hexadecane | iso-hexadecane | WHSV = 3 h−1 | 310 | 30 | 0.33 | 49.7 | [46] |

Table 2.
Previous studies of solid superacid catalysts for hydrocarbon skeletal isomerization.
Table 2.
Previous studies of solid superacid catalysts for hydrocarbon skeletal isomerization.
| Basis of Catalyst Compound | Catalyst | Feed | Product | Catalyst Loading (wt%) | Temp. (°C) | Pressure (bar) | Time (h) | Yield (wt%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Zirconium oxide (ZrO2) | SO4/ZrO2 | Methyl oleate | Methyl iso-stearate | 20 | 250 | 4.4 | 8 | 36 | [47] |
| Pt/SO4/ZrO2 | n-heptane | 3-methyl hexane 2,2-dimethyl pentane 2,3-dimethyl pentane | WHSV = 0.15 h−1 | 250 | 1.01 | 6.7 | 24.7 | [34] | |
| Pt/SO4/ZrO2 | n-hexadecane | iso-hexadecane | WHSV = 18.4 h−1 | 225 | 20.3 | 6 | 0.2 | [48] | |
| Pt/SO4/ZrO2 | n-hexadecane | iso-hexadecane | 16.7 | 160 | 25.8 | 1 | 8.9 | [49] | |
| Pt/WO3/ZrO2 | n-hexadecane | iso-hexadecane | WHSV = 18.4 h−1 | 225 | 20.3 | 6 | 4.1 | [48] | |
| Pd/WO3/ZrO2 | n-hexadecane | iso-hexadecane | WHSV = 1 h−1 | 300 | 21.7 | 6 | 17.5 | [50] | |
| Ni/WO3/ZrO2 | n-hexadecane | iso-hexadecane | WHSV = 1 h−1 | 300 | 21.7 | 6 | 31.7 | [50] | |
| Tin oxide (SnO2) | SO4/SnO2 | n-butane | iso-butane | 0.05 g | 110 | 1.01 | 0.02 | 39.2–44.8 | [51] |
| SO4/SnO2 | n-pentane | 2-methyl butane | 0.8 g | 110 | 0.07 | 3 | 8.0 | [52] | |
| WO3/SnO2 | cycloheptane | Methyl cyclohexane | WHSV = 5.2 h−1 | 140 | 1.01 | 0.19 | 7.6 | [51] | |
| Titanium oxide (TiO2) | SO4/TiO2 | n-hexane | 2-methyl pentane 3-methyl pentane | WHSV = 0.5 h−1 | 350 | 1.01 | 1.5 | 0.74 | [53] |
| SO4/TiO2 | n-hexane | 2-methyl pentane 3-methyl pentane | 5.7 × 103 g.min/mol | 100 | n.a. | 0.83 | 19 | [54] | |
| Pt/SO4/TiO2 | n-hexane | 2-methyl pentane 3-methyl pentane | WHSV = 0.5 h−1 | 350 | 1.01 | 2 | 15.3 | [53] | |
| Aluminum oxide (Al2O3) | SO4/Al2O3 | n-pentane | iso-pentane | 0.8 g | 50 | 0.07 | 3 | 3.7 | [55] |
| Hafnium oxide (HfO2) | Pt/SO4/HfO2 | n-hexadecane | iso-hexadecane | WHSV = 2 h−1 | 210 | 21.6 | 0.5 | 6.5 | [56] |
| Pt/WO3/HfO2 | n-hexadecane | iso-hexadecane | WHSV = 0.5 h−1 | 300 | 21.6 | 2 | 65.1 | [56] |
2. Materials and Methods
2.1. Materials
The chemicals used in this research were tin tetrachloride 98% (Sigma-Aldrich Co., St. Louis, MO, USA), ammonia solution 32% (Merck KGaA, Darmstadt, Germany), ammonium acetate ≥ 98% (Smart Lab Indonesia Co., Tangerang, Indonesia), sulfuric acid 95–97% (Merck KGaA, Darmstadt, Germany), iron(III) nitrate nonahydrate ≥ 98% (Loba Chemie Pvt., Mumbai, India), n-hexane (Merck KGaA, Darmstadt, Germany), sodium hydroxide pellets (Merck KGaA, Darmstadt, Germany), and sodium chloride (Merck KGaA, Darmstadt, Germany). For quantitative analysis of the biodiesel component, the major analytical standard components used in this study were Me-palmitate (>99%), Me-stearate (>99%), Me-oleate (>99%), Me-linoleate (>99%), Me-isopalmitate (>98%), and Me-isostearate (>98%). These standards were purchased from Larodan AB Company (Solna, Sweden). Then, palm–biodiesel feedstock (Me-palmitate = 46.85 wt%; Me-stearate = 5.8 wt%; Me-oleate = 40.86 wt%; Me-linoleate = 5.5 wt%) was supplied by Sinarmas Bio Energy Co., Ltd. (Jakarta, Indonesia).
2.2. Methods
2.2.1. Catalyst Synthesis
Generally, STO was synthesized with the procedure reported by Matsuhashi et al. [52]. It was carried out through two sequential process stages: (1) tin oxide (TO) preparation; and (2) tin oxide sulfation. After sulfation in 3M sulfuric acid, the sulfate-modified solid was then dried at 105 °C for 2 h and calcined at 500 °C for 3 h. The final product was STO. For iron-modified STO (Fe-STO) catalyst, metal impregnation was conducted in the first stage. The iron(III) nitrate nonahydrate (metal precursor) was agitated with tin tetrachloride at stoichiometric iron to a total solid percentage of 3.6%wt in the hydrolysis of tin tetrachloride (Stage 1). Thus, the synthesized solid was sulfated (Stage 2) and then calcined as conducted for the STO catalyst.
2.2.2. Catalyst Characterization
The synthesized TO, STO, and Fe-STO were characterized to evaluate several characteristics of the catalyst, such as thermal decomposition, crystal structure, functional group, component, solid surface textural, and acid sites. Thermal decomposition examination of STO (25 mg) was conducted in air medium at atmospheric pressure using a Linseis STA PT-1600 thermal analyzer (Linseis Messgeraete GmbH, Selb, Germany). This analysis was executed at a temperature range of 30–1000 °C with a heating rate of 10 °C/min. Next, the crystal structure of all samples calcined at 500 °C was analyzed using a Bruker D2 Phaser X-ray Diffractometer (Bruker AXS, Karlsruhe, Germany). The scanning process was carried out with a radiation of Cu-Kα (λ = 1.5406 Å) and in the diffraction angle (2θ) range of 10–80°.
The Fourier Transform Infra-Red (FTIR) analysis was also applied to collect the transmittance spectra of the calcined solid materials. The equipment used in this analysis was a Bruker Spectrometer, Model Alpha with Platinum-ATR (Bruker Optics, Ettlingen, Germany). This analysis was run between wavelengths of 500 and 2000 cm−1. Then, the composition analysis of the prepared solid materials was conducted using Rigaku XRF Equipment, Model NEXCG (Applied Rigaku Technologies, Inc., Cedar Park, TX, USA). Meanwhile, the surface properties of all calcined samples were characterized by the Brunauer–Emmett–Teller (BET) method. The BET measurement equipment was a Micromeritics TriStar II Plus (Micromeritics Instrument Corporation, Norcross, GA, USA), which was operated with isothermal nitrogen at −195.8 °C. In addition, the surface morphology and elemental mapping of the best-performing catalyst was characterized using a SEM-EDS Hitachi SU-3500 (Hitachi High-Tech, Tokyo, Japan).
