End-Users’ Perspectives on Implementation Outcomes of Digital Voice Assistants Delivering a Home-Based Lifestyle Intervention in Older Obese Adults with Type 2 Diabetes Mellitus: A Qualitative Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
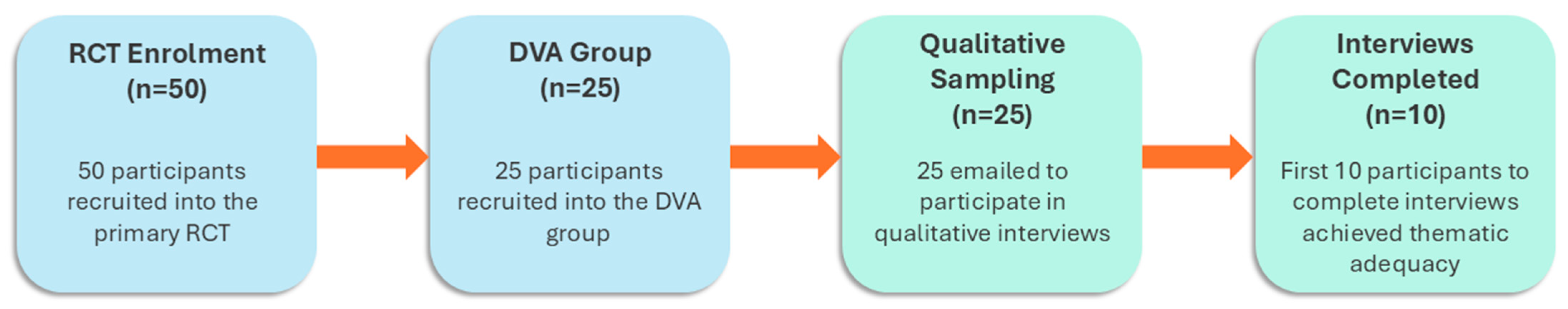
2.2. Participants and Recruitment
2.3. Intervention
2.4. Frameworks
2.5. Data Collection and Analysis
2.6. Researcher Reflexivity
3. Results
3.1. Baseline Demographics
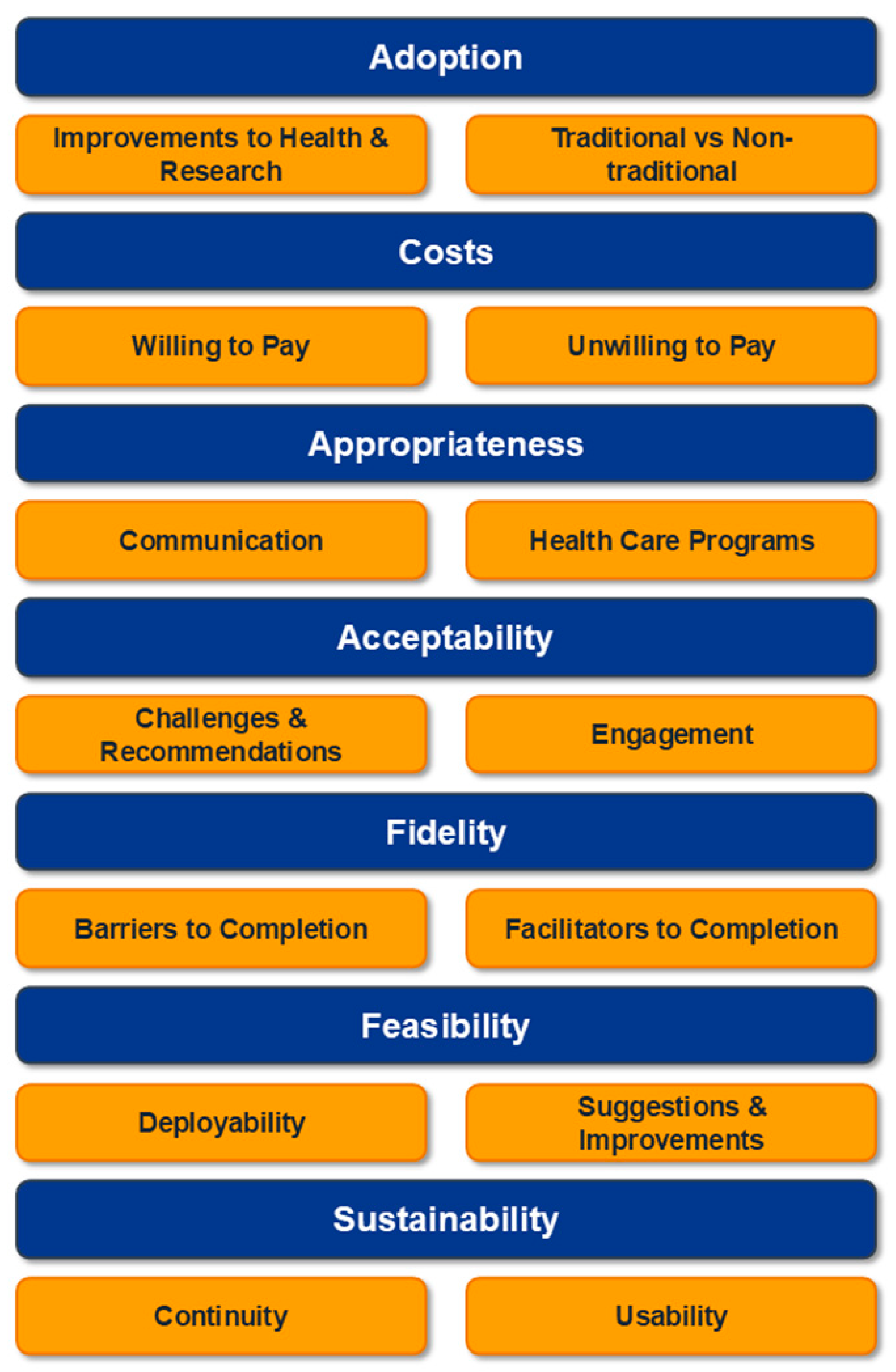
3.2. Qualitative Outcomes
3.2.1. Theme 1: Adoption
‘Health-wise, I felt that it would keep me interested in exercising, and if it could help with diabetes, I really was interested in trying to help myself, because left alone, it’s easy just to fall back into old habits.’(Participant 5; Age 71 years)
‘(Recruitment) through a doctor, or general practitioner… (Because you can) speak to a doctor and get onto a program to help you, making you more aware of the foods, exercise (involved in the program).’(Participant 47; Age 65 years)
‘Whether it’s Facebook or (other forms of social media). Getting permission from some of the Facebook groups would be a good one, like if you had a diabetes or a metabolic website…because you’re not selling anything; you’re actually doing something that’ll help.’(Participant 11; Age 64 years)
‘Diabetes Australia…, or the [National Disability Insurance Scheme (NDIS)] would be valued, perhaps sign up at doctors and the dietitian’s nurse or even podiatrists because often with diabetes, you’re going to podiatrists.’(Participant 5; Age 71 years)
3.2.2. Theme 2: Costs
‘Yes, I would’ve paid for it. I think on a weekly basis, yes, I think if you paid—if you’re a pensioner, I think about 15 a week.’(Participant 5; Age 71 years)
‘Look, I would have paid maybe $50, $60 a month.’(Participant 27; Age 63 years)
‘No, I wouldn’t (pay for the program). In the past I’ve always taken steps to try and mitigate any health problems by reaching out to my doctor and following his or her advice. And I got a feeling I had to do it. I shouldn’t have to do anymore or spend any money.’(Participant 34; Age 75 years)
3.2.3. Theme 3: Appropriateness
‘(The DVA platform) visually and audio-wise, (is) excellent. To incorporate the two, I think it’s very good. I think it’s ideal… and it’s pretty mobile. You can actually unplug it and put it somewhere suitable, for people to actually engage with it, so I think it’s good. Size is a good size, so the 7-inch is more than good enough to see.’(Participant 35; Age 67 years)
3.2.4. Theme 4: Acceptability
‘I was very satisfied… with the information that was being given. (The content delivered) was… reinforcing the ideas that I had in (my) mind of how to go about keeping my blood sugar down, and (completing) the exercises.’(Participant 32; Age 66 years)
