Non-Invasive Multimodal and Multiscale Bioelectrical Sensor System for Proactive Holistic Plant Assessment
Abstract
1. Introduction
- Unified platform development: Integrate multiple electrode configurations and operational modes, enabling both spectroscopy and tomography within a single deployable system.
- Organ-specific optimization: Establish a systematic methodology for identifying optimal frequency ranges and extracting physiologically meaningful features for each organ.
- Physiological validation framework: Correlate bioelectrical features with established reference metrics across all organs.
- Tomographic capability: Develop both 2D and 3D conductivity imaging for spatial physiological assessment.
- Proof-of-concept demonstration: Validate the integrated system on strawberry, establishing a transferable methodology for broader agricultural applications.
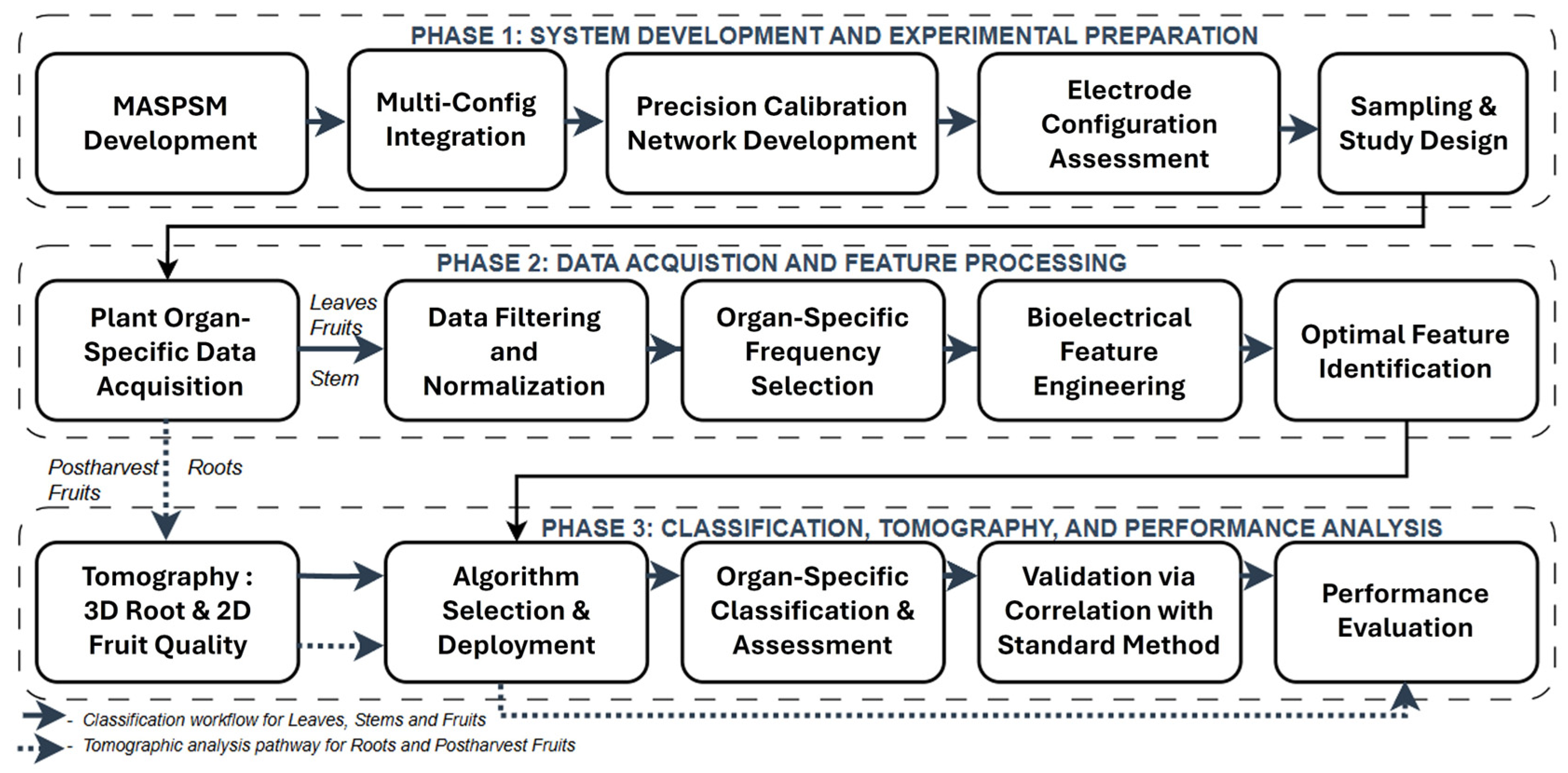
2. Materials and Methods
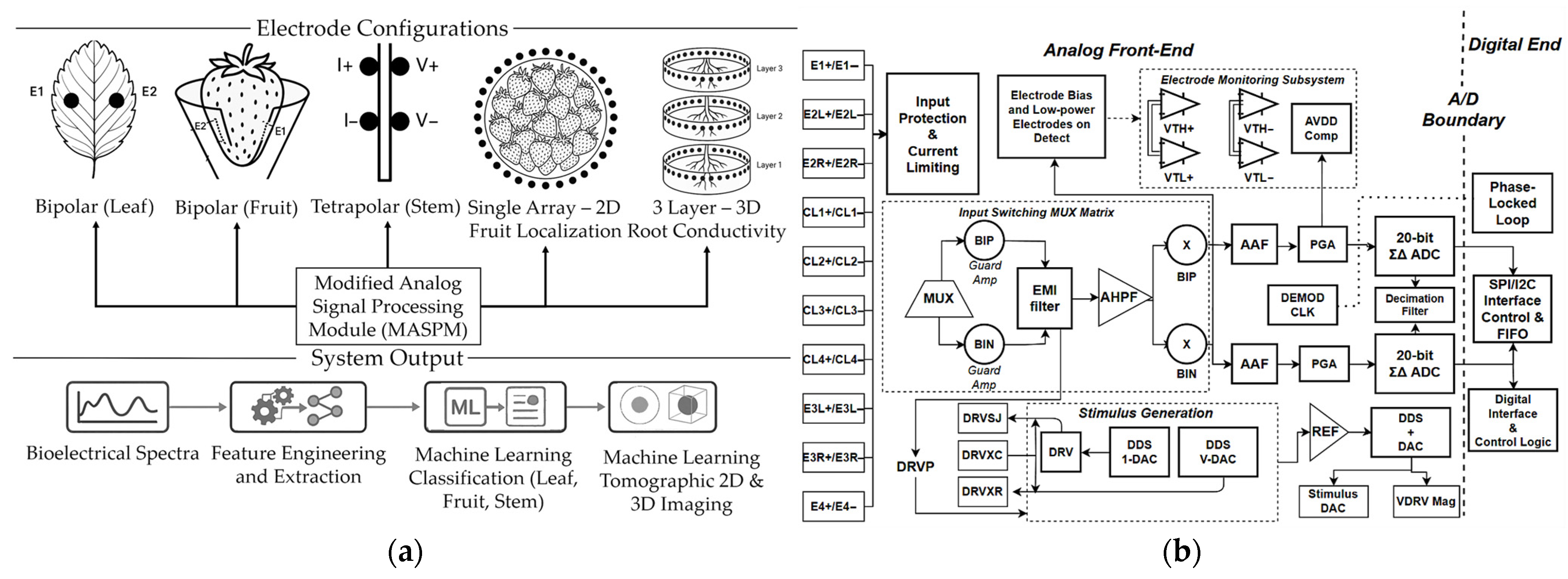
2.1. Modified Analog Signal-Processing Module for Multi-Configuration Integration
2.2. Multimodal Hardware Integration for Spectroscopic and Tomographic Operation
2.3. Development of Calibration Network for Multi-Configuration and Multimodal Operation
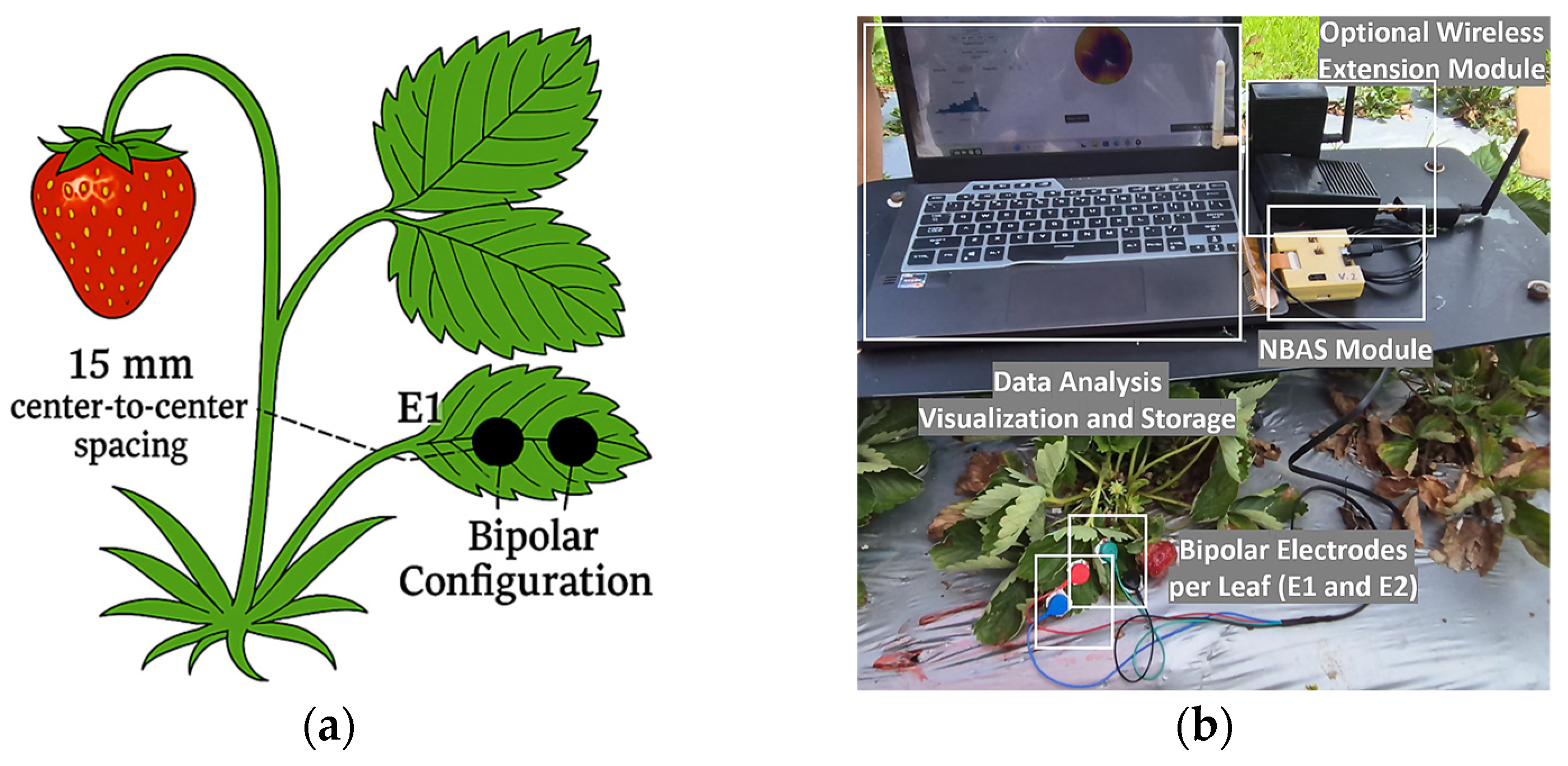
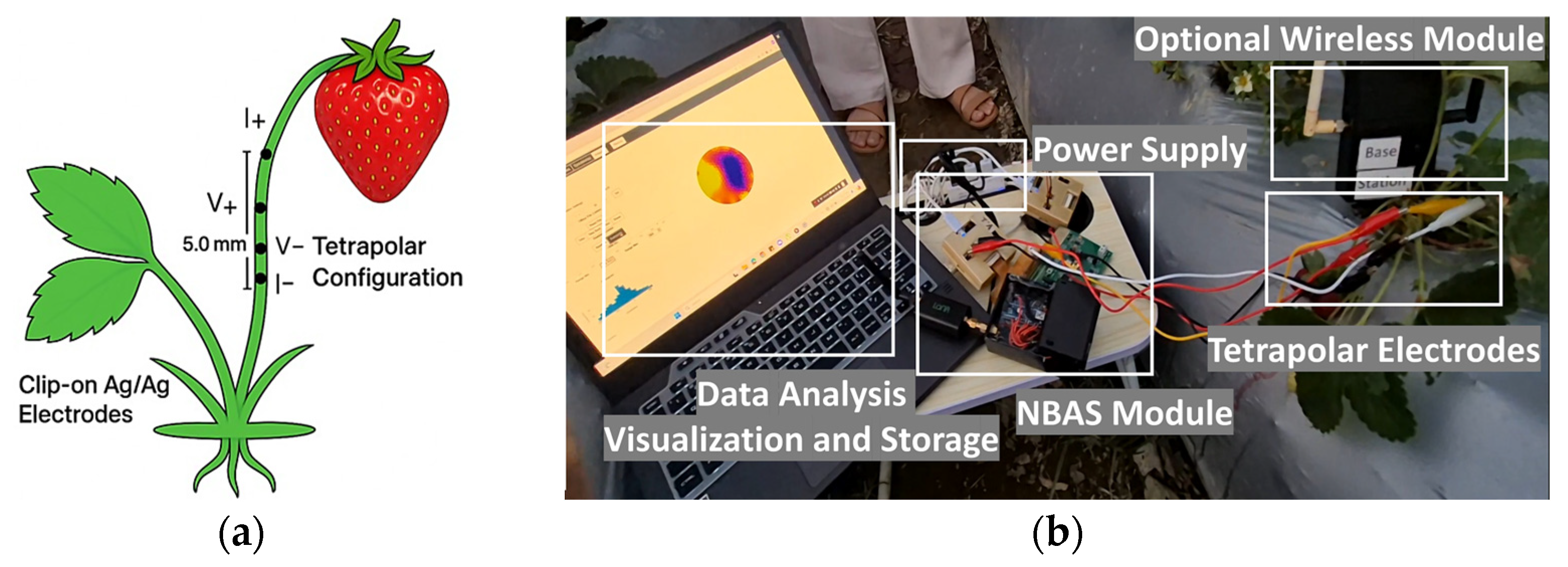
2.4. Electrode Design, Selection, and Performance Evaluation
2.5. Sample Collection and Experimental Design
2.6. Bioelectrical Measurements Organ-Specific Protocols
2.7. Data Preprocessing Filtering and Normalization
2.8. Key Frequency Selection and Statistical Validation of Organ-Specific Ranges
2.9. Bioelectrical Feature Extraction for Physiological Interpretation
2.10. Optimal Parameter Identification and Feature Selection
2.11. Multi-Frequency Tomographic Data Acquisition Method
2.12. Algorithm Selection and Implementation
2.13. System Validation and Physiological Correlation
3. Results
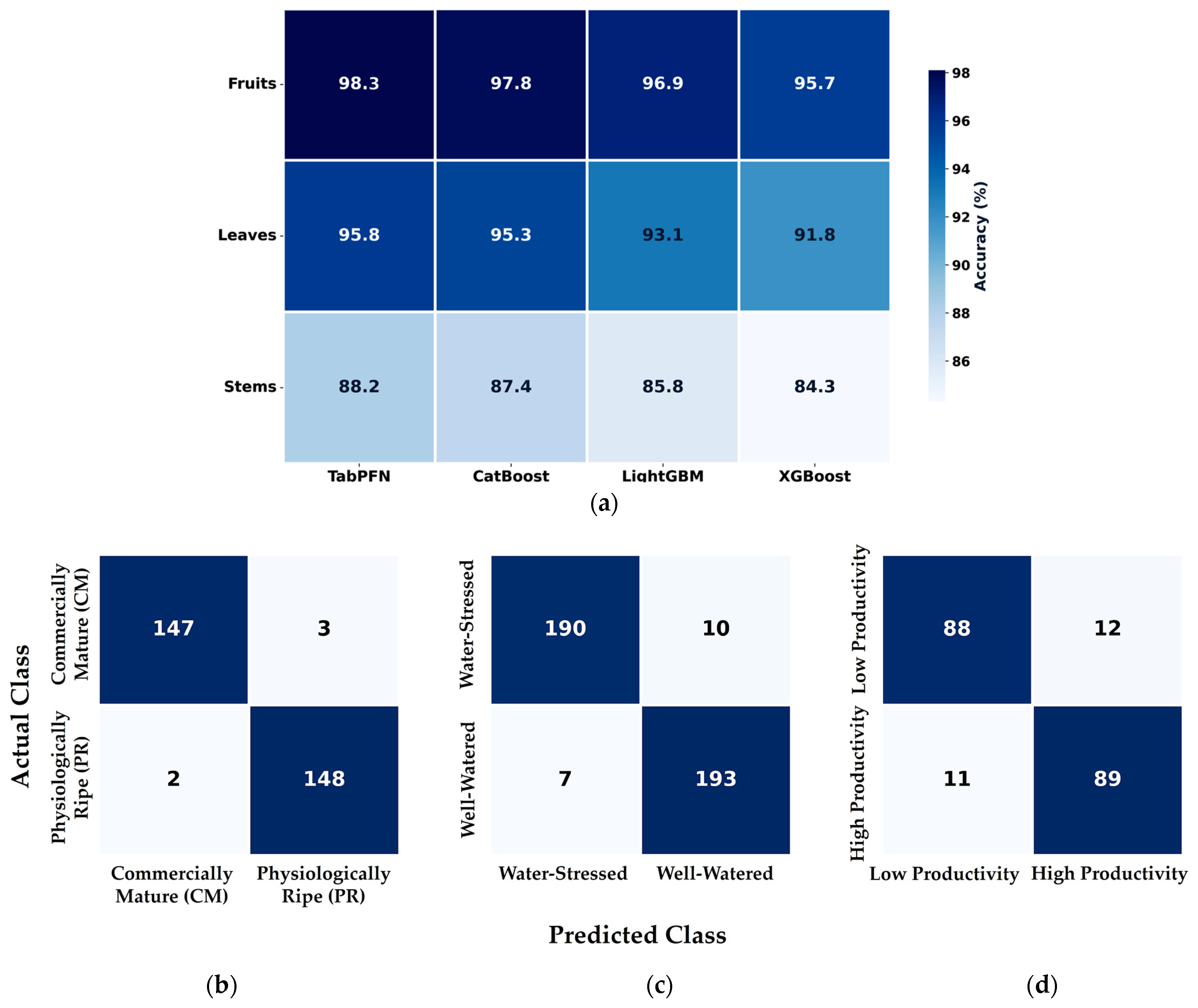
3.1. Comparative Performance of Electrode Types and Configurations for Organ-Specific Measurements
3.2. Optimal Multi-Scale Frequency Across Plant Organs
3.3. Optimal Features for Classification
3.4. Performance Matrix and Confusion Matrices for Classification
3.5. Correlation Results
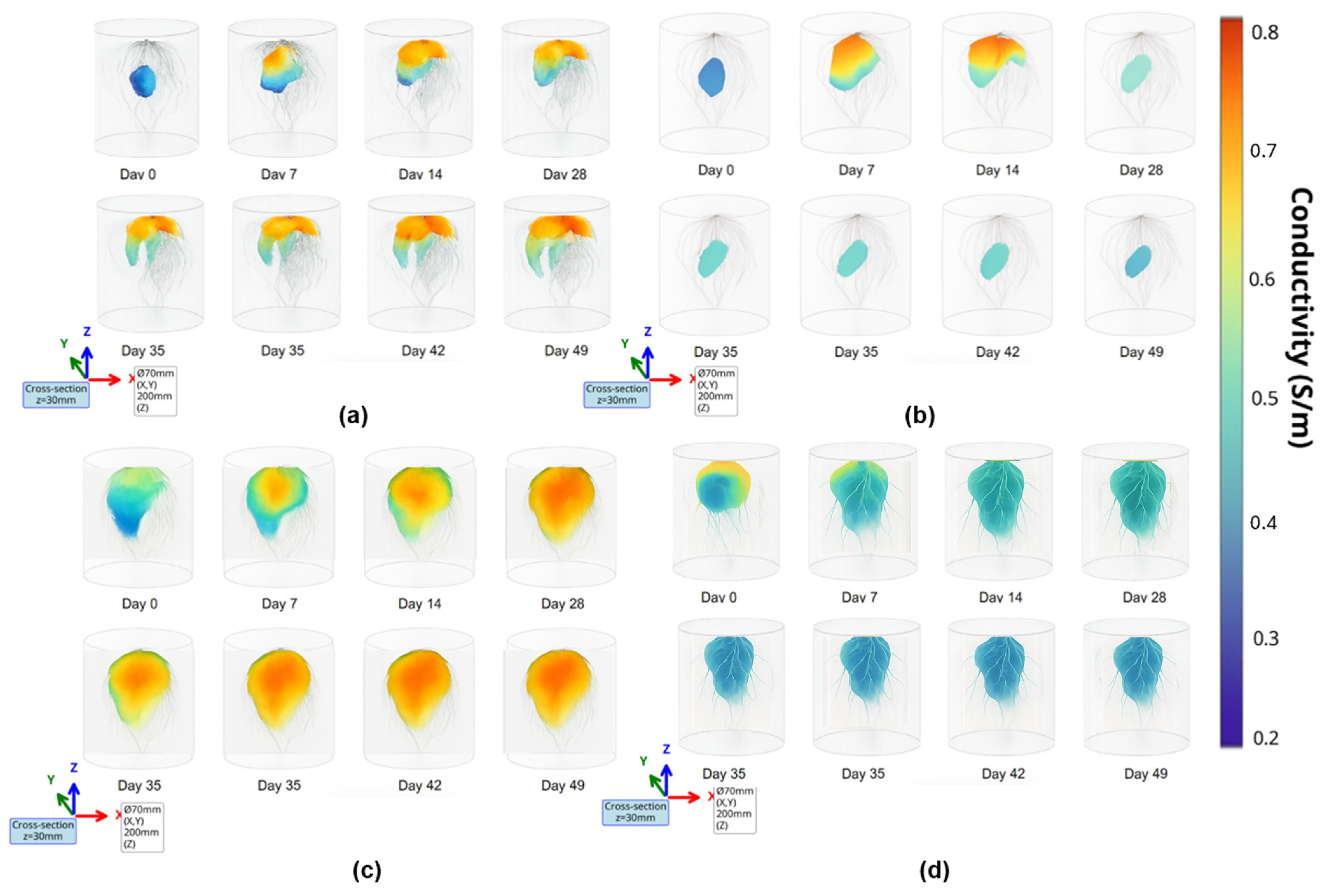
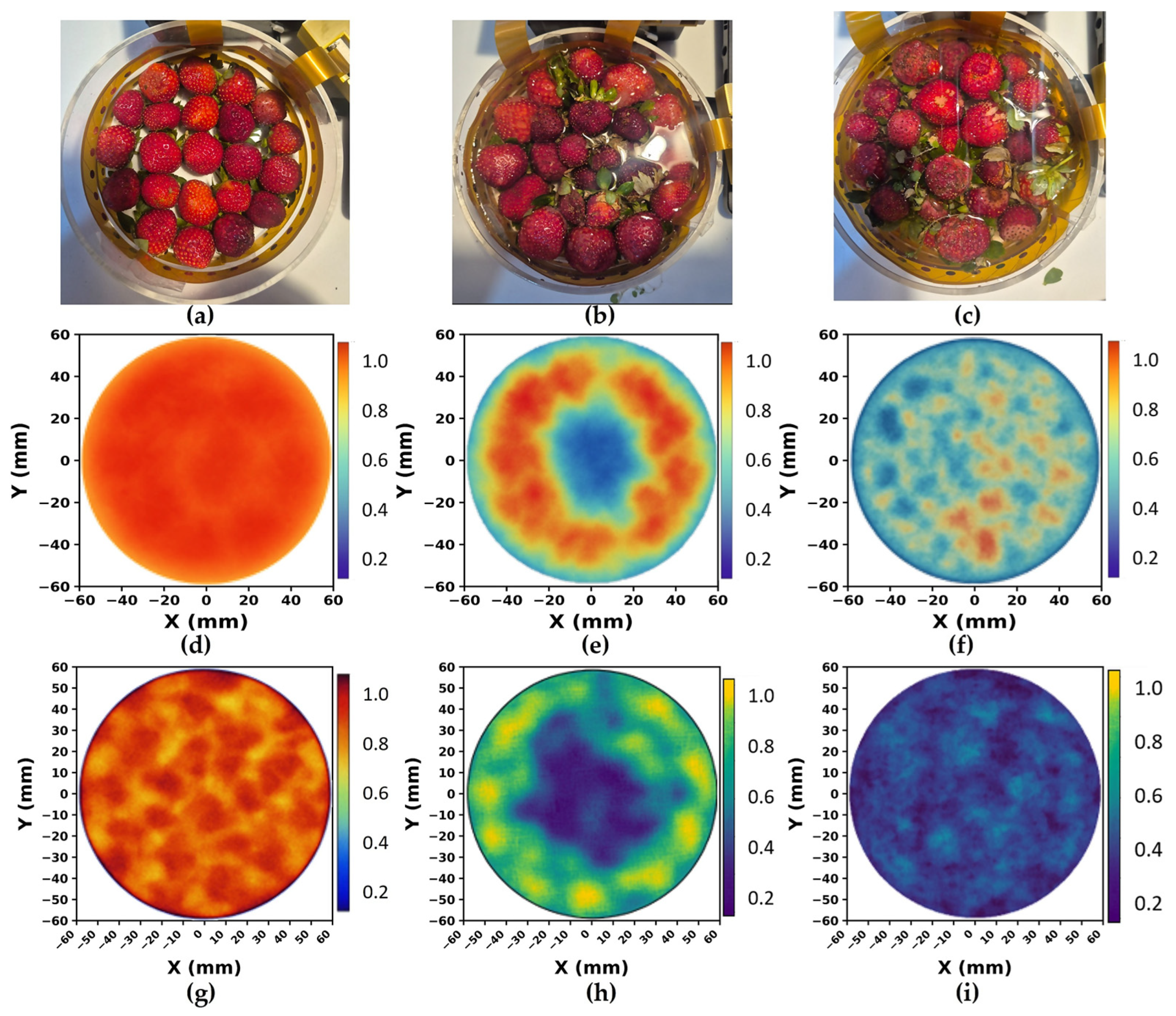
3.6. Tomography Results
4. Discussion
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Organ | Parameter | Instrument/Method | Measurement Uncertainty | Range | References |
|---|---|---|---|---|---|
| Fruit | Sugar content (°Brix) | Atago PAL-3 refractometer (Atago Co., Ltd., Tokyo, Japan) | ±0.2 °Brix | 6–14 °Brix | [95,132,136] |
| Firmness (N) | TA.XT2 texture analyzer (Stable Micro Systems, Godalming, UK) | ±0.15 N | 1.5–4.5 N | [133,136] | |
| Weight (g) | Mettler Toledo MA204 balance (Mettler Toledo, Columbus, OH, USA) | ±0.003 g | 8–25 g | [86,95,136] | |
| Diameter (mm) | Mitutoyo 500-196-30 caliper (Mitutoyo Corporation, Kawasaki, Japan) | ±0.03 mm | 20–40 mm | [90,91,95] | |
| pH | Mettler Toledo FP20 pH meter (Mettler Toledo, Columbus, OH, USA) | ±0.02 pH | 3.2–4.0 | [132,136] | |
| EC (mS/cm) | HI 9033 conductivity meter (Hanna Instruments, Woonsocket, RI, USA) | ±0.05 mS/cm | 2.0–5.0 mS/cm | [95,120,132] | |
| Leaf | RWC (%) | Gravimetric method | ±3% | 65–95% | [68,85,134] |
| Chlorophyll (SPAD) | SPAD-502Plus chlorophyll meter (Konica Minolta, Tokyo, Japan) | ±1.5 units | 25–45 SPAD | [85,88,135] | |
| Thickness (mm) | Mitutoyo 500-196-30 caliper (Mitutoyo Corporation, Kawasaki, Japan) | ±0.03 mm | 0.25–0.45 mm | [68,85,86] | |
| Stem | Diameter (mm) | Mitutoyo CD-6AX caliper (Mitutoyo Corporation, Kawasaki, Japan) | ±0.03 mm | 4–12 mm | [93,125] |
| Sugar content (°Brix) * | Atago PAL-3 refractometer (Atago Co., Ltd., Tokyo, Japan) | ±0.2 °Brix | 6–14 °Brix | [95,132,136] | |
| Firmness (N) * | TA.XT2 texture analyzer (Stable Micro Systems, Godalming, UK) | ±0.15 N | 1.5–4.5 N | [133,136] | |
| Weight (g) * | Mettler Toledo MA204 balance (Mettler Toledo, Columbus, OH, USA) | ±0.003 g | 8–25 g | [86,95,136] | |
| Diameter (mm) * | Mitutoyo 500-196-30 caliper (Mitutoyo Corporation, Kawasaki, Japan) | ±0.03 mm | 20–40 mm | [90,91,95] | |
| pH * | Mettler Toledo FP20 pH meter (Mettler Toledo, Columbus, OH, USA) | ±0.02 pH | 3.2–4.0 | [132,136] | |
| EC (mS/cm) * | HI 9033 conductivity meter (Hanna Instruments, Woonsocket, RI, USA) | ±0.05 mS/cm | 2.0–5.0 mS/cm | [95,120,132] |
| Organ Type (Sample Size, Accuracy) | Rank | Feature Name | Type/Frequency | Relative Weight |
|---|---|---|---|---|
| Fruits (n =300, 98.3% ± 1.0%) | 1 | θ (fruit50kHz) | Phase angle at 50 kHz | 0.187 |
| 2 | τ (fruit50kHz) | Cole-Cole parameter | 0.172 | |
| 3 | L (fruit100kHz) | Inductance at 100 kHz | 0.158 | |
| 4 | Q (fruit50kHz) | Derived at 50 kHz | 0.144 | |
| 5 | α (fruitβ-dispersion) | Dispersion parameter | 0.131 | |
| 6 | (fruit50kHz) | Capacitance at 50 kHz | 0.118 | |
| 7 | R0 (fruit1kHz) | Resistance at 1 kHz | 0.105 | |
| 8 | R∞ (fruit200kHz) | Resistance at 200 kHz | 0.092 | |
| 9 | Z (fruit10k/100k) | Impedance ratio | 0.087 | |
| 10 | Morphological (asymmetry) | Derived geometric | 0.081 | |
| 11 | Spectral centroid (fruitβ-dispersion) | Statistical | 0.074 | |
| 12 | Peak power (fruit50kHz) | Derived | 0.068 | |
| Leaves (n =400, 95.8% ± 1.6%) | 1 | θ (leaf5kHz) | Phase angle at 5 kHz | 0.156 |
| 2 | R0 (leaf0.5kHz) | Resistance at 0.5 kHz | 0.142 | |
| 3 | C (leaf5kHz) | Capacitation at 5 kHz | 0.134 | |
| 4 | τ (leaf5kHz) | Cole-Cole at 5 kHz | 0.128 | |
| 5 | R∞ (leaf50kHz) | Resistance at 50 kHz | 0.121 | |
| 6 | Z (leaf1k/25k) | Impedance ratio | 0.115 | |
| 7 | α (leafα-dispersion) | Dispersion parameter | 0.108 | |
| 8 | Z (ratio leaf5k/50k) | Impedance ratio | 0.102 | |
| 9 | Spectral centroid (leafα-dispersion) | Statistical | 0.096 | |
| 10 | Derivative magnitude (leafα-average) | Derived | 0.089 | |
| Stems (n =200, 88.2% ± 1.9%) | 1 | θ (stem100kHz) | Phase angle at 100 kHz | 0.198 |
| 2 | τ1 (stem100kHz) | Cole-Cole parameter | 0.176 | |
| 3 | τ2 (stem500kHz) | Cole-Cole parameter | 0.149 | |
| 4 | Z (ratio stem1k/250k) | Impedance ratio | 0.132 | |
| 5 | α (stemα-dispersion) | Dispersion parameter | 0.118 | |
| 6 | (stem100kHz) | Capacitance at 100 kHz | 0.097 | |
| 7 | R0 (stem25kHz) | Resistance at 25 kHz | 0.084 | |
| 8 | Coupling factor (stem) | Derived | 0.073 |
| Assessment | Implementation | Organ Coverage | Validation Outcome |
|---|---|---|---|
| Multimodal | Spectroscopy (bipolar/tetrapolar) | Leaves, Fruits, Stems | Accurate classification (95.8%, 98.3%, 88.2%) |