Later, the acidity of all calcined materials was tested using the potentiometric titration and NH3-Temperature Programmed Desorption (TPD) methods. For the potentiometric titration method, the titrant used in this analysis was sodium hydroxide (NaOH) with a concentration of 0.05 M in the 4% sodium chloride solution. The titration steps and acid site determination of this test followed the working procedure and calculation described by Yu et al. [57]. For the NH3-TPD method, the acidity quantification of the samples was carried out using Microtrac MRB Equipment, Model Belcat II (MicrotracBEL Corp., Osaka, Japan). This analysis was operated using helium gas at a flow rate of 30 cm3-STP/min and heating rate of 20 °C/min.
2.2.3. Isomerization Reaction
The catalytic performance of the prepared catalysts (STO and Fe-STO) was investigated in the biodiesel isomerization. This reaction was run at a temperature of 200 °C, atmospheric pressure, stirring rate of 900 rpm, residence time of 12 h, and catalyst loading of 10 wt%. After reaction, the biodiesel product was separated from the solid catalyst. The isomerized biodiesel was then tested for its pour point (PP) and cloud point (CP) using a Phase Technology PCA-70Xi Analyzer (Phase Analyzer Company, Ltd., BC, Canada). This analyzer used an ASTM-D5949 method for PP testing and an ASTM-D5773 method for CP evaluation. Next, the investigated CP data were used to predict CFPP through Equation (1) [58].
Afterwards, the composition analysis for the isomerized biodiesel was quantitatively performed using Perkin Elmer Claurus 600 Gas Chromatograph (GC) with Perkin Elmer Claurus 600T Mass Spectrometer (MS) and a Perkin Elmer Elite-5ms Capillary Column (length = 30 m; inner diameter = 0.25 mm; film thickness = 0.25 μm; max. temperature = 350 °C). This instrument set was manufactured by PerkinElmer, Inc. (Shelton, CT, USA). The component separation in the column was run at a temperature range of 180–260 °C, heating rate of 5 °C/min, injection temperature of 250 °C, and split ratio of 90:1. Based on the composition data, the yield (Y) of fatty ester products was quantified using Equation (2). In this equation, mP,A and mP,B were the mass of products after and before reaction (g), respectively. Then, mR,B referred to the mass of reactants before reaction (g).
Moreover, the data series of component composition, NH3-TPD acidity (ATPD, mmol/g), and BET surface area (SBET, m2/g) were applied to compute turnover frequency (TOF, s−1) using Equations (3)–(5) and isomerization selectivity (Sisomer, %) using Equation (6). In these equations, Rreaction and Ns denoted the reaction rate and the mole of active sites per catalyst surface area, respectively. Np,B and Np,A symbolized the moles of products before and after reaction (mmol), respectively. Then, mcat and treaction referred to the mass of catalyst loading (g) and the total reaction time (s), respectively.
Lastly, the biodiesel feed and products were also tested for their oxidation stability by measuring the induction period. The equipment used for this investigation was a Metrohm 873 Biodiesel Rancimat Oxidative Stability Analyzer (Metrohm AG, Herisau, Switzerland). This analysis was run at a temperature of 110 °C and gas flow of 10 L/h. In addition, the measurement method was ensured to comply with international standards for biodiesel (ASTM-D6751 and EN-14112).
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. Thermal Decomposition Characterization
The thermal decomposition result in the temperature range of 25–1000 °C is illustrated in Figure 4. There were four endothermic decomposition stages detected on the TGA–DTA curve. The first stage was an endothermic curve in the range of 50–200 °C with a mass loss of 2.2 wt%, describing physical water desorption [59,60]. The initial curve of this stage demonstrated a mass gain due to water adsorption. At the beginning of heating, the surrounding water was evaporated and adsorbed onto the solid surface. As the solid temperature increased, the adsorbed water was vaporized again. This case also appeared in the research on sulfated WO3/SnO2 conducted by Alaya and Rabah [59]. The second stage occurred at 200–450 °C with a peak at 265 °C, indicating the release of volatile matters and the decomposition of residual sulfuric acid on the solid surface [51,59]. In this stage, the mass loss percentage was recorded at 4.2 wt%. At this temperature range, the DTA illustration graph did not display an exothermic peak point, which signified TO crystallization. It denoted that the sulfate was evenly distributed on the solid surface and inhibited the formation of TO crystal [59,61].
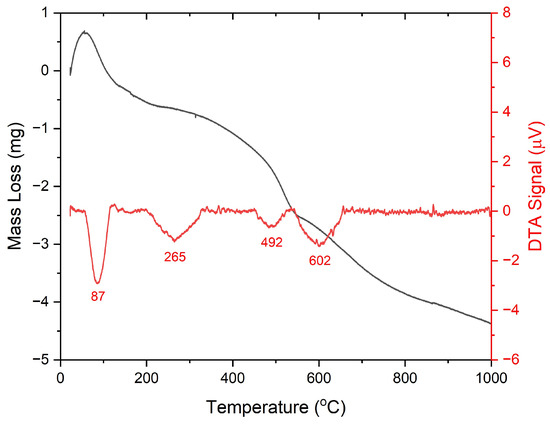
Figure 4.
Thermal decomposition (TGA and DTA) curves of the uncalcined STO.
The third and fourth stages were identified at 450–540 and >540 °C, respectively. These stages represented sulfate decomposition [59], resulting in mass reductions of 3.8 and 7.3 wt%, successively. Overall, the total mass reduction of STO in the temperature range of 25–1000 °C was 17.5 wt%. These results implied that the calcination temperature exceeding 450 °C provided significant removal of sulfate and acid sites. As a result, the material may face challenges in maintaining the stability of catalytic performance and crystalline structure at high temperatures [62]. Consequently, more frequent reactivation may be necessary to sustain high catalytic activity for subsequent reactions. Therefore, it is advisable to calcine and utilize the STO catalysts below 450 °C.
3.1.2. X-Ray Diffraction (XRD)
The results of XRD analysis are demonstrated in Figure 5. The diffractogram of pure SnO2 (TO) presented main intensity peaks at diffraction angles of 26.6, 33.8, and 51.8° with a crystallinity index of 72.8%. It indicated that the synthesized TO had crystal structure of tetragonal cassiterite. This pattern was also found in the pure SnO2 (TO) reported by [59]. Then, sulfate and metal (iron) modification had almost no effect on the diffraction angles of the main peaks, representing no change in the SnO2 crystal structure. However, these modifications hindered SnO2 crystallization [60], so the curve peaks were wider and the crystallinity indexes (STO and Fe-STO) decreased sharply to 53–56%. Additionally, the peak of sulfate crystal was not detected in the diffractograms due to the complete dispersion of sulfate crystal on the solid surface [59].
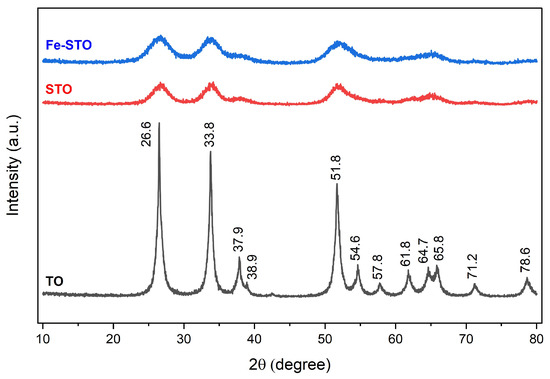
Figure 5.
X-ray diffractograms of the investigated materials (TO, STO, and Fe-STO).
3.1.3. Infra-Red Spectra
The results of infra-red spectra are shown in Figure 6. All investigated materials had vibration bands between 500 and 622 cm−1. This band range was associated with the SnO2 structure in the form of O–Sn–O and Sn–O [59,63]. Modification of sulfate and metal (iron) had an effect on the addition of four vibration band categories. The first category appeared in vibration bands between 890 and 980 cm−1, which were related to the Sn–OH functional group [63]. This appearance was due to the presence of incompletely decomposed hydroxyl groups. The second category consisted of transmittance peaks at 1030–1040 cm−1, assigned to symmetric S=O dan S–O [60]. The third additional functional group was asymmetric S=O dan S–O, proven by infra-red spectra bands at 1130–1160 cm−1 [59,60]. The second and third functional groups were attributed to the dentate sulfate complex structures in the solid material [60]. The last category was the vibration bands between 1500 and 1650 cm−1, denoting the stretching spectra of leftover water [63].
