An Open-Source Neonatal Phototherapy Device
Abstract
1. Introduction
2. Materials and Methods
- (a)
- The structural body including the 3D-printed electronic housings, PVC pipes, and joints connecting the pipes.
- (b)
- The microprocessor, power delivery, and interface electronics.
- (c)
- The light source and heat dissipation assembly.
3. Results
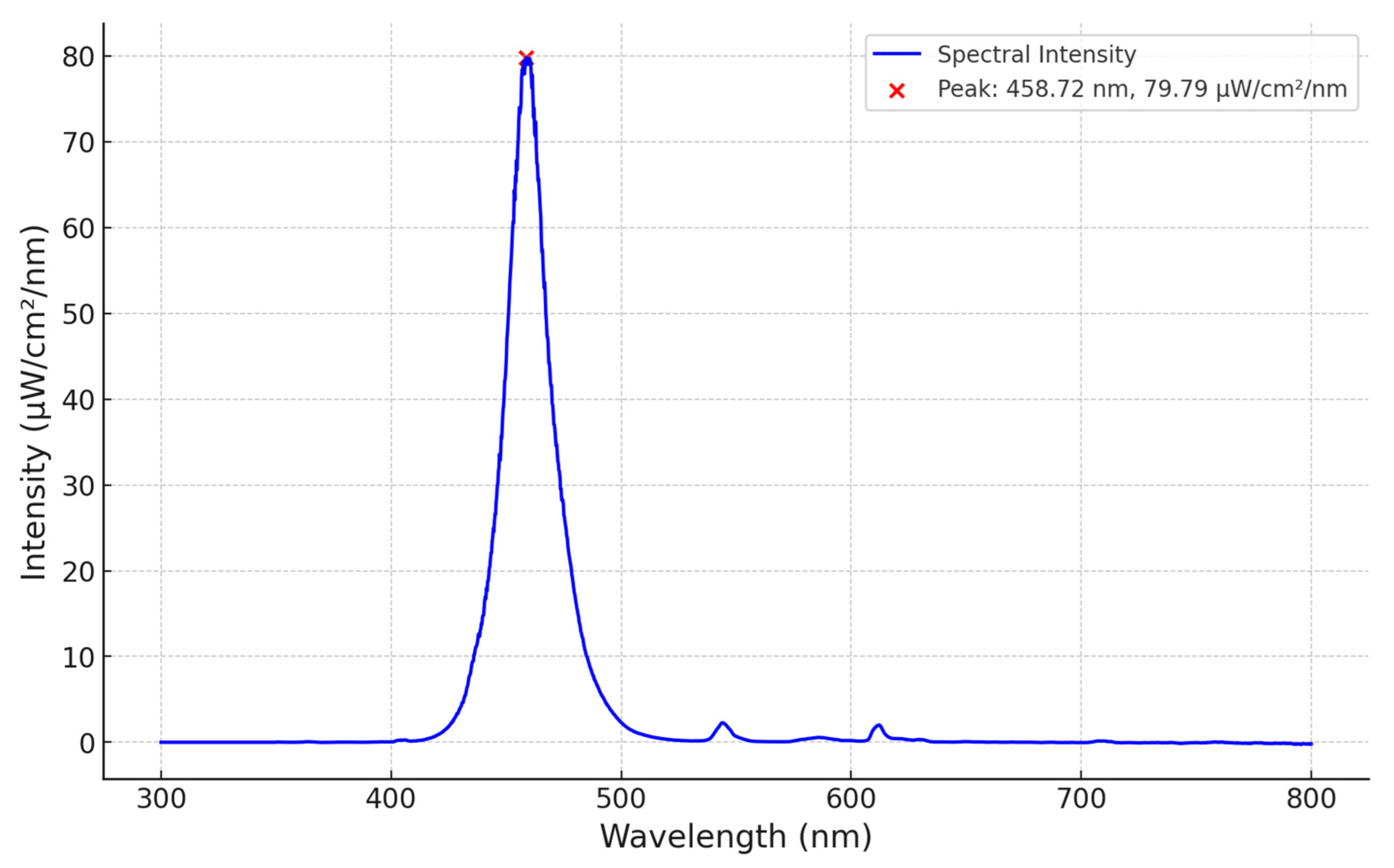
3.1. Spectral Analyses
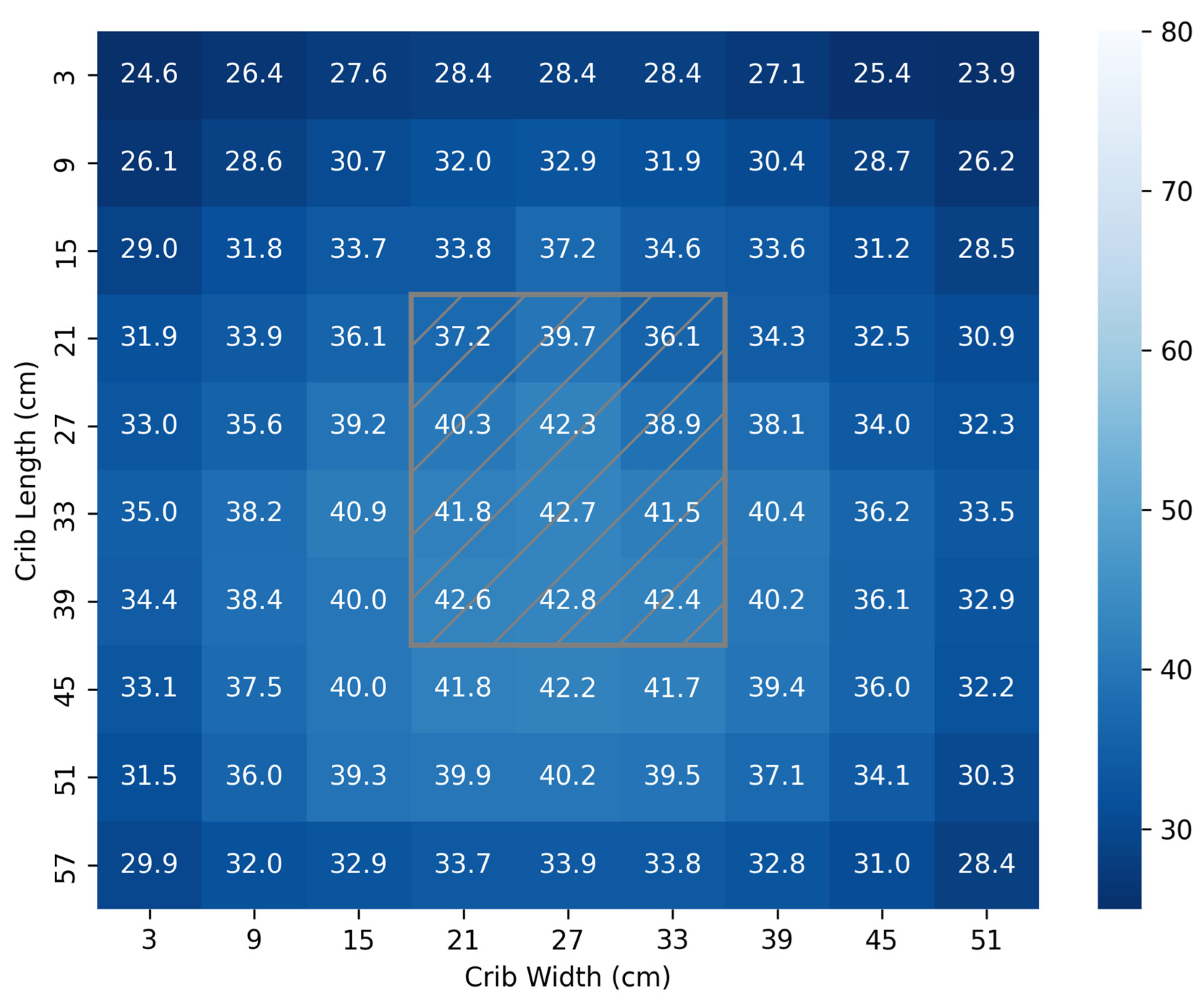
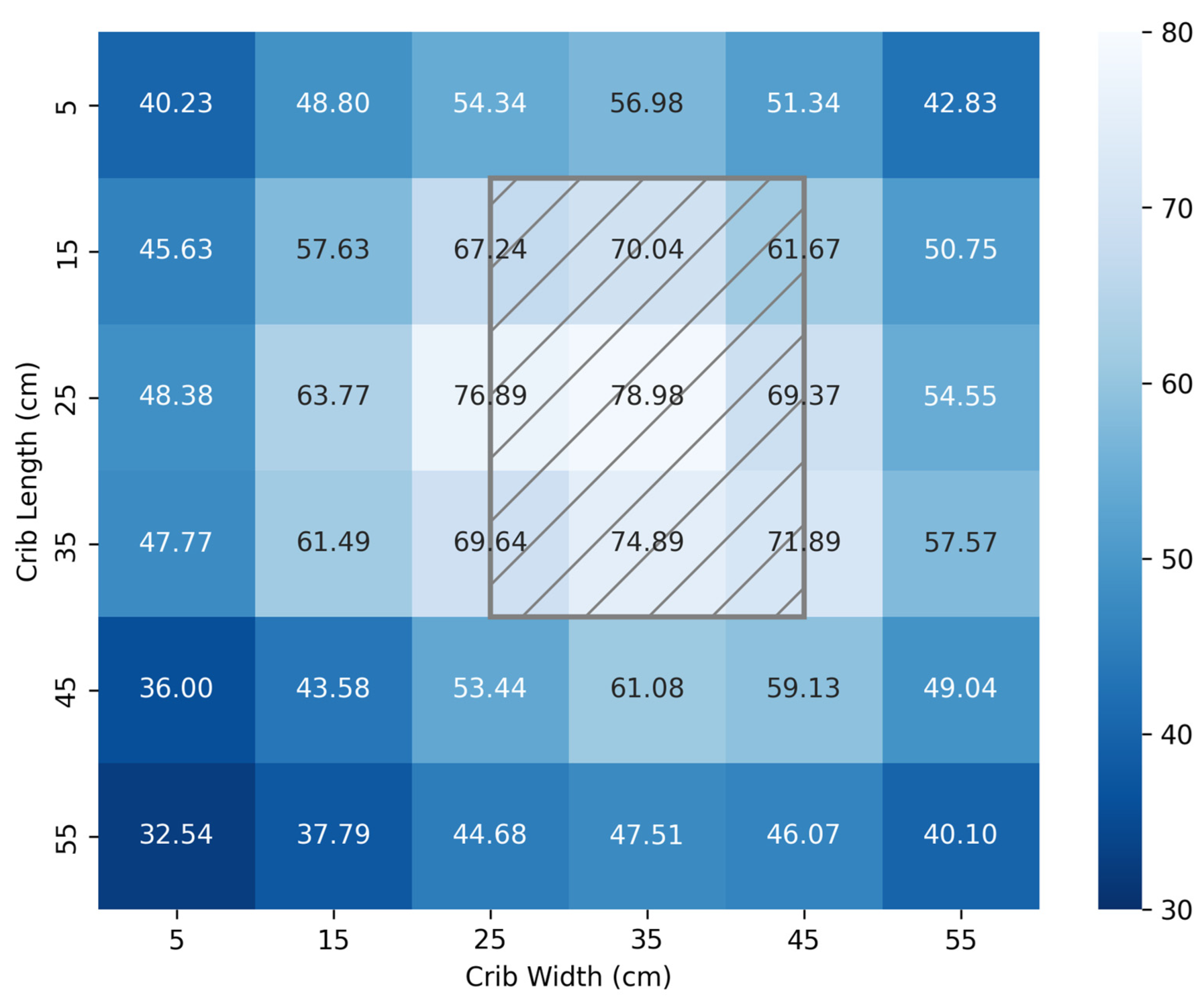
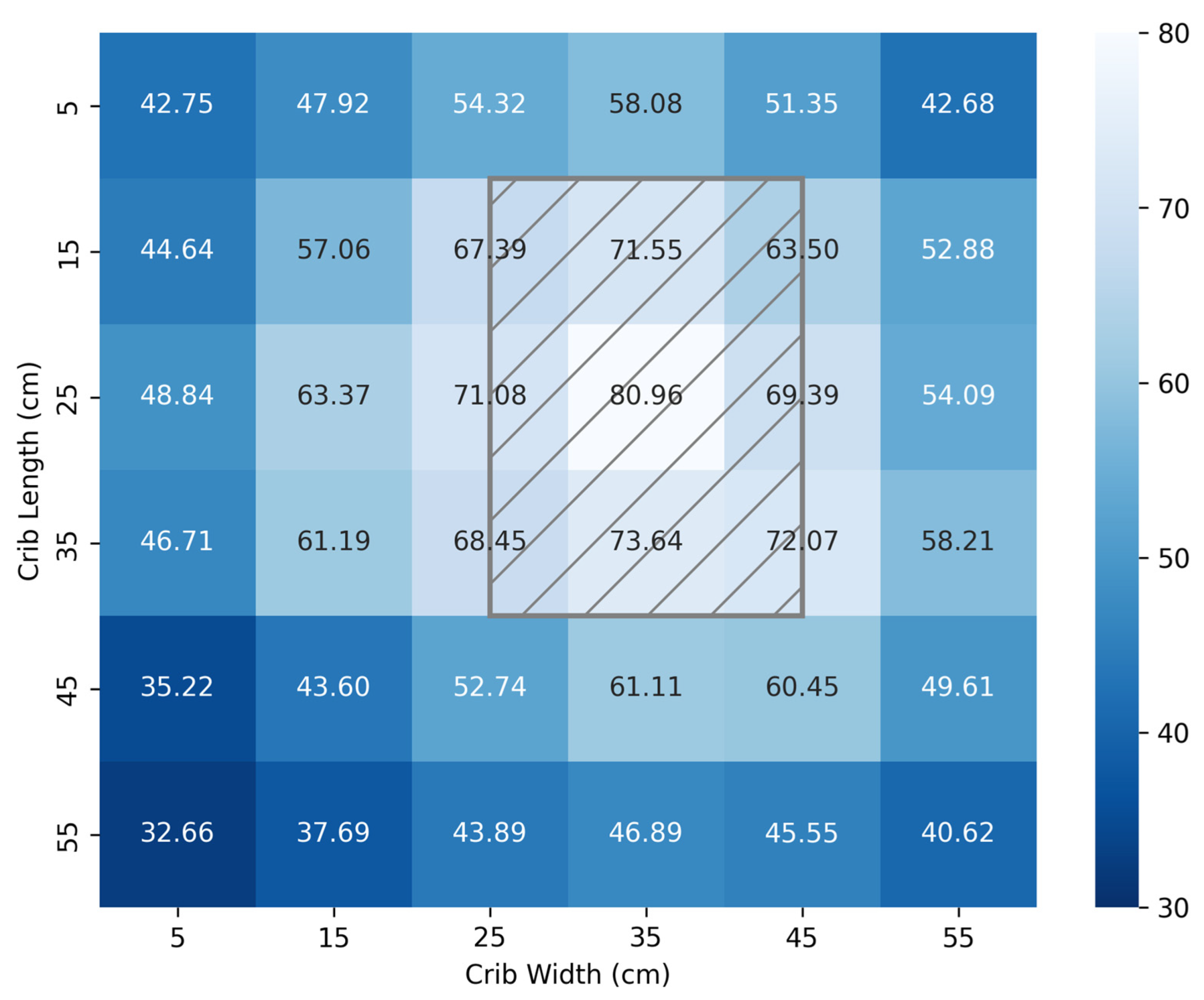
3.2. Irradiance Footprint
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Neonatal Phototherapy Device Bill of Materials
Appendix B. Build Documentation
Appendix B.1. Structural Sub-Assembly