| Tomography (2D & 3D arrays) | Fruits, Roots | Spatial resolution 2.6–3.0 mm; conductivity-based root zone mapping and fruit defect sorting | |
| Multiscale | α-dispersion | Leaves | Captures water status dynamics |
| β-dispersion | Fruits | Market-based fruit quality sorting | |
| Dual dispersion | Stems | Reflects vascular transport and stem productivity | |
| Holistic | Multi-organ protocol on single platform | All four organs | Unified hardware with physiologically validated features |
| Method | Performance Type and Metrics | Detection Type | Key Limitations | References |
|---|---|---|---|---|
| Fruit Quality Assessment | ||||
| Hyperspectral imaging + AI | Classification: 90–99% | Reactive | Equipment cost, surface only, | [24,26,137,140] |
| VIS-NIR spectroscopy | Regression (R2): 0.73–0.91 | Reactive | Cost, Species-specific, Lighting dependent | [27,139] |
| Portable VIS-NIR | Regression (R2): 0.82–0.89 | Reactive | Single species, Lighting dependent, Inconclusive | [27,65,141] |
| Advanced optical spectral | Classification: 85–98% | Reactive | Limited to optical parameters, affected by light | [87,88,138] |
| Thermal imaging | Classification: 73–89% | Reactive | Environment dependent, surface temp only | [28,29,30,83] |
| Chlorophyll fluorescence | Detection: 82–87% | Semi-proactive | Limited to single parameter | [28,29,30,31,32] |
| Electrochemical spectroscopy | Classification: 82–95% | Semi-proactive | Limited features, Limited penetration, | [52,53,64,95] |
| Molecular | Detection: 70–85% | Reactive | Destructive sampling | [33,34,35,36] |
| This Study | Classification: 98.3% | Proactive | Contact required, Low-throughput | Present work |
| Leaf Relative Water Content | ||||
| Spectral indices | Regression (R2): 0.65–0.78 | Semi-proactive | Surface only, Inconclusive in field calibration | [27,88,142] |
| Hyperspectral reflectance | Regression (R2): 0.71–0.83 | Semi-proactive | Equipment cost, Lab conditions only | [24,88,143] |
| Hyperspectral stress | Classification: 83–89% | Semi-proactive | Complex, Equipment cost, Lab conditions only | [24,25,26] |
| Plant-based sensors | Correlation (r): 0.75–0.85 | Monitoring | Fixed installation, Environment dependent | [40,77,144] |
| Thermal imaging | Detection: 73–89% | Semi-proactive | Environment dependent, surface temp only | [28,29,30,31,32] |
| Chlorophyll fluorescence | Classification: 80–85% | Semi-proactive | Dark adaptation, affected by light and radiation | [31,32,147,148,149] |
| Terahertz spectroscopy | Detection: 76–82% | Semi-proactive | Limited features, Limited penetration | [48,49,50] |
| Electrochemical spectroscopy | Classification: 87–90% | Semi-proactive | No feature extraction, Limited features, Limited penetration | [53,67,68,85] |
| Wearable sensors, IoT networks | System dependent | Monitoring | Power/durability, Integration complexity | [37,38,39,40,41] |
| This Study | Classification: 95.8% ± 1.6% | Proactive | Contact required, Low-throughput compared to advanced imaging | Present work |
| Stem Assessment | ||||
| Visual inspection | Accuracy: 70–75% | Reactive | Subjective | [70,92,93] |
| Electrochemical spectroscopy | Classification: 87% | Proactive | Specific to peduncle only | [124,125,126,127,128,129,130,131] |
| Pedicel Assessment | Correlation (r): 0.65–0.72 | Reactive | Subjective Manual measurement, Operator-dependent | [92,93] |
| Sap flow sensors | Correlation (r): 0.70–0.80 | Reactive | Limited point measurement | [77,127,144] |
| This Study | Classification: 88.2% | Proactive | Indirect validation | Present work |
| Root Mapping | ||||
| Electrochemical Tomography | Resolution: 5–10 mm | Sequential | Low resolution | [56,57,94] |
| X-ray micro-CT | Resolution: 0.5–2 mm | Static | Radiation exposure | [43,44,45] |
| MRI | Resolution: 1–3 mm | Time-series | Expensive equipment | [50,51] |
| Ground penetrating radar | Depth: 50–200 mm | Single sweep | Soil dependent | [57,60,94] |
| Conductivity root mapping | Resolution: 8–12 mm | Continuous | Limited penetration | [56,59,60] |
| This Study | Resolution: 2.6–2.8 mm | Real-time | Phantom-grown only | Present work |
| Fruit Defect Detection | ||||
| X-ray imaging | Detection: 85–92% | Static | Radiation required | [44,45] |
| NIR transmittance | Classification: 78–86% | Single scan | Translucent only | [27,65,139] |
| Acoustic analysis | Classification: 75–83% | Pulse response | Noise sensitive | [89,90,91] |
| Previous 2D EIT | Resolution: 4–6 mm | Sequential | Poor boundaries | [56,61,95] |
| MRI internal imaging | Resolution: 1–2 mm | Time-series | Very expensive | [49,50,51] |
| This Study | Resolution: 2.8–3.0 mm | Non-invasive | Binary defect detection only | Present work |
| Bioelectrical Parameter | Brix Content (°Bx) | Firmness (N) | Weight (g) | Diameter (mm) | Juice pH | Juice EC (mS/cm) |
|---|---|---|---|---|---|---|
| θ (fruit50kHz) | 0.923 | 0.912 | 0.687 | 0.654 | 0.843 | 0.896 |
| τ (fruit50kHz) | 0.908 | 0.894 | 0.672 | 0.641 | 0.829 | 0.881 |
| L (fruit100kHz) | 0.896 | 0.887 | 0.698 | 0.665 | 0.856 | 0.874 |
| Q (fruit50kHz) | 0.887 | 0.878 | 0.681 | 0.648 | 0.834 | 0.869 |
| α (fruitβ-dispersion) | 0.874 | 0.865 | 0.663 | 0.631 | 0.821 | 0.856 |
| (fruit50kHz) | 0.869 | 0.858 | 0.656 | 0.624 | 0.812 | 0.847 |
| R0 (fruit1kHz) | 0.856 | 0.847 | 0.649 | 0.617 | 0.798 | 0.834 |
| R∞ (fruit200kHz) | 0.863 | 0.852 | 0.671 | 0.639 | 0.805 | 0.841 |
| Z (fruit10k/100k) | 0.847 | 0.836 | 0.692 | 0.659 | 0.789 | 0.823 |
| Morphological (asymmetry) | 0.834 | 0.823 | 0.678 | 0.645 | 0.776 | 0.812 |
| Spectral centroid (fruitβ-dispersion) | 0.821 | 0.812 | 0.665 | 0.632 | 0.763 | 0.798 |
| Peak power (fruit50kHz) | 0.812 | 0.801 | 0.658 | 0.625 | 0.754 | 0.789 |
| Bioelectrical Parameter | Water Content (%) | Chlorophyll (SPAD) | Thickness (mm) |
|---|---|---|---|
| θ (leaf5kHz) | 0.834 | 0.821 | 0.808 |
| R0 (leaf0.5kHz) | 0.823 | 0.812 | 0.798 |
| C (leaf5kHz) | 0.812 | 0.801 | 0.789 |
| τ (leaf5kHz) | 0.801 | 0.789 | 0.776 |
| R∞ (leaf50kHz) | 0.789 | 0.778 | 0.765 |
| Z (leaf1k/25k) | 0.778 | 0.767 | 0.754 |
| α (leafα-dispersion) | 0.767 | 0.756 | 0.743 |
| Z (ratio leaf5k/50k) | 0.756 | 0.745 | 0.732 |
| Spectral centroid (leafα-dispersion) | 0.745 | 0.734 | 0.721 |
| Derivative magnitude (leafα-average) | 0.734 | 0.723 | 0.712 |
| Parameter | Stem Diameter (mm) | Fruit Brix (°Bx) * | Fruit Firmness (N) * | Fruit Weight (g) * | Fruit Thickness (mm) * | Fruit pH * | Fruit EC (mS/cm) * |
|---|---|---|---|---|---|---|---|
