Vacuum UV (VUV) Photo-Oxidation of Polyethersulfone (PES)
Abstract
:1. Introduction
2. Materials and Methods
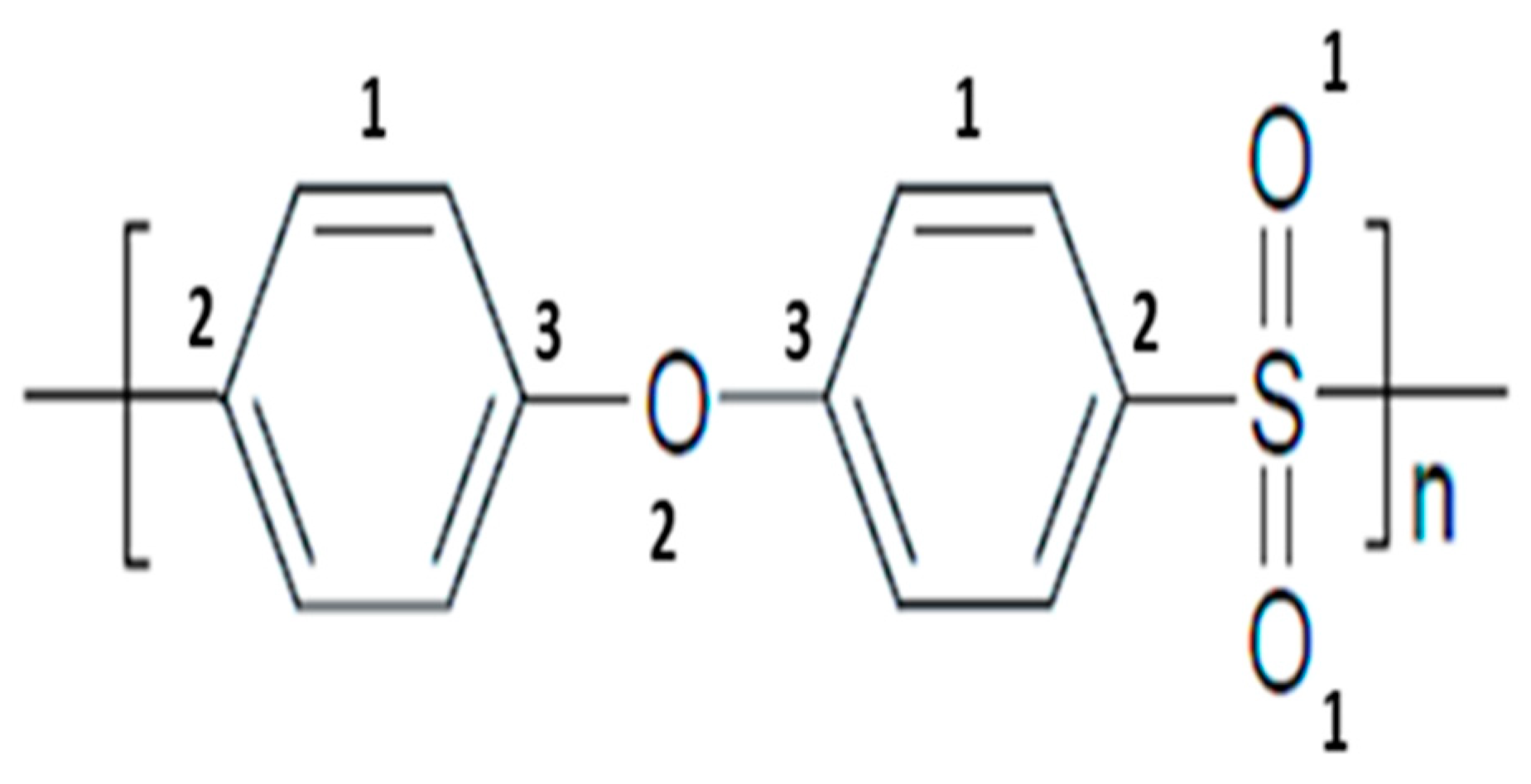
2.1. Materials
2.2. VUV Photo-Oxidation
