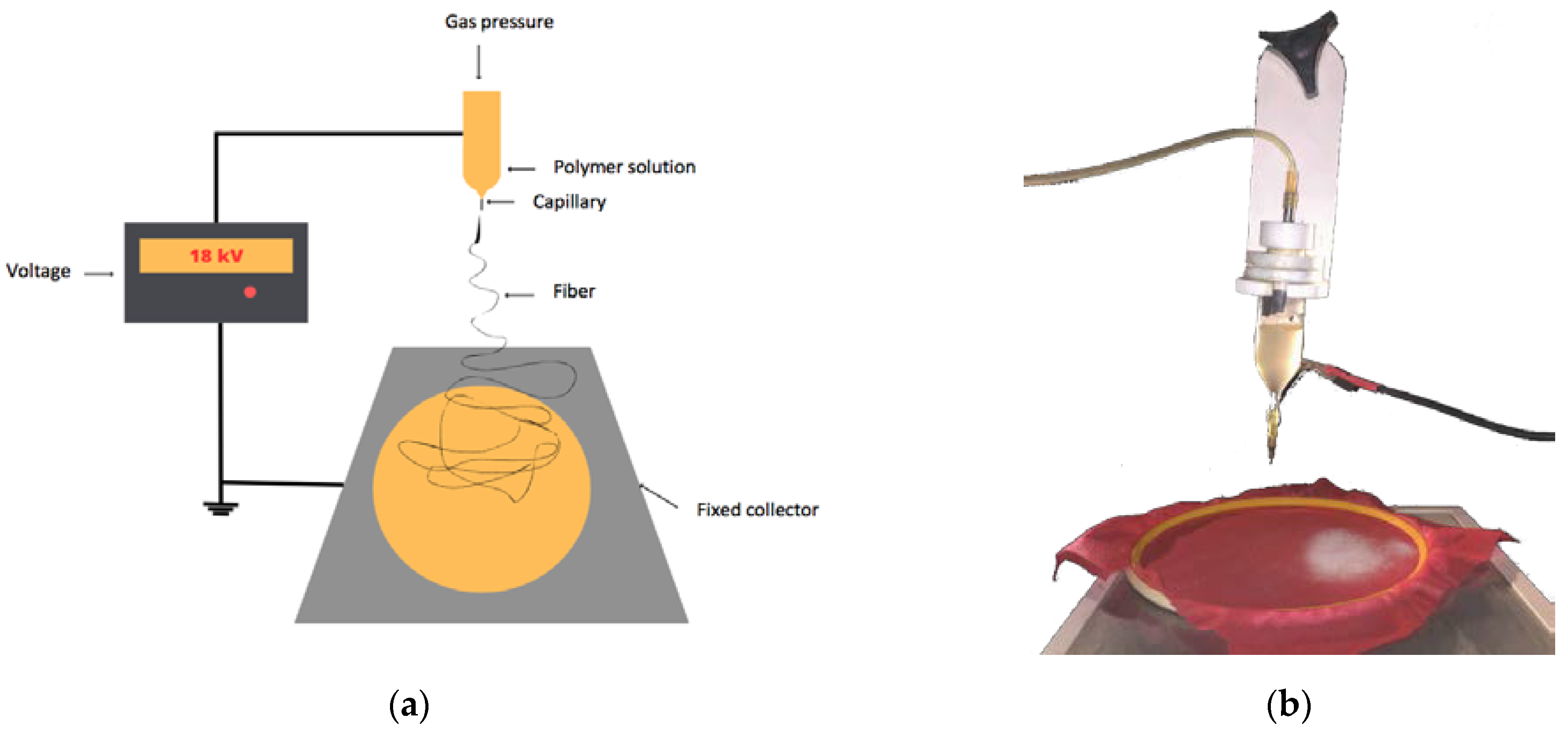
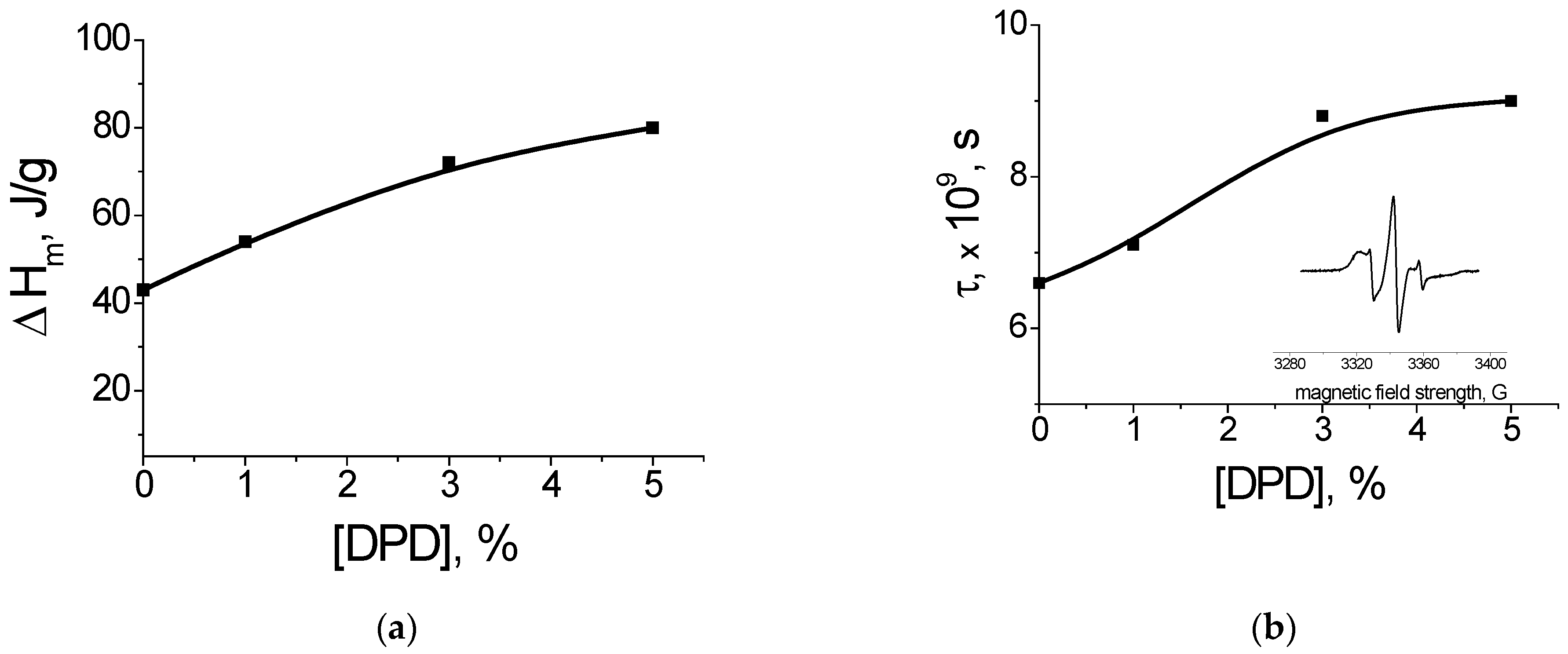3.1. Effect of Solvent Parameters on the Structure of Poly-3-hydroxybutyrate Matrices
One of the most important technological aspects of the formation of polymeric films or fibrous materials is the choice of solvent for the base polymer. Solvents differ not only in their boiling point and molecular weight but also in their degree of polarity. Let us consider the influence of solvent polarity on the supramolecular structure of polyhydroxybutyrate (PHB) matrices. For this purpose, we used the method of differential calorimetric analysis.