‘I’d say the conversations (with the DVA platform) were probably better than (the exercises, which were) the actual other part of the program, so it was good to hear and talk… rather than just sit back and listen. So, yes, I would say that would be a better way of delivering it too, from a conversational point of view.’(Participant 16; Age 63 years)
‘As far as the exercise is concerned, I felt that it was great in the beginning, —but then it became very repetitive in its nature… I didn’t engage as much because it was the same…’(Participant 16; Age 63 years)
‘(The DVA platform) stopped and started, and froze a lot,—people my age find things like that rather annoying because we don’t understand it.’(Participant 47; Age 65 years)
3.2.5. Theme 5: Fidelity
‘To tell you the truth, the reminders that used to come up (assisted me in completing the program). So, we set the reminders at 6:00 o’clock at night and that would prompt you to do it. I think that was a good thing and Alexa (the DVA platform) is great for that.’(Participant 16; Age 63 years)
‘There were two times during this 12-week period when I fell ill…I think this week, I missed out a couple of days… I was on antibiotics and there was no motivation in me to get onto doing the exercises.’(Participant 32; Age 66 years)
3.2.6. Theme 6: Feasibility
‘If you’re in reasonable health, and you can manage the exercises, and as in my case you’re retired or you’re semi-retired… and you feel like you can make the commitment, I think it’s generally doable.’(Participant 48; Age 71 years)
‘(Improving is) just a matter of whether their ability to do the exercises is there and if they’re not getting bored of the exercise. If the exercises are less strenuous than they were expected to be, then they might get bored, so that has to be sorted out.’(Participant 32; Age 66 years)
3.2.7. Theme 7: Sustainability
‘I’m definitely seeing results… besides doing the exercises that come up on the Alexa (the DVA platform), I try to do some crunches now that I’ve already started the exercises. (I) do a few more exercises of my own and I can see the benefits of it.’(Participant 32; Age 66 years)
‘My peripheral neuropathy, and balance (affected me) … particularly at a couple of the stages during the process, I had a couple of dizzy spells, because of the extreme work I was doing… I tried, but some of the (exercises) where I was standing up, I couldn’t balance. That’s my physicality, that’s not your program.’(Participant 38; Age 69 years)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEP | Accredited Exercise Physiologist |
| APD | Accredited Practicing Dietitian |
| ANZCTR | Australian New Zealand Clinical Trials Registry |
| BGLs | Blood glucose levels |
| DVA | Digital voice assistant |
| ESSA | Exercise and Sport Science Australia |
| PRACTIS | Practical Planning for Implementation and Scale-up |
| RCT | Randomised controlled trial |
| RPE | Rating of perceived exertion |
| SRQR | Standards for Reporting Qualitative Research |
