1. Introduction
Corrosion is the degradation of materials in oxidizing environments [
1]. In solutions, it is initiated by the surface reaction of the metallic material with the surrounding electrolyte. The reaction mechanism depends on the material and on the chemical composition of the electrolyte [
2]. The corrosion rate can be significantly reduced by the presence of organic compounds [
3,
4]. Organic compounds may serve as corrosion inhibitors and form an adherent monomolecular film on the metal’s surface [
3]. The protective film may prevent the direct contact of the metal with the electrolyte, thereby reducing the corrosion rate.
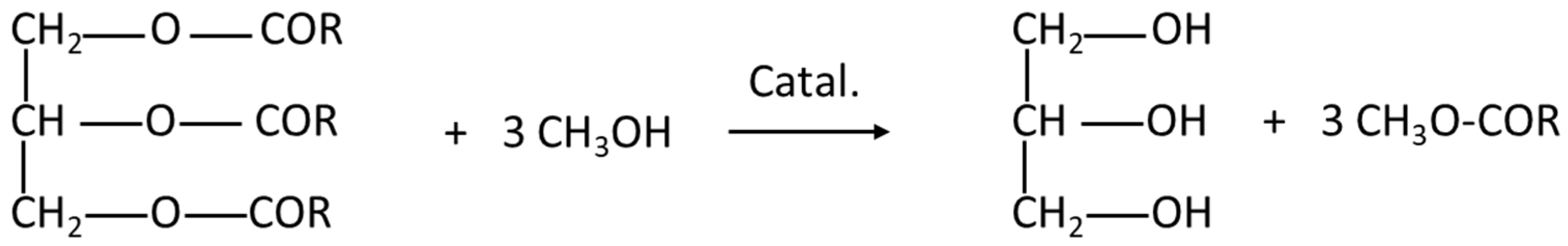
Biodiesel is an environmentally friendly fuel [
5]. It is derived from plants and animal products. Biodiesel is formed by long-chain fatty acid esters. It is produced through transesterification by reacting lipids, such as animal fat or oil, with an alcohol [
5,
6]. The scheme of transesterification is provided in
Figure 1. As a source of lipids, waste cooking oil can be utilized, thereby leading to a sustainable process [
7].
Glycerol (1,2,3-propanetriol) is a by-product of transesterification (
Figure 1). It is produced in substantial amounts: for each 10 kg of biodiesel produced, approximately 1 kg of crude glycerol is generated. Crude glycerol has low economic value as it contains several impurities. Common contaminants include moisture, ash, salts, methanol, soap, fatty acids, and various catalysts [
7]. Crude glycerol is also produced by the soap and tri-glyceride industries. Its chemical composition varies according to the manufacturing industry (
Table 1). The chemical composition of crude glycerol from biodiesel production is dependent on the catalyst and oil source used and the characteristics of the transesterification process. Both alkaline (NaOH) and acidic catalysts (H
2SO
4) have been used. If NaOH is used, sodium salts of fatty acids and sodium chloride are introduced to the crude glycerol solution. The chemical composition of the by-product is also given by the characteristics of the oil used during transesterification. Waste cooking oil, for example, results in a high carbon content in the resulting crude glycerol [
7]. The ash in crude glycerol may originate from the catalyst, as well as from the waste cooking oils used during the transesterification reaction.
The increasing production of biodiesel requires new applications of crude glycerol to be explored. Crude glycerol has been used to produce various chemicals, fuels, and fuel additives. Furthermore, it can be utilized as a livestock feed, in wastewater treatment, and in other applications [
8]. If glycerol is present in an electrodeposition bath, it may increase the corrosion resistance of electrodeposited zinc-based steel coatings [
9]. Glycerol can also be used to produce corrosion-resistant waterborne polyurethane (WPU) coatings via a thermochemical conversion process [
10]. The purification of crude glycerol is costly and energy-demanding. Therefore, direct utilization of non-purified crude glycerol is essential.
Several studies have investigated the effect of glycerol on the corrosion behavior of metallic materials [
11,
12,
13,
14]. It has been found that glycerol can act as a dual-functioning gas hydrate and corrosion inhibitor [
11]. An asset of glycerol is its high viscosity. The viscosity of water–glycerol solutions increases with increasing glycerol concentration [
15]. The increasing viscosity may decrease the diffusion of ions in solution, thereby decreasing the corrosion rate. In the present work, the corrosion activity of crude glycerol was evaluated. If disposed of in the environment, e.g., in ponds or in soil, crude glycerol may initiate the corrosion of steel constructions because of its high content of impurities. Therefore, the corrosion activity of crude glycerol needs to be explored. Previous studies have shown that the effect of water–glycerol solutions on steel corrosion is proportional to their concentration [
12,
13,
14]. Previous studies, however, investigated only very low glycerol concentrations (0.04–1%). It has been suggested by previous authors that further investigations with higher concentrations of glycerol should be performed to validate the corrosion inhibition efficiency [
11]. The corrosion behavior of steel in concentrated crude glycerol has not been investigated yet. Therefore, the corrosion resistance of low-alloy structural steel in crude glycerol from biodiesel production has been studied in the present work. The results are compared with those obtained with tap water.