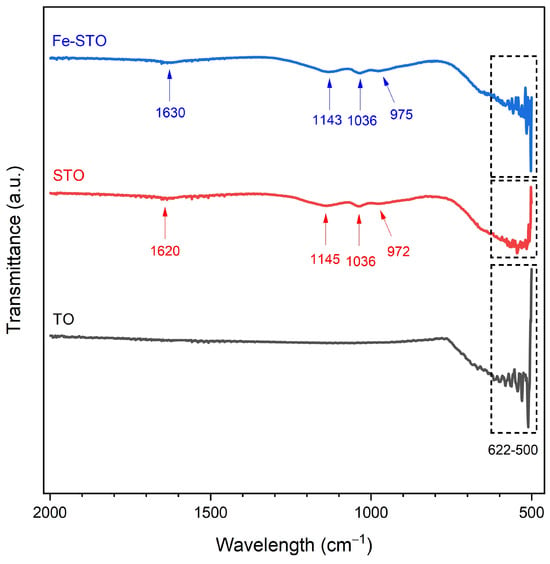
Figure 6.
FTIR spectra of the investigated materials (TO, STO, and Fe-STO).
3.1.4. X-Ray Fluorescence (XRF)
The composition of major oxide compounds and elements is presented in Table 3. Pure TO mostly contained SnO2 and Sn elements with percentages of 93.4 and 73.6 wt%, respectively. The addition of sulfate progressively enhanced SO3 content (5.04 wt%) in the solid (STO). Consequently, it reduced the SnO2 percentage to 90.5 wt%. Moreover, metal (iron) impregnation impressively alleviated SnO2 composition to 85.6 wt% in the material (Fe-STO). Apart from the elevation of metal (iron) content, the percentage of SO3 component in Fe-STO (6.75 wt%) was also greater than STO. This phenomenon was due to the strengthening of sulfate binding by the impregnated metal on the solid surface. Therefore, it was hypothetically predicted to improve the acidity of the catalyst.

Table 3.
Component composition of the synthesized materials (TO, STO, and Fe-STO).
3.1.5. Brunauer–Emmett–Teller (BET)
The hysteresis curves of nitrogen adsorption–desorption are illustrated in Figure 7. The adsorption–desorption curves for all samples did not overlap because of pore obstruction. Based on IUPAC classification, these isotherm curves could be classified into Type IV, representing mesoporous solid materials [64]. This indication was also supported by the pore-blocking loops (Type H2) formed at relative pressures above 0.4 [59]. The sulfate and metal (iron) modification shifted the hysteresis loops to lower relative pressures, indicating smaller pore sizes. Based on the surface properties summarized in Table 4, STO and Fe-STO were proven to have smaller pore diameters than TO. Their pore diameters were recorded at 3.5 and 2.9 nm, respectively. These pore diameters were then compared to the molecular size of the targeted reactants and products.
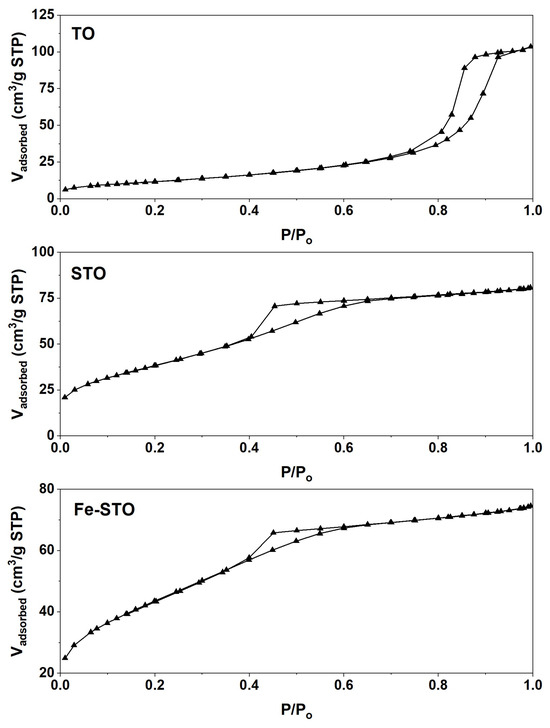
Figure 7.
Nitrogen adsorption–desorption hysteresis curves of the investigated materials (TO, STO, and Fe-STO).

Table 4.
Solid surface properties of the synthesized materials (TO, STO, and Fe-STO).
In this study, the targeted reactants were methyl palmitate and methyl stearate contained in palm biodiesel. Later, the targeted products were their branched-chain isomers. For estimation, the molecular size of stearic acid was used to represent the largest size of these targeted components. As informed by Anwar and Garforth [25], the molecular size of stearic acid (C18:0) was reported at 2.45 × 0.25 nm. Hence, the pore diameters of STO and Fe-STO were larger than the reacting particle size. This condition ensured the pore diffusion of the reacting particles during the reaction. In this stage, the reactant diffuses through the pore from external surface into the active site and vice versa for the synthesized product. Hereafter, the surface area and BET constant of STO and Fe-STO were larger than the pure SnO2 (TO). It implied that the larger surface area provided the larger place for reaction. Therefore, the combination of a larger surface area and appropriate pore diameter encouraged catalysts to have better catalytic performance.
3.1.6. Scanning Electron Microscopy-Energy Dispersive X-Ray Spectroscopy (SEM-EDS)
The material morphology of the finest-performing catalyst (Fe-STO) is demonstrated in Figure 8. The SEM image (Figure 8a) and the EDS elemental mapping (Figure 8b) indicate almost even dispersion of oxygen (O), sulfur, (S), and iron (Fe) elements on the surface. Additionally, the EDS elemental spectrum (Figure 8c) represents the weight percentages of the investigated elements. These results confirmed that the presence of sulfur (10 wt%) and oxygen (20 wt%) indicated the content of sulfate groups in the synthesized material. Sulfate group and solid tin oxide were found to form a dentate sulfate complex as an acid active site [29,60]. Subsequently, the appearance of iron (5 wt%) denoted that this metal was successfully impregnated on the solid surface as a metal active site. Theoretically, each category of these active sites has a specific role in the isomerization reaction mechanism. The details of the reaction mechanism and catalyst role are described in Section 3.3.
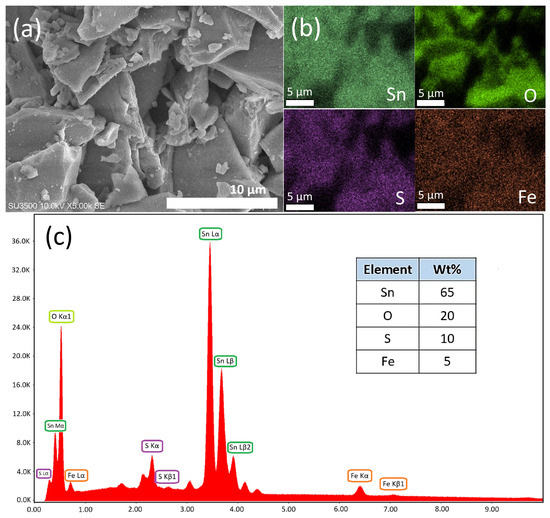
Figure 8.
Material morphology of Fe-STO: (a) SEM image; (b) EDS elemental mapping of Sn-O-S-Fe; and (c) EDS elemental spectrum of Sn-O-S-Fe.