| T2DM | Type 2 diabetes mellitus |
Appendix A. Interview Questions and Prompts
- What made you decide to take part in this project?
- What do you think is the best way to reach older adults with T2DM to engage in lifestyle programs delivered by an Alexa?
- Outside of the research study, how would you have liked to be made aware of the study?
- What do you think makes it harder/easier to reach older adults with T2DM to engage in an exercise and dietary program delivered by an Alexa?
- 3.
- For the purposes of this research study, this project is free to use. Had you not been part of this study, is this something you would have paid for if it was offered to you?
- If yes: how much would you be willing to pay?
- If no: can you describe the reasons why?
- 4.
- As this is a research study, you may have been aware of this project via an email based on engagement with a previous research study. In the future, we plan to offer the program (or recruit) via social media.
- 5.
- How appropriate is this as a way to reach potential participants?
- Are there any advantages/disadvantages in using this recruitment method?
- What would be a more/another appropriate way?
- 6.
- In general, how satisfied were you with this project?
- For example, in terms of the format of the material and content, usability of the Alexa device, conversational based interactions?
- Which aspects did you like the most/least? Can you explain why?
- In what ways could the Alexa delivery be improved?
- 7.
- Were you able to complete all exercise and dietary sessions?
- If yes, what helped you achieve this?
- If no, what prevented you from doing that?
- 8.
- How realistic is it to expect older adults in general to complete all components of the dietary and exercise program delivered by an Alexa?
- Why is that?
- How might this be improved/made easier?
- 9.
- In terms of your experiences receiving lifestyle information in a conversational based format, how appropriate was this as a way to support older adults and communicate relevant exercise and dietary information?
- Was appropriate: what makes this an appropriate way of communicating and reaching older adults?
- Not appropriate: what makes you say this?
- Not appropriate: what do you think is a better way to communicate this information and reach older adults?
- Are there any advantages/disadvantages to this project being available or delivered using a conversational based format?
- 10.
- Did you use the Alexa device to adhere to your lifestyle program for the full 12 weeks?
- If yes, what enabled or encouraged you to maintain usage for this length of time?
- If no, what prevented or discouraged you from continuing to use it?
- 11.
- Do you think you’ll continue to use the Alexa device to adhere to your lifestyle program (i.e., beyond the 12 weeks)?
- Is there anything we can change to help support older adults to continue using the device?
