Abstract
Cognitive fatigue, a state of reduced mental capacity arising from prolonged cognitive activity, poses significant challenges in various domains, from road safety to workplace productivity. Accurately detecting and mitigating cognitive fatigue is crucial for ensuring optimal performance and minimizing potential risks. This paper presents a comprehensive survey of the current landscape in cognitive fatigue detection. We systematically review various approaches, encompassing physiological, behavioral, and performance-based measures, for robust and objective fatigue detection. The paper further analyzes different challenges, including the lack of standardized ground truth and the need for context-aware fatigue assessment. This survey aims to serve as a valuable resource for researchers and practitioners seeking to understand and address the multifaceted challenge of cognitive fatigue detection.
1. Introduction
Cognitive fatigue, arising from prolonged and demanding cognitive activities [1], represents a ubiquitous and significant phenomenon in our modern lives. It involves varying degrees of mental exhaustion that can persist for hours to days, frequently emerging as a rebound effect following periods of mental exertion [2]. This phenomenon can result from a range of mentally taxing activities, from studying for exams and working long hours to engaging in complex problem-solving tasks. Reductions in executive functions, including executive attention [3,4], sustained attention [5,6,7], alternating attention [8], goal-directed attention [9], divided attention [10], response inhibition [11], and planning [12,13], are commonly associated with cognitive fatigue. As cognitive fatigue sets in, individuals often find it increasingly challenging to sustain their focus and perform at their best, which can lead to a cascade of adverse consequences.
Addressing cognitive fatigue clinically remains challenging, mainly due to the lack of well-defined variables that contribute to its understanding [14]. The duration spent on a task is commonly considered a significant predictor of cognitive fatigue [15,16,17,18]. However, it is important to acknowledge that this predictor alone may not fully account for the variability in cognitive fatigue levels. While prolonged task duration can indeed lead to increased cognitive fatigue in many cases [15,19], it is noteworthy that there are instances where extended time spent on a task can paradoxically result in improved performance. This phenomenon can be attributed to the learning effect, wherein individuals develop skills and strategies over time, leading to enhanced task performance despite the potential for cognitive fatigue. Therefore, it is essential for future research to consider additional factors beyond task duration to provide a more comprehensive understanding of cognitive fatigue dynamics.
Cognitive fatigue may be assessed either subjectively or objectively [20], as illustrated in Figure 1. Subjective cognitive fatigue involves an ongoing perception of exhaustion. Objective cognitive fatigue, referred to as fatigability, is measured by the change in cognitive performance relative to a baseline [21]. Subjective fatigue can be further categorized into trait and state components. State fatigue signifies a transient condition that may evolve over time and fluctuate based on both internal and external factors, while trait fatigue characterizes a relatively stable state in an individual and is not likely to undergo significant changes over time [22].
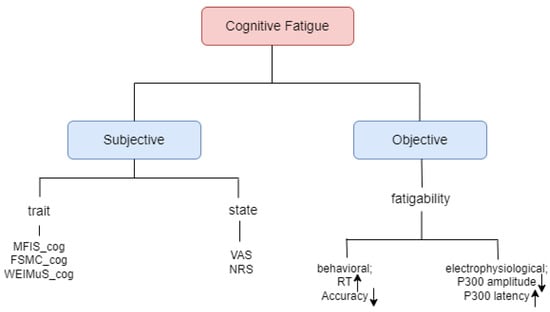
Figure 1.
Cognitive fatigue classification [20].
Assessing subjective trait fatigue can be performed through self-questionnaires, while subjective state fatigue can be measured using visual analog scales (VASs) or numerical rating scales. In contrast, objective fatigue (fatigability) is inherently state-dependent, allowing for an objective assessment through behavioral or electrophysiological parameters. Behavioral fatigue can be evaluated by observing alterations in reaction time and accuracy during simple alertness or vigilance tests conducted over a period. Several studies have demonstrated a trend towards lengthening reaction times [6,23,24,25], alongside declining accuracy [26,27,28,29], as the duration of the task progresses. On the other hand, a study by Pokryszko-Dragan et al. [30] delved into alterations in P300, an event-related potential (ERP) component associated with cognitive processing, in patients. They discovered that prolonged latencies and diminished amplitudes of P300 were linked to heightened subjective cognitive fatigue among the participants. To sum up, cognitive fatigue can be examined both qualitatively as a subjective phenomenon and quantitatively as an objective phenomenon [20]. Studies have been conducted over time to examine both state and trait fatigue across age and gender. The research paper [31] shows that there is no observed correlation between age or gender and trait fatigue. Nevertheless, in the case of state fatigue, an increase in age was linked to a lower level of fatigue.
Cognitive detection techniques, at the intersection of psychology, neuroscience, and technology, have emerged as a pivotal field with profound implications for understanding, monitoring, and enhancing human cognitive processes. These techniques encompass a diverse array of methods and tools designed to assess and measure various facets of cognitive function, including attention, memory, problem-solving, decision-making, and emotional states. In an era marked by unprecedented technological advancements, the development and refinement of cognitive detection techniques have provided a window into the complexities of the human mind, offering insights that extend across numerous domains, from healthcare to education and beyond. The human cognitive system is a marvel of complexity, and its functioning underpins virtually every aspect of daily life. Consequently, the ability to detect, assess, and ultimately enhance cognitive processes holds immense significance. Cognitive detection techniques have evolved from rudimentary observations to sophisticated methodologies that leverage cutting-edge technologies such as neuroimaging, wearable devices, machine learning, and artificial intelligence. These techniques empower researchers, clinicians, educators, and individuals themselves to gain a deeper understanding of cognitive functioning and its dynamic interplay with various external and internal factors.
In this comprehensive exploration of cognitive detection techniques, we delve into the diverse landscape of methods, tools, and applications that characterize this burgeoning field. We examine how cognitive detection techniques have transcended traditional boundaries, fostering interdisciplinary collaborations that offer fresh perspectives on cognition and its associated challenges. As we embark on this journey, it becomes increasingly evident that the integration of cognitive detection techniques into our daily lives has the potential to unlock new dimensions of human potential, fostering well-being, productivity, and overall cognitive enhancement.
2. Impacts on Daily Life
The impact of cognitive fatigue extends to various aspects of human life. In the realm of road safety, cognitive fatigue is a substantial concern. Fatigue among drivers significantly contributes to the occurrence of road accidents [32]. Reaction times become slower, attention wanes, and hazard perception becomes compromised, putting both the fatigued individual and others in potentially life-threatening situations. A study [33] shows that individuals tend to commit more errors as cognitive fatigue increases due to a reduction in perceptual sensitivity. To counteract this, they tend to adopt a more cautious response bias. This underscores the importance of recognizing the signs of cognitive fatigue and taking appropriate measures, such as taking breaks or ensuring adequate rest, to mitigate its impact on driving and public safety.
Beyond road safety, cognitive fatigue has far-reaching implications. It can impair athletic performance [34], affecting an athlete’s ability to make split-second decisions, maintain physical coordination, and execute complex strategies. In professional and personal decision-making, cognitive fatigue can lead to suboptimal choices and hinder problem-solving abilities [35]. Moreover, in the context of health, cognitive fatigue can exert a negative impact on diverse medical conditions, including multiple sclerosis [23,26,36], Parkinson’s disease [37], and traumatic brain injury [2], where it can exacerbate the challenges of coping with these illnesses. A study [38] by Wylie et al. showed that individuals who have suffered a traumatic brain injury (TBI) experience similar levels of cognitive fatigue (CF) as those with multiple sclerosis (MS), but both groups reported higher levels of CF compared to healthy control individuals.
People experiencing cognitive fatigue often find it challenging to focus their attention on tasks for extended periods [1,39] and may become easily distracted [39,40], which can hinder productivity and task completion. Cognitive fatigue can also result in slower information processing, causing individuals to take more time to comprehend and respond to stimuli [41]. Complex problem-solving becomes more arduous, leading to delays and potential errors. This sluggishness in cognitive processing can hinder quick thinking and decision-making, which is particularly problematic in situations requiring rapid responses.
A study conducted by Boksem et al. [9] revealed that cognitive fatigue significantly affects behavior by impairing the efficient allocation of attention among fatigued individuals. However, it is essential to discern the impact of cognitive fatigue on two distinct types of attention: goal-directed and stimulus-driven. While cognitive fatigue notably hampers goal-directed attention, its influence on stimulus-driven attention is relatively limited. This differentiation sheds light on the heightened susceptibility to distractions and reduced adaptability observed in fatigued individuals.
In summary, addressing cognitive fatigue extends beyond mere enhancements in daily productivity; it encompasses the vital aspects of safeguarding well-being, optimizing performance, and promoting public safety. Understanding the signs of cognitive fatigue and implementing effective management strategies are essential for navigating the demands of our modern, complex, and mentally taxing world. By prioritizing strategies to combat cognitive fatigue, individuals can strive for greater resilience, improved decision-making, and enhanced overall quality of life, ultimately contributing to a safer and more productive society.
3. Causes of Cognitive Fatigue
Cognitive fatigue can result from various causes [42]. It typically develops gradually over time as a person engages in prolonged and demanding mental activities. One primary cause is the sustained mental effort required for tasks like studying for exams, working on complex projects, or solving intricate problems. The brain consumes significant energy and resources during such activities, leading to exhaustion if not managed effectively [43]. Another crucial contributor to cognitive fatigue is the lack of adequate sleep. Sleep is essential for the brain to consolidate memories, repair tissues, and remove waste products. When sleep is disrupted or insufficient, these vital processes are compromised, leaving the brain less capable of optimal functioning. Chronic sleep deprivation is a significant factor in persistent cognitive fatigue [44].
Stress and anxiety are emotional states that can also lead to cognitive fatigue. Chronic stress triggers the release of stress hormones like cortisol, which can disrupt normal brain function and result in mental exhaustion. Multitasking, attempting to handle multiple tasks simultaneously, can tax cognitive resources and cause fatigue as the brain constantly switches between tasks [45]. Information overload in our digital age, with constant streams of information from smartphones, social media, and news outlets, can overwhelm the brain, contributing to cognitive fatigue [46]. Fatigue and perceived stress have significant negative effects on participants’ learning and cognitive performance [47].
Physical factors like dehydration, poor nutrition, and sedentary lifestyles also play a role, as the brain relies on a steady supply of nutrients and oxygen for optimal function [48]. Certain medical conditions, including sleep disorders [49], chronic pain [50], neurological disorders [51], and cancer-related fatigue, commonly observed in patients during and after cancer treatments [52,53,54], can directly contribute to cognitive fatigue by impairing the brain’s ability to function effectively. Medications and substances, such as certain antidepressants [55], antihistamines [56], and alcohol [57], can have sedating or cognitive-dulling effects, leading to mental fatigue [58]. Lifestyle choices, like excessive caffeine consumption [59], irregular sleep patterns [49], and a lack of physical activity [60], can disrupt the body’s natural rhythms, which can significantly impact cognitive function, potentially leading to cognitive fatigue. Recognizing the various causes of cognitive fatigue is essential for effective prevention and management. Addressing these causes often involves lifestyle changes, stress management, improving sleep hygiene, and seeking medical attention for underlying conditions when necessary. By identifying and mitigating these factors, individuals can reduce the risk of cognitive fatigue and maintain optimal cognitive function and overall well-being.
4. Measurement and Assessment Techniques
4.1. Physiological Indicators
Physiological markers provide measurable insights into the manifestation of cognitive fatigue. Elevated levels of stress hormones, particularly cortisol, indicate a physiological response to increased mental exertion. Fluctuations in heart rate variability, pupillometry changes, and altered skin conductance reveal the autonomic nervous system’s intricate adjustments during periods of cognitive fatigue. Additionally, variations in respiratory rate, blood pressure, and muscle activity contribute to a comprehensive understanding of the physiological changes associated with cognitive fatigue.
4.1.1. Pupillometry
Pupillometry has evolved into a valuable tool for assessing cognitive and emotional load that extends beyond its initial role in assessing responses to changes in light conditions. As cognitive tasks become more demanding, the pupil’s size consistently increases, a phenomenon observed across a diverse array of cognitive activities such as Stroop interference tasks [61], information retrieval from memory [62], visual search [63], and cognitive control [64], among others. Over the past two decades, there has been a resurgence of pupillometry research driven by its cost-effectiveness and precision, as exemplified in studies by [64,65,66].