3.1.7. Acidity Measurement
The acid amounts of the investigated solids measured by the potentiometric titration and NH3-TPD methods are presented in Table 5. Through both methods, pure SnO2 (TO) was noted to have acid amounts of 0.19 and 0.47 mmol/g, respectively. These values were higher than the acidity reported by Khalaf et al. [60] at 0.11 mmol/g. The sulfation significantly intensified acidity to 1.15 and 0.8 mmol/g, respectively. These results were also higher than the STO material studied by Khalaf et al. [60] at 0.4 mmol/g, SO4/WO3/SnO2 explored by Alaya and Rabah [59] at 0.37 mmol/g, and SO4/ZrO2 synthesized by Chen et al. [65] at 0.16 mmol/g. And then, metal (iron) modification also elevated the acidity more than the basic sulfated SnO2. The acidity values for Fe-STO (1.63 and 1.34 mmol/g) were more excellent than Fe/SO4/ZrO2 prepared by Chen et al. [65] at 0.3 mmol/g. This trend was positively correlated with the trend of SO3 content resulted from the XRF analysis. Moreover, these results represented the superiority in acidity of the synthesized STO and Fe-STO.

Table 5.
Acidity data of the investigated materials (TO, STO, and Fe-STO).
3.2. Catalyst Performance Test
Performance investigation of the synthesized catalysts (STO and Fe-STO) was conducted in the batch biodiesel isomerization. The biodiesel feed and product were evaluated via CFPs investigation, composition analysis, TOF quantification, and oxidation stability measurement.
3.2.1. Cold Flow Properties (CFPs) Investigation
The CFPs investigation results of the isomerized biodiesel are tabulated in Table 6. The STO catalyst was known to slightly reduce biodiesel PP from 12 to 11 °C and increase CP from 15.60 ± 0.10 to 16.45 ± 0.05 °C. The more significant performance was shown by the Fe-STO catalyst. It alleviated both biodiesel PP and CP to 9 and 15.25 ± 0.05 °C, respectively. Compared to the American standard for pure biodiesel (ASTM-D6751) [66], the best PP in this study (9 °C) has complied with the standard value (−15 ≤ PP ≤ 10 °C). Nevertheless, the lowest CP value (15.2 °C) has not met this standard value (−3 ≤ CP ≤ 12 °C). For other standards (European Standard-EN14214, Indonesian Standard-148.K/EK.05/DJE/2024, Malaysian Standard-MS2008:2014, and Brazilian Standard-ANPN°920/2023) [66], there is no value limitation for PP and CP, specifically. As a replacement, these standards regulate biodiesel CFPP. For European and Brazilian Standards, biodiesel producers are only required to report the CFPP of their products. Meanwhile, Indonesian and Malaysian Standards define the maximum value of CFPP at 15 °C. According to Table 6, all calculated CFPP data complied with the standards. The findings suggest that the optimal biodiesel product identified in this study is suitable exclusively for regions that comply with EN-14214, Indonesian, Malaysian, and Brazilian standards. In simple terms, this biodiesel should be applied in areas with an environment temperature above 9 °C. Otherwise, the application of this product leads to fuel solidification, resulting in fuel line clogging [66]. Moreover, this slight improvement may reduce the formation of solidified particles in cold conditions, thus prolonging filter lifespan and lowering the risk of injector damage.

Table 6.
Cold flow properties data of the isomerized biodiesels and several standards (obtained from Pradana et al. [66]).
3.2.2. Composition Analysis
Afterwards, the composition analysis results of the isomerized biodiesel for C16-FAME are visualized in Figure 9. Based on this graph, the investigated catalysts (STO and Fe-STO) were proven to isomerize methyl palmitate into methyl iso-palmitate, but they were still too small. The yields of methyl iso-palmitate (based on methyl palmitate feed) for STO and Fe-STO were calculated at 0.2 ± 0.020 and 0.4 ± 0.028%, respectively. Based on these results, the higher catalyst acidity (Table 5) and surface area (Table 4) had not provided a significant effect in promoting a high yield of branched-chain fatty esters. Theoretically, the higher catalyst acidity and the larger surface area promoted more active sites for reaction, resulting in higher product yields. However, in the isomerization reaction, the activity of acid sites in isomerizing saturated carbon chains depended on the dehydrogenation stage at the beginning of the reaction [25,66]. Then, the dehydrogenation process commonly required a metal catalyst to accelerate the reaction [53]. It was proven in the Fe-STO-catalyzed reaction that the promotion of iron(III) further encouraged the dehydrogenation stage, presenting a higher isomerization yield than the STO catalyst. In other words, the presence of iron metal in the catalyst enhanced its activity in the fatty ester isomerization.
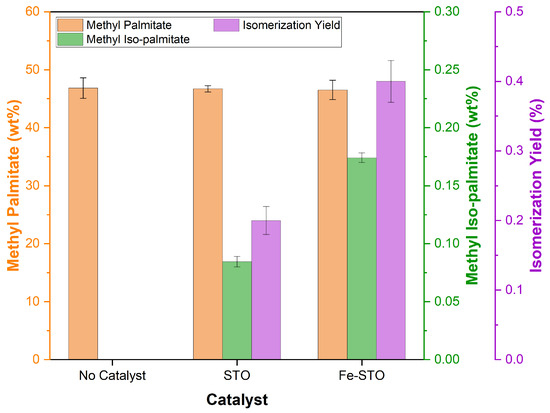
Figure 9.
C16-FAME composition and yield data of the isomerized biodiesels.
Nonetheless, the iron active sites were not yet effective enough in initiating the dehydrogenation process. According to electrode potential, iron(III) has a value of −0.04 V to form an iron component with zero oxidation number [67]. In this condition, iron(III) tends to act as a weak reducing agent, slightly hydrogenating unsaturated fatty esters into more saturated components. In addition, there were no specific oxidizing-agent metal active sites in the Fe-STO catalyst which strongly initiated the dehydrogenation stage. Because of this, the impregnated iron did not present excellent performance on isomerization. Therefore, the exploration of effective impregnated metals, either alone or in combination, still requires intensive attention to significantly improve catalyst activity at atmospheric pressure.
Then, the visualization of the biodiesel component for C18-FAME is presented in Figure 10. The examined catalysts (STO and Fe-STO) were detected to produce small amounts of methyl iso-stearate as the isomerization product of methyl stearate. The quantified yields for STO and Fe-STO were at 0.11 ± 0.003 and 0.11 ± 0.001%, respectively. The branched-chain saturated fatty esters led to the reduction in the PP of biodiesel products. Interestingly, the STO catalyst also tended to catalytically hydrogenate methyl linoleate into methyl oleate. Compared to FAME feedstock, the methyl linoleate composition in the STO-catalyzed biodiesel was reduced by 4.2 wt% and the methyl oleate content enlarged with a yield of 73%. This situation confirmed a slight elevation in the CP of biodiesel product when using an STO catalyst. A different phenomenon occurred in the isomerization reaction with the Fe-STO catalyst. The composition of methyl oleate and linoleate in the product altered insignificantly from the feedstock. For C18-FAME, iron metal modification was specifically a direct isomerization reaction to produce methyl iso-stearate. The weak reduction-oxidation properties of iron(III) promoted a minor effect on the hydrogenation–dehydrogenation process, apart from the absence of a hydrogen source in the reaction. Because of this composition, the isomerized biodiesel using the Fe-STO catalyst reduced PP and CP better than the STO catalyst.