References
- Ye, J.; Wu, Y.; Yang, S.; Zhu, D.; Chen, F.; Chen, J.; Ji, X.; Hou, K. The global, regional and national burden of type 2 diabetes mellitus in the past, present and future: A systematic analysis of the Global Burden of Disease Study 2019. Front. Endocrinol. 2023, 14, 1192629. [Google Scholar] [CrossRef]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef]
- Moore, G.; Durstine, J.L.; Painter, P.; American College of Sports Medicine. Acsm’s Exercise Management for Persons with Chronic Diseases and Disabilities, 4E; Human Kinetics: Champaign, IL, USA, 2016. [Google Scholar]
- Mesinovic, J.; Fyfe, J.J.; Talevski, J.; Wheeler, M.J.; Leung, G.K.; George, E.S.; Hunegnaw, M.T.; Glavas, C.; Jansons, P.; Daly, R.M.; et al. Type 2 diabetes mellitus and sarcopenia as comorbid chronic diseases in older adults: Established and emerging treatments and therapies. Diabetes Metab. J. 2023, 47, 719–742. [Google Scholar] [CrossRef]
- Castaneda, C.; Layne, J.E.; Munoz-Orians, L.; Gordon, P.L.; Walsmith, J.; Foldvari, M.; Roubenoff, R.; Tucker, K.L.; Nelson, M.E. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002, 25, 2335–2341. [Google Scholar] [CrossRef]
- Maiorana, A.; O’Driscoll, G.; Goodman, C.; Taylor, R.; Green, D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res. Clin. Pract. 2002, 56, 115–123. [Google Scholar] [CrossRef]
- Glavas, C.; Mesinovic, J.; Ebeling, P.R.; Sood, S.; George, E.S.; Hunegnaw, M.T.; Zengin, A.; Daly, R.M.; Jansons, P.; Scott, D. Comparing bone and muscle parameters in community-dwelling older adults with obesity, with or without type 2 diabetes mellitus. Bone 2025, 202, 117680. [Google Scholar] [CrossRef] [PubMed]
- Jansons, P.S.; Haines, T.P.; O’Brien, L. Interventions to achieve ongoing exercise adherence for adults with chronic health conditions who have completed a supervised exercise program: Systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Krousel-Wood, M.; Berger, L.; Jiang, X.; Blonde, L.; Myers, L.; Webber, L. Does home-based exercise improve body mass index in patients with type 2 diabetes?: Results of a feasibility trial. Diabetes Res. Clin. Pract. 2008, 79, 230–236. [Google Scholar] [CrossRef]
- Glavas, C.; Mesinovic, J.; Gandham, A.; Cervo, M.M.; Ng, C.-A.; Ebeling, P.R.; George, E.S.; Daly, R.M.; Beck, B.R.; Jansons, P.; et al. Experiences and outcomes of older adults with obesity transitioning from gym-to home-based resistance training due to COVID-19 lockdowns: A mixed-methods analysis of a RCT. BMC Geriatr. 2025, 25, 556. [Google Scholar] [CrossRef]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.; Close, J.C.; Lord, S.R. Exercise to prevent falls in older adults: An updated meta-analysis and best practice recommendations. New South Wales Public Health Bull. 2011, 22, 78–83. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065. [Google Scholar] [CrossRef]
- Barbabella, F.; Melchiorre, M.G.; Quattrini, S.; Papa, R.; Lamura, G.; Richardson, E.; van Ginneken, E. How can Ehealth Improve Care for People with Multimorbidity in Europe? World Health Organization, Regional Office for Europe Copenhagen: Copenhagen, Denmark, 2017. [Google Scholar]
- Nguyen, V.; Ara, P.; Simmons, D.; Osuagwu, U.L. The role of digital health technology interventions in the prevention of type 2 diabetes mellitus: A systematic review. Clin. Med. Insights: Endocrinol. Diabetes 2024, 17, 11795514241246419. [Google Scholar] [CrossRef]
- Kruse, C.; Fohn, J.; Wilson, N.; Patlan, E.N.; Zipp, S.; Mileski, M. Utilization barriers and medical outcomes commensurate with the use of telehealth among older adults: Systematic review. JMIR Med. Inform. 2020, 8, e20359. [Google Scholar] [CrossRef]
- Mirbabaie, M.; Marx, J.; Moellmann, N.; Matt, S. Digital Assistants for Diabetes Treatment: Designing a User Interface to Support Chronic Disease Self-Management. ACM SIGMIS Database DATABASE Adv. Inf. Syst. 2025, 56, 55–79. [Google Scholar] [CrossRef]