Pupillometry was typically limited to controlled laboratory settings or specific scenarios like simulated piloting and driving, requiring subjects to stay still with a restricted field of vision due to potential eye-tracking system limitations [67]. Pupillometry is most suitable for workplaces where employees predominantly sit [68]. However, the use of head-mounted eye-tracking systems offers a promising path toward fully mobile mental workload measurement [69]. Recent technological advancements have made this eye-tracking method more compact by integrating eye-tracking cameras into eye-glass frames, allowing researchers and practitioners to measure pupil size in mobile work settings where individuals have freedom of movement [70].
Pupillometry has found applications in detecting cognitive fatigue during various scenarios. Studies have shown increased pupil diameter in scenarios involving excessive cognitive demands among drivers [67,69], air traffic controllers [71,72], and individuals engaged in simulated piloting tasks [73].
4.1.2. Heart Rate Variability
Heart rate variability (HRV) has become a measure for evaluating fatigue because of its connection to autonomic nervous system activity and its potential to reflect changes related to cognitive fatigue [74]. HRV quantifies the variation in time intervals between heartbeats, which indicates the activity of the system (ANS) [75]. It specifically measures the balance between the sympathetic (fight-or-flight) and parasympathetic (rest-and-digest) branches of the ANS [76,77]. When experiencing fatigue, there is usually an increase in sympathetic activity and a decrease in parasympathetic activity, leading to reduced HRV. Therefore, changes in HRV can provide insights into aspects of cognitive fatigue [78]. Research has indicated that prolonged cognitive tasks or mental exertion can cause a decrease in HRV [79].
Some studies indicate a decline in high-frequency (HF) power during episodes of cognitive fatigue [80,81,82], signaling a dampening of parasympathetic activity. This decline implies a transition towards sympathetic dominance, which correlates with the heightened arousal and stress commonly observed during cognitive fatigue. On the other hand, certain studies [82,83] have reported increased low-frequency (LF) power during cognitive tasks. Elevated LF power is often associated with heightened sympathetic arousal, suggesting an intensified physiological response to cognitive demands.
One of the advantages of using HRV as an indicator for assessing fatigue is its non-invasive nature. Measuring HRV can be performed through methods such as electrocardiography (ECG) and photoplethysmography (PPG). Even using consumer-grade wearables makes it easily accessible for both research purposes and practical applications [77].
Monitoring HRV in real-time makes it possible to assess individuals’ cognitive fatigue levels during tasks. This allows for interventions at the time to optimize performance and reduce the chances of making cognitive fatigue-related errors in different fields like aviation, healthcare, and transportation [84,85]. Analyzing HRV can detect changes in nervous system activity that may not be easily noticeable through other methods. This sensitivity makes it valuable for identifying signs of fatigue before performance declines significantly [86].
However, one important challenge in using HRV as an indicator for assessing fatigue is the variation among individuals. People may have different baseline HRV levels, and their HRV responses to fatigue might vary. This creates difficulty in establishing HRV thresholds for assessing fatigue. Additionally, factors like activity, stress levels, sleep quality, and overall health conditions, apart from cognitive fatigue, can influence HRV. These factors can complicate the interpretation of HRV data when evaluating fatigue [87].
4.1.3. Skin Conductance
Skin conductance, which measures the conductivity of the skin, has been studied as a way to assess fatigue. It is also known as electrodermal activity (EDA) or galvanic skin response (GSR) [88]. Skin conductance is primarily influenced by the activity of sweat glands, which are controlled by the system’s sympathetic branch. When someone experiences cognitive fatigue, changes in their nervous system activity can be seen through skin conductance [89]. As cognitive fatigue sets in, the sympathetic nervous system becomes more active, resulting in increased sweat gland activity and higher levels of skin conductance [90]. Research studies have demonstrated a correlation between skin conductance and cognitive fatigue. For instance, studies conducted by Posada Quintero and Chon [91] showed that prolonged computer tasks leading to fatigue were associated with increased skin conductance levels.
Skin conductance provides real-time data that allow for the noninvasive assessment of cognitive fatigue [85,92]. This can be especially valuable in high-stress environments like aviation, healthcare, and emergency response situations. Skin conductance is a measure that does not rely on self-reporting, reducing biases and improving the accuracy of assessing cognitive fatigue. Measuring skin conductance is a non-intrusive method that participants usually find comfortable, making it applicable in many different situations.
However, relying on skin conductance as an indicator for assessing fatigue has certain limitations. It can be affected by stimuli beyond cognitive fatigue. Emotional arousal, stress, and environmental factors can all influence skin conductance levels, which may lead to misleading interpretations [93,94].
4.1.4. Cortisol Level
Scientists have examined indicators to evaluate cognitive fatigue, and one particular candidate is cortisol, a hormone that is released in response to stress. Cortisol, which is produced by the adrenal glands, plays a role in the body’s stress response system. When we experience stress, cortisol levels increase to help us mobilize resources and deal with the situation [47,95]. Long-term stress or extended periods of exertion have been associated with changes in cortisol secretion, which has prompted investigations into its connection with fatigue. Previous studies have indicated a correlation between levels of cortisol and cognitive fatigue [47,96]. Engaging in prolonged tasks or being exposed to situations often leads to an increase in cortisol production, potentially contributing to mental exhaustion and a decline in cognitive performance. Research using stress-inducing scenarios or continuous cognitive engagement has demonstrated an elevation in cortisol levels, suggesting its association with fatigue [97].
When evaluating cortisol levels, it is typical to analyze parameters such as the cortisol awakening response (CAR) or the area under the curve (AUC), which are calculated from cortisol measurements taken at various points throughout the day [98,99,100]. Together, these metrics contribute to a nuanced evaluation of the interplay between cortisol regulation, stress, and cognitive function, aiding in the assessment of cognitive fatigue levels and their impact on daily performance.
Cortisol levels provide a measure that is closely linked to our body’s response to stress, offering a physiological basis for assessing cognitive fatigue. Furthermore, it can be measured in real-time, enabling the evaluation of cognitive fatigue during tasks or when facing stressful situations [101]. Unlike self-reports, measuring cortisol levels provides a marker that reduces potential biases when assessing cognitive fatigue. However, there are limitations to consider. Cortisol levels can differ greatly among individuals due to factors like the body’s clock, age, and overall health status. This makes it difficult to establish thresholds for determining fatigue. Additionally, cortisol levels tend to vary throughout the day. Stress-related spikes may not necessarily align with fatigue, potentially causing misinterpretation. Moreover, external factors such as caffeine consumption, physical activity, or medications can also impact cortisol levels, further complicating the analysis [102].
4.1.5. Respiratory Rate
The rate at which we breathe, known as the respiratory rate, may be linked to fatigue. Research has shown that when engaging in tasks, people tend to change their breathing patterns. Long periods of activity often lead to an increase in rates as our bodies try to cope with the heightened demands on our brains. This connection between the brain and the respiratory centers in the brainstem means that changes in load can affect our breathing patterns and vice versa. Additionally, individuals experiencing fatigue have been observed to exhibit breathing patterns like an increased respiratory rate or irregular breathing. These changes could potentially serve as indicators of the onset and progression of fatigue [103]. By monitoring our rate in time, we have a promising way of assessing cognitive fatigue promptly and making necessary adjustments or interventions in tasks or environments to minimize its impact [104].
The great thing about measuring the respiratory rate is that it does not need complex methods; it can be easily performed using simple tools like wearable devices or sensors. This accessibility allows for monitoring in multiple settings. Real-time data from measuring the respiratory rate give us feedback so that we can intervene or make adjustments during activities to prevent or alleviate cognitive fatigue effectively [105]. Unlike self-reported measures, which are subjective, the respiratory rate provides an objective physiological marker, reducing the potential biases associated with subjective assessments. However, factors such as fitness, age, and environmental conditions can have an impact on respiratory rate, which can make it difficult to associate it with cognitive fatigue directly. It is important to note that an increased respiratory rate can be caused by factors other than cognitive fatigue, such as physical exertion, stress, or medical conditions [106]. This means that we should not rely solely on respiratory rate to indicate cognitive fatigue.
4.1.6. Blood Pressure
Various studies have shown a connection between blood pressure and cognitive fatigue. It has been observed that fluctuations in blood pressure often occur alongside episodes of fatigue. When we experience increased strain or prolonged mental exertion, changes in both diastolic and systolic blood pressure levels have been recorded. Various studies have revealed a link between cognitive demands and higher blood pressure, indicating that changes in blood pressure might be an indicator of cognitive fatigue [107,108]. Moreover, engaging in tasks can activate the sympathetic nervous system, causing an increase in blood pressure as a physiological response [109]. On the other hand, research suggests that prolonged cognitive fatigue may lead to increased blood pressure due to exhaustion and reduced physiological arousal [16,27]. This two-way relationship between fatigue and blood pressure highlights the potential of using blood pressure as an indicator to assess fatigue [110].
Using blood pressure as an index for assessing fatigue offers significant advantages. Its non-invasive measurement allows for regular monitoring and provides real-time data during tasks or activities. This immediate feedback enables interventions or breaks to manage cognitive fatigue. Furthermore, blood pressure serves as an indicator of one’s state, ensuring impartial measurements and providing a quantitative framework to evaluate levels of cognitive fatigue. However, using blood pressure to gauge fatigue has its limitations. It may be influenced by factors such as stress or physical exertion, which can complicate the interpretation [107]. Additionally, individual variations in responses and changes in the timing of blood pressure in relation to cognitive fatigue symptoms present challenges in determining universal thresholds and precise assessment [110,111]. To address these limitations and improve the accuracy of fatigue evaluation tools, it is crucial to incorporate other markers alongside blood pressure measurements.
4.1.7. Muscle Activity
Research has shown that there is a connection between increased effort and muscle tension, especially in the upper body. When we engage in tasks that require demand, our muscles tend to become tenser, highlighting the close relationship between our cognitive and physiological systems [112]. Studies using electromyography (EMG) have demonstrated increased muscle activity during demanding tasks, suggesting a potential link between cognitive load and muscular response. Moreover, prolonged mental engagement has been associated with muscle fatigue, which can be detected through signal changes. This suggests that as we experience fatigue, changes in muscle activity patterns may serve as an indicator of exhaustion, offering a way to assess cognitive fatigue indirectly by measuring muscle activity [113].
By using muscle activity as an indicator to assess fatigue, we can gain insights into the physical manifestations of mental tiredness without relying solely on subjective self-assessment [114]. Real-time monitoring of muscle activity provides information that allows for interventions to alleviate cognitive fatigue. Additionally, combining muscle activity data with reports enhances our understanding of the relationship between physiological responses and cognitive exhaustion on a more comprehensive level [115]. However, it is important to note that interpreting patterns of muscle activity requires knowledge of electromyography, which limits its applicability without expertise for accurately deciphering complex physiological data.
Moreover, there are diverse ways in which muscles respond to individuals’ difficulties when trying to establish accepted standards for evaluating cognitive fatigue. This can result in variations in how fatigue levels are interpreted among people. Furthermore, the impact of factors like exertion or emotional stress on muscle activity, which goes beyond fatigue, and the presence of physical fatigue add further complexities and potential variables that need to be considered during the assessment process [42].
4.2. Behavioral Indicators
Behavioral markers provide observable signs that offer insights into the behavioral consequences of cognitive fatigue. Indicators of cognitive fatigue include delayed reaction times, reduced accuracy, extended task completion, microsleep episodes, and repetitive behaviors. Self-reported fatigue scores provide subjective information on an individual’s feelings of exhaustion and mental weariness. Furthermore, microsleep episodes, which are defined as brief periods of unplanned and unrecognized sleep, are widespread during cognitive fatigue, impacting attention and performance. Repetitive behaviors, such as tapping fingers or making the same mistakes, frequently emerge as the mind attempts to maintain focus and effectively deal with cognitive demands.
4.2.1. Reaction Time
Reaction time, which refers to the time it takes for a person to respond after being presented with a stimulus, is closely connected to our processes. Research suggests that engaging in prolonged activities can result in fatigue, leading to changes in reaction time [116]. Cognitive fatigue often presents itself in reaction times, indicating a delayed or impaired ability to process information due to mental exhaustion [117]. Studies have found a link between increased fatigue and longer reaction times across various tasks like attention, memory, decision-making, and motor responses [6]. For example, individuals experiencing fatigue may exhibit delayed responses when it comes to tasks that require sustained attention or complex decision-making, highlighting the impact of fatigue on our thinking abilities [118].