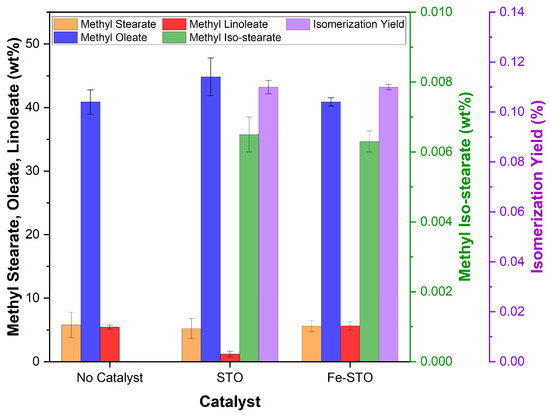
Figure 10.
C18-FAME composition and yield data of the isomerized biodiesels.
For comparison, Zhang et al. [47] studied methyl oleate isomerization using a SO4/ZrO2 catalyst with the highest yield of 36%. This reaction was run at a temperature of 250 °C, pressure of 4.4 bar, catalyst loading of 20 wt%, and residence time of 8 h. Venkatesh et al. [49] reported the branching of n-hexadecane using Pt/SO4/ZrO2 with the best yield of 8.9%, obtained at a temperature of 160 °C, pressure of 25.8 bar, and catalyst loading of 16.7 wt% for 1 h. Busto et al. [48] prepared Pt/SO4/ZrO2 and Pt/WO3/SO4 to accelerate isomerization of n-hexadecane at a temperature of 225 °C and pressure of 20.3 bar for 6 h. The highest yields were 0.2 and 4.1%, respectively. Zhang et al. [50] manufactured Pd/WO3/ZrO2 and Ni/WO3/ZrO2 to catalyze alkane n-C16H34 with the isomerization yields of 17.5 and 31.7%, respectively. These yields were achieved at a temperature of 300 °C and pressure of 21.7 bar for 6 h. According to these previous studies using long-chain hydrocarbon feeds [47,48,49,50], the higher pressure and/or catalyst loading led to a higher yield than this work. The prepared STO and Fe-STO catalysts in this study only performed better when compared to the Pt/SO4/ZrO2 synthesized by Busto et al. [48]. It was due to the longer residence time in this research.
In addition, Hino et al. [51] tested the STO catalyst on the n-butane branching with yields of 39.2–44.8%, reacted at a temperature of 110 °C, atmospheric pressure, and catalyst loading of 0.05 g (reactor volume of 0.05 mL). Hino and coworkers [51] also modified TO with tungstate anion to isomerize cycloheptane with the highest yield of 7.6%. Matsuhashi and his team [52] published their working results on the n-pentane isomerization using an STO catalyst with the best yield of 8.0%, run under vacuum conditions at a temperature of 110 °C and reaction time of 3 h. Enriquez et al. [53] discussed n-hexane isomerization using SO4/TiO2 and Pt/SO4/TiO2 with yields of 0.74 and 15.3%, respectively. Both catalysts were performed at a temperature of 250 °C and atmospheric pressure. Matsuhashi et al. [55] worked on the vacuum isomerization of n-pentane using SO4/Al2O3 with an isomerization yield of 3.7%. Based on these prior findings [51,52,53,55], isomerization of short-chain hydrocarbon presented higher yields at milder temperatures than this study. It implied that biodiesel isomerization required more energy and higher catalyst activity.
3.2.3. Turnover Frequency (TOF) Quantification
The correlation of catalyst performance, acidity, and surface area is quantitatively represented by catalyst turnover frequency (TOF). The calculation results of acid sites, reaction rates, TOFs, and isomerization selectivity for STO and Fe-STO catalysts are summarized in Table 7. The STO catalyst was recorded to have overall and isomerization TOFs of 4.9 × 10−5 and 1.0 × 10−6 s−1, respectively. These values indicated that the STO catalyst had low selectivity for isomerization (2.0%). Later, the overall and isomerization TOFs for the Fe-STO catalyst were 1.3 × 10−6 and 1.2 × 10−6 s, respectively. These results denoted high isomerization selectivity at point of 90.8%. Besides that, the isomerization TOFs of the STO and Fe-STO catalysts were identified at the similar level, confirming the fundamental isomerization TOF for STO-based catalysts. The modification of metals, either alone or in combination, may alter the fundamental value of isomerization TOF, depending on the reduction-oxidation properties of metals.

Table 7.
Calculated acid sites, reaction rates, turnover frequencies, and isomerization selectivity of the prepared catalysts (STO and Fe-STO).
The results also demonstrated that the increase in the acid sites and surface area had almost no effect on the catalyst performance for isomerization. It emphasized that the skeletal isomerization was strongly influenced by the metal catalyst initiating the dehydrogenation process in the first step reaction. And, iron(iii) has not properly enhanced the isomerization reaction rate. Hereafter, the TOF values of the STO-based catalyst in this study were still lower than the TOF values of TO and ZrO2 catalysts for methanol redox reaction (~7.0 × 10−1 and ~3.5 × 10−2 s−1, respectively) [68]. The previous work published by Badlani and Wachs [69] also reported the higher TOF values of TO and ZrO2 catalysts for methanol oxidation reaction (1.9 × 10−1 and 1.3 × 10−1 s−1, respectively). Even though the TOF values have not met expectation, these results implied an opportunity in the further development of STO-based isomerization catalysts.
3.2.4. Oxidation Stability Measurement
Furthermore, biodiesel feed and products were evaluated for their oxidation stability. As shown in Table 8, isomerization using the STO catalyst enhanced the oxidation stability of the biodiesel product. This result was in line with the notable increase in biodiesel saturation level, as depicted in Figure 10. The composition of methyl oleate (C18:1) increased, while the content of methyl linoleate (C18:2) declined. Based on data shown in Figure 2, the oxidation stability of methyl oleate and linoleate is 2.5 and 1 h, respectively. Thus, conversion of methyl linoleate into methyl oleate clearly encouraged improvement in oxidation stability. Subsequently, biodiesel products treated by Fe-STO run into a slight reduction in oxidation stability. This was predominantly due to the presence of branched-chain fatty esters. It affirmed data presented in Figure 3 that the skeletal isomerization products did not significantly affect biodiesel oxidation stability. This situation may not significantly interfere with the operation and storage of biodiesel. Additionally, all samples have complied with all established standards for biodiesel. Amidst the high CFP rule, the American standardization organization, through ASTM-D6751 standard, preferred to lower the limit of oxidation stability parameters (min. at 3 h) [66]. On the other hand, biodiesel standards in Europe, Indonesia, Malaysia, and Brazil have higher minimum limits of 8, 11, 10, and 13 h, respectively [66]. These standards are noted to have moderate regulation for CFP. The differences in the limits of each standard are intended to accommodate the certain application of biodiesel.

Table 8.
Oxidation stability data of the isomerized biodiesels and several standards (obtained from Pradana et al. [66]).
3.3. Theoretical Catalyst Role in the Reaction Mechanism
Generally, the acid-based catalyst has a principal role in the skeletal isomerization process [25]. Based on the previous studies [25,66,70] and proposed by this research, the theoretical catalyst role in the isomerization reaction is demonstrated in Figure 11. It performed protonation on the double bond site of unsaturated hydrocarbon to produce a saturated carbocation compound. Hereinafter, it positively forced the carbocation to undergo a process of carbon chain restructuring. Initially, it directed saturated carbocation to form a protonated intermediate compound. Next, the protonated carbon chain in the intermediate format was branched. In this case, the branching usually occurred at the end of the carbon chain. The branched-chain carbocation was then deprotonated, establishing unsaturated branched-chain hydrocarbon. Figure 11 also visualizes the duties of metal promotion in the catalyst for the reaction mechanism. Firstly, the promoted metal had the function of dehydrogenating saturated carbon chains and creating unstable double bond(s) in hydrocarbon. This step was necessary at the beginning, so that the three acid-catalyzed steps (protonation, branching, and deprotonation) could proceed. Later, the impregnated metal was also aimed to again hydrogenate the unsaturated branched-chain hydrocarbon after deprotonation. For simple understanding, the promoted metal had three sequential purposes: (i) taking hydrogen from straight-chain hydrocarbon; (ii) storing it during the branching process; and (iii) returning it again to the branched-chain hydrocarbon. Therefore, the presence of metal is very useful because it significantly enhanced the catalyst activity in the isomerization reaction.