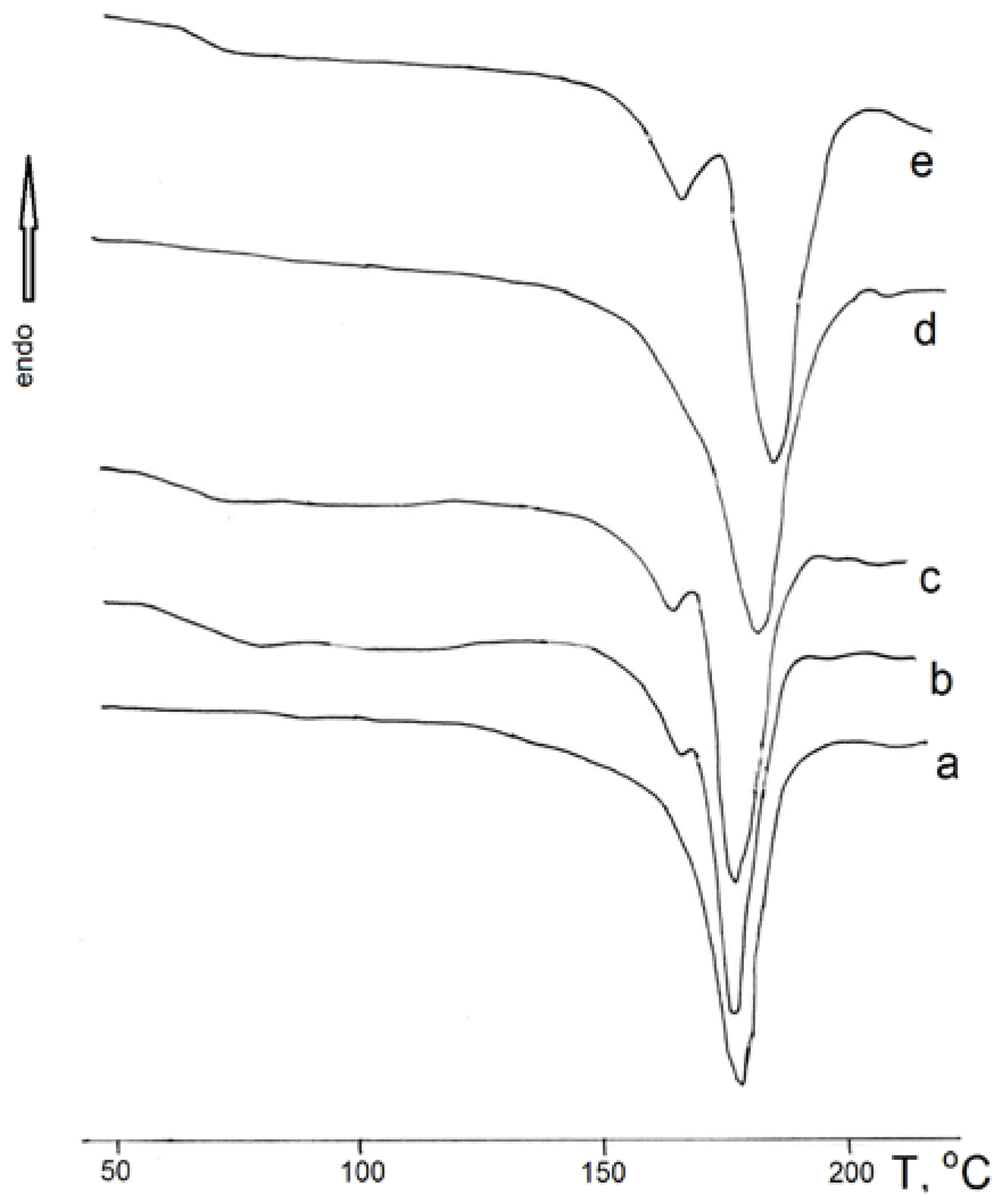
Figure 2 shows typical heating thermograms of PHB films.
From the analysis of the thermograms, it can be observed that the original structure of PHB is disrupted after treatment with the solvents: an amorphous phase appears, characterized by a distinct glass transition, and a fine crystalline modification or regions with disrupted crystallinity emerge. This is evidenced by the appearance of a low-temperature melting peak.
Table 1 presents the thermophysical properties of the PHB films with different solvents and solvent parameters. From the table, it can be seen that with an increase in the solvent’s dipole moment, the crystalline phase of PHB improves (becomes more ordered). This is evidenced by the increase in the polymer’s melting temperature (Tm), as shown in
Figure 3.