| ID | Description | Length (cm) |
|---|---|---|
| A | T-Connector to Light Source Housing | ~45 |
| B | Electronics Housing to T-Connector | ~45 |
| C | Base to Electronics Housing | ~70–130 |
| D–G | Four Legs | ~45 |




Appendix B.2. Electronics Sub-Assembly
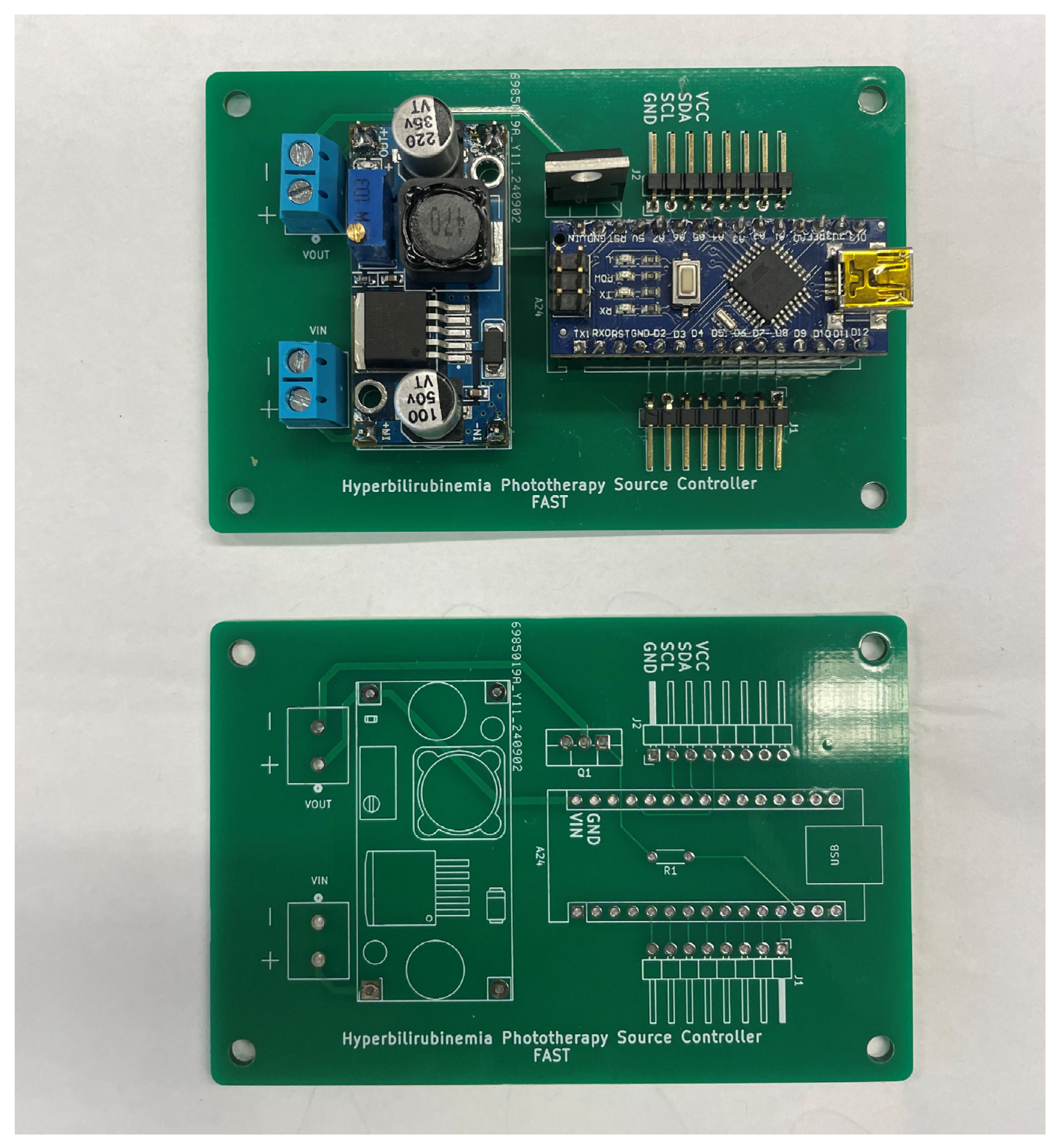
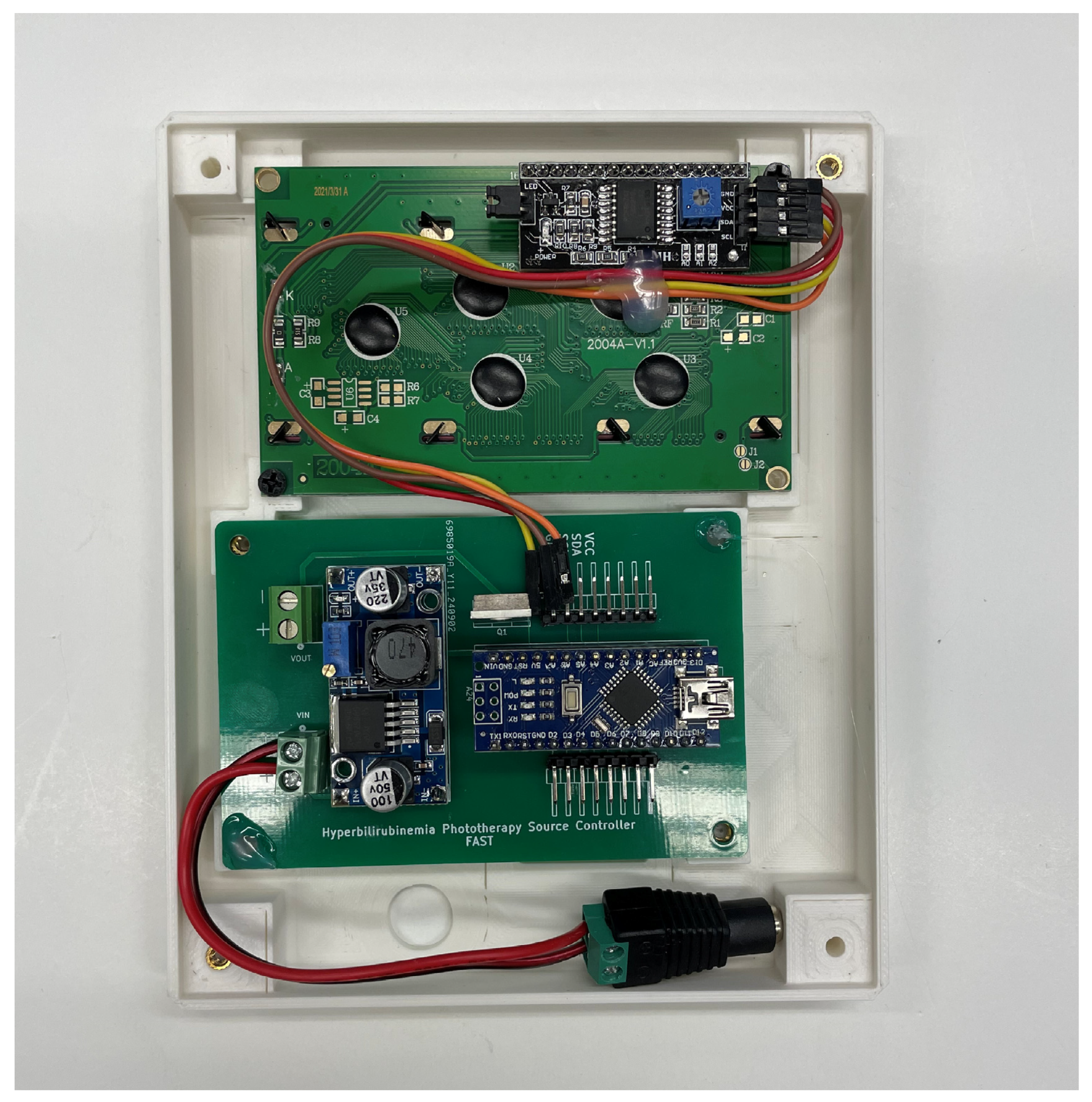
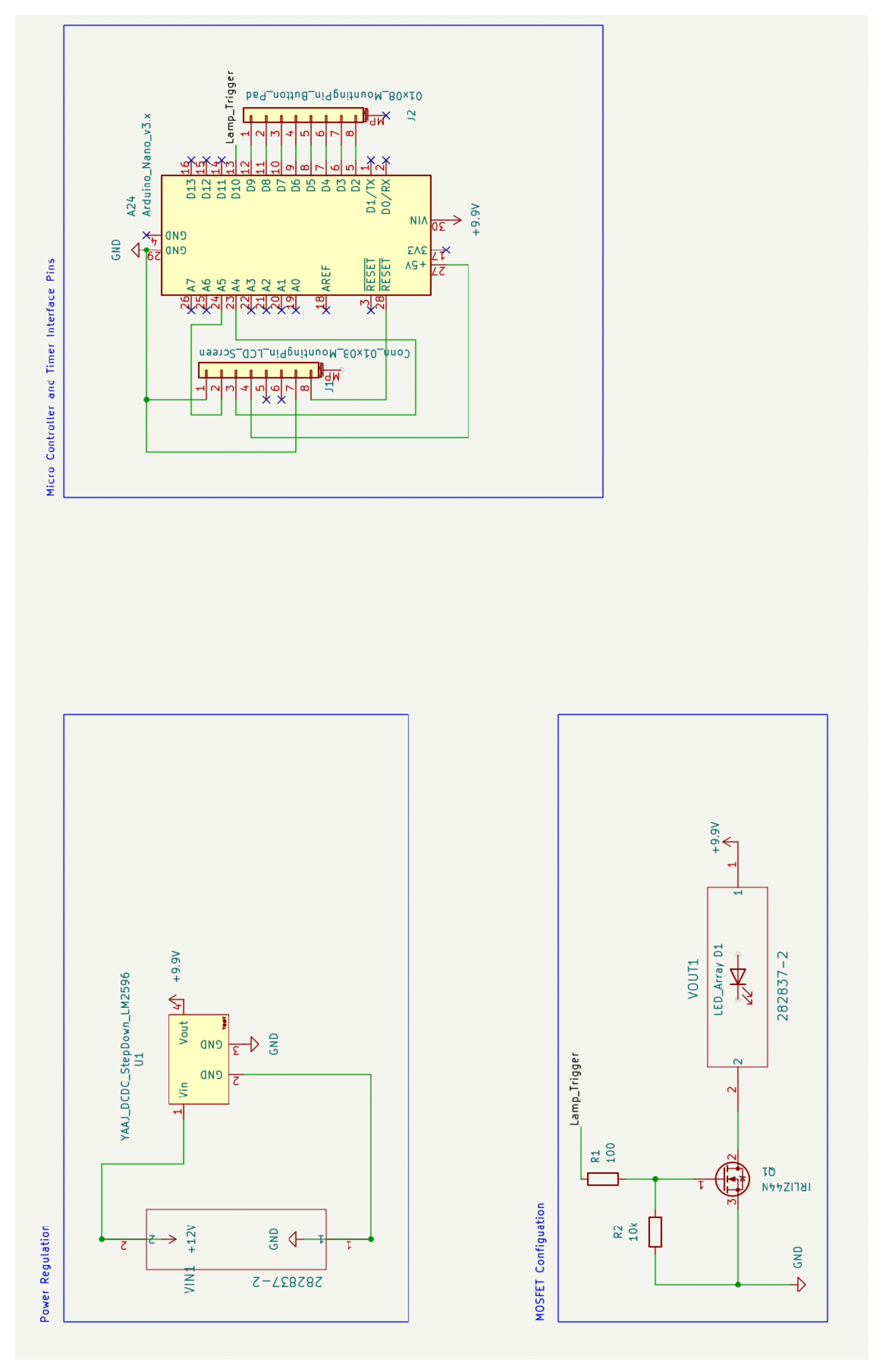

Appendix B.3. Lamp Source Sub-Assembly

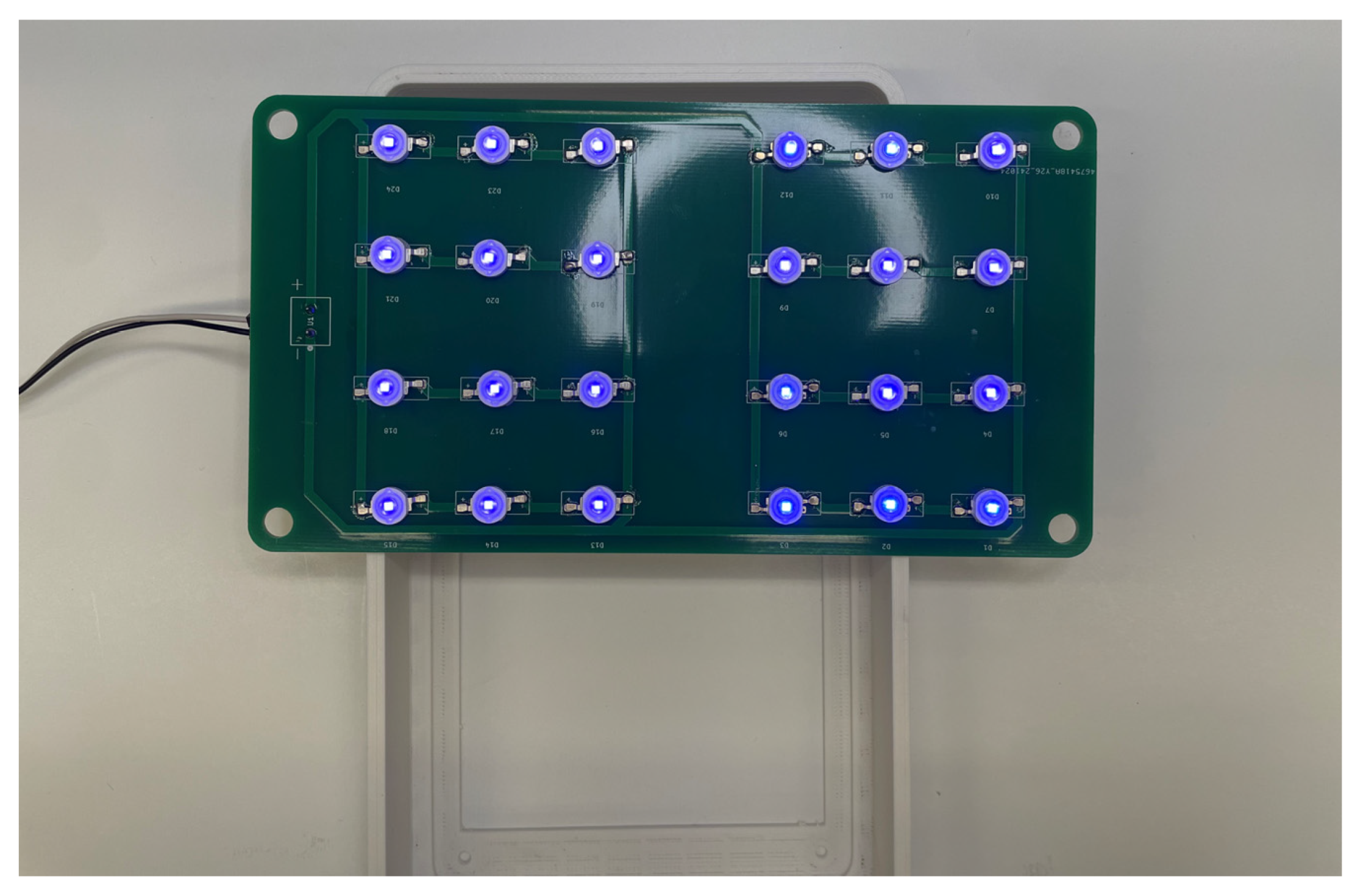

Appendix B.4. Final Assembly

Appendix C. User Interface Guide



References
- Bhutani, V.K.; Zipursky, A.; Blencowe, H.; Khanna, R.; Sgro, M.; Ebbesen, F.; Bell, J.; Mori, R.; Slusher, T.M.; Fahmy, N.; et al. Neonatal Hyperbilirubinemia