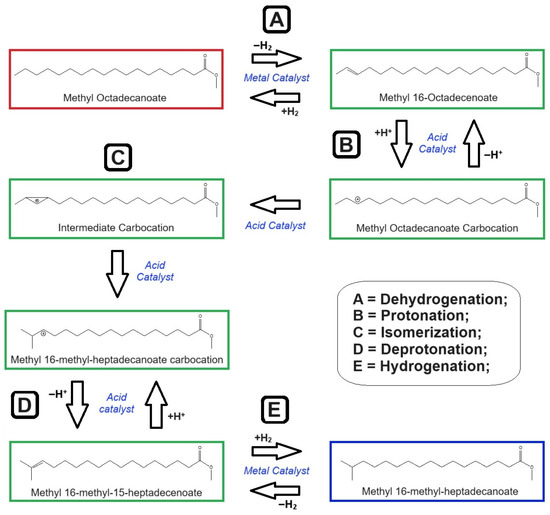
Figure 11.
Theoretical reaction mechanism of skeletal isomerization of methyl octadecenoate using metal-promoted acid catalyst (red = reactant; green = intermediate compounds; blue = final product).
4. Conclusions
This study focused on the skeletal isomerization process to improve biodiesel cold flow properties. This process was reported to require an acid catalyst to facilitate the reaction. Then, STO-based materials appeared as potential acid catalysts due to their excellence in acid strength. The isomerization of biodiesel over STO and Fe-STO catalysts had not met the target for high isomerization yield and remarkable CFPs improvement. However, it proved that the skeletal isomers offered minimum impact on biodiesel oxidation stability. It also confirmed the role of an acid catalyst in the reaction mechanism in terms of protonation, isomerization, and deprotonation. Hereinafter, the metal impregnation was theoretically required to enhance catalyst activity. It initiated the isomerization by dehydrogenating the straight-chain hydrocarbon and terminated the reaction by hydrogenating the branched-chain fatty ester. Finally, this result indicated promising prospect for further performance enhancement and reusability advancement of STO-based isomerization catalyst.
Author Contributions
Conceptualization, Y.S.P., I.G.B.N.M., T.P., T.H.S. and A.I.; methodology, Y.S.P., I.G.B.N.M., T.H.S. and A.I.; software, Y.S.P.; validation, Y.S.P., I.G.B.N.M., T.P., T.H.S. and A.I.; formal analysis, Y.S.P., I.G.B.N.M., T.P. and A.I.; investigation, Y.S.P.; resources, A.I.; data curation, Y.S.P.; writing—original draft preparation, Y.S.P. and A.I.; writing—review and editing, Y.S.P. and A.I.; visualization, Y.S.P.; supervision, I.G.B.N.M., T.P. and A.I.; project administration, A.I.; funding acquisition, A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was jointly funded by: (1) Indonesian Education Scholarship (BPI), Center for Higher Education Funding and Assessment (PPAPT), Ministry of Higher Education, Science, and Technology of the Republic of Indonesia [01198/BPPT/BPI.06/9/2023]; (2) Indonesia Endowment Fund for Education Agency (LPDP), Ministry of Finance of the Republic of Indonesia [01198/BPPT/BPI.06/9/2023]; and (3) Faculty of Industrial Technology, Institut Teknologi Bandung through ITB Community Service Program (PPMI).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank to Department of Bioenergy Engineering and Chemurgy, Institut Teknologi Bandung for research facility support. The authors also gratefully acknowledge to Electron Microscope Laboratory, Research Center for Nanosciences and Nanotechnology, Institut Teknologi Bandung for the support in the surface morphology analysis of the investigated catalysts.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASTM | American Society for Testing and Materials |
| BET | Brunauer-Emmett-Teller |
| CFP | Cold flow properties |
| CFPP | Cold filter plugging point |
| CP | Cloud point |
| EU | European Union |
| FAAE | Fatty acid alkyl ester |
| FAO | Food and Agriculture Organization |
| Fe-STO | Iron-modified sulfated tin oxide |
| FTIR | Fourier Transform Infra-Red |
| OECD | Organisation for Economic Co-operation and Development |
| PP | Pour point |
| SEM-EDS | Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy |
| SFE | Saturated fatty ester |
| STO | Sulfated tin oxide |
| TGA-DTA | Thermogravimetric Analysis-Differential Thermal Analyzer |
| TO | Tin oxide |
| TPD | Temperature Programmed Desorption |
| UFE | Unsaturated fatty ester |
| US | United States |
| WHSV | Weight hourly space velocity |
| XRD | X-ray Diffraction |
| XRF | X-ray Fluorescence |
References
- Leng, L.; Li, W.; Li, H.; Jiang, S.; Zhou, W. Cold Flow Properties of Biodiesel and the Improvement Methods: A Review. Energy Fuels 2020, 34, 10364–10383. [Google Scholar] [CrossRef]
- United Stated Energy Information Administration. International Energy Outlook 2023 with Projections to 2050; United States Department of Energy: Washington, DC, USA, 2023.
- Maghrebi, R.; Buffi, M.; Bondioli, P.; Chiaramonti, D. Isomerization of long-chain fatty acids and long-chain hydrocarbons: A review. Renew. Sustain. Energy Rev. 2021, 149, 111264. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Kusumastuti, Y.; Putri, N.R.E.; Widiyannita, A.M.; Prabasiwi, D.S.; Fadhila, A.N.F.; Suyono, E.A. Enhancing the microalgae Nannochloropsis sp. harvesting by chitosan-based flocculation-sedimentation for biofuel production. AIP Conf. Proc. 2024, 2836, 070004. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Sadewo, B.R.; Haryanto, S.A.; Sudibyo, H. Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production. Open Chem. 2021, 19, 1029–1042. [Google Scholar] [CrossRef]
- Venkatesh, R.; Sharma, A.; Nagarajan, N.; Karthik, K.; Verma, A.; Soudagar, M.E.M.; Vinayagam, M.; Al Obaid, S.; Alharbi, S.A. Cerium oxide activated microalgae growth from grey wastewater for bio-hydrogen extraction via KOH catalyst adopted gasification route: Performance evaluation. Biomass Convers. Biorefin. 2025. [Google Scholar] [CrossRef]
- Al-Jaberi, S.H.H.; Rashid, U.; Al-Doghachi, F.A.J.; Abdulkareem-Alsultan, G.; Taufiq-Yap, Y.H. Synthesis of MnO-NiO-SO4−2/ZrO2 solid acid catalyst for methyl ester production from palm fatty acid distillate. Energy Convers. Manag. 2017, 139, 166–174. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Dewi, R.N.; Di Livia, K.; Arisa, F.; Rochmadi; Cahyono, R.B.; Budiman, A. Advancing biodiesel production from microalgae Spirulina sp. By a simultaneous extraction-transesterification process using palm oil as a co-solvent of methanol. Open Chem. 2020, 18, 833–842. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Chong, J.H.; Yuniarto, A.; Hadibarata, T. The current scenario and challenges of biodiesel production in Asian countries: A review. Bioresour. Technol. Rep. 2020, 12, 100608. [Google Scholar] [CrossRef]
- Pydimalla, M.; Husaini, S.; Kadire, A.; Kumar Verma, R. Sustainable biodiesel: A comprehensive review on feedstock, production methods, applications, challenges and opportunities. Mater. Today Proc. 2023, 92, 458–464. [Google Scholar] [CrossRef]
- Venu, H.; Soudagar, M.E.M.; Kiong, T.S.; Razali, N.M.; Wei, H.R.; Khan, T.M.Y.; Almakayeel, N.; Kalam, M.A.; Cuce, E. Performance and emission prediction using ANN (artificial neural network) on H2-assisted Garcinia gummi-gutta biofuel doped with nano additives. Sci. Rep. 2025, 15, 5911. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Masruri, W.; Azmi, F.A.; Suyono, E.A.; Sudibyo, H.; Rochmadi, R. Extractive-transesterification of Microalgae Arthrospira sp. Using Methanol-Hexane Mixture as solvent. Int. J. Renew. Energy Res. 2018, 8, 1499–1507. [Google Scholar]
- Prakoso, T.; Tanaka, A.; Hirotsu, T.; Udomsap, P.; Chollacoop, N.; Goto, S.; Indarto, A. Oxidation stability of biodiesel fuel produced from Jatropha Curcas L using Rancimat and PetroOXY method. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 501–506. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development (OECD); Food and Agriculture Organization (FAO); OECD Agriculture Statistics (Database). OECD-FAO Agricultural Outlook 2023–2032. 2024. Available online: https://data-explorer.oecd.org/ (accessed on 26 July 2024).