Concurrently, the specific heat capacity (DCp) of PHB decreases. This may be related to a decrease in the proportion of the amorphous phase in the polymer.
The PHB molecule contains a complex ester group in its main chain that is capable of interacting with polar solvent molecules. As a result, the conformation of macromolecules in both the crystalline and amorphous regions of the polymer can change under the influence of solvent molecules. The structure of the crystallites is stabilized, and the macromolecules in the amorphous phase lose their flexibility. These effects were observed by other authors [
14], who studied the influence of water molecules on PHB films. It was found that the interaction with polar water molecules leads to the formation of hydrogen bonds between neighboring chains, resulting in the stabilization of the polymer’s crystalline structure. The hydrogen bonding network formed at the crystallite boundaries enhances the orientation of crystallites in the polymer matrix.
In the present study, among the considered solvents, which have a dipole moment lower or higher (dichloroethane) than that of water (1.84 D) [
15], these effects may occur to a lesser extent due to the presence of PHB films with defective crystalline structures, which exhibit lower melting temperatures, as shown in
Figure 1.
In conclusion, an increase in solvent polarity corresponds to an increased ability of the solvent to interact with polar groups of PHB. As a result of this interaction, the crystallites are improved, and the amorphous phase of the polymer is densified during film formation.
This study also revealed that the changes in the Gibbs melting energy of the PHB crystalline phase and the cohesion energy density of the solvent molecules are well-correlated in a certain sequence, as shown in
Table 1.
Cohesion energy density is known to characterize the degree of intermolecular interaction in a substance [
16]. From
Table 1, it follows that decreased intermolecular interaction in the solvent leads to an increase in disorder in the crystalline phase of the PHB films, characterized by a decrease in the Gibbs melting energy. In other words, the lower the level of intermolecular interaction in the solvent is, the higher the mobility of its molecules is, and consequently, the higher their rate of transition to the vapor phase (evaporation) is. Due to the different rates of phase transition of molecules in the considered solvents, the formation of PHB films proceeds differently. This is reflected in the degree of completion of the crystallization process and the parameters of the polymer’s crystalline structure.
Based on the conducted research, it can be concluded that with an increase in the polarity of the solvent, there is an increase and improvement in the crystalline structure, and the proportion of the amorphous phase of PHB decreases. With a decrease in the degree of intermolecular interaction in the solvent, the proportion of defective crystalline phase in PHB films increases. It was found that the highest-quality films are obtained from chloroform. Films obtained from dioxane have a non-homogeneous, heterogeneous structure. In the course of the experimental work on solvent combination, it was found that high-quality films can be obtained from dioxane by re-dissolving the films from chloroform. Films from dichloroethane were characterized by increased brittleness and heterogeneity. Combining solvents did not improve the quality of the films. The films based on formic acid were not cohesive and disintegrated into fragments after the desorption of the solvent. Additionally, formic acid accelerates the hydrolysis processes of PHB. Therefore, based on the analysis of the film morphology, chloroform was chosen as a good solvent for obtaining homogeneous film materials for the study of water sorption–diffusion properties. In addition to solvent characteristics, important factors for fiber structure formation include polymer solution parameters. The fiber morphology primarily determines the geometric parameters of the monofilament nonwoven fibrous material. Furthermore, in this study, we attempted to summarize the influences of the main technological characteristics of the polymer solution on the electroforming and structure of polyhydroxybutyrate (PHB) fibers.
3.2. Influence of the Main Technological Characteristics on the Structure of the Fibrous Matrices of Poly-3-hydroxybutyrate
As a large number of factors are responsible for various stages of electrospinning, researchers and technologists are faced with the task of identifying the most significant and dominating characteristics that have the greatest impact on the fiber’s structure, morphology, porosity, and geometry. This is typical of various polymer systems. For example, a solution of natural chitosan or chitosan lactate with polyethylene oxide is perfectly formed with the addition of ethyl alcohol, but the technological parameters of electrospinning have little effect on the morphology of the material. The viscosity of the spinning solution is determined by the polymer concentration as well as the interpolymer interactions [
17]. In this regard, we will now consider some key physicochemical characteristics of polymer solutions of PHB intended for electrospinning.
The geometric parameters of monofilaments primarily determine the morphology of the nonwoven fibrous material (packing density, filament orientation, defectiveness, etc.). At the same time, they affect a number of performance properties, including diffusion, physical–mechanical and biomedical properties, etc. The geometry of the fiber mainly depends on the polymer’s molecular weight, viscosity, electrical conductivity, and solution flow rate (productivity).
In
Figure 4a,b, the dependencies of the PHB fiber diameter on the productivity of the electroforming process are presented for 5% and 7% polymer solutions in chloroform.
As can be seen in
Figure 4, the fiber diameter does not depend on the performance of the electrospinning process at different concentrations of PHB in solution. Apparently, as a result of the presence of strong intermolecular interaction in the PHB solution, as evidenced by a high degree of crystallinity (60–80%), an increase in the productivity of fiber formation does not lead to a change in the degree of crystallinity or free intermolecular volume.
Figure 4 shows the dependence of the fiber diameter on the molecular weight of PHB for the 7% solutions in chloroform.
As shown in
Figure 5, there is an increase in the diameter of the fiber with an increase in the molecular weight of the medium-viscosity PHB. The increase in diameter with an increase in the length of the macromolecular chains of PHB is quite natural and is explained by the increase in the number of intermolecular engagements in the polymer solution, which prevent the stretching of the jet during electroforming. The mesh of physical meshes formed in the solution interferes with orientation processes and increases the viscoelastic properties of the polymer system. The formation of a network of intramolecular entanglements, as well as the growth of the crystalline phase, will lead to an increase in the mechanical characteristics of the fibers. This aspect will be considered by us in a separate scientific publication.