2. Materials and Methods
In the present work, the corrosion behavior of S355MC steel was studied. S355MC is a low-alloy structural steel [
16]. Its chemical composition is shown in
Table 2.
The steel plates were cut on an ISOMET 5000 saw into slices of approx. 15 × 15 mm. The slices were then mounted on non-conductive translucent Transoptik resin at a temperature of 177 °C. An isostatic pressure of 145 bar was used during mounting. The pressing time was 3 min. The obtained cylindrical samples had a diameter of 30 mm and a height of 10 mm. The samples were metallographically prepared by grinding and polishing to remove the naturally occurring oxide layer and eliminate surface irregularities. Grinding was carried out on a Phoenix 400 grinder at 200 rotations per minute. Grinding was carried out successively on silicon carbide sandpapers with grades of 80, 240, 600, and 1200. Cooling was provided with running water. After grinding, the samples were further polished with a monodisperse diamond suspension with a grain size of 9 µm. Cooling during polishing was ensured using Metadifluid wetting agent. Finally, a hole was drilled on the other side of the sample with a manual drilling machine to ensure electrical contact with the steel during corrosion experiments.
The corrosion experiments were conducted in crude glycerol and tap water. The corrosion testing was carried out in a 0.5 L KMZ 5 glass vessel. A three-electrode setup was used. The system consisted of working, reference, and counter electrodes. A polished sample’s surface was used as the working electrode. The reference electrode was an Ag/AgCl wire immersed in a saturated KCl solution. The counter electrode was platinum foil. The course of the reaction was controlled by a potentiostat. At the beginning of the experiment, the