- Glavas, C.; Scott, D.; Sood, S.; George, E.S.; Daly, R.M.; Gvozdenko, E.; de Courten, B.; Jansons, P. Exploring the Feasibility of Digital Voice Assistants for Delivery of a Home-Based Exercise Intervention in Older Adults With Obesity and Type 2 Diabetes Mellitus: Randomized Controlled Trial. JMIR Aging 2024, 7, e53064. [Google Scholar] [CrossRef]
- Hordern, M.D.; Dunstan, D.W.; Prins, J.B.; Baker, M.K.; Singh, M.A.F.; Coombes, J.S. Exercise prescription for patients with type 2 diabetes and pre-diabetes: A position statement from Exercise and Sport Science Australia. J. Sci. Med. Sport 2012, 15, 25–31. [Google Scholar] [CrossRef]
- Rouleau, G.; Wu, K.; Ramamoorthi, K.; Boxall, C.; Liu, R.H.; Maloney, S.; Zelmer, J.; Scott, T.; Larsen, D.; Wijeysundera, H.C.; et al. Mapping Theories, Models, and Frameworks to Evaluate Digital Health Interventions: Scoping Review. J. Med. Internet Res. 2024, 26, e51098. [Google Scholar] [CrossRef]
- Koorts, H.; Eakin, E.; Estabrooks, P.; Timperio, A.; Salmon, J.; Bauman, A. Implementation and scale up of population physical activity interventions for clinical and community settings: The PRACTIS guide. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 51. [Google Scholar] [CrossRef]
- Nilsen, E.R.; Stendal, K.; Gullslett, M.K. Implementation of eHealth Technology in Community Health Care: The complexity of stakeholder involvement. BMC Health Serv. Res. 2020, 20, 395. [Google Scholar] [CrossRef]
- Proctor, E.; Silmere, H.; Raghavan, R.; Hovmand, P.; Aarons, G.; Bunger, A.; Griffey, R.; Hensley, M. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm. Policy Ment. Health Ment. Health Serv. Res. 2011, 38, 65–76. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Reflecting on reflexive thematic analysis. Qual. Res. Sport Exerc. Health 2019, 11, 589–597. [Google Scholar] [CrossRef]
- Hudson, J.L.; Moon, Z.; Hughes, L.D.; Moss-Morris, R. Engagement of stakeholders in the design, evaluation, and implementation of complex interventions. In The Handbook of Behavior Change; Cambridge University Press: Cambridge, UK, 2020; pp. 349–360. [Google Scholar]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for reporting qualitative research: A synthesis of recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef]
- Jansons, P.; Fyfe, J.; Via, J.D.; Daly, R.M.; Gvozdenko, E.; Scott, D. Barriers and enablers for older adults participating in a home-based pragmatic exercise program delivered and monitored by Amazon Alexa: A qualitative study. BMC Geriatr. 2022, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Jansons, P.S.; Robins, L.; Haines, T.P.; O’Brien, L. Barriers and enablers to ongoing exercise for people with chronic health conditions: Participants’ perspectives following a randomized controlled trial of two interventions. Arch. Gerontol. Geriatr. 2018, 76, 92–99. [Google Scholar] [CrossRef]
- BuddyLink. BuddyLink VIPA Therapeutic Program 2023. Available online: https://www.buddylink.au (accessed on 16 August 2024).
- LiveVR. TeleTrainer 02: Amazon; 2023. Available online: https://www.amazon.com.au/LiveVR-TeleTrainer-02/dp/B09CQ8LK7H/ref=sr_1_1?crid=T9UNQDHAA8DF&keywords=Teletrainer&qid=1677322735&s=digital-skills&sprefix=teletrainer%2Calexa-skills%2C260&sr=1-1] (accessed on 16 August 2024).
- Williams, N. The Borg rating of perceived exertion (RPE) scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef]
- Colagiuri, S.; Dickinson, S.; Girgis, S.; Colagiuri, R. National Evidence Based Guideline for Blood Glucose Control in Type 2 Diabetes. Available online: https://www.diabetesaustralia.com.au/wp-content/uploads/National-Evidence-Based-Guideline-for-Blood-Glucose-Control-in-Type-2-Diabetes.pdf (accessed on 10 October 2025).
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef]
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef]
- TranscribeMe. Fast & Accurate Human Transcription Services 2024. Available online: https://www.transcribeme.com/ (accessed on 16 August 2024).