| θ (stem100kHz) | 0.712 | 0.698 | 0.584 | 0.521 | 0.487 | 0.634 | 0.671 |
| τ1 (stem100kHz) | 0.698 | 0.685 | 0.571 | 0.508 | 0.474 | 0.621 | 0.658 |
| τ2 (stem500kHz) | 0.685 | 0.672 | 0.558 | 0.495 | 0.461 | 0.608 | 0.645 |
| Z (ratio stem1k/250k) | 0.672 | 0.659 | 0.545 | 0.482 | 0.448 | 0.595 | 0.632 |
| α (stemα-dispersion) | 0.659 | 0.646 | 0.532 | 0.469 | 0.435 | 0.582 | 0.619 |
| (stem100kHz) | 0.646 | 0.633 | 0.519 | 0.456 | 0.422 | 0.569 | 0.606 |
| R0 (stem25kHz) | 0.633 | 0.620 | 0.506 | 0.443 | 0.409 | 0.556 | 0.593 |
| Coupling factor (stem) | 0.620 | 0.607 | 0.493 | 0.430 | 0.396 | 0.543 | 0.580 |
| Process | Hardware Implementation |
|---|---|
| Signal Generation | AD9833 DDS + AD835 multiplier |
| Electrode Routing | ADG714, ADG1404, ADG1408/1409 |
| Input Protection | SMAJ5.0A TVS + EMI filtering |
| Signal Conditioning | 3-stage RC networks: HPF + BPF + LPF |
| Current Source | Howland: OPA2376/AD5171/AD5663 |
| Voltage Buffering | OPA333 with guard drive |
| Instrumentation | INA333 + sense resistors |
| Reference Elements | 4.7 kΩ (H-bridge), calibration resistors |
| Data Acquisition | ADS1256 (24-bit) + OPA4188 + REF5025 |
| System Control | STM32H755 + XC7A35RT FPGA |
| Power System | Linear ±12 V (LM7812/7912) |
References
- Food and Agriculture Organization. The State of Food Security and Nutrition in the World 2023; FAO: Rome, Italy, 2023; pp. 1–250. [Google Scholar]
- Taha, M.F.; Ahmed, S.M.; Hassan, R.K.; Al-Rashid, M.A.; Ibrahim, N.H.; Mahmoud, A.S. Emerging Technologies for Precision Crop Management Towards Agriculture 5.0: A Comprehensive Overview. Agriculture 2025, 15, 582. [Google Scholar] [CrossRef]
- Rahman, M.A.; Singh, P.; Kumar, V.; Patel, S.; Sharma, A.; Gupta, R. Ensuring food security through rapid and in-field detection of diseases in food crops using real time and portable sensors. Anal. Biochem. 2025, 705, 115925. [Google Scholar] [CrossRef] [PubMed]
- Alhabsi, A.; Ling, Y.; Crespi, M.; Reddy, A.S.N.; Mahfouz, M. Alternative Splicing Dynamics in Plant Adaptive Responses to Stress. Annu. Rev. Plant Biol. 2025, 76, 687–717. [Google Scholar] [CrossRef]
- Kumar, M.; Saifi, Z.; Krishnananda, S.D. Decoding the physiological response of plants to stress using deep learning for forecasting crop loss due to abiotic, biotic, and climatic variables. Sci. Rep. 2023, 13, 8598. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-Stage Detection of Biotic and Abiotic Stress on Plants by Chlorophyll Fluorescence Imaging Analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Aina, O.; Bakare, O.O.; Fadaka, A.O.; Keyster, M.; Klein, A. Plant biomarkers as early detection tools in stress management in food crops: A review. Planta 2024, 259, 60. [Google Scholar] [CrossRef]
- Trifilò, P.; Abate, E.; Petruzzellis, F.; Azzarà, M.; Nardini, A. Critical water contents at leaf, stem and root level leading to irreversible drought-induced damage in two woody and one herbaceous species. Plant Cell Environ. 2022, 46, 119–132. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhang, X.; Li, Y.; Wang, Y. Stress resilience in plants: The complex interplay between heat stress memory and resetting. New Phytol. 2025, 203, 203–217. [Google Scholar]
- IPCC. Climate Change 2023: Synthesis Report; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Ali, Q.; Rahman, S.; Ahmed, M.; Hassan, A.; Khan, R.; Malik, S. Research advances and applications of biosensing technology for the diagnosis of pathogens in sustainable agriculture. Environ. Sci. Pollut. Res. 2021, 28, 9002–9019. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, S.; Patel, N.; Singh, K.; Kumar, A.; Sharma, R.; Gupta, M. Advancements in Plant Diagnostic and Sensing Technologies. Adv. Sens. Res. 2025, 4, 2500045. [Google Scholar] [CrossRef]
- Khakimov, A.; Salakhutdinov, I.; Omolikov, A.; Utaganov, S. Traditional and current-prospective methods of agricultural plant diseases detection: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 951, 012002. [Google Scholar] [CrossRef]
- Attaluri, S.; Dharavath, R. Novel plant disease detection techniques-a brief review. Mol. Biol. Rep. 2023, 50, 9677–9690. [Google Scholar] [CrossRef]
- Ang, M.C.-Y.; Lew, T.T.S. Non-destructive Technologies for Plant Health Diagnosis. Front. Plant Sci. 2022, 13, 884454. [Google Scholar] [CrossRef] [PubMed]
- Okere, E.E.; Arendse, E.; Nieuwoudt, H.; Fawole, O.A.; Perold, W.J.; Opara, U.L. Non-Invasive Methods for Predicting the Quality of Processed Horticultural Food Products, with Emphasis on Dried Powders, Juices and Oils: A Review. Foods 2021, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Roper, J.M.; Garcia, J.F.; Tsutsui, H. Emerging Technologies for Monitoring Plant Health In Vivo. ACS Omega 2021, 6, 5101–5107. [Google Scholar] [CrossRef] [PubMed]
- Burynin, D.A. Screening in Plant Factories: A Review of Non-Invasive Plant Monitoring Techniques for Closed Regulated Agroecosystems. Agroengineering 2022, 6, 70–75. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and Future of Plant Stress Detection: An Overview From Remote Sensing to Positron Emission Tomography. Front. Plant Sci. 2021, 11, 609155. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, F.; Wang, M.; Zhang, Y.; Fu, S. Flexible wearable sensors for crop monitoring: A review. Front. Plant Sci. 2024, 15, 1406074. [Google Scholar] [CrossRef]
- Alejandrino, J.D.; Concepcion, R.; Culaba, A.; Dadios, E. Root Phenotyping Revolution: Science-based Policy Recommendations Amidst Green Revolution 2.0’s Transition from Invasive to Non-Invasive Tomography. In Proceedings of the 2021 IEEE 13th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Manila, Philippines, 28–30 November 2021; pp. 1–6. [Google Scholar]
- Coatsworth, P.; Gonzalez-Macia, L.; Collins, A.S.P.; Bozkurt, T.; Güder, F. Continuous monitoring of chemical signals in plants under stress. Nat. Rev. Chem. 2023, 7, 7–25. [Google Scholar] [CrossRef]
- Savary, S. A Global Assessment of the State of Plant Health. Plant Dis. 2023, 107, 3649–3665. [Google Scholar] [CrossRef]
- Wan, G.; Liu, Y.; Zhang, H.; Wang, X.; Chen, M.; Li, S. Hyperspectral imaging technology for nondestructive identification of quality deterioration in fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2025, 65, 1–30. [Google Scholar] [CrossRef]
- Dabek, A.; Mantovani, L.; Mirabella, S.; Vignati, M.; Cinquemani, S. Advancements in Non-Destructive Detection of Biochemical Traits in Plants Through Spectral Imaging-Based Algorithms: A Systematic Review. Algorithms 2025, 18, 255. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Chen, Y.; Liu, H.; Zhang, K.; Zhao, M. Trend analysis of the application of multispectral technology in plant yield prediction: A bibliometric visualization analysis (2003–2024). Front. Sustain. Food Syst. 2025, 9, 1513690. [Google Scholar] [CrossRef]