open-circuit potential (OCP) was measured for 30 min. Subsequently, a linear polarization in the range of −1200 mV to 0 mV vs. Ag/AgCl was conducted. The polarization was performed using a sweeping rate of 1 mV/s. In this way, a polarization curve was obtained, from which the corrosion current density and corrosion potential were determined by Tafel extrapolation [
1]. The corrosion rate of steel in mm/year was calculated from the corrosion current density using Faraday’s law.
The steel microstructures were studied using a Zeiss NEOPHOT 2 optical microscope. The microscope was equipped with a CCD camera. A 2% Nital solution was used for etching. The microstructures of the steel were also investigated using a JEOL JSM-7600F scanning electron microscope (SEM, JEOL Ltd., Tokyo, Japan). The back-scattered electron mode (BSE) was used for imaging. The chemical composition of the corrosion products was studied with an Oxford Instruments (Bucks, UK) energy-dispersive X-ray spectrometer (EDS) using a Si(Li) detector X-Max 50 mm2 integrated into a SEM. The spectrometer was operated using INCA software (Oxford Instruments NanoAnalysis, Bucks, UK). The surface topography of the corroded samples was also inspected by a Zeiss LSM 700 confocal laser scanning microscope (CLSM). The topographical resolution of the surface was determined using ZEN 2009 software.
3. Results and Discussion
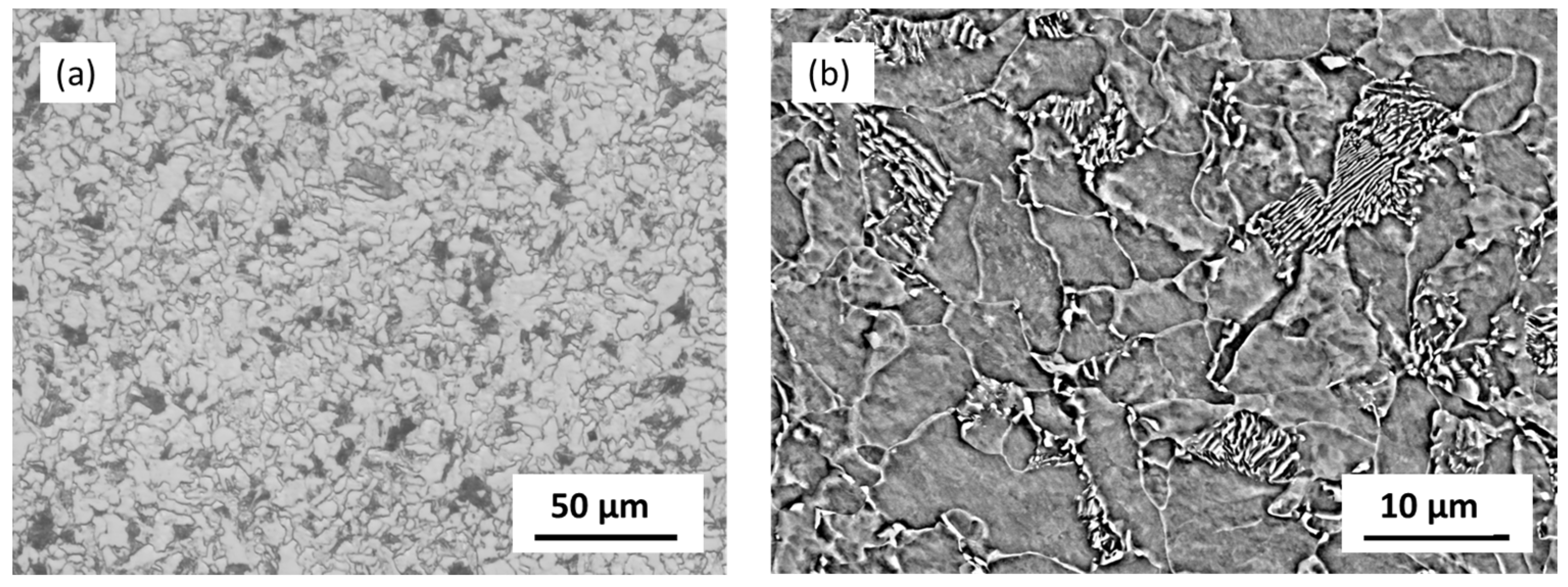
S355MC is a low-carbon structural steel with moderate strength and good weldability [
16]. Steels of this grade are used to construct tanks, wagons, and pipelines. The microstructure of S355MC is presented in
Figure 2. It has a ferrite–pearlite microstructure. Pearlite has a lamellar microstructure. It is composed of alternating layers of ferrite and cementite. The lamellar structure of pearlite is visible in the SEM image (