and Rhesus Disease of the Newborn: Incidence and Impairment Estimates for 2010 at Regional and Global Levels. Pediatr. Res. 2013, 74, 86–100. [Google Scholar] [CrossRef]
- Diala, U.M.; Usman, F.; Appiah, D.; Hassan, L.; Ogundele, T.; Abdullahi, F.; Satrom, K.M.; Bakker, C.J.; Lee, B.W.; Slusher, T.M. Global Prevalence of Severe Neonatal Jaundice among Hospital Admissions: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3738. [Google Scholar] [CrossRef]
- Kasirer, Y.; Kaplan, M.; Hammerman, C. Kernicterus on the Spectrum. NeoReviews 2023, 24, e329–e342. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Kaplan, M.; Hansen, T.W.R. Neonatal Hyperbilirubinaemia: A Global Perspective. Lancet Child Adolesc. Health 2018, 2, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Slusher, T.M.; Zamora, T.G.; Appiah, D.; Stanke, J.U.; Strand, M.A.; Lee, B.W.; Richardson, S.B.; Keating, E.M.; Siddappa, A.M.; Olusanya, B.O. Burden of Severe Neonatal Jaundice: A Systematic Review and Meta-Analysis. BMJ Paediatr. Open 2017, 1, e000105. [Google Scholar] [CrossRef] [PubMed]
- Satrom, K.M.; Farouk, Z.L.; Slusher, T.M. Management Challenges in the Treatment of Severe Hyperbilirubinemia in Low- and Middle-Income Countries: Encouraging Advancements, Remaining Gaps, and Future Opportunities. Front. Pediatr. 2023, 11, 1001141. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Ogunlesi, T.A.; Slusher, T.M. Why Is Kernicterus Still a Major Cause of Death and Disability in Low-Income and Middle-Income Countries? Arch. Dis. Child. 2014, 99, 1117–1121. [Google Scholar] [CrossRef]
- neoBLUE® LED Phototherapy System. Available online: https://natus.com/sensory/neoblue-led-phototherapy-system/ (accessed on 24 November 2024).
- neoBLUE® Compact LED Phototherapy System for Newborn Jaundice. Available online: https://natus.com/sensory/neoblue-compact/ (accessed on 24 November 2024).
- Pocket Nurse® Infant Phototherapy Unit for Simulation. Available online: https://emrn.ca/pocket-nurse-infant-phototherapy-unit-for-simulation/ (accessed on 24 November 2024).
- Bistos BT-400 Neonatal Blue LED Phototherapy Equipment. Available online: https://medlabamerica.com/products/bistos-bt-400-neonatal-blue-led-phototherapy-equipment (accessed on 12 September 2025).
- Bistos BT-550 Infant Warmer w/LCD Display. Available online: https://medlabamerica.com/products/bistos-bt-550-infant-warmer (accessed on 12 September 2025).
- Coker, A.; Achi, C.; Idowu, O.; Olayinka, O.; Oturu, K. Remanufacture of Medical Equipment: A Viable Sustainable Strategy for Medical Waste Reduction. In Proceedings of the International Conference on Remanufacturing, Brighton, UK, 24–25 May 2021; Available online: https://www.researchgate.net/publication/361969995_Remanufacture_of_Medical_Equipment_A_viable_sustainable_strategy_for_medical_waste_reduction (accessed on 12 September 2025).