Using reaction time as an indicator for assessing fatigue offers advantages. Firstly, measuring reaction time is relatively simple and non-invasive, making it convenient for both lab-based research and real-world settings. Additionally, because reaction time is sensitive to changes caused by fatigue, researchers and professionals can track changes in performance over time and take early measures when needed [119]. Furthermore, analyzing reaction time provides data that allow for comparisons between individuals and different experimental conditions. This quantitative aspect helps make assessments of fatigue more objective and aids in establishing evaluation methods across different populations and contexts [113,120].
However, despite how useful it is, using reaction time as a way to assess fatigue has its limitations. First and foremost, reaction time alone may not solely indicate fatigue; other factors, like motivation, arousal levels, and individual differences, can also impact reaction time, which could potentially complicate the interpretation of results [120]. Additionally, while reaction time does capture changes in how our brains process information, it might not fully capture the aspects of cognitive fatigue, such as personal experiences or specific areas of thinking that are affected [119]. This limitation emphasizes the importance of an assessment that considers measures in order to gain a well-rounded understanding of cognitive fatigue.
4.2.2. Accuracy
The relationship between accuracy and cognitive fatigue is quite complex. Research suggests that as fatigue increases, accuracy tends to decrease. This is because fatigue can negatively impact attention, working memory, decision-making abilities, and reaction times. As a result, tasks that require engagement may be prone to errors and reduced accuracy [28,121]. As we become mentally exhausted, we tend to make errors, have lapses in judgment, and struggle with accuracy when completing tasks. Various studies using tasks like reaction time tests, memory assessments, and decision-making exercises consistently demonstrate that errors rise as fatigue accumulates [122,123]. A study conducted by Boksem and his colleagues [124] found that prolonged cognitive tasks led to decreased accuracy due to increased fatigue. Similarly, research on the effects of sleep deprivation by Van Dongen et al. [125] demonstrated a decline in accuracy across tasks. These studies highlight a connection between fatigue and diminished accuracy.
Using accuracy as an indicator for assessing fatigue offers advantages. Firstly, it provides a quantifiable measure that allows for evaluation across different individuals and cognitive tasks [126]. This objectivity enhances comparability and consistency when evaluating performance, enabling researchers and professionals to track changes over time or compare interventions. Additionally, accuracy often serves as a warning sign of fatigue, allowing for timely interventions and adjustments to mitigate its impact. Monitoring real-time accuracy levels during tasks provides a way to proactively address fatigue, potentially preventing more significant declines in performance [123].
Relying only on accuracy to assess fatigue has its limitations, despite its usefulness. The context in which tasks are performed can greatly affect accuracy, with task complexity, novelty, and familiarity skewing interpretations of fatigue levels. Furthermore, individual differences in performance can complicate the establishment of a threshold for assessing fatigue based on accuracy. Contextual factors like stress, environmental conditions, and task complexity can also independently affect error rates, introducing confounding variables that make it difficult to attribute changes to cognitive fatigue [127]. Managing these factors becomes crucial for interpreting error rates when assessing cognitive fatigue.
4.2.3. Task Completion Time
Task completion time often correlates with cognitive fatigue levels. Prolonged engagement in cognitive tasks can lead to reduced attention, impaired decision-making, and slower information processing, ultimately elongating the time required to complete tasks. Studies, such as those employing reaction time tests or complex cognitive tasks, have shown a direct association between increased cognitive fatigue and prolonged task completion time [43]. Moreover, cognitive fatigue adversely affects executive functions, memory, and attentional resources, impacting task efficiency. As cognitive fatigue escalates, individuals may experience diminished cognitive abilities, leading to errors, decreased accuracy, and a heightened need for breaks during task execution. These factors collectively contribute to a lengthened task completion time [128,129].
Task completion time serves as an objective measure, offering a quantifiable metric that enables straightforward comparisons across individuals and tasks. Moreover, its cost-effectiveness and non-invasive nature make it easily implementable in various settings, enabling continuous monitoring of cognitive fatigue without significant resource investment [130]. However, the multifaceted nature of cognitive fatigue poses challenges. Task completion time alone is an incomplete indicator of cognitive fatigue, as it can be affected by factors such as emotional state, sleep quality, and individual differences. Additionally, the dependency of task completion time on the nature and complexity of tasks introduces variability, making it challenging to use as a universal index for cognitive fatigue. Furthermore, external factors like motivation or environmental distractions can confound the accuracy of task completion time as a sole measure of cognitive fatigue, limiting its reliability [131]. Considering these limitations, integrating task completion time within a comprehensive framework may enhance its utility in assessing cognitive fatigue.
4.2.4. Self-Reported Fatigue Scales
Self-reported measures of fatigue have the potential to be tools for assessing fatigue, but it is important to examine their effectiveness, advantages, and limitations thoroughly. These measures, such as the Multidimensional Fatigue Inventory (MFI) [132,133,134], Fatigue Severity Scale (FSS) [135,136,137], and Visual Analog Scale (VAS) [138], aim to capture experiences of fatigue that encompass mental and emotional aspects. These scales correlate with cognitive fatigue due to their ability to capture perceived tiredness, mental weariness, and decreased motivation, all of which are components of cognitive fatigue. Research has shown correlations between scores on self-report measures of fatigue and performance on tasks, suggesting that self-report measures could be useful as indicators of cognitive fatigue [139].
One advantage of self-report measures is their accessibility and ease of use, making them suitable for application in multiple research settings. They provide insights into an individual’s experience by capturing dimensions of fatigue, including its cognitive aspects, that may not be evident through objective measures alone. Moreover, these measures allow for the tracking of fatigue levels over time, which can help evaluate the effectiveness of interventions or treatments. However, relying on perceptions introduces variability due to differences in how people report and perceive their own fatigue levels. This subjective nature presents difficulties when it comes to ensuring accuracy and dependability, which can have an impact on the reliability of assessments. Additionally, the absence of specific indicators for fatigue and reliance on individuals’ feelings on self-reported scales restrict their precision and validity. External factors like mood, motivation, and contextual influences can also introduce bias in self-reporting, potentially leading to inaccuracies in fatigue evaluations [140,141].
4.2.5. Microsleep Episodes
Episodes of microsleep, which are involuntary periods of sleep lasting from a fraction of a second to seconds, have been linked to cognitive fatigue [142]. These episodes are often characterized by lapses in attention and awareness. Research indicates that individuals who experience fatigue are more prone to experiencing microsleep, especially when engaging in prolonged tasks that require sustained attention [143]. On the other hand, cognitive fatigue is associated with feelings of tiredness, reduced motivation, and impaired cognitive abilities [144,145]. Studies have shown that cognitive fatigue can negatively impact attention, memory, decision-making, and reaction times [42]. The occurrence of episodes aligns with these impairments, suggesting a possible connection between the two phenomena.
Microsleep episodes provide a measurable way to assess fatigue. They give data about the frequency and duration of attention lapses, which allow for the monitoring and early detection of cognitive decline [146]. Detecting microsleep in time also provides feedback, helping to identify critical periods of fatigue. This valuable insight can lead to interventions that prevent errors or accidents in safe environments [147]. However, relying on episodes to assess cognitive fatigue has limitations. Contextual factors like task characteristics and environmental conditions can influence the occurrence of microsleep, making it challenging to differentiate between fatigue-related episodes and those caused by other factors. Subjective interpretation is also a drawback when using technologies that require judgment to distinguish between microsleep and momentary lapses in attention [148,149].
4.2.6. Repetitive Behaviors
Repetitive actions cover a range of behaviors that people often engage in, such as pacing, tapping fingers, or following rituals. These behaviors are commonly seen as coping mechanisms when dealing with stress, anxiety, or cognitive overload. Research has shown a link between the occurrence of actions and cognitive fatigue. When individuals experience fatigue, they may resort to actions as a way to regulate themselves or handle the overwhelming mental demands. This suggests that repetitive behaviors can be used as an indicator to assess someone’s state [150].
There are advantages to using repetitive behaviors as a potential measure of cognitive fatigue. Firstly, they provide a measurable aspect that allows for observation and recording, enhancing objectivity in assessment. This tangibility makes it easier for clinical practitioners and researchers to measure and evaluate behaviors consistently [151]. Additionally, these behaviors often occur before obvious signs of fatigue appear, providing an opportunity for early detection and intervention. This system for detection could help provide support and strategies to minimize the impact of mental exhaustion on a person’s daily functioning. Additionally, since repetitive behaviors can be easily observed in situations and age groups without methods, they offer a relatively convenient and cost-effective way to assess cognitive fatigue.
However, there are limitations to using behaviors as an indicator for assessing cognitive fatigue. One significant challenge is the nature of interpreting these behaviors. The definition of what qualifies as a behavior and its intensity may vary among individuals, cultural backgrounds, and contexts, which can lead to variations in assessment results. Moreover, while repetitive behaviors can indicate fatigue, they are not specific enough because factors like anxiety or habitual tendencies can also cause these behaviors. This lack of specificity raises concerns about attributing these behaviors to cognitive fatigue, which could potentially lead to misinterpretations during assessments. Additionally, contextual variations in how repetitive behaviors manifest complicate their interpretation; therefore, it is crucial to consider cultural factors and individual differences when developing assessment protocols [151,152].
5. Neurophysiological Approaches
Neurophysiological approaches are pivotal in the realm of cognitive neuroscience, particularly in unraveling the complex dynamics of cognitive fatigue. These methods provide an invaluable window into the brain’s functioning, revealing the intricate patterns of neural activity that underpin mental exhaustion. Among the most prominent techniques in this field are electroencephalography (EEG), functional magnetic resonance imaging (fMRI), and functional near-infrared spectroscopy (fNIRS), each offering unique insights into how our brains respond to prolonged cognitive demands. This section delves into the contributions of these methodologies, shedding light on their effectiveness in detecting and understanding the neurophysiological underpinnings of cognitive fatigue.
Recent studies indicate that the neural mechanisms driving mental fatigue during cognitive tasks are more complex than previously believed, challenging the traditional understanding that mental fatigue arises solely from diminished activity in brain regions relevant to the task. Accumulating evidence suggests the existence of mental facilitation and inhibition systems, which intricately modulate cognitive task performance by regulating the activity of task-relevant brain regions. These systems are essential components of the neural mechanisms underlying mental fatigue, significantly influencing cognitive task execution [153].
EEG stands as a pivotal tool in cognitive fatigue research, offering real-time insights into brain activity alterations due to fatigue. This technique measures the brain’s electrical activity, with a particular focus on fluctuating patterns within specific frequency bands. EEG signals provide fundamental information about brain states and cognitive function, where the analysis of different frequency bands in the EEG can reveal insights into alertness, attention, workload, and emotion [154]. Research indicates that cognitive fatigue manifests as distinct changes in these frequencies: a notable increase in theta waves (4–7 Hz) and a concurrent decrease in alpha waves (8–12 Hz). These alterations are considered biomarkers of mental fatigue and cognitive strain. For instance, theta waves, commonly associated with drowsy or meditative states, tend to escalate as the brain struggles with prolonged cognitive demands. Conversely, alpha waves, which are linked to a relaxed but alert state, diminish, reflecting a reduction in cognitive vigilance. Studies have consistently shown that cognitive fatigue is marked by specific alterations in these frequencies, such as increased theta and decreased alpha activities, which are indicative of mental strain and exhaustion [155].
Studies using EEG have shown varying impacts of mental fatigue on the central nervous system. For example, Li et al. [156] found that mental arithmetic tasks induce mental fatigue, with the alpha frequency band’s division into alpha1 and alpha2 being crucial in fatigue studies. Different types of mental fatigue produced different alterations of the EEG variables, such as beta, alpha, and theta power densities on various electrodes. These alterations reflected the deterioration of multi-modal and high-level information processing in the brain due to mental fatigue [157]. Additionally, Trejo et al. [158] demonstrated that EEG could track mental fatigue development over time, with theta and alpha power increases correlating with mood and behavioral changes. A study by Wang et al [159] developed a real-time EEG-based system for detecting cognitive fatigue while driving. The system used power spectrum density (PSD) and sample entropy (SE) methods to detect cognitive fatigue in real-time.
fMRI, known for its high spatial resolution, is a tool that tracks brain activity by detecting changes associated with blood flow. It has proven particularly effective in identifying brain regions most affected by cognitive fatigue, as it can observe reduced activity in the fronto-parietal network during prolonged cognitive tasks, indicating fatigue [7].
fNIRS is a non-invasive optical brain monitoring technique that measures blood oxygenation changes, similar to fMRI. fNIRS estimates the concentration of hemoglobin from changes in the absorption of near-infrared light, allowing the measurement of cortical hemodynamic activity [160]. It offers the advantage of being less restrictive and more suitable for real-time monitoring compared to EEG and fMRI. However, it has a lower spatial resolution and limited depth of recording. Moreover, the effectiveness of the method is constrained by inherent variations among individuals. For instance, data quality is inherently lower for individuals with thick, dark hair compared to those who are bald. Similarly, variations in skin pigmentation, influencing light penetration through the skin, can result in systematic differences in data quality. Despite this, fNIRS is a promising tool for multimodal imaging studies, as it can be easily combined with other imaging modalities like fMRI and EEG to capitalize on the strengths of each method [161]. This study [162] highlights how fNIRS can be effectively used to assess mental workload and fatigue in operational settings. A system combining fNIRS with EEG has been developed to measure cognitive fatigue in pilots during both flight simulation and actual flight, utilizing metrics such as the EEG engagement ratio and wavelet coherence fNIRS-based metrics to classify fatigue levels [163].