Figure 2b).
The corrosion experiments on S355MC were carried out at room temperature. Crude glycerol solution and tap water were used for corrosion testing. The chemical composition of crude glycerol is shown in
Table 3. Crude glycerol from biodiesel production was used. It was supplied as a cloudy yellow–brown viscous liquid, in which solid black particles were floating (
Figure 3).
Glycerol is a sugar alcohol [
17]. Crude glycerol is used as a livestock feed [
18]. As such, it is commonly diluted with drinking water from the tap. The quality and chemical composition of tap water in Slovakia is defined by the regulation on water quality [
19] and is constantly monitored by the government. Therefore, tap water was used as the reference solution.
The hydrogen exponent (pH) and conductivity of the crude glycerol and drinking water were determined with a hand-held glass electrode multimeter. The experimental data are presented in
Table 4. The drinking water was neutral. The glycerol solution was slightly acidic. The electrical conductivity of glycerol was significantly higher than that of water. This observation indicates an increased quantity of ionized substances in the glycerol solution and a relatively high degree of pollution compared with drinking water.
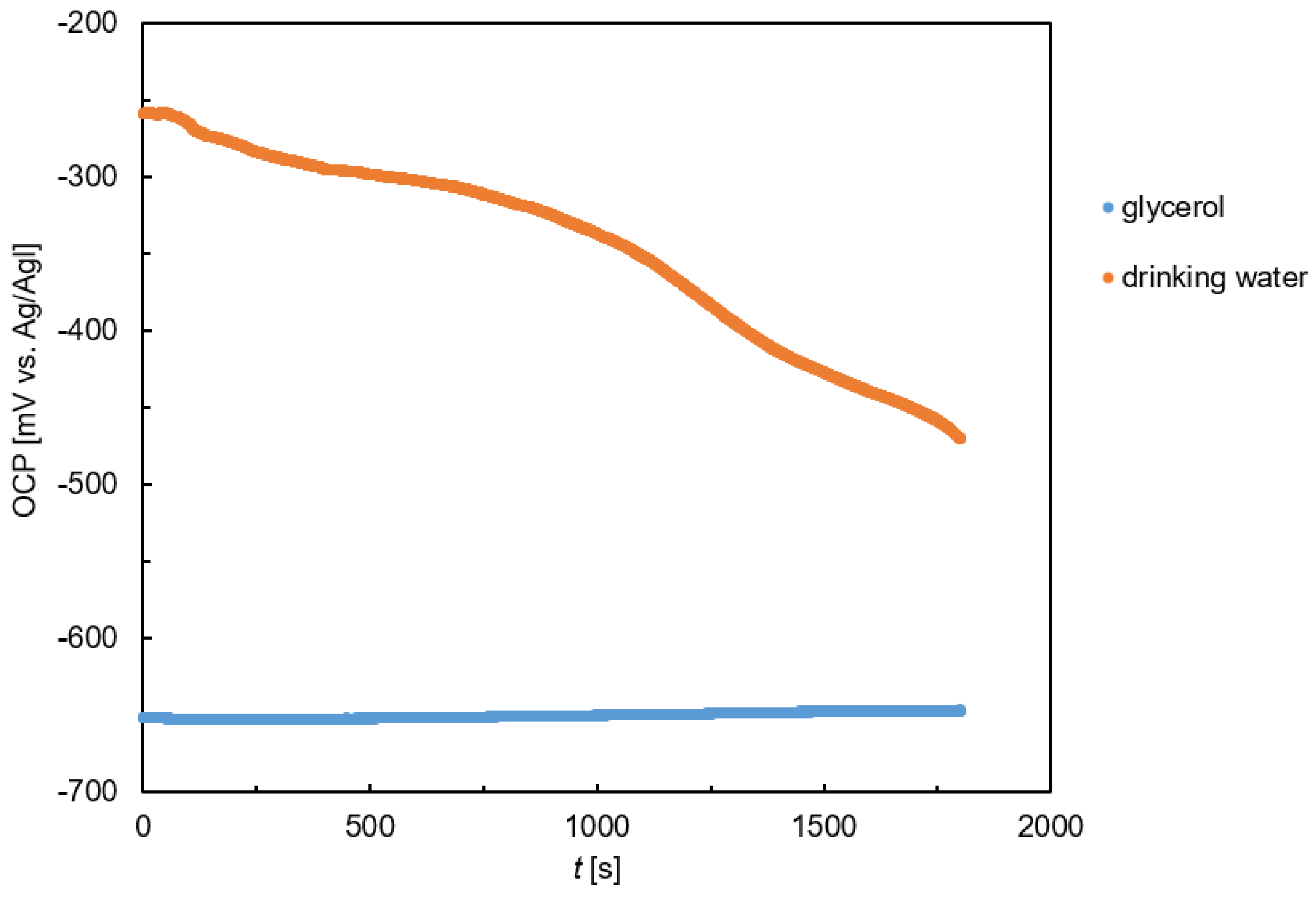
The corrosion testing of S355MC was carried out in a 0.5 L KMZ 5 glass vessel using electrochemical techniques. The OCP of the steel was measured first. The results for the glycerol solution and drinking water are shown in