- Organisation for Economic Co-Operation and Development (OECD); Food and Agriculture Organization (FAO). OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Isah, A.G.; Faruk, A.A.; Musa, U.; Garba, U.M.; Alhassan, M.; Abdullahi, U.B.; Damian, A.T. Oxidation stability and cold flow properties of biodiesel synthesized from castor oil: Influence of alkaline catalysts type and purification techniques. Mater. Today Proc. 2022, 57, 748–752. [Google Scholar] [CrossRef]
- Liu, W.; Lu, G.; Yang, G.; Bi, Y. Improving oxidative stability of biodiesel by cis-trans isomerization of carbon-carbon double bonds in unsaturated fatty acid methyl esters. Fuel 2019, 242, 133–139. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Impact of cold flow properties of biodiesel on engine performance. Renew. Sustain. Energy Rev. 2014, 31, 650–656. [Google Scholar] [CrossRef]
- Riyadi, T.W.B.; Spraggon, M.; Herawan, S.G.; Idris, M.; Paristiawan, P.A.; Putra, N.R.; Faizullizam R, M.; Silambarasan, R.; Veza, I. Biodiesel for HCCI engine: Prospects and challenges of sustainability biodiesel for energy transition. Results Eng. 2023, 17, 100916. [Google Scholar] [CrossRef]
- Sia, C.B.; Kansedo, J.; Tan, Y.H.; Lee, K.T. Evaluation on biodiesel cold flow properties, oxidative stability and enhancement strategies: A review. Biocatal. Agric. Biotechnol. 2020, 24, 101514. [Google Scholar] [CrossRef]
- Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A. Methods for improving the cold flow properties of biodiesel with high saturated fatty acids content: A review. Renew. Sustain. Energy Rev. 2017, 72, 774–790. [Google Scholar] [CrossRef]
- Rasimoglu, N.; Temur, H. Cold flow properties of biodiesel obtained from corn oil. Energy 2014, 68, 57–60. [Google Scholar] [CrossRef]
- Amran, N.A.; Bello, U.; Hazwan Ruslan, M.S. The role of antioxidants in improving biodiesel’s oxidative stability, poor cold flow properties, and the effects of the duo on engine performance: A review. Heliyon 2022, 8, e09846. [Google Scholar] [CrossRef]
- Lugito, G.; Pamungkas, A.Y.; Realdi, M.N.D.; Alam, A.K.; Egiyawati, C.; Pradana, Y.S.; Adhi, T.P.; Soerawidjaja, T.H.; Makertihartha, I.G.B.N.; Mohtar, W.H.M.W.; et al. Biodiesel Stability Enhancement Through Catalytic Transfer Hydrogenation Using Glycerol as Hydrogen Donor. Eng 2025, 6, 94. [Google Scholar] [CrossRef]
- Anwar, A.; Garforth, A. Challenges and opportunities of enhancing cold flow properties of biodiesel via heterogeneous catalysis. Fuel 2016, 173, 189–208. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. A Comprehensive Evaluation of the Melting Points of Fatty Acids and Esters Determined by Differential Scanning Calorimetry. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Moser, B.R. Comparative oxidative stability of fatty acid alkyl esters by accelerated methods. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 699–706. [Google Scholar] [CrossRef]
- Knothe, G.; Matheaus, A.C.; Ryan, T.W. Cetane numbers of branched and straight-chain fatty esters determined in an ignition quality tester q. Fuel 2003, 82, 971–975. [Google Scholar] [CrossRef]
- Varala, R.; Narayana, V.; Kulakarni, S.R.; Khan, M.; Alwarthan, A.; Adil, S.F. Sulfated tin oxide (STO)—Structural properties and application in catalysis: A review. Arab. J. Chem. 2016, 9, 550–573. [Google Scholar] [CrossRef]
- Sierra-Cantor, J.F.; Gimello, O.; Guerrero-Fajardo, C.A.; Di Renzo, F.; Petitjean, H.; Riviere, M.; Gérardin, C.; Tanchoux, N. Synthesis of alkyl-branched fatty acids by isomerization on micro-mesoporous ferrierite-based zeolitic materials. Appl. Catal. B 2024, 344, 123602. [Google Scholar] [CrossRef]
- Misra, P.; Alvarez-Majmutov, A.; Chen, J. Isomerization catalysts and technologies for biorefining: Opportunities for producing sustainable aviation fuels. Fuel 2023, 351, 128994. [Google Scholar] [CrossRef]
- Arata, K. Solid Superacids. Adv. Catal. 1990, 37, 165–211. [Google Scholar] [CrossRef]
- Arata, K.; Matsuhashi, H.; Hino, M.; Nakamura, H. Synthesis of solid superacids and their activities for reactions of alkanes. Catal. Today 2003, 81, 17–30. [Google Scholar] [CrossRef]
- Oloye, F.F.; Aliyev, R.; Anderson, J.A. Hydroisomerisation of n-heptane over Pt/sulfated zirconia catalyst at atmospheric pressure. Fuel 2018, 222, 569–573. [Google Scholar] [CrossRef]
- Žula, M.; Grilc, M.; Likozar, B. Hydrocracking, hydrogenation and hydro-deoxygenation of fatty acids, esters and glycerides: Mechanisms, kinetics and transport phenomena. Chem. Eng. J. 2022, 444, 136564. [Google Scholar] [CrossRef]
- Sarker, M.I.; Moreau, R.A.; Ngo, H. Comparison of Various Phosphine Additives in Zeolite Based Catalytic Isomerization of Oleic Acid. Eur. J. Lipid Sci. Technol. 2018, 120, 1800070. [Google Scholar] [CrossRef]
- Sarker, M.I.; Latona, R.J.; Moreau, R.A.; Micheroni, D.; Jones, K.C.; Ngo, H. Convenient and Environmentally Friendly Production of Isostearic Acid with Protonic Forms of Ammonium Cationic Zeolites. Eur. J. Lipid Sci. Technol. 2017, 119, 1700262. [Google Scholar] [CrossRef]
- Ngo, H.L. Lewis base additives improve the zeolite Ferrierite-catalyzed synthesis of isostearic acids synthesis of isostearic acids. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 613–619. [Google Scholar] [CrossRef]
- Ngo, H.L.; Hoh, E.; Foglia, T.A. Improved synthesis and characterization of saturated branched-chain fatty acid isomers. Eur. J. Lipid Sci. Technol. 2012, 114, 213–221. [Google Scholar] [CrossRef]
- Reaume, S.J.; Ellis, N. Use of hydroisomerization to reduce the cloud point of saturated fatty acids and methyl esters used in biodiesel production. Biomass Bioenergy 2013, 49, 188–196. [Google Scholar] [CrossRef]
- Ngo, H.L.; Nunez, A.; Lin, W.; Foglia, T.A. Zeolite-catalyzed isomerization of oleic acid to branched-chain isomers. Eur. J. Lipid Sci. Technol. 2007, 109, 214–224. [Google Scholar] [CrossRef]
- Hodgson, W.R.; Lok, C.M.; Roberts, G.; Koetsier, W.T. Fatty Acid Isomerisation. Patent EP0774451B2, 11 September 2002. [Google Scholar]