In the process of electrospinning, the electric field creates free ionic charges that migrate along the polymer solution jet from one electrode to another. In organic nonionic polymer solutions with low dielectric permittivity, the concentration of such ions is extremely low, which determines the low values of the electrical conductivity of the molding system. Such systems include solutions of PHB, which is known to be highly soluble in chloroform, dichloroethane and some other nonionic organic solvents [
18].
The flow of the primary jet of the polymer solution between two oppositely charged electrodes is associated with two processes: the interaction of an electric field with the polymer solution and the evaporation of the solvent, resulting in the formation of an ultrathin fiber. The first process is largely determined by the electrical conductivity of the polymer solution. In this case, the electromechanical effect on the formed polymer jet sets the conditions for changing the diameter and degree of defect of the resulting ultrathin fiber [
19]. The second process is determined by the intermolecular interaction between the polymer and the solvent, as well as the cohesion energy of the solvent. When the solvent diffuses from the fiber, micro- and nanoporous systems are usually formed (
Figure 5b).
The effect of electrical conductivity and viscosity on the electrospinning process of a binary solution of PHB in chloroform was studied (
Figure 6). In particular, it was found that the low electrical conductivity of the PHB solution prevents the formation of fibers of uniform thickness, as demonstrated in
Figure 6a.
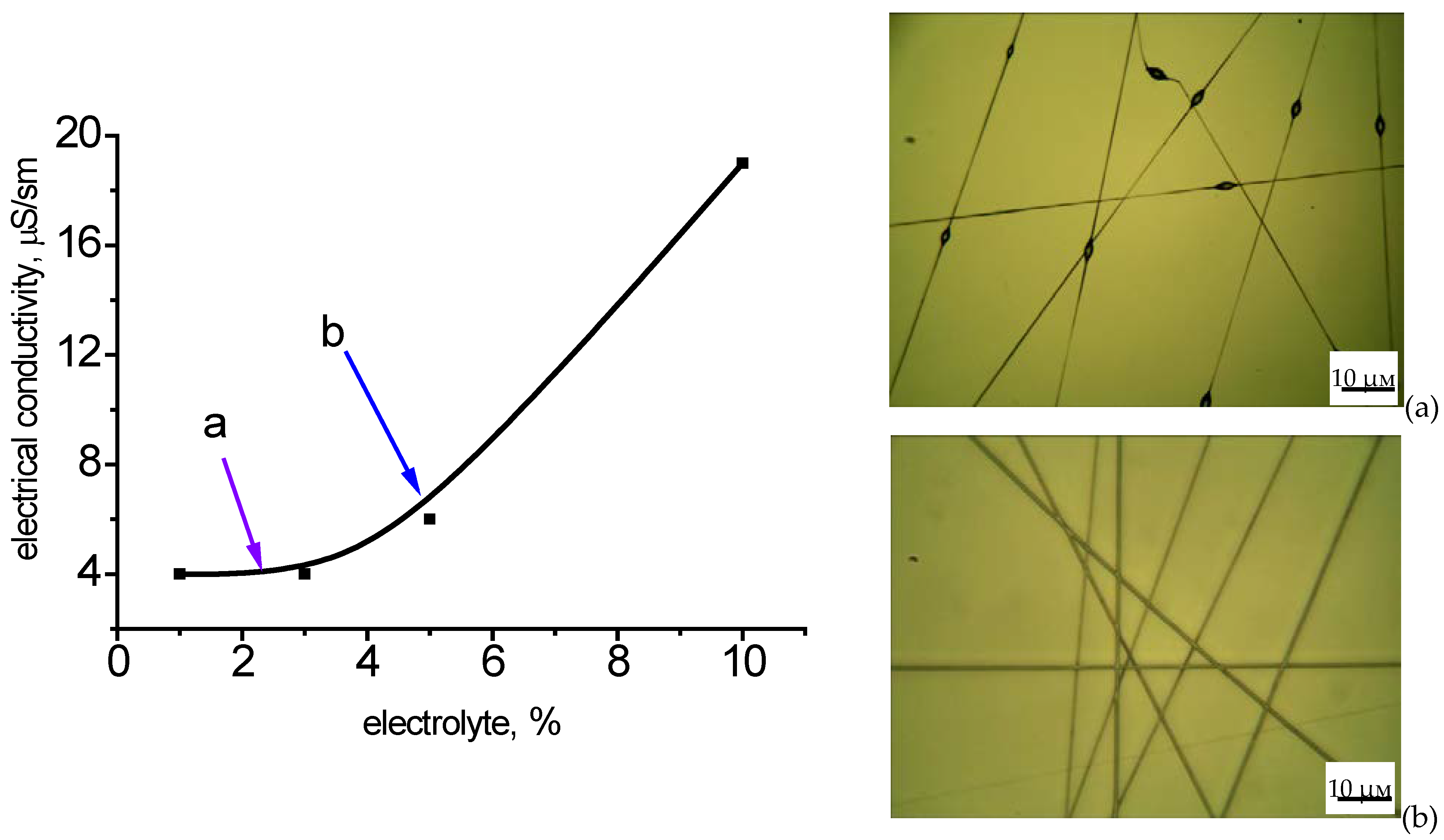
The electrical conductivity can be regulated by the introduction of an electrolyte into the solution for electrospinning.