Figure 4. The OCP of S355MC steel in glycerol was close to −650 mV (vs. Ag/AgCl). It was stable over time. The OCP of steel in drinking water was significantly higher. At the beginning of the experiment, the OCP of S355MC steel in drinking water was approximately −250 mV (vs. Ag/AgCl). The OCP in drinking water decreased during the experiment. After 30 min of exposure, it dropped down to −470 mV (vs. Ag/AgCl). The significant decrease in the open circuit potential indicates faster corrosion kinetics occurring in drinking water compared with glycerol.
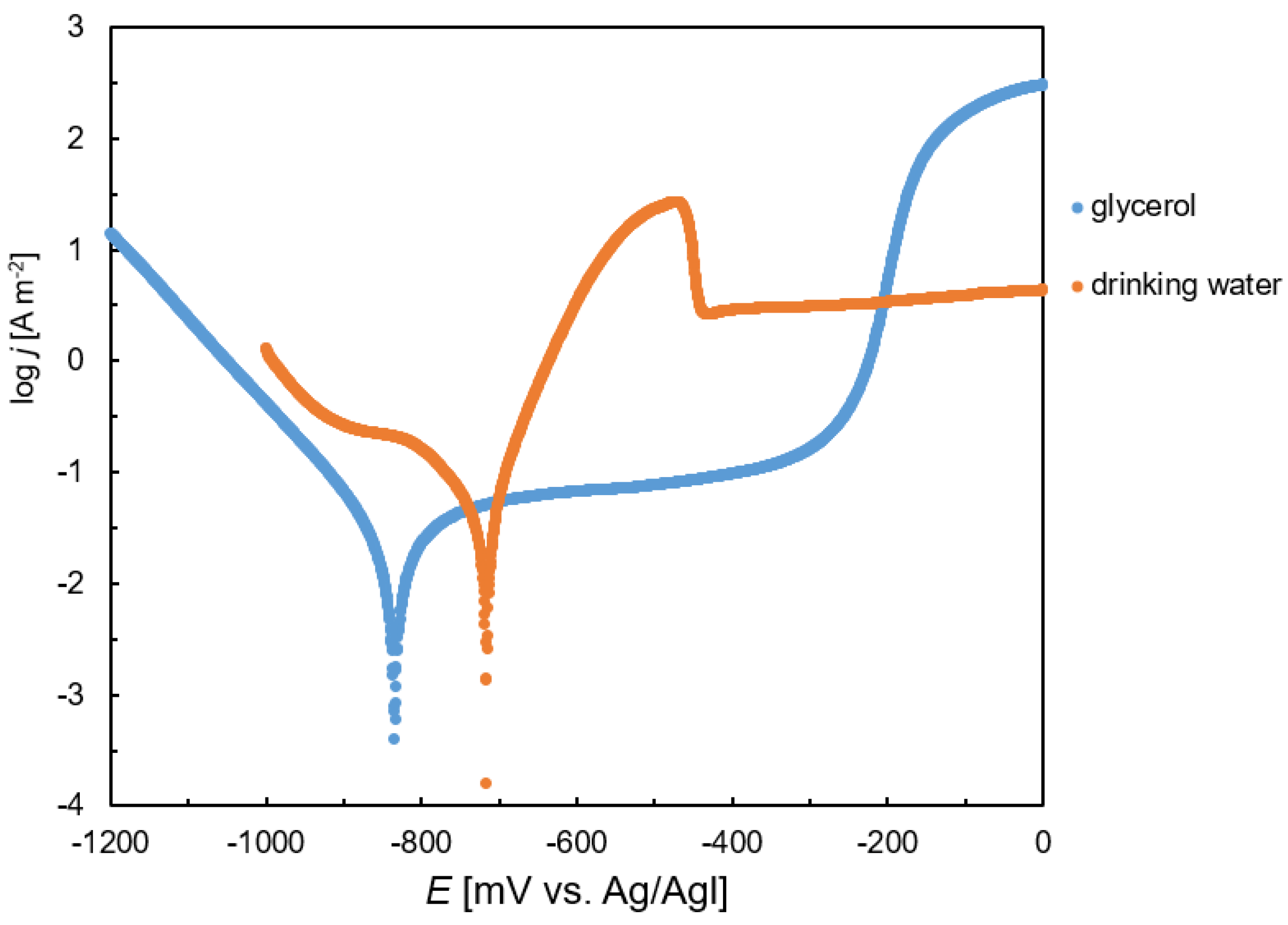
After 30 min of OCP measurement, a potentiodynamic experiment was initiated. The steel electrode’s potential linearly increased from −1200 mV to 0 mV vs. Ag/AgCl using a sweeping rate of 1 mV/s. The resulting polarization curves of the steel in crude glycerol and tap water are compared in
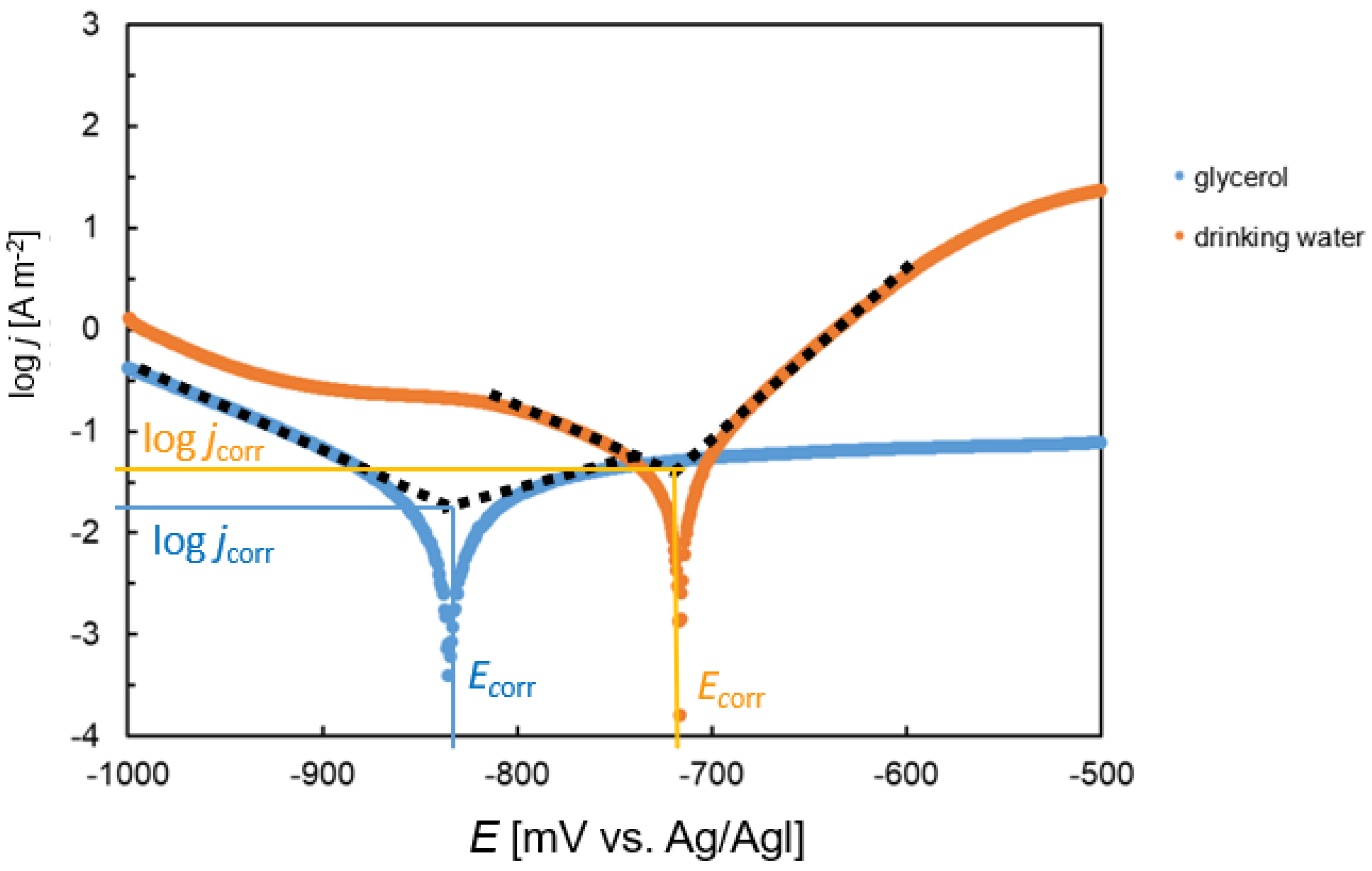
Figure 5. A single minimum was recorded on each curve, which corresponded to the transition of the steel from the immune to the active state. The position of this minimum determined the corrosion potential. On the polarization curve of the steel in glycerol, a broad plateau characterized by a steady current density value was found. It was observed at potentials higher than the corrosion potential. The plateau indicates the rapid transition of the steel to a passive state. In drinking water, on the other hand, a sharp increase in the current density was observed after overcoming the corrosion potential. This behavior indicates faster corrosion kinetics compared with glycerol. Steel was also slightly passivated in drinking water; however, the passivation occurred at considerably higher potentials (~−450mV vs. Ag/AgCl,
Figure 5). Furthermore, the passivation current density was significantly higher compared with the passivation current in glycerol.
The region of the corrosion minimum was used to determine the corrosion current. The procedure of Tafel extrapolation is shown in