- Foglia, T.A.; Perlstein, T.; Nakano, Y.; Maerker, G. Process for The Preparation of Branched Chain Fatty Acids and Esters. Patent US4371469A, 1 February 1983. [Google Scholar]
- Dhar, A.; Vekariya, R.L.; Sharma, P. Kinetics and mechanistic study of n-alkane hydroisomerization reaction on Pt-doped γ-alumina catalyst. Petroleum 2017, 3, 489–495. [Google Scholar] [CrossRef]
- Jahangiri, M.; Salehirad, F.; Alijani, S. Preparation of Pt/Al2O3-Cl Catalyst and Investigation of Operating Variables Effects on Isomerization Reaction. J. Chem. Pet. Eng. 2018, 52, 13–21. [Google Scholar] [CrossRef]
- Regali, F.; Liotta, L.F.; Venezia, A.M.; Montes, V.; Boutonnet, M.; Järås, S. Effect of metal loading on activity, selectivity and deactivation behavior of Pd/silica-alumina catalysts in the hydroconversion of n-hexadecane. Catal. Today 2014, 223, 87–96. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Steichen, D. Skeletal Isomerization of Alkyl Esters and Derivatives Prepared Therefrom. Patent US6946567B2, 20 September 2005. [Google Scholar]
- Busto, M.; Dosso, L.A.; Vera, C.R.; Grau, J.M. Composite catalysts of Pt/SO42−-ZrO2 and Pt/WO3-ZrO2 for producing high octane isomerizate by isomerization-cracking of long paraffins. Fuel Process. Technol. 2012, 104, 128–135. [Google Scholar] [CrossRef]
- Venkatesh, K.R.; Hu, J.; Wang, W.; Holder, G.D.; Tierney, J.W.; Wender, I. Hydrocracking and Hydroisomerization of Long-Chain Alkanes and Polyolefins over Metal-Promoted Anion-Modified Zirconium Oxides. Energy Fuels 1996, 10, 1163–1170. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Tierney, J.W.; Wender, I. Anion-modified zirconia: Effect of metal promotion and hydrogen reduction on hydroisomerization of n-hexadecane and Fischer-Tropsch waxes. Fuel Process. Technol. 2001, 69, 59–71. [Google Scholar] [CrossRef]
- Hino, M.; Takasaki, S.; Furuta, S.; Matsuhashi, H.; Arata, K. meta-Stannic acid as an effective support for the preparation of sulfated and tungstated stannias. Appl. Catal. A Gen. 2007, 321, 147–154. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Miyazaki, H.; Arata, K. The Preparation of Solid Superacid of Sulfated Tin Oxide with Acidity Higher Than Sulfated Zirconia. Chem. Lett. 2001, 30, 452–453. [Google Scholar] [CrossRef]
- Enríquez, J.M.H.; Lajas, L.A.C.; Alamilla, R.G.; Martín, E.Á.S.; Alamilla, P.G.; Handy, E.B.; Galindo, G.C.; Serrano, L.A.G. Synthesis of Solid Acid Catalysts Based on TiO2-SO4 and Pt/TiO2-SO4 Applied in n-Hexane Isomerization. Open J. Met. 2013, 3, 34–44. [Google Scholar] [CrossRef]
- Noda, L.K.; De Almeida, R.M.; Probst, L.F.D.; Gonçalves, N.S. Characterization of sulfated TiO2 prepared by the sol-gel method and its catalytic activity in the n-hexane isomerization reaction. J. Mol. Catal. A Chem. 2005, 225, 39–46. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Sato, D.; Arata, K. Influence of Calcination Temperature on The Surface Acidity of The Solid Superacid of Sulfated Alumina. React. Kinet. Catal. Lett. 2004, 81, 183–188. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Linganiso, L.Z.; Jacobs, G.; Keogh, R.A.; Shafer, W.D.; Davis, B.H. Hydroisomerization of n-Hexadecane Over Anion Modified Pt/HfO2 Catalysts. Catal. Lett. 2012, 142, 1180–1189. [Google Scholar] [CrossRef]
- Yu, K.; Kumar, N.; Aho, A.; Roine, J.; Heinmaa, I.; Murzin, D.Y.; Ivaska, A. Determination of acid sites in porous aluminosilicate solid catalysts for aqueous phase reactions using potentiometric titration method. J. Catal. 2016, 335, 117–124. [Google Scholar] [CrossRef]
- Dunn, R.O. Cold flow properties of biodiesel: A guide to getting an accurate analysis. Biofuels 2015, 6, 115–128. [Google Scholar] [CrossRef]
- Alaya, M.N.; Rabah, M.A. Some physico-chemical properties and catalytic activity of sulfate ion supported on WO3/SnO2 catalyst. Arab. J. Chem. 2017, 10, S439–S449. [Google Scholar] [CrossRef]
- Khalaf, H.A.; Mansour, S.E.; El-Madani, E.A. The influence of sulfate contents on the surface properties of sulfate-modified tin(IV) oxide catalysts. J. Assoc. Arab. Univ. Basic Appl. Sci. 2011, 10, 15–20. [Google Scholar] [CrossRef]
- Khder, A.S.; Ahmed, A.I. Selective nitration of phenol over nanosized tungsten oxide supported on sulfated SnO2 as a solid acid catalyst. Appl. Catal. A Gen. 2009, 354, 153–160. [Google Scholar] [CrossRef]
- Joao, K. Thermo-Gravimetric Analysis in the Investigation of Catalysts: Insights and Innovations. J. Chromatogr. Sep. Tech. 2024, 15, 1000597. [Google Scholar] [CrossRef]
- Akkera, H.S.; Mann, V.; Varalakshmi, B.N.; Ploloju, M.; Kambhala, N.; Venkatesh, G. Effect of Sr-doped on physical and photoluminescence properties of SnO2 transparent conducting oxide thin films. J. Mater. Sci. Mater. Electron. 2023, 34, 1044. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chen, W.H.; Ko, H.H.; Sakthivel, A.; Huang, S.J.; Liu, S.H.; Lo, A.Y.; Tsai, T.C.; Liu, S.B. A solid-state NMR, FT-IR and TPD study on acid properties of sulfated and metal-promoted zirconia: Influence of promoter and sulfation treatment. Catal. Today 2006, 116, 111–120. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Makertihartha, I.G.B.N.; Indarto, A.; Prakoso, T.; Soerawidjaja, T.H. A Review of Biodiesel Cold Flow Properties and Its Improvement Methods: Towards Sustainable Biodiesel Application. Energies 2024, 17, 4543. [Google Scholar] [CrossRef]
- Arning, M.D.; Minteer, S.D. Electrode Potentials. Handbook of Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Wachs, I.E. Number of surface sites and turnover frequencies for oxide catalysts. J. Catal. 2022, 405, 462–472. [Google Scholar] [CrossRef]
- Badlani, M.; Wachs, I.E. Methanol: A “Smart” Chemical Probe Molecule. Catal. Lett. 2001, 75, 137–149. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Kaka khel, T.A.; Azkaar, M.; Engblom, S.; Murzin, D.Y. Catalytic Hydroisomerization of Long-Chain Hydrocarbons for the Production of Fuels. Catalysts 2018, 8, 534. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).