- Zahir, S.A.D.M.; Omar, A.F.; Jamlos, M.F.; Azmi, M.A.M.; Muncan, J. A review of visible and near-infrared (Vis-NIR) spectroscopy application in plant stress detection. Sens. Actuators A Phys. 2022, 338, 113468. [Google Scholar] [CrossRef]
- Sharma, N.; Tarade, P.; Rathi, R.; Biradar, A.; Jahirabadkar, S. Stress Analysis of ‘Ashwagandha, a Medicinal Plant’ Using Thermal Imaging. In Proceedings of the International Conference on Advances in Electrical Technology (ICAET), Mumbai, India, 10–11 January 2025; pp. 1–8. [Google Scholar]
- Smigaj, M.; Gaulton, R.; Suárez, J.C.; Barr, S.L. Thermal Infrared Remote Sensing of Stress Responses in Forest Environments: A Review of Developments, Challenges, and Opportunities. Curr. For. Rep. 2023, 9, 424–443. [Google Scholar] [CrossRef]
- Omia, E.; Bae, H.; Park, E.; Kim, M.S.; Baek, I.; Kabenge, I.; Cho, B.-K. Remote Sensing in Field Crop Monitoring: A Comprehensive Review of Sensor Systems, Data Analyses and Recent Advances. Remote Sens. 2023, 15, 354. [Google Scholar] [CrossRef]
- Grishina, A.; Sherstneva, O.; Mysyagin, S.; Brilkina, A.; Vodeneev, V. Detecting Plant Infections: Prospects for Chlorophyll Fluorescence Imaging. Agronomy 2024, 14, 2600. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J.; Ndikuryayo, F.; Chen, Y.; Liu, G.; Yang, W.-C. Advances in Fluorescence-based Probes for Abiotic Stress Detection in Plants. ACS Sens. 2025, 10, 2474–2486. [Google Scholar] [CrossRef]
- Shukla, A.; Yadav, D.; Mishra, U.K.; Dipake, S.; Mishra, R. Revolutionizing Plant Virus Detection: A Review on Molecular Breakthroughs and their Implications. Int. J. Plant Soil Sci. 2023, 35, 339–350. [Google Scholar] [CrossRef]
- Alsharksi, A.N.; Sirekbasan, S.; Gürkök-Tan, T.; Mustapha, A. From Tradition to Innovation: Diverse Molecular Techniques in the Fight Against Infectious Diseases. Diagnostics 2024, 14, 2876. [Google Scholar] [CrossRef]
- Trippa, D.; Scalenghe, R.; Basso, M.F.; Panno, S.; Davino, S.; Molinatto, G.; Brugaletta, V.; Mauceri, A.; Mercati, F.; Martinelli, F. Next-generation methods for early disease detection in crops. Pest Manag. Sci. 2024, 80, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ahmar, S.; Sohail, M.A.; Kamran, M.; Ali, M.; Saleem, M.H.; Rizwan, M.; Ahmed, A.M.; Mora-Poblete, F.; Júnior, A.T.A.; et al. Advances, limitations, and prospects of biosensing technology for detecting phytopathogenic bacteria. Chemosphere 2022, 296, 133773. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Z.; Chen, Y.; Wu, Y.; Li, X.; Wang, S.; Liu, Z.; Sun, W.; Hu, Z. Wearable Sensors for Plants: Status and Prospects. Biosensors 2025, 15, 53. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Wang, X.; Chen, L.; Liu, Y.; Zhou, H.; Yang, S.; Zhao, Q. Exploration of the Plant World: Application and Innovation of Plant-Wearable Sensors for Real-Time Detection. Crit. Rev. Anal. Chem. 2025, 55, 1–17. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Li, J.; Gao, Y.; Liu, C.; Hao, G. Wearable sensor supports in-situ and continuous monitoring of plant health in precision agriculture era. Plant Biotechnol. J. 2024, 22, 14283. [Google Scholar] [CrossRef]
- Prithviraj, S.S.; Raksha, G.; Sahana, N.J.; Sayuj, P.N.; Faisal, P. Smart Plant Monitoring System: A Review of Approaches for Monitoring Condition of Plants. Int. J. Adv. Res. Sci. Commun. Technol. 2024, 13, 685–692. [Google Scholar] [CrossRef]
- Sharma, R.; Vaidya, P.; Sharma, B. A Review on Plant Growth Monitoring using Artificial Intelligence and the Internet of Things. In Proceedings of the 2024 11th International Conference on Computing for Sustainable Global Development (INDIACom), New Delhi, India, 28 February–1 March 2024. [Google Scholar]
- Gou, C.; Zhu, P.; Meng, Y.; Yang, F.; Xu, Y.; Xia, P.; Chen, J.; Li, J. Machine and Deep Learning: Artificial Intelligence Application in Biotic and Abiotic Stress Management in Plants. Front. Biosci. 2024, 29, 20. [Google Scholar] [CrossRef]
- Jackulin, C.; Murugavalli, S. A comprehensive review on detection of plant disease using machine learning and deep learning approaches. Meas. Sens. 2022, 24, 100441. [Google Scholar] [CrossRef]
- Karahara, I.; Yamauchi, D.; Uesugi, K.; Mineyuki, Y. Three-dimensional visualization of plant tissues and organs by X-ray micro-computed tomography. Microscopy 2023, 72, 310–325. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, R.; Mine, Y.; Zhang, X.; Song, Z.; Meng, W.; Zhou, H. Applications of Computed Tomography (CT) in environmental soil and plant sciences. Soil Tillage Res. 2023, 226, 105574. [Google Scholar] [CrossRef]
- Saletnik, A.; Saletnik, B.; Zaguła, G.; Puchalski, C. Raman Spectroscopy for Plant Disease Detection in Next-Generation Agriculture. Sustainability 2024, 16, 5474. [Google Scholar] [CrossRef]
- Zavafer, A.; Ball, M.C. Good vibrations: Raman spectroscopy enables insights into plant biochemical composition. Funct. Plant Biol. 2023, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Peretti, R. Terahertz technology for biophotonics: A general overview and recent advances in THz spectroscopy toward biology. In Proceedings of the SPIE Optical Metrology, Munich, Germany, 26–29 June 2023. [Google Scholar]
- Greco, M.; Cinquemani, S.; Malavasi, M.; Martellosio, A.; Resta, V.; Alghisi, D.; Beccalli, R.; Chiesa, M.; Crespi, E.; D’Adda, F.; et al. Studies of leaf water content in smart agriculture using THz technologies: A review. J. Smart Environ. Green Comput. 2022, 2, 46–57. [Google Scholar] [CrossRef]
- Anand, A.; Sharma, A.; Kaur, H.; Khandelwal, M.; Singh, G.; Barnwal, S.; Rath, A.; Mohan, S.; Shah, R. Profiling of Plant Derived Natural Constituents by Using Magnetic Resonance Techniques. Concepts Magn. Reson. Part A 2022, 2022, 5705637. [Google Scholar] [CrossRef]
- Blystone, S.; Nuixe, M.; Traoré, A.S.; Cochard, H.; Picon-Cochard, C.; Pagès, G. Towards portable MRI in the plant sciences. Plant Methods 2024, 20, 31. [Google Scholar] [CrossRef]
- Alejandrino, J.; Concepcion, R.; Dadios, E. Ripening Degree Assessment of Organic Strawberry Based on Electrical Impedance Spectroscopy with Machine Learning. In Proceedings of the 2024 6th International Conference on Applied Electromagnetics, Signal Processing & Communication (AGRETA), Bhubaneswar, India, 26–28 September 2024; pp. 61–66. [Google Scholar]