Figure 6. The cathodic and anodic parts of the curve were approximated by straight lines. Subsequently, the corrosion current density (
jcorr) and corrosion potential (
Ecorr) were determined from the line intersection points.
The polarization resistance,
Rp, was determined according to the Stern–Geary method [
20]. The experimental electrochemical parameters determined in the present work are given in
Table 5. Each experiment was repeated three times. The standard deviations were smaller than 5%.
The corrosion current densities were used to calculate the corrosion rate of low-alloy S355MC steel. The following formula, based on Faraday’s law, was used:
In Equation (1),
vcorr represents the corrosion rate,
jcorr is the corrosion current density,
z is the number of electrons exchanged in the corrosion reaction,
Mm is the molar mass of Fe,
ρ is the alloy density, and
F is the Faraday constant (
F = 96485 C mol
−1). The following values were used in the calculation:
z = 2,
ρ = 7874 kg m
−3, M
m = 55.85 × 10
−3 kg mol
−1. The calculated corrosion rates are given in
Table 5.
The corrosion rate of S355MC steel in drinking water (4.61 × 10
−2 mm/year) corresponded to previously reported results for low-alloy steels [
21]. The corrosion rate in glycerol was 2.06 × 10
−2 mm/year. This value was approximately 2.2 times smaller compared with that of drinking water. The corrosion rate of crude glycerol is comparable to that in the results obtained by Sivabalan et al. [
11] The authors studied the corrosion behavior of mild X52 steel in 3.5 wt. % NaCl solutions using the weight loss method. The concentrations of glycerol were 0.04–1 wt. %. The authors observed corrosion rates of 2–7 × 10
−2 mm/year [
11]. The corrosion rate of mild X52 steel decreased with increasing glycerol concentration.