Research suggests that functional connectivity among various brain regions, including the striatum of the basal ganglia, the dorsolateral prefrontal cortex (DLPFC), the dorsal anterior cingulate cortex (dACC), the ventro-medial prefrontal cortex (vmPFC), and the anterior insula, varies with the levels of cognitive fatigue. For instance, a study by Wylie et al. [164] found that as cognitive fatigue increased, functional connectivity between these regions and other frontal areas decreased, while connectivity with more posterior regions increased. Furthermore, the striatum, DLPFC, insula, and vmPFC were identified as central nodes or hubs within the fatigue network.
Together, these studies showcase the evolution and current capabilities of neurophysiological techniques for detecting cognitive fatigue. Comparing EEG, fMRI, and fNIRS, each method presents unique advantages and limitations in cognitive fatigue research. EEG offers real-time monitoring of brain activity, making it ideal for dynamic or longitudinal studies. However, its spatial resolution is limited compared to fMRI. In contrast, fMRI provides detailed spatial mapping of brain activity, which is essential for localizing specific brain regions affected by fatigue. Nonetheless, fMRI is less suited for real-time or long-duration monitoring due to its restrictive nature and susceptibility to movement artifacts. fNIRS strikes a balance, offering portability and the capability for real-time monitoring, though its spatial resolution and depth of penetration are inferior to fMRI.
6. Machine Learning and AI
Cognitive fatigue detection is a multidisciplinary field that benefits significantly from the integration of machine learning and artificial intelligence (AI) techniques. These technologies provide the foundation for developing predictive models and systems that can effectively monitor and detect cognitive fatigue. In this section, we explore the various ways in which machine learning and AI have been employed in this context and provide examples of specific models and techniques.
The analysis of electroencephalography (EEG) signals is a popular method for detecting cognitive fatigue. The integration of EEG with advanced machine learning techniques, especially deep learning approaches, has demonstrated considerable promise in this realm. A study of particular note employed a long short-term memory (LSTM) network—a variant of recurrent neural networks—to analyze EEG signals for predicting cognitive load [165]. This methodology surpassed the performance of other machine learning models such as random forest, AdaBoost, support vector machine, and extreme gradient boosting, attaining a remarkable accuracy of 87.1% in cognitive load recognition. In a separate investigation, EEG signals were coupled with convolutional neural networks (CNNs) to detect cognitive fatigue among construction workers [166]. Another significant study highlighted the efficacy of machine learning algorithms, particularly random forests, in conjunction with functional near-infrared spectroscopy (fNIRS) data. This approach successfully classified four levels of cognitive workload with an exceptional accuracy rate of approximately 99.99% [167]. Additionally, the application of CNNs in conjunction with EEG data has proven effective in identifying mental fatigue during language comprehension tasks, especially when combining frequency and entropy features [167]. These recent studies collectively underscore the prevalent use of EEG signals and machine learning for identifying or classifying cognitive fatigue and mental load across various domains. However, this field is not without its challenges. Key issues include the need for artifact removal from EEG data and the limitations posed by dataset sizes. Despite these hurdles, the synergy of EEG data and machine learning techniques has consistently shown its effectiveness in detecting and classifying cognitive fatigue.
The utilization of machine learning techniques to analyze eye movement and pupil dilation has emerged as a reliable method for detecting cognitive fatigue. Extensive research has been conducted in this domain, particularly in identifying cognitive fatigue or mental load through eye-tracking integrated with machine learning methodologies [168,169]. A noteworthy study in this area applied pupillometry alongside machine learning techniques, such as classification methods and convolutional neural networks (CNNs), to evaluate the cognitive workload of ultrasound operators. This was achieved by observing variations in pupil diameter during routine scans, which correlated with the complexity of ultrasound tasks and the operator’s level of expertise [170]. Another significant research effort employed machine learning algorithms, including k-nearest neighbors (KNN), random forest, and multilayer perceptron (MLP), to gauge cognitive workload intensities. This study leveraged eye-tracking data collected during a digit symbol test. The findings enhanced our understanding of brain functionality and mental fatigue, improved the quality of classifications with a reduced set of features, and offered insights into different levels of cognitive workload [171]. Additionally, a recent publication introduced COLET, an eye-tracking dataset designed for cognitive workload estimation. This study analyzed eye movements from 47 individuals engaged in tasks of varying complexity and employed machine learning for predicting workload levels. It revealed notable impacts of multitasking and time pressure on eye movement characteristics and achieved an accuracy of up to 88% in estimating workload levels [172].
Apart from EEG signals and eye movement, a fusion of physiological sensors and behavioral biometrics has been used to detect or classify cognitive fatigue. To obtain a more holistic perspective on cognitive fatigue, researchers have explored the fusion of data from various physiological sensors. Electrocardiogram (ECG), electrodermal (EDA), and electromyography (EMG) sensors provide valuable input. Machine learning and deep learning techniques like random forest, gradient boosting, and long short-term memory (LSTM) networks are then used to fuse and analyze these multimodal data, offering a comprehensive view of the user’s cognitive state [173,174,175,176]. Behavioral biometrics, specifically keystroke dynamics, have shown promise in identifying cognitive fatigue. Machine learning models can analyze changes in typing patterns, providing an indirect but informative indicator of the cognitive state [177,178].
Recognizing the emergence of immersive virtual reality (VR) in the realm of cognitive fatigue detection is paramount, as it offers a unique platform for conducting controlled experiments, enabling researchers to simulate various cognitive tasks and environmental conditions. Research conducted by Siravenha et al. [179] employed a residual multilayer perceptron (MLP) network known as ResMLPNet to evaluate its efficacy in the intricate task of classifying mental fatigue from cognitive electrophysiology data. The data were gathered during VR training sessions designed to replicate real-world scenarios encountered by excavator operators in the mining industry. In their study [180], Kamińska et al. developed a data acquisition protocol involving alternating sequences of relaxing and stressful scenarios presented via a VR interactive simulation. Following this, stress levels were classified using a convolutional neural network (CNN), and the classification performance was compared with that of traditional machine learning algorithms.
These examples illustrate the versatility of machine learning and AI techniques in the context of cognitive fatigue detection. Table 1 provides a comprehensive summary of machine learning techniques and their associated features utilized in cognitive fatigue detection over the last decade. It outlines their individual accuracies, the features utilized, and the classification methods applied, providing insights into the methodologies employed. The selection process involved considering techniques that have been utilized frequently or cited prominently in the literature, as well as those that have demonstrated promising results in cognitive fatigue detection tasks. While each method has its unique strengths and limitations, the integration of these approaches is crucial for developing comprehensive systems for real-time cognitive fatigue assessment. It is essential to note that the cited research articles represent only a fraction of the vast body of work in this field, emphasizing the growing importance and potential of AI-driven cognitive fatigue detection.

Table 1.
Cognitive fatigue detection techniques.
7. Research Gaps and Future Directions
Despite progress in cognitive fatigue detection, several research gaps hinder practical implementation. Cognitive fatigue arises from a complex interplay of factors, encompassing physical, social, environmental, and emotional influences [199]. While research has explored individual features like heart rate [200,201], eye movement [202], and EEG signals [203,204], a holistic approach integrating diverse features remains elusive. Datasets like Cogbeacon [205] showcase the multi-faceted nature of fatigue, but pinpointing individual triggers proves challenging due to inter-personal differences. This complexity necessitates sophisticated analysis techniques to disentangle and prioritize relevant features for robust fatigue detection.
Current fatigue detection often relies on bulky sensors like EEG headsets or eye-tracking glasses [163,206]. Such tools often restrict applicability to controlled lab settings, limiting real-world feasibility. Recent advancements in miniaturized sensors and wearables have shown promise [189,207], but challenges remain in balancing data quality with user comfort. Furthermore, the precision and reliability of identifying cognitive fatigue through wearables are lower compared to the detection of physical fatigue, primarily due to the influence of concurrent factors like emotions or exercise [105]. Research should prioritize the development of unobtrusive, portable sensing systems that capture diverse physiological and behavioral markers seamlessly during daily activities. Furthermore, cognitive fatigue manifests differently across contexts. Repetitive tasks like assembly lines induce distinct fatigue patterns compared to complex decision-making in high-risk environments like aviation [208,209]. This variability demands context-aware fatigue detection models that adapt to various task demands and environmental factors. Future research needs to address this challenge by building scenario-specific models trained on data collected in real-world settings representing diverse occupations and activities.
Exploring the integration of data from diverse sources, including physiological, behavioral, and environmental inputs, through the application of machine learning algorithms facilitates the creation of a comprehensive overview of individual fatigue states. This approach offers a more nuanced understanding by combining various data streams. In the realm of personalized fatigue models, the development of adaptive models becomes crucial to account for inherent differences in individuals’ responses to fatigue. By tailoring interventions based on personal sensitivities and fatigue profiles, these models enhance the effectiveness of fatigue management strategies, recognizing and addressing the unique needs of each individual. Efficient fatigue detection systems should be designed to consider the broader context of user activity, environmental factors, and task demands. This contextual awareness enables the provision of accurate and timely fatigue alerts, taking into account the specific circumstances influencing an individual’s cognitive state. Emphasizing research on miniaturized, wearable sensors is pivotal for unobtrusive fatigue monitoring. These sensors aim to seamlessly integrate into daily life, facilitating continuous and non-invasive monitoring of fatigue. The unobtrusive nature of these technologies enhances user compliance and allows for more naturalistic data collection.
Addressing the identified research gaps requires collaborative efforts across various disciplines, including neuroscience, computer science, engineering, and human factors psychology. Bridging these interdisciplinary gaps is crucial for advancing the development of robust, personalized, and context-aware cognitive fatigue detection systems. Ultimately, such systems have the potential to enhance safety, productivity, and overall well-being across diverse populations and scenarios.
8. Limitations of the Study
This survey on cognitive fatigue detection faces several limitations inherent to its scope and methodology. Given the vastness of the field, it is challenging to encompass every study or aspect comprehensively, potentially resulting in overlooked or omitted methodologies. Moreover, the survey may exhibit bias towards published research, potentially excluding relevant but unpublished studies or gray literature. Variability in the quality and reliability of included sources, such as small sample sizes or subjective measures, could impact the survey’s conclusions. Additionally, temporal constraints may limit the survey to studies published up to a certain date, potentially missing recent developments. Lastly, generalized interpretations of findings from individual studies may overlook nuanced or context-specific factors crucial to fully understanding cognitive fatigue detection.
9. Conclusions
In conclusion, cognitive fatigue stands as a pervasive and impactful aspect of modern life, gradually diminishing mental resources and affecting energy, motivation, and concentration. Linked to reductions in various executive functions, cognitive fatigue poses challenges to sustaining focus and optimal performance. The classification of cognitive fatigue, both subjectively and objectively, provides a nuanced understanding through trait and state components. The intersection of psychology, neuroscience, and technology has given rise to cognitive detection techniques, offering a profound window into the complexities of the human mind. These evolving methodologies, fueled by cutting-edge technologies, hold immense potential to enhance our comprehension of cognitive processes and contribute to well-being, productivity, and overall cognitive advancement in various domains of life. The integration of these techniques into daily life marks a promising journey toward unlocking new dimensions of human potential.