Figure 6 shows the dependence of electrical conductivity on the concentration of the electrolyte and that of the diameter of the fibers on the electrical conductivity of the PHB solution in chloroform. The concentration of the electrolyte has a great impact on the electrical conductivity of the solution, which leads to changes in the diameter of fibers (
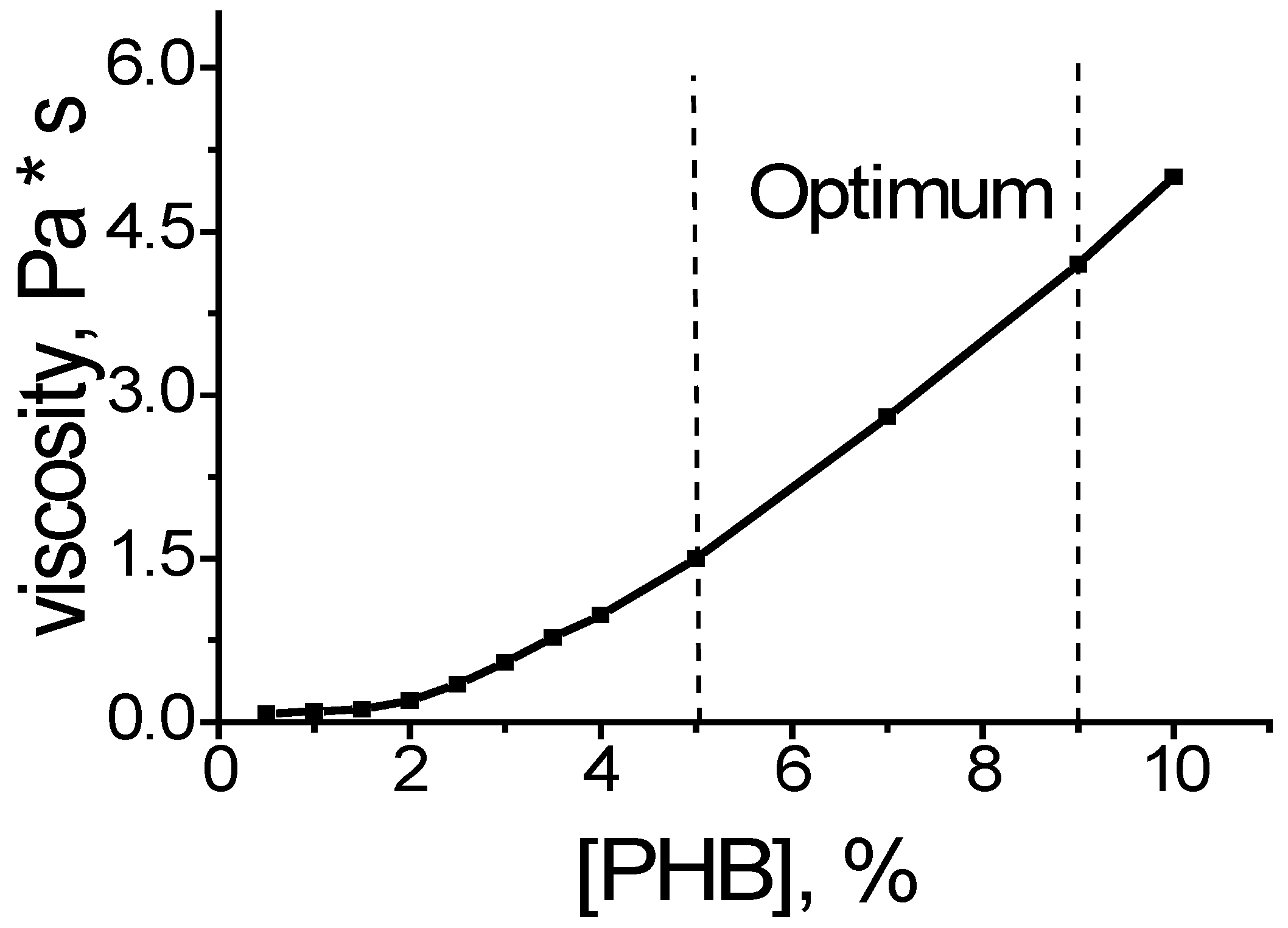
Figure 7). These dependencies are exponential. The initial section is characterized by a low level of indicators, which is reflected in the formation of a defective fiber structure (thickening). In the case of low electrical conductivity, the fiber is not formed at all, but droplet formation occurs. When the optimal level of indicators is reached, smooth fibers are formed. These dependencies are quite closely correlated with the dependence of the viscosity of the PHB solution on the concentration of the polymer (
Figure 8). This correlation is primarily related to the properties of the polymer solution at the polymer–air interface. The surface tension determines the conditions for the extraction of the jet and its disintegration into separate fragments (droplets).
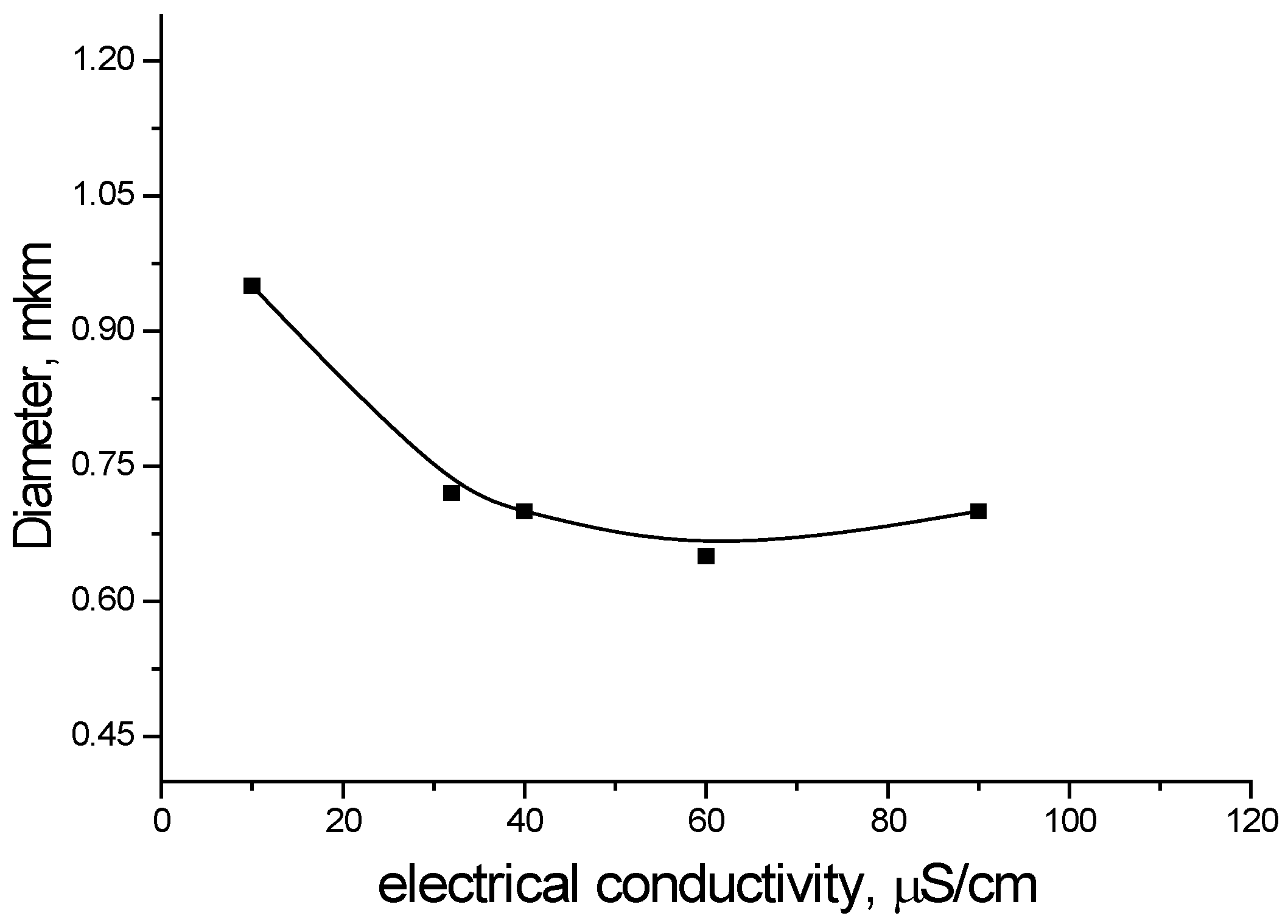
The dependence of the average diameter on the electrical conductivity is characterized by an exponential descending curve (