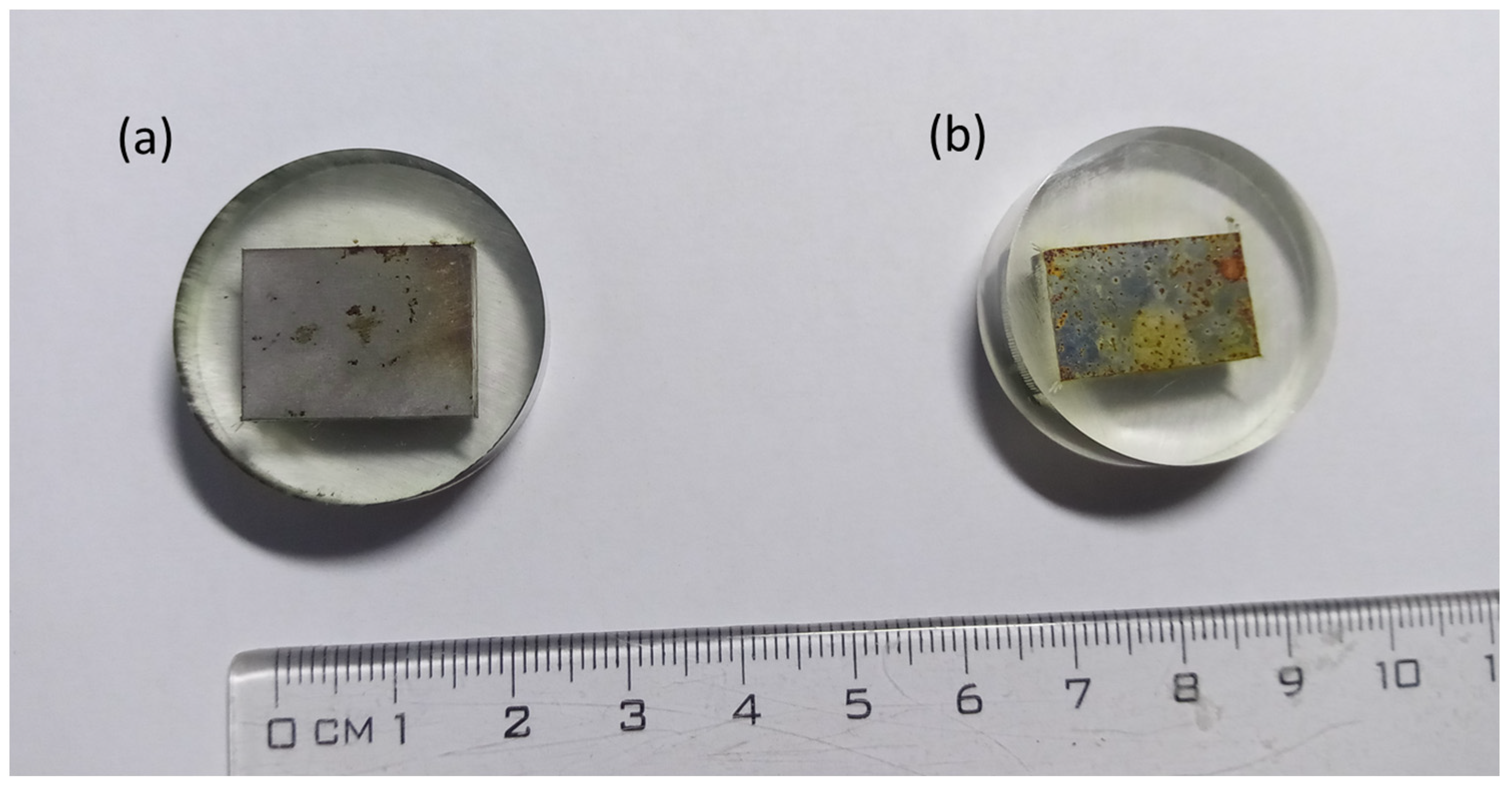
Glycerol is an organic substance. As such, it may adsorb on the surface of steel and act as a corrosion inhibitor. A comparison of the appearance of the samples after the corrosion test is shown in
Figure 7. The surface of the steel exposed to 65% glycerol solution was covered with an adherent layer of corrosion products. Conversely, the steel exposed to drinking water exhibited an orange, discontinuous, flaking layer of corrosion products. This observation also points to the better corrosion resistance of steel in glycerol compared with that in drinking water.
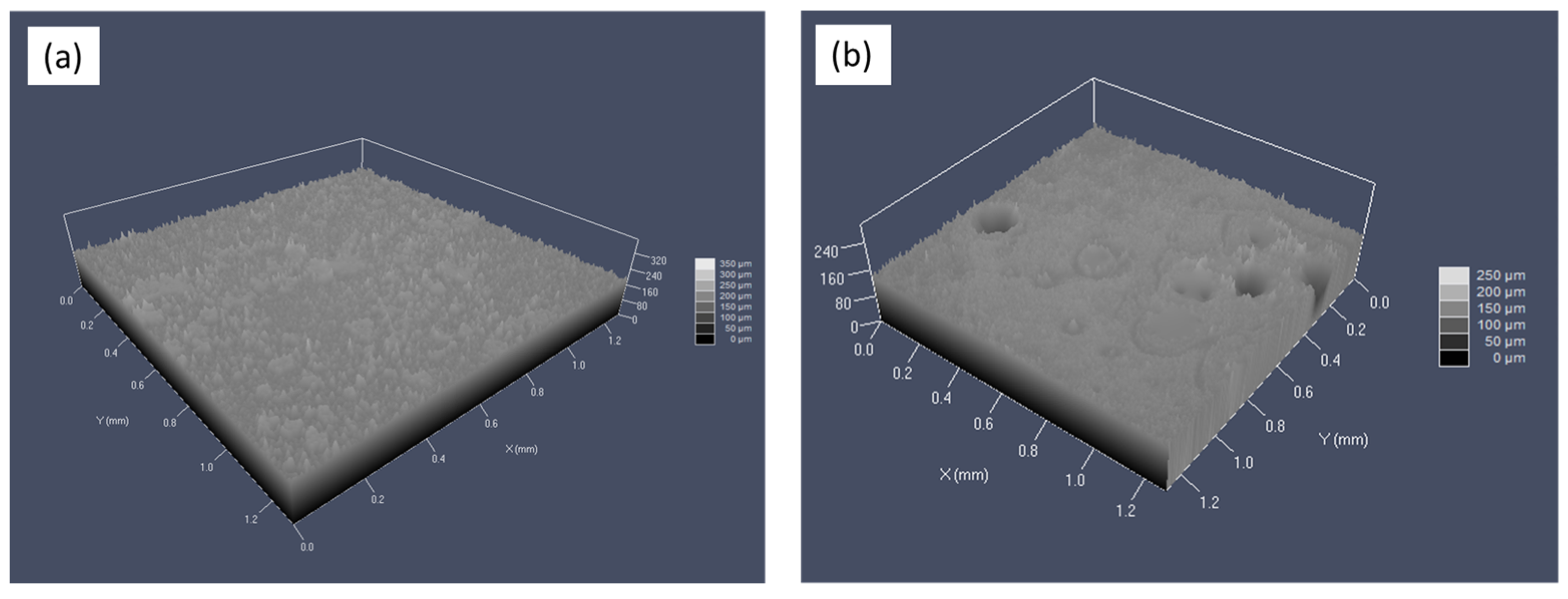
The post-corroded specimens were also inspected by confocal laser scanning microscopy. Three-dimensional images of the samples’ surfaces after corrosion are compared in
Figure 8. It can be observed that the surface of the steel exposed to tap water contained numerous pits (
Figure 8b). The surface of the steel exposed to glycerol, on the other hand, was not affected by pitting corrosion (
Figure 8a).
To further investigate the identity of the corrosion products, an SEM/EDS analysis was performed. The microstructure of post-corroded surfaces is presented in
Figure 9 and
Figure 10. The BSE mode was employed to provide elemental resolution. The dark areas correspond to corrosion products. It can be observed that the surface of the steel exposed to tap water was not fully covered by corrosion products (
Figure 9). The corrosion products were preferentially located at pits. The products were not adherent and spalled off the surface.
The microstructure of the steel exposed to the glycerol solution is presented in
Figure 10. The surface of the steel was significantly less affected by corrosion. In the microstructure, grains of ferrite and lamellar pearlite can be observed. The microstructure resembles the microstructure prior to corrosion (
Figure 2b). This observation indicates that the surface was significantly less affected by corrosion. It is possible that an adsorption layer of glycerol formed on the surface, protecting the underlying substrate against corrosion.
The chemical compositions of corrosion products were studied by EDS. The results are compared in