Author Contributions
Conceptualization, E.K. and H.R.P.; writing—original draft, E.K., H.R.P., S.N., A.H., A.R., H.R.N., and A.J.; writing—review and editing: E.K., H.R.P., S.N., A.H., A.R., H.R.N., A.J., and G.R.W.; supervision, F.M.; project administration, F.M.; funding acquisition, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the National Science Foundation grant 2226164.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boksem, M.A.; Tops, M. Mental fatigue: Costs and benefits. Brain Res. Rev. 2008, 59, 125–139. [Google Scholar] [CrossRef]
- Wylie, G.R.; Flashman, L.A. Understanding the interplay between mild traumatic brain injury and cognitive fatigue: Models and treatments. Concussion 2017, 2, CNC50. [Google Scholar] [CrossRef]
- Van Der Linden, D.; Frese, M.; Sonnentag, S. The impact of mental fatigue on exploration in a complex computer task: Rigidity and loss of systematic strategies. Hum. Factors 2003, 45, 483–494. [Google Scholar] [CrossRef]
- Holtzer, R.; Shuman, M.; Mahoney, J.R.; Lipton, R.; Verghese, J. Cognitive fatigue defined in the context of attention networks. Aging Neuropsychol. Cogn. 2010, 18, 108–128. [Google Scholar] [CrossRef]
- Dorrian, J.; Roach, G.D.; Fletcher, A.; Dawson, D. Simulated train driving: Fatigue, self-awareness and cognitive disengagement. Appl. Ergon. 2007, 38, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Langner, R.; Steinborn, M.B.; Chatterjee, A.; Sturm, W.; Willmes, K. Mental fatigue and temporal preparation in simple reaction-time performance. Acta Psychol. 2010, 133, 64–72. [Google Scholar] [CrossRef]
- Lim, J.; Wu, W.c.; Wang, J.; Detre, J.A.; Dinges, D.F.; Rao, H. Imaging brain fatigue from sustained mental workload: An ASL perfusion study of the time-on-task effect. Neuroimage 2010, 49, 3426–3435. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, D.; Frese, M.; Meijman, T.F. Mental fatigue and the control of cognitive processes: Effects on perseveration and planning. Acta Psychol. 2003, 113, 45–65. [Google Scholar] [CrossRef]
- Boksem, M.A.; Meijman, T.F.; Lorist, M.M. Effects of mental fatigue on attention: An ERP study. Cogn. Brain Res. 2005, 25, 107–116. [Google Scholar] [CrossRef]
- Van der Linden, D.; Eling, P. Mental fatigue disturbs local processing more than global processing. Psychol. Res. 2006, 70, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Endo, H.; Kizuka, T. Mental fatigue and impaired response processes: Event-related brain potentials in a Go/NoGo task. Int. J. Psychophysiol. 2009, 72, 204–211. [Google Scholar] [CrossRef]
- Lorist, M.M.; Klein, M.; Nieuwenhuis, S.; De Jong, R.; Mulder, G.; Meijman, T.F. Mental fatigue and task control: Planning and preparation. Psychophysiology 2000, 37, 614–625. [Google Scholar] [CrossRef]
- Lorist, M.M. Impact of top-down control during mental fatigue. Brain Res. 2008, 1232, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sandry, J.; Genova, H.M.; Dobryakova, E.; DeLuca, J.; Wylie, G. Subjective cognitive fatigue in multiple sclerosis depends on task length. Front. Neurol. 2014, 5, 214. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, P.L. (Ed.) 100 years without resting. In Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications; American Psychological Association: Washington, DC, USA, 2011; pp. 11–43. [Google Scholar]
- DeLuca, J. Fatigue, cognition, and mental effort. In Fatigue as a Window to the Brain; MIT Press: Cambridge, MA, USA, 2005; Volume 37. [Google Scholar]
- Kanfer, R. Determinants and consequences of subjective cognitive fatigue. In Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications; Ackerman, P.L., Ed.; American Psychological Association: Washington, DC, USA, 2011; pp. 189–207. [Google Scholar]
- Van Dongen, H.; Belenky, G.; Krueger, J.M. Investigating the temporal dynamics and underlying mechanisms of cognitive fatigue. In Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications; Ackerman, P.L., Ed.; American Psychological Association: Washington, DC, USA, 2011; pp. 127–147. [Google Scholar]
- Jensen, J.L.; Berry, D.A.; Kummer, T.A. Investigating the effects of exam length on performance and cognitive fatigue. PLoS ONE 2013, 8, e70270. [Google Scholar] [CrossRef] [PubMed]
- Linnhoff, S.; Fiene, M.; Heinze, H.J.; Zaehle, T. Cognitive fatigue in multiple sclerosis: An objective approach to diagnosis and treatment by transcranial electrical stimulation. Brain Sci. 2019, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Genova, H.M.; Rajagopalan, V.; DeLuca, J.; Das, A.; Binder, A.; Arjunan, A.; Chiaravalloti, N.; Wylie, G. Examination of cognitive fatigue in multiple sclerosis using functional magnetic resonance imaging and diffusion tensor imaging. PLoS ONE 2013, 8, e78811. [Google Scholar] [CrossRef] [PubMed]
- Claros-Salinas, D.; Bratzke, D.; Greitemann, G.; Nickisch, N.; Ochs, L.; Schröter, H. Fatigue-related diurnal variations of cognitive performance in multiple sclerosis and stroke patients. J. Neurol. Sci. 2010, 295, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Fiene, M.; Rufener, K.S.; Kuehne, M.; Matzke, M.; Heinze, H.J.; Zaehle, T. Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. J. Neurol. 2018, 265, 607–617. [Google Scholar] [CrossRef]
- Junior, A.F.; Chierotti, P.; Gabardo, J.M.; Giovanini, B.; Okano, A.H.; Buzzachera, C.F.; Okazaki, V.H.; Okuno, N.M.; Altimari, L.R. Residual effects of mental fatigue on subjective fatigue, reaction time and cardiac responses. Rev. Psicol. Deporte. 2020, 29, 27–34. [Google Scholar]
- Bailey, A.; Channon, S.; Beaumont, J. The relationship between subjective fatigue and cognitive fatigue in advanced multiple sclerosis. Mult. Scler. J. 2007, 13, 73–80. [Google Scholar] [CrossRef]
- Bryant, D.; Chiaravalloti, N.D.; DeLuca, J. Objective measurement of cognitive fatigue in multiple sclerosis. Rehabil. Psychol. 2004, 49, 114. [Google Scholar] [CrossRef]
- Yung, M.; Manji, R.; Wells, R.P. Exploring the relationship of task performance and physical and cognitive fatigue during a daylong light precision task. Hum. Factors 2017, 59, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, S.A.; Venkatesan, S.A.; Shankar, G.; Samivel, B.; Ranganathan, L.N. A study of cognitive fatigue in Multiple Sclerosis with novel clinical and electrophysiological parameters utilizing the event related potential P300. Mult. Scler. Relat. Disord. 2016, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pokryszko-Dragan, A.; Zagrajek, M.; Slotwinski, K.; Bilinska, M.; Gruszka, E.; Podemski, R. Event-related potentials and cognitive performance in multiple sclerosis patients with fatigue. Neurol. Sci. 2016, 37, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Wylie, G.R.; Pra Sisto, A.J.; Genova, H.M.; DeLuca, J. Fatigue across the lifespan in men and women: State vs. trait. Front. Hum. Neurosci. 2022, 16, 790006. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Adalarasu, K.; Gupta, A. EEG based analysis of cognitive fatigue during simulated driving. Int. J. Ind. Syst. Eng. 2011, 7, 135–149. [Google Scholar] [CrossRef]
- Wylie, G.R.; Yao, B.; Sandry, J.; DeLuca, J. Using signal detection theory to better understand cognitive fatigue. Front. Psychol. 2021, 11, 579188. [Google Scholar] [CrossRef]
- Pageaux, B.; Lepers, R. The effects of mental fatigue on sport-related performance. Prog. Brain Res. 2018, 240, 291–315. [Google Scholar]
- Smith, M.R.; Zeuwts, L.; Lenoir, M.; Hens, N.; De Jong, L.M.; Coutts, A.J. Mental fatigue impairs soccer-specific decision-making skill. J. Sport. Sci. 2016, 34, 1297–1304. [Google Scholar] [CrossRef]
- Chen, M.H.; Wylie, G.R.; Sandroff, B.M.; Dacosta-Aguayo, R.; DeLuca, J.; Genova, H.M. Neural mechanisms underlying state mental fatigue in multiple sclerosis: A pilot study. J. Neurol. 2020, 267, 2372–2382. [Google Scholar] [CrossRef]
- Havlikova, E.; Rosenberger, J.; Nagyova, I.; Middel, B.; Dubayova, T.; Gdovinova, Z.; Van Dijk, J.P.; Groothoff, J.W. Impact of fatigue on quality of life in patients with Parkinson’s disease. Eur. J. Neurol. 2008, 15, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Wylie, G.R.; Genova, H.M.; Yao, B.; Chiaravalloti, N.; Román, C.A.; Sandroff, B.M.; DeLuca, J. Evaluating the effects of brain injury, disease and tasks on cognitive fatigue. Sci. Rep. 2023, 13, 20166. [Google Scholar] [CrossRef] [PubMed]
- Hockey, G.R.J. A motivational control theory of cognitive fatigue. In Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications; Ackerman, P.L., Ed.; American Psychological Association: Washington, DC, USA, 2011; pp. 167–187. [Google Scholar]
- Csathó, A.; van der Linden, D.; Hernádi, I.; Buzás, P.; Kalmar, G. Effects of mental fatigue on the capacity limits of visual attention. J. Cogn. Psychol. 2012, 24, 511–524. [Google Scholar] [CrossRef]
- Diamond, B.J.; Johnson, S.K.; Kaufman, M.; Graves, L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch. Clin. Neuropsychol. 2008, 23, 189–199. [Google Scholar] [CrossRef]
- McMorris, T.; Barwood, M.; Hale, B.J.; Dicks, M.; Corbett, J. Cognitive fatigue effects on physical performance: A systematic review and meta-analysis. Physiol. Behav. 2018, 188, 103–107. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, C.; Schücker, L.; Hagemann, N.; Strauss, B. Cognitive fatigue effects on physical performance during running. J. Sport Exerc. Psychol. 2014, 36, 375–381. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Robbins, K.T.; Rao, K.; Malone, J.; Seiz, A.; Reminger, S.; Markwell, S.J.; Burra, V. Factors associated with fatigue, sleep, and cognitive function among patients with head and neck cancer. Head Neck J. Sci. Spec. Head Neck 2008, 30, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Kudesia, R.S.; Pandey, A.; Reina, C.S. Doing more with less: Interactive effects of cognitive resources and mindfulness training in coping with mental fatigue from multitasking. J. Manag. 2022, 48, 410–439. [Google Scholar] [CrossRef]
- Goswami, S. Analysing effects of information overload on decision quality in an online environment. J. Manag. Res. 2015, 15, 231–245. [Google Scholar]
- Palmer, L.; Economou, P.; Cruz, D.; Abraham-Cook, S.; Huntington, J.; Maris, M.; Makhija, N.; Welsh, T.; Maley, L. The relationship between stress, fatigue, and cognitive functioning. Coll. Stud. J. 2014, 48, 198–211. [Google Scholar]
- Ochi, G.; Kuwamizu, R.; Suwabe, K.; Fukuie, T.; Hyodo, K.; Soya, H. Cognitive fatigue due to exercise under normobaric hypoxia is related to hypoxemia during exercise. Sci. Rep. 2022, 12, 9835. [Google Scholar] [CrossRef]
- Waters, F.; Bucks, R.S. Neuropsychological effects of sleep loss: Implication for neuropsychologists. J. Int. Neuropsychol. Soc. 2011, 17, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.; Iadecola, C.; Petersen, R.C.; Breteler, M.M.; Nyenhuis, D.L.; Black, S.E.; Powers, W.J.; DeCarli, C.; Merino, J.G.; Kalaria, R.N.; et al. National Institute of Neurological Disorders and Stroke–Canadian stroke network vascular cognitive impairment harmonization standards. Stroke 2006, 37, 2220–2241. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Milzer, M.; Weiß, C.; Reinke, P.; Grapp, M.; Steindorf, K. Cancer-related fatigue: Benefits of information booklets to improve patients’ knowledge and empowerment. Support. Care Cancer 2022, 30, 4813–4821. [Google Scholar] [CrossRef] [PubMed]