- Jansons, P.; Dalla Via, J.; Daly, R.M.; Fyfe, J.J.; Gvozdenko, E.; Scott, D. Delivery of home-based exercise interventions in older adults facilitated by Amazon Alexa: A 12-week feasibility trial. J. Nutr. Health Aging 2022, 26, 96–102. [Google Scholar] [CrossRef]
- Valera Román, A.; Pato Martínez, D.; Lozano Murciego, Á.; Jiménez-Bravo, D.M.; de Paz, J.F. Voice assistant application for avoiding sedentarism in elderly people based on IoT technologies. Electronics 2021, 10, 980. [Google Scholar] [CrossRef]
- Wei, C.; Finkelstein, J. Comparison of Alexa voice and audio video interfaces for home-based physical telerehabilitation. In AMIA Annual Symposium Proceedings; American Medical Informatics Association: Rockville, MD, USA, 2022; p. 496. [Google Scholar]
- Davies, L.; LeClair, K.L.; Bagley, P.; Blunt, H.; Hinton, L.; Ryan, S.; Ziebland, S. Face-to-face compared with online collected accounts of health and illness experiences: A scoping review. Qual. Health Res. 2020, 30, 2092–2102. [Google Scholar] [CrossRef]
- Wilson, J.; Heinsch, M.; Betts, D.; Booth, D.; Kay-Lambkin, F. Barriers and facilitators to the use of e-health by older adults: A scoping review. BMC Public Health 2021, 21, 1556. [Google Scholar] [CrossRef]
- Harris, M.T.; Blocker, K.A.; Rogers, W.A. Older adults and smart technology: Facilitators and barriers to use. Front. Comput. Sci. 2022, 4, 835927. [Google Scholar] [CrossRef]
- Cajita, M.I.; Hodgson, N.A.; Lam, K.W.; Yoo, S.; Han, H.-R. Facilitators of and barriers to mHealth adoption in older adults with heart failure. Comput. Inform. Nurs. CIN 2018, 36, 376. [Google Scholar] [CrossRef]
- Chen, K.; Chan, A.H.S. Use or non-use of gerontechnology—A qualitative study. Int. J. Environ. Res. Public Health 2013, 10, 4645–4666. [Google Scholar] [CrossRef]
- Cimperman, M.; Brenčič, M.M.; Trkman, P.; Stanonik, M.d.L. Older adults’ perceptions of home telehealth services. Telemed. e-Health 2013, 19, 786–790. [Google Scholar] [CrossRef]
- Gentili, A.; Failla, G.; Melnyk, A.; Puleo, V.; Tanna, G.L.D.; Ricciardi, W.; Cascini, F. The cost-effectiveness of digital health interventions: A systematic review of the literature. Front. Public Health 2022, 10, 787135. [Google Scholar] [CrossRef]
- Puleo, V.; Gentili, A.; Failla, G.; Melnyk, A.; Di Tanna, G.; Ricciardi, W.; Cascini, F. Digital health technologies: A systematic review of their cost-effectiveness. Eur. J. Public Health 2021, 31 (Suppl. 3), ckab164. 273. [Google Scholar] [CrossRef]
- Duong, T.; Olsen, Q.; Menon, A.; Woods, L.; Wang, W.; Varnfield, M.; Jiang, L.; Sullivan, C. Digital Health Interventions to Prevent Type 2 Diabetes Mellitus: Systematic Review. J. Med. Internet Res. 2025, 27, e67507. [Google Scholar] [CrossRef] [PubMed]
- Karvela, M.; Golden, C.T.; Bell, N.; Martin-Li, S.; Bedzo-Nutakor, J.; Bosnic, N.; DeBeaudrap, P.; de Mateo-Lopez, S.; Alajrami, A.; Qin, Y.; et al. Assessment of the impact of a personalised nutrition intervention in impaired glucose regulation over 26 weeks: A randomised controlled trial. Sci. Rep. 2024, 14, 5428. [Google Scholar] [CrossRef]
- Kitazawa, M.; Takeda, Y.; Hatta, M.; Horikawa, C.; Sato, T.; Osawa, T.; Ishizawa, M.; Suzuki, H.; Matsubayashi, Y.; Fujihara, K.; et al. Lifestyle intervention with smartphone app and isCGM for people at high risk of type 2 diabetes: Randomized trial. J. Clin. Endocrinol. Metab. 2024, 109, 1060–1070. [Google Scholar] [CrossRef]
- Solis-Navarro, L.; Gismero, A.; Fernández-Jané, C.; Torres-Castro, R.; Solá-Madurell, M.; Bergé, C.; Pérez, L.M.; Ars, J.; Martín-Borràs, C.; Vilaró, J.; et al. Effectiveness of home-based exercise delivered by digital health in older adults: A systematic review and meta-analysis. Age Ageing 2022, 51, afac243. [Google Scholar]
- Chambers, R.; Beaney, P. The potential of placing a digital assistant in patients’ homes. Br. J. Gen. Pract. 2020, 70, 8–9. [Google Scholar] [CrossRef]