Figure 7). With increasing electrical conductivity, the average diameter decreases to a certain value, and then there is a tendency towards some growth. This growth is determined by an increase in the viscosity of the solution due to the high concentration of the electrolyte.
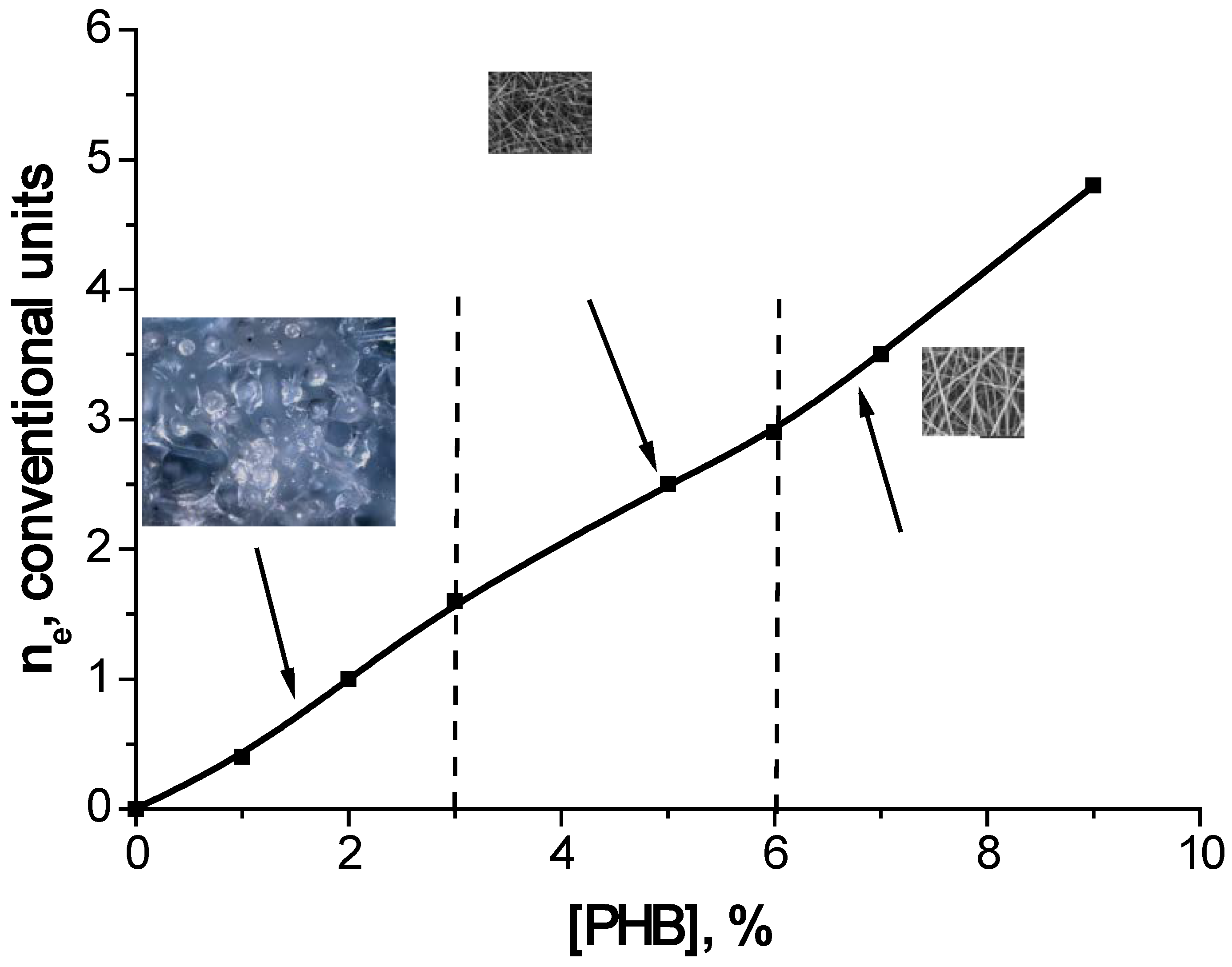
A gradual increase in the concentration of PHB in the solution leads to the emergence and increase in the number of intermolecular knots of engagement and interlacing of the polymer chains, which allows the polymer solution to stretch into a thin jet and form a fiber at an appropriate concentration (
Figure 9). At a low polymer concentration, the intermolecular entanglements are not sufficient to form a stable jet, and it breaks up into individual droplets. As shown in
Figure 9, this occurs in the concentration range of 1–3 wt.% PHB. In the transition region of concentrations of 3–6 wt.% PHB, the lack of molecular meshing does not lead to the disintegration of the jet but to the formation of fibers of complex geometry with alternating cylindrical sections and teardrop-shaped thickenings. At these concentrations, the polymer solution exhibits a sufficiently high value of surface tension, which does not cause the jet to disintegrate into separate fragments. At an optimal concentration of PHB in solution over 6 wt.%, the number of molecular meshes is large enough to form smooth cylindrical fibers. It should be noted that with an increase or decrease in the molecular weight of the polymer, this dependence shifts to the region of lower or higher concentrations.