- Ademe, B.W.; Tebeje, B.; Molla, A. Availability and Utilization of Medical Devices in Jimma Zone Hospitals, Southwest Ethiopia: A Case Study. BMC Health Serv. Res. 2016, 16, 287. [Google Scholar] [CrossRef]
- De Maria, C.; Di Pietro, L.; Ravizza, A.; Lantada, A.D.; Ahluwalia, A.D. Chapter 2—Open-Source Medical Devices: Healthcare Solutions for Low-, Middle-, and High-Resource Settings. In Clinical Engineering Handbook, 2nd ed.; Iadanza, E., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 7–14. ISBN 978-0-12-813467-2. [Google Scholar]
- Otero, J.; Pearce, J.M.; Gozal, D.; Farré, R. Open-Source Design of Medical Devices. Nat. Rev. Bioeng. 2024, 2, 280–281. [Google Scholar] [CrossRef]
- Pearce, J.M. Distributed Manufacturing of Open Source Medical Hardware for Pandemics. J. Manuf. Mater. Process. 2020, 4, 49. [Google Scholar] [CrossRef]
- Gibb, A. Building Open Source Hardware: DIY Manufacturing for Hackers and Makers; Addison-Wesley Professional: Boston, MA, USA, 2014; ISBN 978-0-13-337390-5. [Google Scholar]
- Oberloier, S.; Pearce, J.M. General Design Procedure for Free and Open-Source Hardware for Scientific Equipment. Designs 2018, 2, 2. [Google Scholar] [CrossRef]
- Jones, R.; Haufe, P.; Sells, E.; Iravani, P.; Olliver, V.; Palmer, C.; Bowyer, A. RepRap—The Replicating Rapid Prototyper. Robotica 2011, 29, 177–191. [Google Scholar] [CrossRef]
- Sells, E.; Bailard, S.; Smith, Z.; Bowyer, A.; Olliver, V. RepRap: The Replicating Rapid Prototyper: Maximizing Customizability by Breeding the Means of Production. In Handbook of Research in Mass Customization and Personalization; World Scientific Publishing Company: Singapore, 2009; pp. 568–580. ISBN 978-981-4280-25-9. [Google Scholar]
- Selvaraj, A.; Kulkarni, A.; Pearce, J.M. Open-Source 3-D Printable Autoinjector: Design, Testing, and Regulatory Limitations. PLoS ONE 2023, 18, e0288696. [Google Scholar] [CrossRef]
- Newharvest-Incubator-Perfusion/README.Md at Main IRNAS/Newharvest-Incubator-Perfusion. Available online: https://github.com/IRNAS/newharvest-incubator-perfusion/blob/main/README.md (accessed on 1 December 2024).
- Collins, J.T.; Knapper, J.; Stirling, J.; Mduda, J.; Mkindi, C.; Mayagaya, V.; Mwakajinga, G.A.; Nyakyi, P.T.; Sanga, V.L.; Carbery, D.; et al. Robotic Microscopy for Everyone: The OpenFlexure Microscope. Biomed Opt Express 2020, 11, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. Cut Costs with Open-Source Hardware. Nature 2014, 505, 618. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. Economic Savings for Scientific Free and Open Source Technology: A Review. HardwareX 2020, 8, e00139. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, V.K.; the Committee on Fetus and Newborn. Phototherapy to Prevent Severe Neonatal Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2011, 128, e1046–e1052. [Google Scholar] [CrossRef]
- Bhutani, V.K.; Wong, R.J.; Turkewitz, D.; Rauch, D.A.; Mowitz, M.E.; Barfield, W.D.; Eichenwald, E.; Ambalavanan, N.; Guillory, C.; Committee on Fetus & Newborn. Phototherapy to Prevent Severe Neonatal Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation: Technical Report. Pediatrics 2024, 154, e2024068026. [Google Scholar] [CrossRef]
- Itoh, S.; Okada, H.; Kuboi, T.; Kusaka, T. Phototherapy for Neonatal Hyperbilirubinemia. Pediatr. Int. 2017, 59, 959–966. [Google Scholar] [CrossRef]
- Ansong-Assoku, B.; Shah, S.D.; Adnan, M.; Ankola, P.A. Neonatal Jaundice. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kumar, P.; Chawla, D.; Deorari, A. Light-Emitting Diode Phototherapy for Unconjugated Hyperbilirubinaemia in Neonates. Cochrane Database Syst. Rev. 2011, 2011, CD007969. [Google Scholar] [CrossRef]
- Abe, S.; Fujioka, K. Can Exchange Transfusion Be Replaced by Double-LED Phototherapy? Open Med. 2021, 16, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.R.; Lu, H.; McClary, J.; Marasch, J.; Nock, M.L.; Ryan, R.M. Evaluation of Intravenous Immunoglobulin Administration for Hyperbilirubinemia in Newborn Infants with Hemolytic Disease. Children 2023, 10, 496. [Google Scholar] [CrossRef]
- Tan, K.L. Comparison of the Effectiveness of Phototherapy and Exchange Transfusion in the Management of Nonhemolytic Neonatal Hyperbilirubinemia. J. Pediatr. 1975, 87, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Givans, J.T.M.; Waswa, A.; Madete, J.; Pearce, J.M. Open-Source Light Calibration System for Hyperbilirubinemia Phototherapy Treatments 2025. Available online: https://doi.org/10.1101/2025.08.01.25332669 (accessed on 24 October 2025).
- Photo Therapy Unit,w/Access. Available online: https://supply.unicef.org/s0002032.html (accessed on 16 November 2024).
- Wentworth, S.D. Neonatal Phototherapy–Today’s Lights, Lamps and Devices. Infant 2005, 1, 14–19. [Google Scholar]
- SR2. Available online: https://www.oceanoptics.com/spectrometer/sr2/ (accessed on 12 November 2024).