- Daly, R.M.; Gianoudis, J.; Hall, T.; Mundell, N.L.; Maddison, R. Feasibility, usability, and enjoyment of a home-based exercise program delivered via an exercise app for musculoskeletal health in community-dwelling older adults: Short-term prospective pilot study. JMIR Mhealth Uhealth 2021, 9, e21094. [Google Scholar] [CrossRef]
- Kerr, D.; Ahn, D.; Waki, K.; Wang, J.; Breznen, B.; Klonoff, D.C. Digital interventions for self-management of type 2 diabetes mellitus: Systematic literature review and meta-analysis. J. Med. Internet Res. 2024, 26, e55757. [Google Scholar] [CrossRef]
- Jones, V.K.; Hanus, M.; Yan, C.; Shade, M.Y.; Blaskewicz Boron, J.; Maschieri Bicudo, R. Reducing loneliness among aging adults: The roles of personal voice assistants and anthropomorphic interactions. Front. Public Health 2021, 9, 750736. [Google Scholar] [CrossRef]
- Pitardi, V.; Marriott, H.R. Alexa, she’s not human but… Unveiling the drivers of consumers’ trust in voice-based artificial intelligence. Psychol. Mark. 2021, 38, 626–642. [Google Scholar] [CrossRef]
- Graham, S.A.; Stein, N.; Shemaj, F.; Branch, O.H.; Paruthi, J.; Kanick, S.C. Older adults engage with personalized digital coaching programs at rates that exceed those of younger adults. Front. Digit. Health 2021, 3, 642818. [Google Scholar] [CrossRef]
- Kuerbis, A.; Mulliken, A.; Muench, F.; Moore, A.A.; Gardner, D. Older Adults and Mobile Technology: Factors that Enhance and Inhibit Utilization in the Context of Behavioral Health. 2017. Available online: https://academicworks.cuny.edu/hc_pubs/301/ (accessed on 6 November 2025).
- Kusal, S.; Patil, S.; Choudrie, J.; Kotecha, K.; Mishra, S.; Abraham, A. AI-based conversational agents: A scoping review from technologies to future directions. IEEE Access 2022, 10, 92337–92356. [Google Scholar] [CrossRef]
- Balsa, J.; Félix, I.; Cláudio, A.P.; Carmo, M.B.; Silva, I.C.E.; Guerreiro, A.; Guedes, M.; Henriques, A.; Guerreiro, M.P. Usability of an intelligent virtual assistant for promoting behavior change and self-care in older people with type 2 diabetes. J. Med. Syst. 2020, 44, 130. [Google Scholar] [CrossRef]
- Cheng, A.; Raghavaraju, V.; Kanugo, J.; Handrianto, Y.P.; Shang, Y. Development and evaluation of a healthy coping voice interface application using the Google home for elderly patients with type 2 diabetes. In Proceedings of the 2018 15th IEEE Annual Consumer Communications & Networking Conference (CCNC), Las Vegas, NV, USA, 12–15 January 2018; IEEE: Piscataway, NJ, USA, 2018. [Google Scholar]
- Peres, K.; Zamudio-Rodriguez, A.; Dartigues, J.-F.; Amieva, H.; Lafitte, S. Prospective pragmatic quasi-experimental study to assess the impact and effectiveness of an innovative large-scale public health intervention to foster healthy ageing in place: The SoBeezy program protocol. BMJ Open 2021, 11, e043082. [Google Scholar] [CrossRef]
- Striegl, J.; Gollasch, D.; Loitsch, C.; Weber, G. Designing vuis for social assistance robots for people with dementia. In Proceedings of Mensch und Computer 2021; Association for Computing Machinery: New York, NY, USA, 2021; pp. 145–155. [Google Scholar]
- Chen, C.; Johnson, J.G.; Charles, K.; Lee, A.; Lifset, E.T.; Hogarth, M.; Moore, A.A.; Farcas, E.; Weibel, N. Understanding barriers and design opportunities to improve healthcare and QOL for older adults through voice assistants. In Proceedings of the 23rd International ACM SIGACCESS Conference on Computers and Accessibility, Virtual, 18–22 October 2021; pp. 1–16. [Google Scholar]
- Van Acker, J.; Maenhout, L.; Compernolle, S. Older adults’ user engagement with mobile health: A systematic review of qualitative and mixed-methods studies. Innov. Aging 2023, 7, igad007. [Google Scholar] [CrossRef]
- Daniels, K.; Bonnechère, B. Harnessing digital health interventions to bridge the gap in prevention for older adults. Front. Public Health 2024, 11, 1281923. [Google Scholar] [CrossRef]
- Kraaijkamp, J.J.; van Dam van Isselt, E.F.; Persoon, A.; Versluis, A.; Chavannes, N.H.; Achterberg, W.P. eHealth in geriatric rehabilitation: Systematic review of effectiveness, feasibility, and usability. J. Med. Internet Res. 2021, 23, e24015. [Google Scholar] [CrossRef] [PubMed]