Since the studied fibrous materials are recommended for use in medicine as matrices for the prolonged delivery of drugs, antiseptics and tissue engineering, next, we will consider the effects of these substances on the fiber structure. For the model experiment, we selected substances with terminal hydroxyl groups and a complex containing a metal halide. Intermolecular interactions of these components with the polar groups of PHB can be expected in the process of fiber formation.
3.3. Influence of Polar Molecules on the Structure of the Fibrous Matrices of Poly-3-hydroxybutyrate
The presence of polar functional groups or complexes with metal in a chemical compound (medicinal, biologically active and antiseptic substances) should affect not only the technological characteristics of the molding solution but also the processes of fiber structure formation. As an illustration, we chose two different substances: dipiidamol (DPD), containing terminal -OH groups, and the iron (III) chlorine terrafenylporphyrin (FeClTPP), containing ferric chloride (3+) as part of the complex.
Figure 10 shows the structural formulas of these compounds.
Since the presence of foreign substances is prohibited in biomedical materials, the electrolyte was not introduced into the molding solution. However, as our research has shown, the introduction of a medicinal substance, antiseptic or other ingredient containing polar groups of atoms into the molding solution can improve the technological parameters of the solution. This was demonstrated on the PHB-DPD and PHB-FeClTPP fibrous matrices.
Figure 11 shows that with an increase in the concentration of DPD, the geometry of the fibers changes from transitional (cylinders-drops) to smooth and cylindrical. At the same time, the fibers themselves have a porous structure. The average diameter of the fiber is 2–5 microns, and the pore size ranges from 0.05 to 0.2 microns.
The supramolecular structure of PHB changes with the increasing DPD concentration.
Figure 12a shows the dependence of the enthalpy of melting of PHB on the concentration of DPD (DSC method). With an increase in the content of DPD, an increase in the enthalpy of melting of PHB is observed, which indicates an increase in the degree of crystallinity. DPD particles are effective nuclei of crystallization. At the same time, the average size of the crystallites practically does not change, as indicated by the invariance of the melting temperature (168–169 °C).
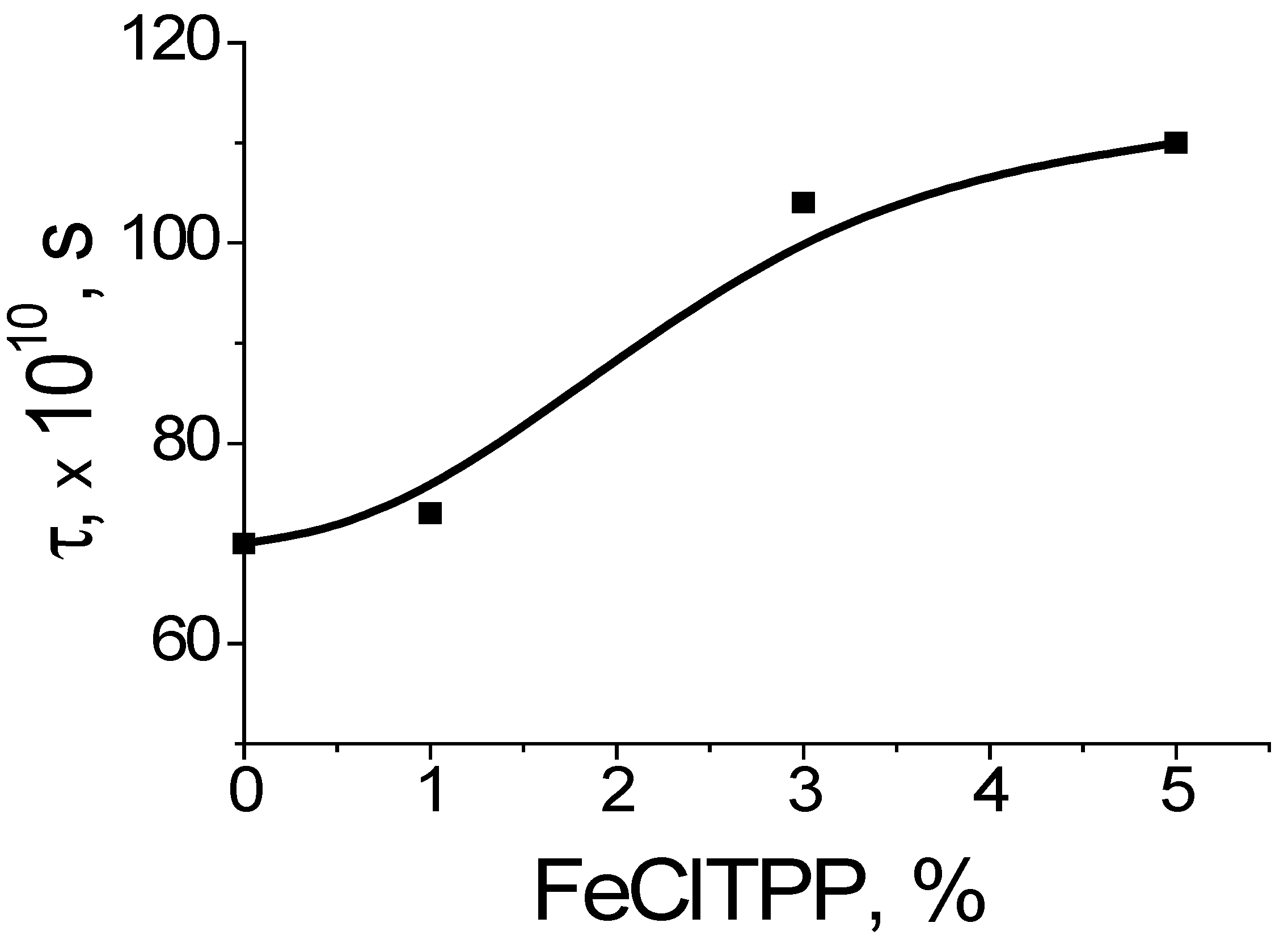
The analysis of the amorphous phase of PHB fibers via the EPR method using a probe (TEMPO) (
Figure 12b) showed the presence of a superposition of the spectra of the probe rotation speeds, showing a disequilibrium of the structure of the amorphous phase in the fibers: the presence of regions with different packing densities of macromolecule segments. The dependence of the average correlation time on the concentration of DPD is shown in
Figure 12b. It can be seen that with an increase in the concentration of DPD, the correlation time increases. This allows us to highlight the compaction of the amorphous phase in the fibers. This dependence correlates with the DSC data, which show an increase in crystallinity (
Figure 12a).