- Hamed, S.; Altana, A.; Lugli, P.; Petti, L.; Ibba, P. Supervised classification and circuit parameter analysis of electrical bioimpedance spectroscopy data of water stress in tomato plants. Comput. Electron. Agric. 2024, 226, 109347. [Google Scholar] [CrossRef]
- Van Haeverbeke, M.; De Baets, B.; Stock, M. Plant impedance spectroscopy: A review of modeling approaches and applications. Front. Plant Sci. 2023, 14, 1187573. [Google Scholar] [CrossRef]
- La, Z.; Wang, Y.; Chen, X.; Li, J.; Zhang, M.; Liu, H.; Zhou, S.; Yang, L. Electrical Signal Characterization of Aloe vera Var. Chinensis Using Non-Parametric and Parametric Signal Analysis. Appl. Sci. 2025, 15, 1708. [Google Scholar] [CrossRef]
- Custodio, J.M.; Concepcion, R.; Alejandrino, J.; Relano, R.-J.; Bandala, A.; Mirjalili, S. Patterns and Positions of Concentric Ring Electrodes for Electrical Impedance Tomography. In Proceedings of the 2023 IEEE 15th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Manila, Philippines, 28–30 November 2023. [Google Scholar]
- Basak, R.; Wahid, K.A. A Rapid, Low-Cost, and High-Precision Multifrequency Electrical Impedance Tomography Data Acquisition System for Plant Phenotyping. Remote Sens. 2022, 14, 3214. [Google Scholar] [CrossRef]
- Alejandrino, J.D.; Concepcion, R.S., II; Bandala, A.A.; Sybingco, E.; Vicerra, R.R.P.; Dadios, E.P. Non-Invasive Plant Root Tomography Through Optimized Sonar Array Transducer Antenna Design Using Genetic Swarm Metaheuristic. J. Adv. Comput. Intell. Intell. Inform. 2024, 28, 59–66. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Li, X.; Chen, J.; Liu, Y.; Zhang, H. A New Portable Electrical Impedance Tomography System for Measuring Two-Dimensional Stem Water Content Distribution. Agric. For. Meteorol. 2022, 325, 109161. [Google Scholar] [CrossRef]
- Mary, B.; Vanella, D.; Consoli, S.; Cassiani, G. Imaging of the electrical activity in the root zone under limited-water-availability stress: A laboratory study for Vitis vinifera. Biogeosciences 2023, 20, 4625–4650. [Google Scholar] [CrossRef]
- Calvo, S.; Barezzi, M.; Demarchi, D.; Garlando, U. In-vivo proximal monitoring system for plant water stress and biological activity based on stem electrical impedance. In Proceedings of the 2023 9th International Workshop on Advances in Sensors and Interfaces (IWASI), Monopoli, Italy, 8–9 June 2023; pp. 80–85. [Google Scholar]
- Mohsen, M.; Said, L.A.; Madian, A.H.; Radwan, A.G.; Elwakil, A.S. Fractional-Order Bio-Impedance Modeling for Interdisciplinary Applications: A Review. IEEE Access 2021, 9, 33158–33168. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Keblawy, A.; Akhtar, N.; Elwakil, A.S. Electrical Impedance Spectroscopy in Plant Biology. In Sustainable Agriculture Reviews 52; Springer: Cham, Switzerland, 2021; pp. 395–416. [Google Scholar]
- Alejnikov, A.F.; Cheshkova, A.F.; Mineev, V.V. Choice of impedance parameter of strawberry tissue for detection of fungal diseases. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 032005. [Google Scholar] [CrossRef]
- Serrano-Finetti, E.; Castillo, E.; Alejos, S.; León, M. Toward Noninvasive Monitoring of Plant Leaf Water Content by Electrical Impedance Spectroscopy. Comput. Electron. Agric. 2023, 209, 107907. [Google Scholar] [CrossRef]
- Alejandrino, J.; Concepcion, R., II; Dadios, E. Impedance-Based Assessment of Ipomoea Batatas Leaf Water Status Using Hybrid Mutual Dependence and Variance Inflation Factor Regression Analysis. In Intelligent Computing and Optimization, Proceedings of the 7th International Conference on Intelligent Computing and Optimization 2023 (ICO2023), Phnom Penh, Cambodia, 26–27 October 2023; Lecture Notes in Networks and Systems; Springer: Singapore, 2024; pp. 139–150. [Google Scholar]
- Kang, J.; Park, Y.J.; Lee, J.; Wang, S.H.; Park, D.S. Flexible PI-Based Plant Drought Stress Sensor for Real-Time Monitoring System in Smart Farm. Electronics 2018, 7, 114. [Google Scholar]
- Basak, R.; Wahid, K.A.; Dinh, A.; Soolanayakanahally, R.; Fotouhi, R.; Mehr, A.S. Rapid and Efficient Determination of Relative Water Contents of Crop Leaves Using Electrical Impedance Spectroscopy in Vegetative Growth Stage. Remote Sens. 2020, 12, 1753. [Google Scholar] [CrossRef]
- Hamed, S.; Ibba, P.; Altana, A.; Lugli, P.; Petti, L. Towards Tomato Plant Iron Stress Monitoring Through Bioimpedance and Circuit Analysis. In Proceedings of the 2023 IEEE Conference on AgriFood Electronics (CAFE), Torino, Italy, 25–27 September 2023; pp. 20–24. [Google Scholar]
- Alejandrino, J.; Concepcion, R.; Bandala, A.; Sybingco, E.; Dadios, E.; Vicerra, R.R. Non-Invasive Bioelectrical Characterization of Strawberry Peduncles for Post-Harvest Physiological Maturity Classification. AgriEngineering 2025, 7, 223. [Google Scholar] [CrossRef]
- Altana, A.; Hamed, S.; Lugli, P.; Petti, L.; Ibba, P. Monitoring Iron Stress in Tomato Plants Through Bioimpedance and Machine-Learning-Enhanced Classification Based on Circuit Component Analysis. IEEE Trans. AgriFood Electron. 2024, 2, 190–197. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.; Ortiz-Melendez, A.; Guevara-Gonzalez, R.; Torres-Pacheco, I.; Herrera-Ruiz, G.; Contreras-Medina, L.; Prado-Olivarez, J.; Ocampo-Velazquez, R. An Analysis of Electrical Impedance Measurements Applied for Plant N Status Estimation in Lettuce (Lactuca sativa). Sensors 2014, 14, 11492–11503. [Google Scholar] [CrossRef]
- Tran, D.; Dutoit, F.; Najdenovska, E.; Wallbridge, N.; Plummer, C.; Mazza, M.; Raileanu, L.E.; Camps, C. Electrophysiological assessment of plant status outside a Faraday cage using supervised machine learning. Sci. Rep. 2019, 9, 17073. [Google Scholar] [CrossRef]
- Alejandrino, J.D.; Concepcion, R.; Bandala, A.; Dadios, E. Bioimpedance Spectroscopy Tool with Embedded Hybrid PCA-SVM Model for Post-Harvest Carrot Decay Detection. In Proceedings of the 2023 IEEE 15th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Manila, Philippines, 28–30 November 2023; pp. 1–6. [Google Scholar]
- Cooper, R.L.; Thomas, M.A.; McLetchie, D.N. Impedance Measures for Detecting Electrical Responses during Acute Injury and Exposure of Compounds to Roots of Plants. Methods Protoc. 2022, 5, 56. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, Y.; Choi, H. Early detection of plant stress using the internal electrical conductivity of Capsicum annuum in response to temperature and salinity stress. Plant Growth Regul. 2021, 95, 359–371. [Google Scholar] [CrossRef]