- Robben, M.; Hajighasemi, A.; Nasr, M.S.; Veerla, J.P.; Alsup, A.M.; Rout, B.; Shang, H.H.; Fowlds, K.; Malidarreh, P.B.; Koomey, P.; et al. The state of applying artificial intelligence to tissue imaging for cancer research and early detection. arXiv 2023, arXiv:2306.16989. [Google Scholar] [CrossRef]
- Shang, H.H.; Nasr, M.S.; Veerla, J.P.; Malidarreh, P.B.; Saurav, M.; Hajighasemi, A.; Huber, M.; Moleta, C.; Makker, J.; Luber, J.M. Histopathology Slide Indexing and Search: Are We There Yet? arXiv 2023, arXiv:2306.17019. [Google Scholar]
- Goldberg, J. Adverse cognitive effects of psychotropic medications. In Cognitive Dysfunction in Bipolar Disorder: A Guide for Clinicians; American Psychiatric Publishing: Washington, DC, USA, 2008; pp. 137–158. [Google Scholar]
- Kay, G.G. The effects of antihistamines on cognition and performance. J. Allergy Clin. Immunol. 2000, 105, S622–S627. [Google Scholar] [CrossRef]
- Gorelick, P.B. The status of alcohol as a risk factor for stroke. Stroke 1989, 20, 1607–1610. [Google Scholar] [CrossRef]
- Zeigler, D.W.; Wang, C.C.; Yoast, R.A.; Dickinson, B.D.; McCaffree, M.A.; Robinowitz, C.B.; Sterling, M.L. The neurocognitive effects of alcohol on adolescents and college students. Prev. Med. 2005, 40, 23–32. [Google Scholar] [CrossRef]
- Van Boxtel, M.; Schmitt, J.; Bosma, H.; Jolles, J. The effects of habitual caffeine use on cognitive change: A longitudinal perspective. Pharmacol. Biochem. Behav. 2003, 75, 921–927. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; Van Bockxmeer, F.M.; Xiao, J.; Greenop, K.R.; Almeida, O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 2008, 300, 1027–1037. [Google Scholar] [CrossRef]
- Laeng, B.; Ørbo, M.; Holmlund, T.; Miozzo, M. Pupillary stroop effects. Cogn. Process. 2011, 12, 13–21. [Google Scholar] [CrossRef]
- Wierda, S.M.; van Rijn, H.; Taatgen, N.A.; Martens, S. Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution. Proc. Natl. Acad. Sci. USA 2012, 109, 8456–8460. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.; Troscianko, T.; Gilchrist, I.D. Effort during visual search and counting: Insights from pupillometry. Q. J. Exp. Psychol. 2007, 60, 211–229. [Google Scholar] [CrossRef] [PubMed]
- van der Wel, P.; Van Steenbergen, H. Pupil dilation as an index of effort in cognitive control tasks: A review. Psychon. Bull. Rev. 2018, 25, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Sirois, S.; Brisson, J. Pupillometry. Wiley Interdiscip. Rev. Cogn. Sci. 2014, 5, 679–692. [Google Scholar] [CrossRef]
- Mathôt, S. Pupillometry: Psychology, physiology, and function. J. Cogn. 2018, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Recarte, M.A.; Nunes, L.M. Mental workload while driving: Effects on visual search, discrimination, and decision making. J. Exp. Psychol. Appl. 2003, 9, 119–137. [Google Scholar] [CrossRef]
- Marinescu, A.C.; Sharples, S.; Ritchie, A.C.; Sanchez Lopez, T.; McDowell, M.; Morvan, H.P. Physiological parameter response to variation of mental workload. Hum. Factors 2018, 60, 31–56. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Viirre, E.; Strychacz, C.; Chase, B.; Jung, T.P. Task performance and eye activity: Predicting behavior relating to cognitive workload. Aviat. Space Environ. Med. 2007, 78, B176–B185. [Google Scholar] [PubMed]
- Vansteenkiste, P.; Zeuwts, L.; van Maarseveen, M.; Cardon, G.; Savelsbergh, G.; Lenoir, M. The implications of low quality bicycle paths on the gaze behaviour of young learner cyclists. Transp. Res. Part F Traffic Psychol. Behav. 2017, 48, 52–60. [Google Scholar] [CrossRef]
- Ahlstrom, U.; Friedman-Berg, F.J. Using eye movement activity as a correlate of cognitive workload. Int. J. Ind. Ergon. 2006, 36, 623–636. [Google Scholar] [CrossRef]
- Truschzinski, M.; Betella, A.; Brunnett, G.; Verschure, P.F. Emotional and cognitive influences in air traffic controller tasks: An investigation using a virtual environment? Appl. Ergon. 2018, 69, 1–9. [Google Scholar] [CrossRef]
- Causse, M.; Peysakhovich, V.; Fabre, E.F. High working memory load impairs language processing during a simulated piloting task: An ERP and pupillometry study. Front. Hum. Neurosci. 2016, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Elliott, T.; Knowles, M.; Howard, R. Heart rate variability in relation to cognition and behavior in neurodegenerative diseases: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101539. [Google Scholar] [CrossRef]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Regulation 2016, 37, 125. [Google Scholar]
- Alugubelli, N.; Abuissa, H.; Roka, A. Wearable Devices for Remote Monitoring of Heart Rate and Heart Rate Variability—What We Know and What Is Coming. Sensors 2022, 22, 8903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, X. Estimating mental fatigue based on electroencephalogram and heart rate variability. Pol. J. Med Phys. Eng. 2010, 16, 67–84. [Google Scholar] [CrossRef]
- Melo, H.M.; Nascimento, L.M.; Takase, E. Mental fatigue and heart rate variability (HRV): The time-on-task effect. Psychol. Neurosci. 2017, 10, 428–436. [Google Scholar] [CrossRef]
- Mizuno, K.; Tanaka, M.; Yamaguti, K.; Kajimoto, O.; Kuratsune, H.; Watanabe, Y. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav. Brain Funct. 2011, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Mizuno, K.; Tajima, S.; Sasabe, T.; Watanabe, Y. Central nervous system fatigue alters autonomic nerve activity. Life Sci. 2009, 84, 235–239. [Google Scholar] [CrossRef]
- Tanaka, M.; Mizuno, K.; Yamaguti, K.; Kuratsune, H.; Fujii, A.; Baba, H.; Matsuda, K.; Nishimae, A.; Takesaka, T.; Watanabe, Y. Autonomic nervous alterations associated with daily level of fatigue. Behav. Brain Funct. 2011, 7, 46. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, C.X.; Yu, X.L. Automatic recognition of cognitive fatigue from physiological indices by using wavelet packet transform and kernel learning algorithms. Expert Syst. Appl. 2009, 36, 4664–4671. [Google Scholar] [CrossRef]
- Goumopoulos, C.; Potha, N. Mental fatigue detection using a wearable commodity device and machine learning. J. Ambient Intell. Humaniz. Comput. 2023, 14, 10103–10121. [Google Scholar] [CrossRef]
- Adão Martins, N.R.; Annaheim, S.; Spengler, C.M.; Rossi, R.M. Fatigue monitoring through wearables: A state-of-the-art review. Front. Physiol. 2021, 12, 2285. [Google Scholar] [CrossRef]
- Mendes, W.B. Assessing autonomic nervous system activity. Methods Soc. Neurosci. 2009, 118, 21. [Google Scholar]
- Ishaque, S.; Khan, N.; Krishnan, S. Trends in heart-rate variability signal analysis. Front. Digit. Health 2021, 3, 639444. [Google Scholar] [CrossRef]
- Heaton, K.J.; Williamson, J.R.; Lammert, A.C.; Finkelstein, K.R.; Haven, C.C.; Sturim, D.; Smalt, C.J.; Quatieri, T.F. Predicting changes in performance due to cognitive fatigue: A multimodal approach based on speech motor coordination and electrodermal activity. Clin. Neuropsychol. 2020, 34, 1190–1214. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Yoda, T.; Sugiura, K.; Horiguchi, A.; Iwanaga, K.; Katsuura, T. Use of frequency domain analysis of skin conductance for evaluation of mental workload. J. Physiol. Anthropol. 2008, 27, 173–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayres, P.; Lee, J.Y.; Paas, F.; van Merriënboer, J.J. The validity of physiological measures to identify differences in intrinsic cognitive load. Front. Psychol. 2021, 12, 702538. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.F.; Kong, Y.; Nguyen, K.; Tran, C.; Beardslee, L.; Chen, L.; Guo, T.; Cong, X.; Feng, B.; Chon, K.H. Using electrodermal activity to validate multilevel pain stimulation in healthy volunteers evoked by thermal grills. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 319, R366–R375. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.; Guedes, J.C.; Vaz, M.P.; Pombo, E.; Fernandes, R.J.; Costa, J.T.; Baptista, J.S. Non-invasive physiological monitoring for physical exertion and fatigue assessment in military personnel: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 8815. [Google Scholar] [CrossRef] [PubMed]
- Prokasy, W. Electrodermal Activity in Psychological Research; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Boucsein, W. Electrodermal Activity; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- Österberg, K.; Karlson, B.; Hansen, Å. Cognitive performance in patients with burnout, in relation to diurnal salivary cortisol: Original research report. Stress 2009, 12, 70–81. [Google Scholar] [CrossRef]
- Bohnen, N.; Houx, P.; Nicolson, N.; Jolles, J. Cortisol reactivity and cognitive performance in a continuous mental task paradigm. Biol. Psychol. 1990, 31, 107–116. [Google Scholar] [CrossRef]
- Nicolson, N.A. Measurement of cortisol. Handb. Physiol. Res. Methods Health Psychol. 2008, 1, 37–74. [Google Scholar]
- Klaassen, E.B.; de Groot, R.H.; Evers, E.A.; Nicolson, N.A.; Veltman, D.J.; Jolles, J. Cortisol and induced cognitive fatigue: Effects on memory activation in healthy males. Biol. Psychol. 2013, 94, 167–174. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Yoneyama, T.; Ikuji, T.; Tsuchiya, H.; Sugo, T. Cortisol awakening response is associated with fatigue following a single bench press exercise. J. Phys. Educ. Sport 2022, 22, 1990–1998. [Google Scholar]
- Shin, J.; Kim, S.; Yoon, T.; Joo, C.; Jung, H.I. Smart Fatigue Phone: Real-time estimation of driver fatigue using smartphone-based cortisol detection. Biosens. Bioelectron. 2019, 136, 106–111. [Google Scholar] [CrossRef]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, M.; Vlemincx, E.; Von Leupoldt, A.; Mittelstädt, J.M.; Van den Bergh, O. Respiratory changes in response to cognitive load: A systematic review. Neural Plast. 2016, 2016, 8146809. [Google Scholar] [CrossRef]
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and human performance: An updated framework. Sport. Med. 2023, 53, 7–31. [Google Scholar] [CrossRef]
- Kodithuwakku Arachchige, S.N.; Burch V, R.F.; Chander, H.; Turner, A.J.; Knight, A.C. The use of wearable devices in cognitive fatigue: Current trends and future intentions. Theor. Issues Ergon. Sci. 2022, 23, 374–386. [Google Scholar] [CrossRef]
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The importance of respiratory rate monitoring: From healthcare to sport and exercise. Sensors 2020, 20, 6396. [Google Scholar] [CrossRef]
- Novak, V.; Hajjar, I. The relationship between blood pressure and cognitive function. Nat. Rev. Cardiol. 2010, 7, 686–698. [Google Scholar] [CrossRef]
- Alipour, H.; Goldust, M. The association between blood pressure components and cognitive functions and cognitive reserve. Clin. Exp. Hypertens. 2016, 38, 95–99. [Google Scholar] [CrossRef]
- Brosschot, J.F.; Verkuil, B.; Thayer, J.F. Exposed to events that never happen: Generalized unsafety, the default stress response, and prolonged autonomic activity. Neurosci. Biobehav. Rev. 2017, 74, 287–296. [Google Scholar] [CrossRef]
- Forte, G.; Casagrande, M. Effects of blood pressure on cognitive performance in aging: A systematic review. Brain Sci. 2020, 10, 919. [Google Scholar] [CrossRef]