Table 6. In the case of the steel oxidized in tap water, a high concentration of oxygen was found in the corrosion product. The chemical composition of the corrosion product corresponded to Fe
2O
3 (hematite). Hematite forms flaky corrosion products with an orange appearance, commonly referred to as rust. In the case of steel exposed to crude glycerol, a high concentration of carbon was found on the alloy’s surface. The presence of carbon indicated that an adsorbed layer of an organic compound, presumably glycerol, was formed on the sample’s surface. In glycerol, the steel was passivated rapidly (
Figure 3). The protective film was, therefore, most likely composed of metal–glycerol complexes.
Previous studies have investigated the effects of crude glycerol on the corrosion behavior of mild steel in hydrochloric acid [
12,
13]. An overall maximum inhibition efficiency of 98% was reached at a 70 h residence time and a 1% inhibitor concentration [
12]. Glycerol was also found to inhibit the corrosion reaction of copper in aerated NaCl solutions (0.5 M) [
14]. The inhibition efficiency increased with increasing glycerol concentration. The corrosion inhibition is probably related to the adsorption of glycerol molecules on the metal’s surface. The density functional theory calculations indicated that adsorption happened mainly through oxygen atoms [
22]. The protective crude glycerol layer became thicker as the concentration of the inhibitor increased.
The present paper provides the first results on the corrosion behavior of low-alloy steel in crude glycerol. Crude glycerol is a byproduct of several industries—biodiesel production, the soap industry, and the fatty acid industry. The present paper indicates that this byproduct could be utilized as a corrosion inhibitor directly and without purification. The results are of practical importance and might be useful for various technologies. The corrosion inhibition effect of crude glycerol might be utilized in closed-loop heating and cooling systems, transportation, or lubrication. A larger study on the effect of crude glycerol on the corrosion behavior of mild steel is planned. We plan to study different water–glycerol mixtures. Furthermore, we plan to include a better controlled reference environment—an aqueous NaCl solution—and study different salt concentrations. The results are planned to be reported in a full paper.