- Reiners, F.; Sturm, J.; Bouw, L.J.; Wouters, E.J. Sociodemographic factors influencing the use of eHealth in people with chronic diseases. Int. J. Environ. Res. Public Health 2019, 16, 645. [Google Scholar] [CrossRef]
- Chesser, A.; Burke, A.; Reyes, J.; Rohrberg, T. Navigating the digital divide: A systematic review of eHealth literacy in underserved populations in the United States. Inform. Health Soc. Care 2016, 41, 1–19. [Google Scholar] [CrossRef]
- Hasnan, S.; Aggarwal, S.; Mohammadi, L.; Koczwara, B. Barriers and enablers of uptake and adherence to digital health interventions in older patients with cancer: A systematic review. J. Geriatr. Oncol. 2022, 13, 1084–1091. [Google Scholar] [CrossRef]
- Choi, N.G.; DiNitto, D.M. The digital divide among low-income homebound older adults: Internet use patterns, eHealth literacy, and attitudes toward computer/Internet use. J. Med. Internet Res. 2013, 15, e93. [Google Scholar] [CrossRef]
- Hennink, M.M.; Kaiser, B.N.; Marconi, V.C. Code saturation versus meaning saturation: How many interviews are enough? Qual. Health Res. 2017, 27, 591–608. [Google Scholar] [CrossRef]
- Malterud, K.; Siersma, V.D.; Guassora, A.D. Sample size in qualitative interview studies: Guided by information power. Qual. Health Res. 2016, 26, 1753–1760. [Google Scholar] [CrossRef]

| DVA (n = 10) | |
|---|---|
| Age—mean (SD) | 67 (4) |
| Gender (female)—n (%) | 5 (50%) |
| Parents’ country of birth—n (%) | |
| Australia | 0 (0%) |
| Other | 5 (50%) |
| Not Answered | 5 (50%) |
| Highest level of education—n (%) | |
| Secondary/high school | 1 (10%) |
| Technical or further educational institution | 4 (40%) |
| University or other higher educational institution | 5 (50%) |
| Current employment status—n (%) | |
| Employed/self-employed full-time | 4 (40%) |
| Employed/self-employed part-time | 2 (20%) |
| Unemployed | 0 (0%) |
| Retired | 3 (30%) |
| Home duties | 0 (0%) |
| Pension (including disability or sole pension) | 1 (10%) |
| Medical conditions—n (%) | |
| Coronary heart disease a | 2 (20%) |
| Hypertension | 6 (60%) |
| Hypercholesterolaemia | 2 (20%) |
| Asthma | 3 (30%) |
| Chronic bronchitis or emphysema | 1 (10%) |
| Osteoarthritis | 1 (10%) |
| Other major illness b | 4 (40%) |
| Reported chronic health conditions other than T2DM | 10 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glavas, C.; Ma, J.; Sood, S.; George, E.S.; Daly, R.M.; Gvozdenko, E.; de Courten, B.; Scott, D.; Jansons, P. End-Users’ Perspectives on Implementation Outcomes of Digital Voice Assistants Delivering a Home-Based Lifestyle Intervention in Older Obese Adults with Type 2 Diabetes Mellitus: A Qualitative Analysis. Technologies 2025, 13, 511. https://doi.org/10.3390/technologies13110511
Glavas C, Ma J, Sood S, George ES, Daly RM, Gvozdenko E, de Courten B, Scott D, Jansons P. End-Users’ Perspectives on Implementation Outcomes of Digital Voice Assistants Delivering a Home-Based Lifestyle Intervention in Older Obese Adults with Type 2 Diabetes Mellitus: A Qualitative Analysis. Technologies. 2025; 13(11):511. https://doi.org/10.3390/technologies13110511
Chicago/Turabian StyleGlavas, Costas, Jiani Ma, Surbhi Sood, Elena S. George, Robin M. Daly, Eugene Gvozdenko, Barbora de Courten, David Scott, and Paul Jansons. 2025. "End-Users’ Perspectives on Implementation Outcomes of Digital Voice Assistants Delivering a Home-Based Lifestyle Intervention in Older Obese Adults with Type 2 Diabetes Mellitus: A Qualitative Analysis" Technologies 13, no. 11: 511. https://doi.org/10.3390/technologies13110511
APA StyleGlavas, C., Ma, J., Sood, S., George, E. S., Daly, R. M., Gvozdenko, E., de Courten, B., Scott, D., & Jansons, P. (2025). End-Users’ Perspectives on Implementation Outcomes of Digital Voice Assistants Delivering a Home-Based Lifestyle Intervention in Older Obese Adults with Type 2 Diabetes Mellitus: A Qualitative Analysis. Technologies, 13(11), 511. https://doi.org/10.3390/technologies13110511