For comparison, we examined the structure of heterogeneous fibrous matrices based on PHB and FeClTPP with high antibacterial properties. This compound is a derivative of chlorophyll and has a high antibacterial effect due to its specific reaction with oxygen [
20].
Figure 10b shows the structural formulas of one of the FeClTPP samples.
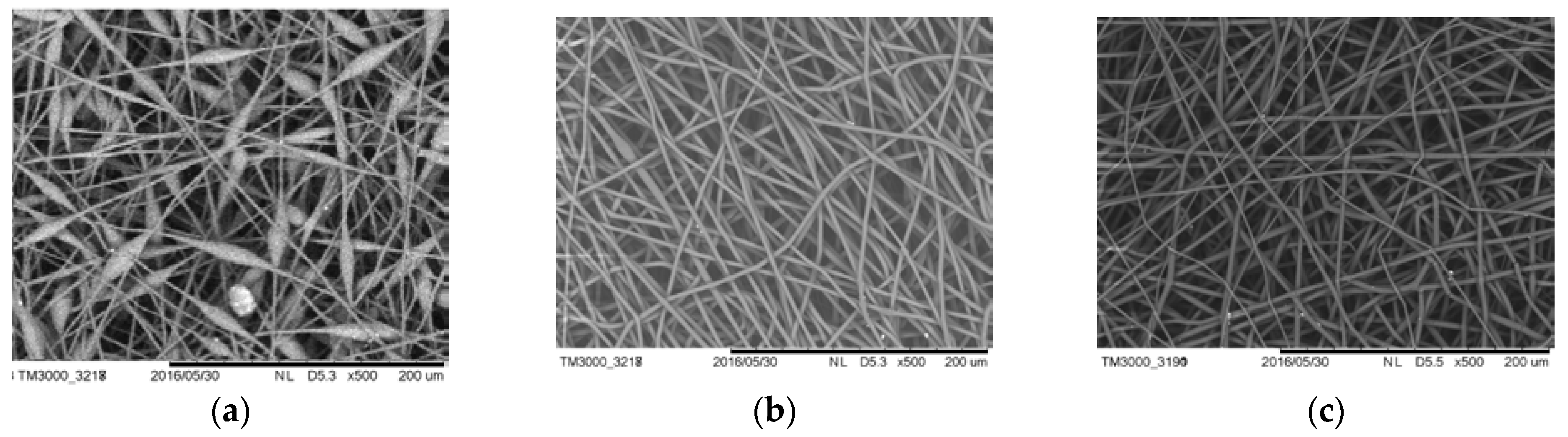
Figure 13 shows SEM images of the electrospun PHB-FeClTPP materials.
As in the case of DPD, the addition of 1–5 wt.% FeClTPP improves the quality of the polymeric solution for electrospinning process, which leads to the formation of smooth fibers with an average diameter of 2–4 microns. The strong polarity of this complex may cause its active interaction with oxygen-containing groups of PHB during the formation of fibers.
Table 2 shows the results of the study of the fibers using the DSC method.
Table 2 shows that the increase in the concentration of FeClTPP leads to an increase in the degree of crystallinity of PHB. In this case, a typical mechanism of nucleation of the crystalline phase is observed. Upon the cooling of the material, the inhibition of crystallization processes in PHB is visible, which is associated with the strong intermolecular interaction and high viscosity of PHB in the melted state.
Studying the crystalline structure of PHB via the X-ray method showed that with an increase in the concentration of FeClTPP, the longitudinal size of the crystallites and the long period of the crystalline phase of PHB increased (
Figure 14). This observation is consistent with the overall increase in the degree of crystallinity measured using the DSC method (
Table 2). This is in good agreement with the patterns obtained earlier when adding the DPA.
The results of the EPR study of the PHB- FeClTPP fibers are shown in
Figure 15.
The introduction of FeClTPP into PHB leads to the compaction of the amorphous phase of the polymer during the formation of the fiber. The proportion of dense regions increases with an increase in the concentration of FeClTPP. Apparently, the disequilibrium of the fiber is caused by a large number of mesomorphic structures (unfinished crystals), the formation of which occurs as a result of the intermolecular interactions of polar groups of PHB with strongly polar FeClTPP complexes. Adhesive bonds prevent the realization and complete passage of the crystallization process of the polymer matrix.
Both DPD and FeClTPP are highly soluble in chloroform and are distributed evenly in the fiber on the molecular level. In the case of polar nanoparticles, a different structural formation of PHB can be expected due to the insolubility of the particles in chloroform and their agglomeration in the fiber during the formation of the supramolecular structure. Our task was to study the effects of titanium and silicon oxide nanoparticles on the formation of the crystal structure of the fiber.