- Goldstein, F.C.; Levey, A.I.; Steenland, N.K. High blood pressure and cognitive decline in mild cognitive impairment. J. Am. Geriatr. Soc. 2013, 61, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, A.; Knyazeva, V. Effects of cognitive loading on the development of muscle fatigue. Neurosci. Behav. Physiol. 2017, 47, 960–966. [Google Scholar] [CrossRef]
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The effects of mental fatigue on physical performance: A systematic review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.K.; Parasuraman, R. Effects of mental fatigue on the development of physical fatigue: A neuroergonomic approach. Hum. Factors 2014, 56, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Trejo, L.J.; Kochavi, R.; Kubitz, K.; Montgomery, L.D.; Rosipal, R.; Matthews, B. Measures and models for predicting cognitive fatigue. In Proceedings of the Biomonitoring for Physiological and Cognitive Performance during Military Operations, SPIE, Orlando, FL, USA, 30 March–1 April 2005; Volume 5797, pp. 105–115. [Google Scholar]
- Wascher, E.; Rasch, B.; Sänger, J.; Hoffmann, S.; Schneider, D.; Rinkenauer, G.; Heuer, H.; Gutberlet, I. Frontal theta activity reflects distinct aspects of mental fatigue. Biol. Psychol. 2014, 96, 57–65. [Google Scholar] [CrossRef]
- Rozand, V.; Lebon, F.; Papaxanthis, C.; Lepers, R. Effect of mental fatigue on speed–accuracy trade-off. Neuroscience 2015, 297, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Möckel, T.; Beste, C.; Wascher, E. The effects of time on task in response selection-an ERP study of mental fatigue. Sci. Rep. 2015, 5, 10113. [Google Scholar] [CrossRef]
- Kuratsune, D.; Tajima, S.; Koizumi, J.; Yamaguti, K.; Sasabe, T.; Mizuno, K.; Tanaka, M.; Okawa, N.; Mito, H.; Tsubone, H.; et al. Changes in reaction time, coefficient of variance of reaction time, and autonomic nerve function in the mental fatigue state caused by long-term computerized Kraepelin test workload in healthy volunteers. World J. Neurosci. 2012, 2, 19445. [Google Scholar] [CrossRef]
- Migliaccio, G.M.; Di Filippo, G.; Russo, L.; Orgiana, T.; Ardigò, L.P.; Casal, M.Z.; Peyré-Tartaruga, L.A.; Padulo, J. Effects of mental fatigue on reaction time in sportsmen. Int. J. Environ. Res. Public Health 2022, 19, 14360. [Google Scholar] [CrossRef]
- Nikishkova, I.; Kutikov, D.; Kutikov, O.; Kizurina, J. Peculiarities of an assessment of a cognitive efficacy in multiple sclerosis. Ukrains’kyi Visnyk Psykhonevrolohii 2020, 28, 21–26. [Google Scholar] [CrossRef]
- Korkmaz, S.G.; Korkmaz, O.; Aydemir, Ö. Detection of Cognitive Fatigue Based on Mathematical and Auditory Tasks using Gamma Band of EEG Signals. Eur. J. Sci. Technol. 2022, 6–15. [Google Scholar] [CrossRef]
- Fang, D.; Jiang, Z.; Zhang, M.; Wang, H. An experimental method to study the effect of fatigue on construction workers’ safety performance. Saf. Sci. 2015, 73, 80–91. [Google Scholar] [CrossRef]
- Boksem, M.A.; Meijman, T.F.; Lorist, M.M. Mental fatigue, motivation and action monitoring. Biol. Psychol. 2006, 72, 123–132. [Google Scholar] [CrossRef]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Tian, H. Cognitive Fatigue in Memory Task Error of Middle School Students based on Brain Evoked Potential. Neuroquantology 2018, 16, 669–675. [Google Scholar] [CrossRef]
- Arévalo-Romero, C.; Costa-Cordella, S.; Rojas-Líbano, D. The role of contextual factors on neurocognitive processing: A systematic review with meta-analysis of the effect of response types in cognitive tasks. J. Cogn. Psychol. 2023, 35, 763–794. [Google Scholar] [CrossRef]
- Poliakoff, E.; Smith-Spark, J. Everyday cognitive failures and memory problems in Parkinson’s patients without dementia. Brain Cogn. 2008, 67, 340–350. [Google Scholar] [CrossRef]
- Maaß, S.C.; Riemer, M.; Wolbers, T.; Rijn, H. Timing deficiencies in amnestic Mild Cognitive Impairment: Disentangling clock and memory processes. Behav. Brain Res. 2019, 373, 112110. [Google Scholar] [CrossRef]
- Filipas, L.; Gallo, G.; Pollastri, L.; Torre, A.L. Mental fatigue impairs time trial performance in sub-elite under 23 cyclists. PLoS ONE 2019, 14, e0218405. [Google Scholar] [CrossRef] [PubMed]
- Herlambang, M.B.; Taatgen, N.; Cnossen, F. The Role of Motivation as a Factor in Mental Fatigue. Hum. Factors 2019, 61, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, L.; Gyllensten, A.L.; Hansson, L. A Psychometric Study of the Multidimensional Fatigue Inventory to Assess Fatigue in Patients with Schizophrenia Spectrum Disorders. Community Ment. Health J. 2015, 51, 377–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, K.; Yu, B.Y.; ping Yung, K.; Yeung, W.; Ng, T.H.; Ho, F. Assessment of fatigue using the Multidimensional Fatigue Inventory in patients with major depressive disorder. Compr. Psychiatry 2014, 55 7, 1671–1678. [Google Scholar] [CrossRef]
- Lin, J.M.S.; Brimmer, D.J.; Maloney, E.M.; Nyarko, E.; Belue, R.; Reeves, W. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Popul. Health Metrics 2009, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Ferentinos, P.; Kontaxakis, V.; Havaki-kontaxaki, B.; Dikeos, D.; Lykouras, L. Psychometric evaluation of the Fatigue Severity Scale in patients with major depression. Qual. Life Res. 2011, 20, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ozyemisçi-Taskiran, O.; Batur, E.; Yuksel, S.; Cengiz, M.; Karataş, G. Validity and reliability of fatigue severity scale in stroke. Top. Stroke Rehabil. 2018, 26, 122–127. [Google Scholar] [CrossRef]
- Lerdal, A. Fatigue severity scale. In Encyclopedia of Quality of Life and Well-Being Research; Springer: Dordrecht, The Netherlands, 2021; pp. 1–5. [Google Scholar]
- Jamison, R.; Gracely, R.; Raymond, S.; Levine, J.; Katz, N. Comparative study of electronic vs. paper VAS ratings: A randomized, crossover trial using healthy volunteers. PAIN 2002, 99, 341–347. [Google Scholar] [CrossRef]
- Mallinson, T.; Cella, D.; Cashy, J.; Holzner, B. Giving meaning to measure: Linking self-reported fatigue and function to performance of everyday activities. J. Pain Symptom Manag. 2006, 31 3, 229–241. [Google Scholar] [CrossRef]
- Poller, D.N.; Perez-Machado, M. When is it time to stop working due to fatigue? A simple human factors (HF) self-assessment test. J. Clin. Pathol. 2020, 73, 523. [Google Scholar] [CrossRef]
- Elbers, R.G.; Rietberg, M.B.; Van Wegen, E.E.; Verhoef, J.; Kramer, S.F.; Terwee, C.B.; Kwakkel, G. Self-report fatigue questionnaires in multiple sclerosis, Parkinson’s disease and stroke: A systematic review of measurement properties. Qual. Life Res. 2012, 21, 925–944. [Google Scholar] [CrossRef]
- Sharma, P.; Justus, J.C.; Poudel, G.R. Sensors and Systems for Monitoring Mental Fatigue: A systematic review. arXiv 2023, arXiv:2307.01666. [Google Scholar]
- Wang, Q.; Wang, H.; Zhao, C.; Yang, J. Driver fatigue detection technology in active safety systems. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011; pp. 3097–3100. [Google Scholar]
- Kim, S.; Cranor, B.D.; Ryu, Y.S. Fatigue: Working under the influence. In Proceedings of the XXIst Annual International Occupational Ergonomics and Safety Conference, Dallas, TX, USA, 11–12 June 2009; Volume 317322. [Google Scholar]
- Phillips, R.O. A review of definitions of fatigue—And a step towards a whole definition. Transp. Res. Part F Traffic Psychol. Behav. 2015, 29, 48–56. [Google Scholar] [CrossRef]
- Shen, J.; Barbera, J.; Shapiro, C.M. Distinguishing sleepiness and fatigue: Focus on definition and measurement. Sleep Med. Rev. 2006, 10, 63–76. [Google Scholar] [CrossRef]
- Dawson, D.; Searle, A.K.; Paterson, J.L. Look before you (s) leep: Evaluating the use of fatigue detection technologies within a fatigue risk management system for the road transport industry. Sleep Med. Rev. 2014, 18, 141–152. [Google Scholar] [CrossRef]
- De Fazio, R.; Mattei, V.; Al-Naami, B.; De Vittorio, M.; Visconti, P. Methodologies and wearable devices to monitor biophysical parameters related to sleep dysfunctions: An overview. Micromachines 2022, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lodewijks, G. Detecting fatigue in car drivers and aircraft pilots by using non-invasive measures: The value of differentiation of sleepiness and mental fatigue. J. Saf. Res. 2020, 72, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Dirnberger, G.; Duregger, C.; Trettler, E.; Lindinger, G.; Lang, W. Fatigue in a simple repetitive motor task: A combined electrophysiological and neuropsychological study. Brain Res. 2004, 1028, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Searle, B.L.; Spathis, D.; Constantinides, M.; Quercia, D.; Mascolo, C. Anticipatory detection of compulsive body-focused repetitive behaviors with wearables. In Proceedings of the 23rd International Conference on Mobile Human-Computer Interaction, Virtual Event, 27 September–1 October 2021; pp. 1–15. [Google Scholar]
- Gilchrist, K.H.; Hegarty-Craver, M.; Christian, R.B.; Grego, S.; Kies, A.C.; Wheeler, A.C. Automated detection of repetitive motor behaviors as an outcome measurement in intellectual and developmental disabilities. J. Autism Dev. Disord. 2018, 48, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Tanaka, M.; Watanabe, Y. Neural mechanisms of mental fatigue. Rev. Neurosci. 2014, 25, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Vora, N.R.; Hajighasemi, A.; Reynolds, C.T.; Radmehr, A.; Mohamed, M.; Saurav, J.R.; Aziz, A.; Veerla, J.P.; Nasr, M.S.; Lotspeich, H.; et al. Real-Time Diagnostic Integrity Meets Efficiency: A Novel Platform-Agnostic Architecture for Physiological Signal Compression. arXiv 2023, arXiv:2312.12587. [Google Scholar]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Nguyen, H. Regional brain wave activity changes associated with fatigue. Psychophysiology 2012, 49, 574–582. [Google Scholar] [CrossRef]
- Li, G.; Huang, S.; Xu, W.; Jiao, W.; Jiang, Y.; Gao, Z.; Zhang, J. The impact of mental fatigue on brain activity: A comparative study both in resting state and task state using EEG. BMC Neurosci. 2020, 21, 20. [Google Scholar] [CrossRef]
- Tanaka, M.; Shigihara, Y.; Ishii, A.; Funakura, M.; Kanai, E.; Watanabe, Y. Effect of mental fatigue on the central nervous system: An electroencephalography study. Behav. Brain Funct. 2012, 8, 48. [Google Scholar] [CrossRef]
- Trejo, L.J.; Kubitz, K.; Rosipal, R.; Kochavi, R.L.; Montgomery, L.D. EEG-based estimation and classification of mental fatigue. Psychology 2015, 6, 55452. [Google Scholar] [CrossRef]
- Wang, H.; Dragomir, A.; Abbasi, N.; Li, J.; Thakor, N.; Bezerianos, A. A novel real-time driving fatigue detection system based on wireless dry EEG Cogn. Cogn. Neurodynamics 2018, 12, 365–376. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Scarapicchia, V.; Brown, C.; Mayo, C.; Gawryluk, J.R. Functional magnetic resonance imaging and functional near-infrared spectroscopy: Insights from combined recording studies. Front. Hum. Neurosci. 2017, 11, 419. [Google Scholar] [CrossRef]
- Ayaz, H.; Shewokis, P.A.; Bunce, S.; Izzetoglu, K.; Willems, B.; Onaral, B. Optical brain monitoring for operator training and mental workload assessment. Neuroimage 2012, 59, 36–47. [Google Scholar] [CrossRef]