- Trevisanello, L.-R.; Meneghini, M.; Mura, G.; Sanna, C.; Buso, S.; Spiazzi, G.; Vanzi, M.; Meneghesso, G.; Zanoni, E. Thermal Stability Analysis of High Brightness LED during High Temperature and Electrical Aging. Proc. SPIE Int. Soc. Opt. Eng. 2007, 6669, 666913. [Google Scholar]
- Wang, J.; Guo, G.; Li, A.; Cai, W.-Q.; Wang, X. Challenges of Phototherapy for Neonatal Hyperbilirubinemia (Review). Exp. Ther. Med. 2021, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Sisson, T.R.C. Molecular Basis of Hyperbilirubinemia and Phototherapy. J. Investig. Dermatol. 1981, 77, 158–161. [Google Scholar] [CrossRef]
- Sisson, T.R.C. Visible Light Therapy of Neonatal Hyperbilirubinemia. In Photochemical and Photobiological Reviews: Volume 1; Smith, K.C., Ed.; Springer US: Boston, MA, USA, 1976; pp. 241–268. ISBN 978-1-4684-2574-1. [Google Scholar]
- Hansen, T.W.R. Kernicterus: An International Perspective. Semin. Neonatol. 2002, 7, 103–109. [Google Scholar] [CrossRef]
- Das, S.; van Landeghem, F.K.H. Clinicopathological Spectrum of Bilirubin Encephalopathy/Kernicterus. Diagnostics 2019, 9, 24. [Google Scholar] [CrossRef]
- Muchowski, K.E. Evaluation and Treatment of Neonatal Hyperbilirubinemia. Am. Fam. Physician 2014, 89, 873–878. [Google Scholar]
- Porter, M.L.; Dennis, B.L. Hyperbilirubinemia in the Term Newborn. Am. Fam. Physician 2002, 65, 599–607. [Google Scholar]
- Maisels, M.J.; McDonagh, A.F. Phototherapy for Neonatal Jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.G.; Ethan, M.T.; Hendrik, J.V.; Tina, M.S. Neonatal Hyperbilirubinemia in Low-Income African Countries. Int. J. Pediatr. Res. 2021, 7, 073. [Google Scholar] [CrossRef]
- Slusher, T.M.; Zipursky, A.; Bhutani, V.K. A Global Need for Affordable Neonatal Jaundice Technologies. Semin. Perinatol. 2011, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Cline, B.K.; Vreman, H.J.; Faber, K.; Lou, H.; Donaldson, K.M.; Amuabunosi, E.; Ofovwe, G.; Bhutani, V.K.; Olusanya, B.O.; Slusher, T.M. Phototherapy Device Effectiveness in Nigeria: Irradiance Assessment and Potential for Improvement. J. Trop. Pediatr. 2013, 59, 321–325. [Google Scholar] [CrossRef]

| Device | Cost (CAD) | Ref. |
|---|---|---|
| NEOBLUE OVERHEAD W/ROLLSTAND | 11,685.00 | [8] |
| NEOBLUE COMPACT LED PHOTOTHERAPY SYSTEM | 4315.00 | [9] |
| Pocket Nurse Infant Phototherapy Unit | 2046.02 | [10] |
| Bistos BT-400 Neonatal Blue LED Phototherapy Equipment | 1265.39 | [11] |
| Bistos BT-550 Infant Warmer w/LCD Display | 6481.13 | [12] |
| Device | Distance to Patient (cm) | Footprint Area (Length × Width, cm2) | Spectrum, Total (nm) | Bandwidth (nm) | Peak (nm) | Footprint Irradiance (μW/cm2/nm) Max | |||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | |||||||
| LED | neoBLUE | 30 | 1152 (48 × 24) | 420–540 | 20 | 462 | 12 | 37 | 30 ± 7 |
| PortaBed | ≥5 | 1740 (30 × 58) | 425–540 | 27 | 463 | 14 | 70 | 67 ± 8 | |
| FAST NPTD | 30–45 | 3600 (60 × 54) | 420–500 | 22 | 459 | 23.9 | 43 | 35 ± 4 | |
| Fluorescent | BiliLITE CW/BB | 45 | 2928 (48 × 61) | 380–720 | 68 | 578 | 6 | 10 | 10 ± 1 |
| BiliLITE BB | 45 | 2928 (48 × 61) | 400–550 | 55 | 437 | 11 | 17 | 17 ± 2 | |
| BiliLITE TL52 | 45 | 2928 (48 × 61) | 400–550 | 35 | 437 | 8 | 13 | 13 ± 2 | |
| BiliBed | 0 | 693 (21 × 33) | 400–580 | 80 | 450 | 4 | 8 | 8 ± 2 | |
| Halogen | MiniBiliite | 45 | 490 (25 diam) | 350–800 | 190 | 500 | <1 | 3 | 3 ± 1 |
| Phototherapy Lite | 45 | 490 (25 diam) | 370–850 | 200 | 590 | <1 | 17 | 7 ± 5 | |
| Distance from Light (cm) | 3D-Printed Light Meter (µW/cm2/nm) | MTTS Light Meter (µW/cm2/nm) |
|---|---|---|
| 10 | 34.28 | 35 |
| 15 | 17.64 | 21 |
| 20 | 13.11 | 14 |
| 25 | 10.40 | 10 |
| 30 | 9.60 | 7 |
| 40 | 4.68 | 4 |
| 50 | 3.26 | 3 |
| 60 | 2.65 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Givans, J.; Waswa, A.; Nyambura, J.; Njoroge, G.; Macharia, G.; Madete, J.; Pearce, J.M. An Open-Source Neonatal Phototherapy Device. Technologies 2025, 13, 499. https://doi.org/10.3390/technologies13110499
Givans J, Waswa A, Nyambura J, Njoroge G, Macharia G, Madete J, Pearce JM. An Open-Source Neonatal Phototherapy Device. Technologies. 2025; 13(11):499. https://doi.org/10.3390/technologies13110499
Chicago/Turabian StyleGivans, Joshua, Augustine Waswa, Janiffer Nyambura, Gidraf Njoroge, Gordon Macharia, June Madete, and Joshua M. Pearce. 2025. "An Open-Source Neonatal Phototherapy Device" Technologies 13, no. 11: 499. https://doi.org/10.3390/technologies13110499
APA StyleGivans, J., Waswa, A., Nyambura, J., Njoroge, G., Macharia, G., Madete, J., & Pearce, J. M. (2025). An Open-Source Neonatal Phototherapy Device. Technologies, 13(11), 499. https://doi.org/10.3390/technologies13110499