- Laarabi, S. Characterization of Short-Term Stress Applied to the Root System by Electrical Impedance Measurement in the First Leaf of Corn (Zea mays L.) and Pumpkin (Cucurbita maxima L.). Am. J. Plant Sci. 2014, 5, 1285–1295. [Google Scholar] [CrossRef]
- Sneha, M.; Afijth Ravindranath, N.; Jayaraman, V. Electrochemical Investigations of Plant Response to Salt Stress: Insights Through Impedance Studies of OECT-Based Biosensor. ChemistrySelect 2024, 9, e202302928. [Google Scholar]
- Jócsák, I.; Droppa, M.; Horváth, G.; Bóka, K.; Vozáry, E. Electrical impedance measurement on plants: A review with some insights to other fields. Theor. Exp. Plant Physiol. 2019, 31, 359–375. [Google Scholar] [CrossRef]
- Alejandrino, J.D.; Concepcion, R.; Bandala, A.; Dadios, E. Detection of Chilling Injury in Cucumis Sativus Using Bioimpedance Spectroscopy and Machine Learning Algorithms. In Proceedings of the 2023 IEEE 15th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Manila, Philippines, 28–30 November 2023; pp. 1–6. [Google Scholar]
- Reynolds, J.; Taggart, M.; Lobaton, E.; Daniele, M.; Rufty, T.; Bozkurt, A. An Environmental Station with Bioimpedance Capabilities for Agricultural Deployment. In Proceedings of the 2020 IEEE SENSORS, Rotterdam, The Netherlands, 25–28 October 2020. [Google Scholar]
- Zheng, C.; Abd-Elrahman, A.; Whitaker, V. Remote Sensing and Machine Learning in Crop Phenotyping and Management, with an Emphasis on Applications in Strawberry Farming. Remote Sens. 2021, 13, 531. [Google Scholar] [CrossRef]
- Wu, D. Detecting Plant Stress and Phenotyping in Live California Crops Using Thermal Imaging Technique. In Proceedings of the 2024 5th International Conference on Smart Sensors and Application (ICSSA), Kuala Lumpur, Malaysia, 24–25 September 2024; pp. 1–6. [Google Scholar]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.-K. Chlorophyll Fluorescence Imaging for Early Detection of Drought and Heat Stress in Strawberry Plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, H.; Zhou, J.; Fu, X.; Ye, H.; Nguyen, H.T. Relationship among Electrical Signals, Chlorophyll Fluorescence, and Root Vitality of Strawberry Seedlings under Drought Stress. Agronomy 2022, 12, 1428. [Google Scholar] [CrossRef]
- Ullah, I.; Dawar, K.; Tariq, M.; Sharif, M.; Fahad, S.; Asghar, M.A.; Ahmad, M.; Abbas, A.; Refat, M.E. High-temperature stress in strawberry: Understanding physiological, biochemical and molecular responses. Planta 2024, 260, 118. [Google Scholar] [CrossRef]
- Raj, R.; Cosgun, A.; Kulić, D. Strawberry Water Content Estimation and Ripeness Classification Using Hyperspectral Sensing. Agronomy 2022, 12, 425. [Google Scholar] [CrossRef]
- Poobalasubramanian, M.; Park, E.-S.; Faqeerzada, M.A.; Kim, T.; Kim, M.S.; Baek, I.; Cho, B.-K. Identification of Early Heat and Water Stress in Strawberry Plants Using Chlorophyll-Fluorescence Indices Extracted via Hyperspectral Images. Sensors 2022, 22, 8706. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Brillante, L.; Benelli, A.; Bini, F.; Serni, E.; Nati, C.; Caruso, G.; Papini, R.; Gucci, R. Image analysis to predict the maturity index of strawberries. Adv. Hortic. Sci. 2023, 37, 83–87. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Han, Q.; Zhang, Z.; Kong, D.; Zou, X. Strawberry Detection and Ripeness Classification Using YOLOv8+ Model and Image Processing Method. Agriculture 2024, 14, 751. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Chen, Y.; Wu, Y.; Zhang, X. Strawberry ripeness classification method in facility environment based on red color ratio of fruit rind. Comput. Electron. Agric. 2023, 214, 108313. [Google Scholar] [CrossRef]
- Çezik, F.; Saraçoğlu, O. The Effect of Pedicel Length and Post-Harvest Calcium Chloride Application on the Storage Life of Strawberry Fruit. Appl. Fruit Sci. 2024, 66, 843–853. [Google Scholar] [CrossRef]
- Barezzi, M.; Cum, F.; Garlando, U.; Martina, M.; Demarchi, D. On the impact of the stem electrical impedance in neural network algorithms for plant monitoring applications. In Proceedings of the 2022 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Perugia, Italy, 3–5 November 2022; pp. 80–85. [Google Scholar]
- Corona-Lopez, D.D.J.; Sommer, S.; Rolfe, S.A.; Podd, F.; Grieve, B.D. Electrical impedance tomography as a tool for phenotyping plant roots. Plant Methods 2019, 15, 49. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Abera, B.D.; Cantarella, G.; Petti, L.; Lugli, P. Bio-impedance and circuit parameters: An analysis for tracking fruit ripening. Postharvest Biol. Technol. 2020, 159, 110978. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Wang, H.; Zhang, X.; Chen, L.; Li, M.; Zhou, S. Shape Reconstruction with Multiphase Conductivity for Electrical Impedance Tomography Using Improved Convolutional Neural Network Method. IEEE Sens. J. 2021, 21, 9277–9287. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Y.; Zhang, L.; Liu, H.; Chen, X.; Li, Z.; Zhao, M. Imaging Algorithm and Optimal Measurement Protocol for 3D Impedance Tomography on 2D High Density Micro Electrode Array. IEEE Sens. J. 2024, 24, 24452–24465. [Google Scholar] [CrossRef]
- Dimas, C.; Alimisis, V.; Uzunoglu, N.; Sotiriadis, P. Advances in Electrical Impedance Tomography Inverse Problem Solution Methods: From Traditional Regularization to Deep Learning. IEEE Access 2024, 12, 47976–48015. [Google Scholar] [CrossRef]
- Bera, T.K.; Nagaraju, J. Gold electrode sensors for electrical impedance tomography (EIT) studies. In Proceedings of the 2011 IEEE Sensors Applications Symposium, San Antonio, TX, USA, 22–24 February 2011. [Google Scholar]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Inkjet-Printed Flexible Gold Electrode Arrays for Bioelectronic Interfaces. Adv. Funct. Mater. 2016, 26, 1004–1013. [Google Scholar] [CrossRef]
- Lo Presti, D.; Romano, A.; Massaroni, C.; D’Abbraccio, J.; Massari, L.; Caponero, M.A.; Oddo, G.; Formica, D.; Schena, E. Current understanding, challenges and perspective on portable systems applied to plant monitoring and precision agriculture. Biosens. Bioelectron. 2023, 222, 115005. [Google Scholar] [CrossRef]
- Sun, J.-Q.; Zhao, X.-Z.; Liang, C.-Y.; Yang, Z.-X.; Liu, Y.; Qi, D.-P. The monitoring of plant physiology and ecology: From materials to flexible devices. Chin. J. Anal. Chem. 2023, 51, 100211. [Google Scholar] [CrossRef]
- Bera, T.K.; Nagaraju, J. A Gold Sensors Array for Imaging The Real Tissue Phantom in Electrical Impedance Tomography. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012083. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y. A silver/silver chloride woven electrode with convex based on electrical impedance tomography. J. Text. Inst. 2020, 112, 1067–1079. [Google Scholar] [CrossRef]