- Dehais, F.; Dupres, A.; Di Flumeri, G.; Verdiere, K.; Borghini, G.; Babiloni, F.; Roy, R. Monitoring pilot’s cognitive fatigue with engagement features in simulated and actual flight conditions using an hybrid fNIRS-EEG passive BCI. In Proceedings of the 2018 IEEE international conference on systems, man, and cybernetics (SMC), Miyazaki, Japan, 7–10 October 2018; pp. 544–549. [Google Scholar]
- Wylie, G.; Yao, B.; Genova, H.; Chen, M.; DeLuca, J. Using functional connectivity changes associated with cognitive fatigue to delineate a fatigue network. Sci. Rep. 2020, 10, 21927. [Google Scholar] [CrossRef]
- Yoo, G.; Kim, H.; Hong, S. Prediction of Cognitive Load from Electroencephalography Signals Using Long Short-Term Memory Network. Bioengineering 2023, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Gu, B.; Cao, S.; Fang, D. Identifying mental fatigue of construction workers using EEG and deep learning. Autom. Constr. 2023, 151, 104887. [Google Scholar] [CrossRef]
- Khan, M.A.; Asadi, H.; Hoang, T.; Lim, C.P.; Nahavandi, S. Measuring Cognitive Load: Leveraging fNIRS and Machine Learning for Classification of Workload Levels. In International Conference on Neural Information Processing; Springer: Singapore, 2023; pp. 313–325. [Google Scholar]
- Souchet, A.D.; Philippe, S.; Lourdeaux, D.; Leroy, L. Measuring visual fatigue and cognitive load via eye tracking while learning with virtual reality head-mounted displays: A review. Int. J. Hum.-Comput. Interact. 2022, 38, 801–824. [Google Scholar] [CrossRef]
- Bafna, T.; Hansen, J.P. Mental fatigue measurement using eye metrics: A systematic literature review. Psychophysiology 2021, 58, e13828. [Google Scholar] [CrossRef]
- Sharma, H.; Drukker, L.; Papageorghiou, A.T.; Noble, J.A. Machine learning-based analysis of operator pupillary response to assess cognitive workload in clinical ultrasound imaging. Comput. Biol. Med. 2021, 135, 104589. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, M.; Plechawska-Wójcik, M.; Tokovarov, M. Interpretable machine learning models for three-way classification of cognitive workload levels for eye-tracking features. Brain Sci. 2021, 11, 210. [Google Scholar] [CrossRef]
- Ktistakis, E.; Skaramagkas, V.; Manousos, D.; Tachos, N.S.; Tripoliti, E.; Fotiadis, D.I.; Tsiknakis, M. COLET: A dataset for COgnitive workLoad estimation based on eye-tracking. Comput. Methods Programs Biomed. 2022, 224, 106989. [Google Scholar] [CrossRef] [PubMed]
- Ul Alam, M.A. Activity-Aware Deep Cognitive Fatigue Assessment using Wearables. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Mexico City, Mexico, 1–5 November 2021; pp. 7433–7436. [Google Scholar] [CrossRef]
- Kanal, V. Kopos: A Framework to Study and Detect Physical and Cognitive Fatigue Concurrently; The University of Texas at Arlington: Arlington, TX, USA, 2021. [Google Scholar]
- Ding, Y.; Cao, Y.; Duffy, V.G.; Wang, Y.; Zhang, X. Measurement and identification of mental workload during simulated computer tasks with multimodal methods and machine learning. Ergonomics 2020, 63, 896–908. [Google Scholar] [CrossRef]
- Jaiswal, A.; Zaki Zadeh, M.; Hebri, A.; Ramesh Babu, A.; Makedon, F. A Smart Sensor Suit (SSS) to Assess Cognitive and Physical Fatigue with Machine Learning. In International Conference on Human-Computer Interaction; Springer: Cham, Switzerland, 2023; pp. 120–134. [Google Scholar]
- Acien, A.; Morales, A.; Vera-Rodriguez, R.; Fierrez, J.; Mondesire-Crump, I.; Arroyo-Gallego, T. Detection of Mental Fatigue in the General Population: Feasibility Study of Keystroke Dynamics as a Real-world Biomarker. JMIR Biomed. Eng. 2022, 7, e41003. [Google Scholar] [CrossRef]
- Pimenta, A.; Carneiro, D.; Novais, P.; Neves, J. Monitoring mental fatigue through the analysis of keyboard and mouse interaction patterns. In Proceedings of the Hybrid Artificial Intelligent Systems: 8th International Conference, HAIS 2013, Salamanca, Spain, 11–13 September 2013; Proceedings 8; pp. 222–231. [Google Scholar]
- Siravenha, A.C.; Reis, M.N.; Cordeiro, I.; Tourinho, R.A.; Gomes, B.D.; Carvalho, S.R. Residual mlp network for mental fatigue classification in mining workers from brain data. In Proceedings of the 2019 8th Brazilian Conference on Intelligent Systems (BRACIS), Salvador, BA, Brazil, 15–18 October 2019; pp. 407–412. [Google Scholar]
- Kamińska, D.; Smółka, K.; Zwoliński, G. Detection of mental stress through EEG signal in virtual reality environment. Electronics 2021, 10, 2840. [Google Scholar] [CrossRef]
- Li, P.; Jiang, W.; Su, F. Single-channel EEG-based mental fatigue detection based on deep belief network. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 367–370. [Google Scholar]
- Mu, Z.; Hu, J.; Min, J. Driver fatigue detection system using electroencephalography signals based on combined entropy features. Appl. Sci. 2017, 7, 150. [Google Scholar] [CrossRef]
- Savaş, B.K.; Becerikli, Y. Real time driver fatigue detection system based on multi-task ConNN. IEEE Access 2020, 8, 12491–12498. [Google Scholar] [CrossRef]
- Ansari, S.; Naghdy, F.; Du, H.; Pahnwar, Y.N. Driver mental fatigue detection based on head posture using new modified reLU-BiLSTM deep neural network. IEEE Trans. Intell. Transp. Syst. 2021, 23, 10957–10969. [Google Scholar] [CrossRef]
- Hu, J. Automated detection of driver fatigue based on AdaBoost classifier with EEG signals. Front. Comput. Neurosci. 2017, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Shi, T.; Wang, F.; Ma, S. Real-time EEG-based detection of fatigue driving danger for accident prediction. Int. J. Neural Syst. 2015, 25, 1550002. [Google Scholar] [CrossRef] [PubMed]
- Zorzos, I.; Kakkos, I.; Miloulis, S.T.; Anastasiou, A.; Ventouras, E.M.; Matsopoulos, G.K. Applying Neural Networks with Time-Frequency Features for the Detection of Mental Fatigue. Appl. Sci. 2023, 13, 1512. [Google Scholar] [CrossRef]
- Cos, C.A.; Lambert, A.; Soni, A.; Jeridi, H.; Thieulin, C.; Jaouadi, A. Enhancing Mental Fatigue Detection through Physiological Signals and Machine Learning Using Contextual Insights and Efficient Modelling. J. Sens. Actuator Netw. 2023, 12, 77. [Google Scholar] [CrossRef]
- Butkevičiūtė, E.; Michalkovič, A.; Bikulčienė, L. Ecg signal features classification for the mental fatigue recognition. Mathematics 2022, 10, 3395. [Google Scholar] [CrossRef]
- Wang, J.; Shi, J.; Xu, Y.; Zhong, H.; Li, G.; Tian, J.; Xu, W.; Gao, Z.; Jiang, Y.; Jiao, W.; et al. A New Strategy for Mental Fatigue Detection Based on Deep Learning and Respiratory Signal. In Proceedings of the 11th International Conference on Computer Engineering and Networks, Beijing, China, 9–11 December 2022; pp. 543–552. [Google Scholar]
- Karim, E.; Pavel, H.R.; Jaiswal, A.; Zadeh, M.Z.; Theofanidis, M.; Wylie, G.; Makedon, F. An EEG-based Cognitive Fatigue Detection System. In Proceedings of the 16th International Conference on PErvasive Technologies Related to Assistive Environments, Corfu, Greece, 5–7 July 2023; pp. 131–136. [Google Scholar]
- Ramírez-Moreno, M.A.; Carrillo-Tijerina, P.; Candela-Leal, M.O.; Alanis-Espinosa, M.; Tudón-Martínez, J.C.; Roman-Flores, A.; Ramírez-Mendoza, R.A.; Lozoya-Santos, J.d.J. Evaluation of a fast test based on biometric signals to assess mental fatigue at the workplace—A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 11891. [Google Scholar] [CrossRef]
- Jaiswal, A.; Ramesh Babu, A.; Zaki Zadeh, M.; Wylie, G.; Makedon, F. Detecting Cognitive Fatigue in Subjects with Traumatic Brain Injury from FMRI Scans Using Self-Supervised Learning. In Proceedings of the 16th International Conference on PErvasive Technologies Related to Assistive Environments, Corfu, Greece, 5–7 July 2023; pp. 83–90. [Google Scholar]
- Mu, Z.; Hu, J.; Yin, J. Driving fatigue detecting based on EEG signals of forehead area. Int. J. Pattern Recognit. Artif. Intell. 2017, 31, 1750011. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Umer, W.; Wang, H.; Xing, X.; Zhao, S.; Hou, J. Identification and classification of construction equipment operators’ mental fatigue using wearable eye-tracking technology. Autom. Constr. 2020, 109, 103000. [Google Scholar] [CrossRef]
- Pavel, H.R.; Karim, E.; Jaiswal, A.; Acharya, S.; Nale, G.; Theofanidis, M.; Makedon, F. Assessment of Cognitive Fatigue from Gait Cycle Analysis. Technologies 2023, 11, 18. [Google Scholar] [CrossRef]
- Chai, R.; Tran, Y.; Craig, A.; Ling, S.H.; Nguyen, H.T. Enhancing accuracy of mental fatigue classification using advanced computational intelligence in an electroencephalography system. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1318–1341. [Google Scholar]
- Zadeh, M.Z.; Babu, A.R.; Lim, J.B.; Kyrarini, M.; Wylie, G.; Makedon, F. Towards cognitive fatigue detection from functional magnetic resonance imaging data. In Proceedings of the 13th ACM International Conference on PErvasive Technologies Related to Assistive Environments, Virtual, 30 June–3 July 2020; pp. 1–2. [Google Scholar]
- Price, E.; Moore, G.; Galway, L.; Linden, M. Towards mobile cognitive fatigue assessment as indicated by physical, social, environmental, and emotional factors. IEEE Access 2019, 7, 116465–116479. [Google Scholar] [CrossRef]
- Lee, K.F.A.; Gan, W.S.; Christopoulos, G. Biomarker-informed machine learning model of cognitive fatigue from a heart rate response perspective. Sensors 2021, 21, 3843. [Google Scholar] [CrossRef]
- Lee, K.F.A.; Chan, E.; Car, J.; Gan, W.S.; Christopoulos, G. Lowering the sampling rate: Heart rate response during cognitive fatigue. Biosensors 2022, 12, 315. [Google Scholar] [CrossRef]
- Schweizer, T.; Wyss, T.; Gilgen-Ammann, R. Detecting Soldiers’ Fatigue Using Eye-Tracking Glasses: Practical Field Applications and Research Opportunities. Mil. Med. 2021, 187, e1330–e1337. [Google Scholar] [CrossRef]
- Ren, Z.; Li, R.; Chen, B.; Zhang, H.; Ma, Y.; Wang, C.; Lin, Y.; Zhang, Y. EEG-based driving fatigue detection using a two-level learning hierarchy radial basis function. Front. Neurorobot. 2021, 15, 618408. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, T.; Dogan, S.; Subasi, A. EEG-based driving fatigue detection using multilevel feature extraction and iterative hybrid feature selection. Biomed. Signal Process. Control 2021, 68, 102591. [Google Scholar] [CrossRef]
- Papakostas, M.; Rajavenkatanarayanan, A.; Makedon, F. CogBeacon: A Multi-Modal Dataset and Data-Collection Platform for Modeling Cognitive Fatigue. Technologies 2019, 7, 46. [Google Scholar] [CrossRef]
- Tag, B.; Vargo, A.W.; Gupta, A.; Chernyshov, G.; Kunze, K.; Dingler, T. Continuous alertness assessments: Using EOG glasses to unobtrusively monitor fatigue levels In-the-Wild. In Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems, Scotland, UK, 4–9 May 2019; pp. 1–12. [Google Scholar]
- Tjolleng, A.; Jung, K.; Hong, W.; Lee, W.; Lee, B.; You, H.; Son, J.; Park, S. Classification of a Driver’s cognitive workload levels using artificial neural network on ECG signals. Appl. Ergon. 2017, 59, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Tiwari, A.; Routray, A. Analysis of cognitive fatigue using EEG parameters. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 2554–2557. [Google Scholar]
- Sieber, W.K.; Chen, G.X.; Krueger, G.P.; Lincoln, J.E.; Menéndez, C.C.; O’Connor, M.B. Research gaps and needs for preventing worker fatigue in the transportation and utilities industries. Am. J. Ind. Med. 2022, 65, 857–866. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).