- Sasaki, S.; Yokota, K.; Hanagata, N. Study of salt permeation process into Vigna angularis using Ag/AgCl electrodes. Bioelectrochemistry 2004, 64, 29–31. [Google Scholar] [CrossRef]
- Ehosioke, S.; Garré, S.; Huisman, J.A.; Kemna, A.; Nguyen, F. Sensing the electrical properties of roots: A review. Vadose Zone J. 2020, 19, e20082. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A Review of Imaging Techniques for Plant Phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Kessouri, P.; Furman, A.; Huisman, J.A.; Martin, T.; Mellage, A.; Ntarlagiannis, D.; Bücker, M.; Ehosioke, S.; Fernandez, P.; Flores-Orozco, A.; et al. Induced polarization applied to biogeophysics: Recent advances and future prospects. Near Surf. Geophys. 2019, 17, 595–621. [Google Scholar] [CrossRef]
- Meder, F.; Saar, S.; Taccola, S.; Filippeschi, C.; Mattoli, V.; Mazzolai, B. Ultraconformable, Self-Adhering Surface Electrodes for Measuring Electrical Signals in Plants. Adv. Mater. Technol. 2021, 6, 2001182. [Google Scholar] [CrossRef]
- Son, D.; Park, J.; Lee, S.; Kim, J.J.; Chung, S. Integrating non-invasive VIS-NIR and bioimpedance spectroscopies for stress classification of sweet basil (Ocimum basilicum L.) with machine learning. Biosens. Bioelectron. 2024, 263, 116579. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Freitas, V.M.S.; Vidotto, L.H.B.; Schleder, G.R.; de Oliveira, R.A.G.; Rocha, J.F.C.; Kubota, L.T.; Vieira, L.C.S.; Tolentino, H.C.N.; Neckel, I.T.; et al. Biocompatible Wearable Electrodes on Leaves toward the On-Site Monitoring of Water Loss from Plants. ACS Appl. Mater. Interfaces 2022, 14, 22989–23001. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Wang, L.; Chen, M.; Li, S.; Liu, J. A Highly Sensitive, Low Creep Hydrogel Sensor for Plant Growth Monitoring. Sensors 2024, 24, 6197. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Ranatunga, D.R.A.; Shtessel, Y.B.; Volkova, M.I.; Markin, V.S.; Jana, S. Electrotonic potentials in Aloe vera L.: Effects of intercellular and external electrodes arrangement. Bioelectrochemistry 2017, 113, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gurovich, L.A. Real-time Plant Water Potential Assessment Based on Electrical Signalling in Higher Plants. In Proceedings of the 2009 ASABE Annual International Meeting, Reno, NV, USA, 21–24 June 2009. [Google Scholar]
- Xu, H.; Chen, L.; Zhang, Y.; Wang, M.; Li, J.; Zhou, S. A plant-friendly wearable sensor for reducing interfacial abiotic stress effects and growth monitoring. J. Mater. Chem. A 2024, 12, 30012–30021. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Liu, M.; Chen, X.; Li, Y. Mandarin orange ripening assessment using bioimpedance spectroscopy with commercial ECG electrodes. J. Food Eng. 2024, 358, 111654. [Google Scholar]
- Kluza, M.; Karpiel, I.; Duch, K.; Komorowski, D.; Siecinski, S. An Assessment of the Freshness of Fruits and Vegetables Through the Utilization of Bioimpedance Spectroscopy (BIS)—A Preliminary Study. Foods 2025, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Z.; Li, M.; Chen, Y.; Zhang, X.; Liu, H. High-Adhesive Flexible Electrodes and Their Manufacture: A Review. Micromachines 2021, 12, 1505. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Chang, Z.; Zhao, J.; Wang, X.; Xian, J. Detection of Localized Damage in Tomato Based on Bioelectrical Impedance Spectroscopy. Agronomy 2024, 14, 1822. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Abdelhalim, A.; Cantarella, G.; Petti, L.; Lugli, P. Bio-impedance spectroscopy for the identification of strawberry ripeness. Sci. Rep. 2021, 11, 11409. [Google Scholar]
- Chen, W.; Liu, X.; Zhang, Y.; Wang, H.; Li, Z. Flexible screen-printed electrodes for real-time fruit quality monitoring during storage. Food Control 2024, 145, 109456. [Google Scholar]
- Patel, R.; Kumar, S.; Singh, A.; Sharma, M.; Gupta, N. Applications of chemically modified screen-printed electrodes in food analysis and quality monitoring: A review. RSC Adv. 2024, 14, 27957–27976. [Google Scholar] [CrossRef]
- Singh, S.; Wang, J.; Cinti, S. Review—An Overview on Recent Progress in Screen-Printed Electroanalytical (Bio)Sensors. ECS Sens. Plus 2022, 1, 023401. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, H.; Chen, L.; Li, M. Supervised classification and circuit parameter analysis of electrical bioimpedance spectroscopy for tomato water stress detection. Comput. Electron. Agric. 2024, 223, 109087. [Google Scholar]
- Garlando, U.; Bar-On, L.; Ros, P.M.; Sanginario, A.; Calvo, S.; Martina, M.; Avni, A.; Shacham-Diamand, Y.; Demarchi, D. Analysis of in Vivo Plant Stem Impedance Variations in Relation with External Conditions Daily Cycle. In Proceedings of the 2021 IEEE International Symposium on Circuits and Systems (ISCAS), Daegu, Republic of Korea, 22–28 May 2021; pp. 1–5. [Google Scholar]
- Dotan, T.; Jog, A.; Kadan-Jamal, K.; Avni, A.; Shacham-Diamand, Y. In Vivo Plant Bio-Electrochemical Sensor Using Redox Cycling. Biosensors 2023, 13, 219. [Google Scholar] [CrossRef]
- Gurovich, L.; Hermosilla, P. Electric signalling in fruit trees in response to water applications and light–darkness conditions. J. Plant Physiol. 2009, 166, 290–300. [Google Scholar] [CrossRef]
- Kozlova, E.; Yudina, L.; Sukhova, E.; Sukhov, V. Analysis of Electrome as a Tool for Plant Monitoring: Progress and Perspectives. Plants 2025, 14, 1500. [Google Scholar] [CrossRef]
- Volkov, A. Plant Electrophysiology; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Rajkai, K.; Végh, K.R.; Nacsa, T. Electrical capacitance of roots in relation to plant electrodes, measuring frequency and root media. Acta Agron. Hung. 2005, 53, 197–210. [Google Scholar] [CrossRef]
- Ríos-Rojas, L.; Tapia, F.; Gurovich, L.A. Electrophysiological assessment of water stress in fruit-bearing woody plants. J. Plant Physiol. 2014, 171, 799–806. [Google Scholar] [CrossRef]
- Hernanz, D.; Recamales, Á.F.; Meléndez-Martínez, A.J.; González-Miret, M.L.; Heredia, F.J. Assessment of the differences in the phenolic composition of five strawberry cultivars grown in two different soilless systems. J. Agric. Food Chem. 2007, 55, 1846–1852. [Google Scholar] [CrossRef]
- Døving, A.; Måge, F. Methods of testing strawberry fruit firmness. Acta Agric. Scand. B Soil Plant Sci. 2002, 52, 43–51. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]
- Hanhineva, K.; Beleggia, R.; Dai, N.; Rogachev, I.; Nikiforova, V.J.; Fernie, A.R.; Aharoni, A. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol. 2018, 148, 730–750. [Google Scholar]
- Huang, Y.; Ren, Z.; Li, D.; Liu, X. Phenotypic techniques and applications in fruit trees: A review. Plant Methods 2020, 16, 107. [Google Scholar] [CrossRef]
- Aline, U.; Bhattacharya, T.; Faqeerzada, M.A.; Kim, M.S.; Baek, I.; Cho, B.-K. Advancement of non-destructive spectral measurements for the quality of major tropical fruits and vegetables: A review. Front. Plant Sci. 2023, 14, 1240361. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Wieme, J.; Mollazade, K.; Malounas, I.; Zude-Sasse, M.; Zhao, M.; Gowen, A.; Argyropoulos, D.; Fountas, S.; Van Beek, J. Application of hyperspectral imaging systems and artificial intelligence for quality assessment of fruit, vegetables and mushrooms: A review. Biosyst. Eng. 2022, 222, 156–176. [Google Scholar] [CrossRef]
- Ecarnot, M.; Bączyk, P.; Tessarotto, L.; Chervin, C. Rapid phenotyping of the tomato fruit model, Micro-Tom, with a portable VIS–NIR spectrometer. Plant Physiol. Biochem. 2013, 70, 159–163. [Google Scholar] [CrossRef]
- Ballester, C.; Zarco-Tejada, P.J.; Nicolás, E.; Alarcón, J.J.; Fereres, E.; Intrigliolo, D.S.; Gonzalez-Dugo, V. Evaluating the performance of xanthophyll, chlorophyll and structure-sensitive spectral indices to detect water stress in five fruit tree species. Precis. Agric. 2017, 19, 178–193. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Zhang, Y.-H.; Han, Z.-M.; Liu, X.-Y.; Jian, Y.-F.; Hu, C.-G.; Dian, Y.-Y. Evaluating the Performance of Hyperspectral Leaf Reflectance to Detect Water Stress and Estimation of Photosynthetic Capacities. Remote Sens. 2021, 13, 2160. [Google Scholar] [CrossRef]
- Scalisi, A.; Bresilla, K.; Grilo, F. Continuous determination of fruit tree water-status by plant-based sensors. Italus Hortus 2018, 25, 39–50. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; Su, Y.; Li, H.; Fang, L.; Xing, D. Plant’s electrophysiological information manifests the composition and nutrient transport characteristics of membrane proteins. Plant Signal. Behav. 2021, 16, 1918867. [Google Scholar] [CrossRef]
- Okajima, M.; Nakagawa, H.; Sugiyama, M. Possibility of directly sensing plant stress under environment temperature changes using electrochemical impedance spectroscopy. Jpn. J. Appl. Phys. 2023, 62, 088002. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, R.; McCabe, M. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat. Agronomy 2017, 7, 61. [Google Scholar] [CrossRef]
- Cendrero-Mateo, M.; Carmo-Silva, A.; Porcar-Castell, A.; Hamerlynck, E.; Papuga, S.; Moran, M.S. Dynamic response of plant chlorophyll fluorescence to light, water and nutrient availability. Funct. Plant Biol. 2015, 42, 746–757. [Google Scholar] [CrossRef]
- Reynolds, J.; Taggart, M.; Lobaton, E.; Daniele, M.; Rufty, T.; Bozkurt, A. Rapid drought stress detection in plants using bioimpedance measurements and analysis. IEEE Trans. AgriFood Electron. 2023, 1, 135–144. [Google Scholar] [CrossRef]
- Afzal, A.; Duiker, S.; Watson, J. Leaf thickness and electrical capacitance as measures of plant water status. Trans. ASABE 2017, 60, 1063–1074. [Google Scholar] [CrossRef]


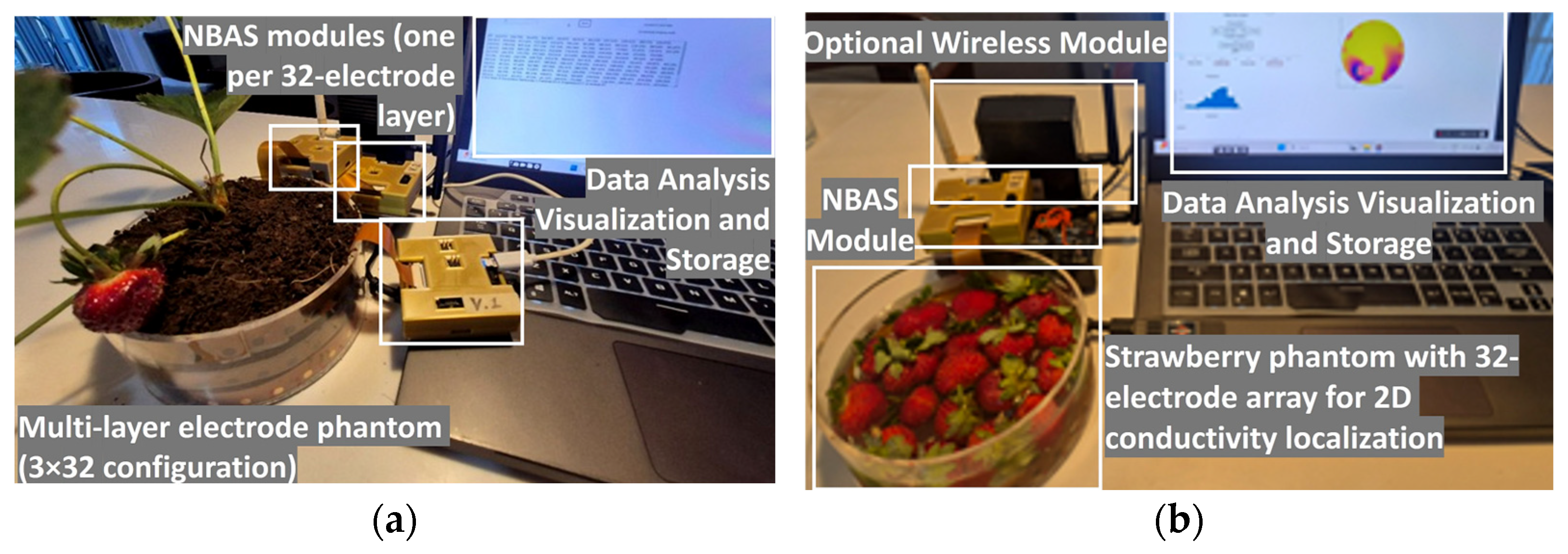
| Organ | Electrode Type | Configuration | Signal Quality (SNR, dB) | Contact Resistance(Ω) | Repeatability (CV, %) | Contact Area (mm2) |
|---|---|---|---|---|---|---|
| Leaf | Hydrogel Patch | Bipolar | 48.2 ± 1.8 | 950 ± 120 | 2.1 ± 0.4 | 3.14 |
| Tetrapolar | 46.1 ± 1.9 | 1050 ± 140 | 2.6 ± 0.5 | 3.14 | ||
| Clip Ag/AgCl | Bipolar | 42.8 ± 2.3 | 1280 ± 170 | 3.4 ± 0.7 | 3.14 | |
| Tetrapolar | 39.6 ± 2.6 | 1450 ± 195 | 4.1 ± 0.9 | 2.83 | ||
| Fruit | Conical Screen Printed | Bipolar | 47.8 ± 1.6 | 890 ± 115 | 2.3 ± 0.4 | 7.07 |
| Tetrapolar | 45.2 ± 1.8 | 1020 ± 135 | 2.7 ± 0.5 | 7.07 | ||
| Adhesive Patch | Bipolar | 41.3 ± 2.2 | 1320 ± 185 | 3.5 ± 0.8 | 2.83 | |
| Tetrapolar | 38.9 ± 2.5 | 1480 ± 210 | 4.2 ± 0.9 | 3.14 | ||
| Stem | Clip-on Ag/AgCl | Bipolar | 42.3 ± 2.1 | 1180 ± 165 | 3.3 ± 0.7 | 7.07 |
| Tetrapolar | 46.8 ± 1.7 | 920 ± 125 | 2.4 ± 0.5 | 5.31 | ||
| Clamp Ag/AgCl | Bipolar | 35.6 ± 2.8 | 1580 ± 245 | 5.1 ± 1.2 | 4.91 | |
| Tetrapolar | 40.1 ± 2.4 | 1350 ± 190 | 3.8 ± 0.9 | 2.83 |
| Organ | Frequency (kHz) | Cohen’s d | Mutual Information |
|---|---|---|---|
| Leaf | 0.5 | 1.34 | 0.847 |
| 1.0 | 1.28 | 0.823 | |
| 5.0 | 1.67 | 0.912 | |
| 10.0 | 1.45 | 0.876 | |
| 25.0 | 1.23 | 0.798 | |
| 50.0 | 1.18 | 0.765 | |
| Fruit | 1.0 | 1.12 | 0.734 |
| 10.0 | 1.41 | 0.856 | |
| 50.0 | 1.78 | 0.934 | |
| 75.0 | 1.65 | 0.898 | |
| 100.0 | 1.52 | 0.867 | |
| 200.0 | 1.34 | 0.812 | |
| Stem | 1.0 | 0.98 | 0.687 |
| 25.0 | 1.15 | 0.743 | |
| 100.0 | 1.34 | 0.798 | |
| 250.0 | 1.28 | 0.776 | |
| 500.0 | 1.22 | 0.754 | |
| 1000.0 | 1.08 | 0.712 |
| Application | Frequency Range | Penetration Depth (mm) | Spatial Resolution (mm) | Sensitivity | Performance Index |
|---|---|---|---|---|---|
| 3D Root Conductivity Mapping | 0.01–0.1 kHz | 70 ± 8 | 6.2 ± 0.8 | 0.85 | 0.68 |
| 0.1–10 kHz | 48 ± 3 | 3.8 ± 0.3 | 0.71 | 0.92 | |
| 10–100 kHz | 22 ± 3 | 2.7 ± 0.3 | 0.34 | 0.42 | |
| 2D Fruit Conductivity Localization | 0.1–1 kHz | 55 ± 6 | 5.1 ± 0.6 | 0.78 | 0.72 |
| 1–50 kHz | 28 ± 2 | 3.1 ± 0.2 | 0.62 | 0.94 | |
| 50–500 kHz | 12 ± 2 | 2.1 ± 0.3 | 0.23 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alejandrino, J.; Dadios, E.; Vicerra, R.R.; Bandala, A.; Sybingco, E.; Gan Lim, L.; Naguib, R.; Concepcion, R., II. Non-Invasive Multimodal and Multiscale Bioelectrical Sensor System for Proactive Holistic Plant Assessment. Technologies 2025, 13, 496. https://doi.org/10.3390/technologies13110496
Alejandrino J, Dadios E, Vicerra RR, Bandala A, Sybingco E, Gan Lim L, Naguib R, Concepcion R II. Non-Invasive Multimodal and Multiscale Bioelectrical Sensor System for Proactive Holistic Plant Assessment. Technologies. 2025; 13(11):496. https://doi.org/10.3390/technologies13110496
Chicago/Turabian StyleAlejandrino, Jonnel, Elmer Dadios, Ryan Rhay Vicerra, Argel Bandala, Edwin Sybingco, Laurence Gan Lim, Raouf Naguib, and Ronnie Concepcion, II. 2025. "Non-Invasive Multimodal and Multiscale Bioelectrical Sensor System for Proactive Holistic Plant Assessment" Technologies 13, no. 11: 496. https://doi.org/10.3390/technologies13110496
APA StyleAlejandrino, J., Dadios, E., Vicerra, R. R., Bandala, A., Sybingco, E., Gan Lim, L., Naguib, R., & Concepcion, R., II. (2025). Non-Invasive Multimodal and Multiscale Bioelectrical Sensor System for Proactive Holistic Plant Assessment. Technologies, 13(11), 496. https://doi.org/10.3390/technologies13110496