2.3. X-ray Photoelectron Spectroscopy (XPS)
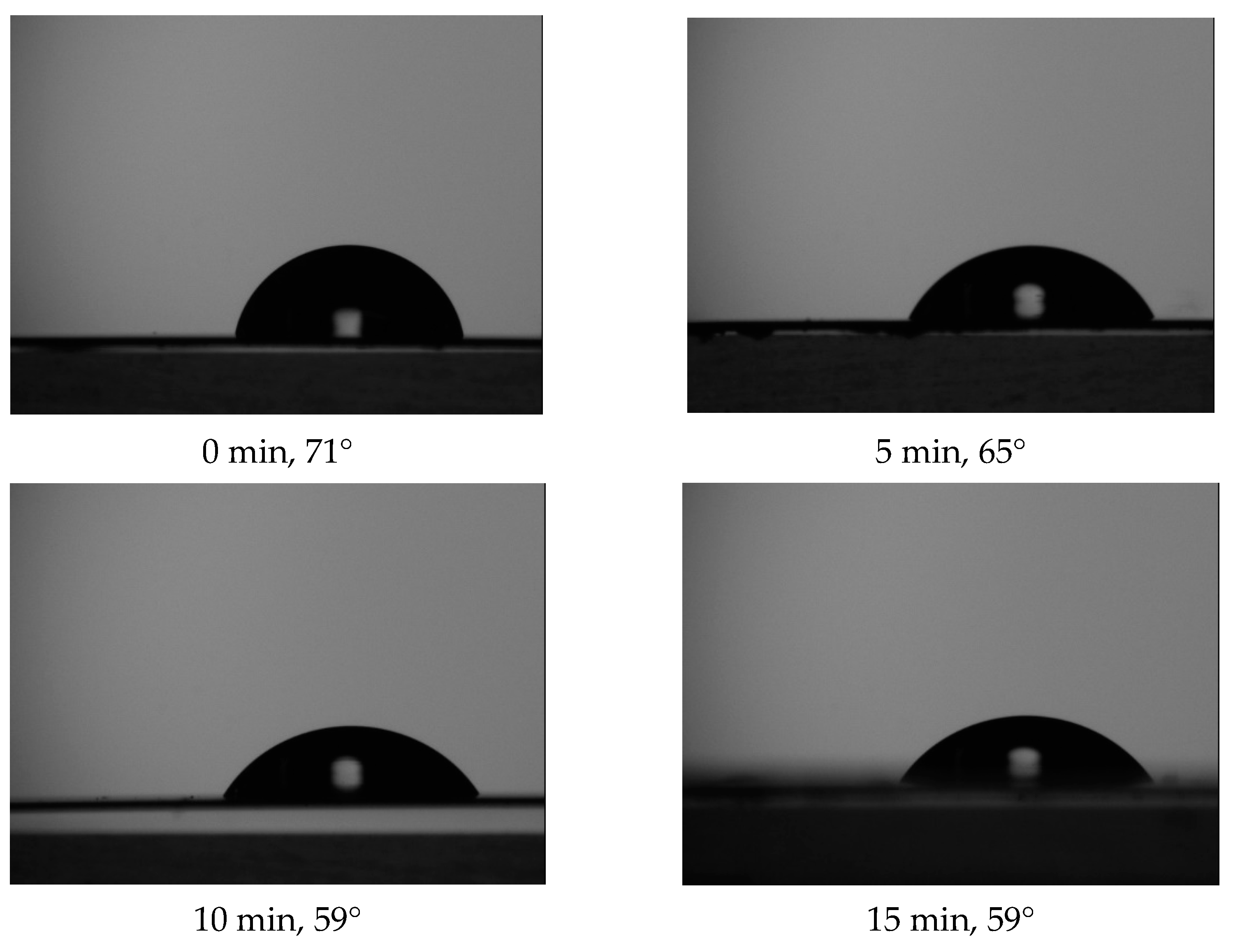
2.4. Contact Angle (CA) Goniometry
2.5. Scanning Electron Microscopy (SEM)
3. Results
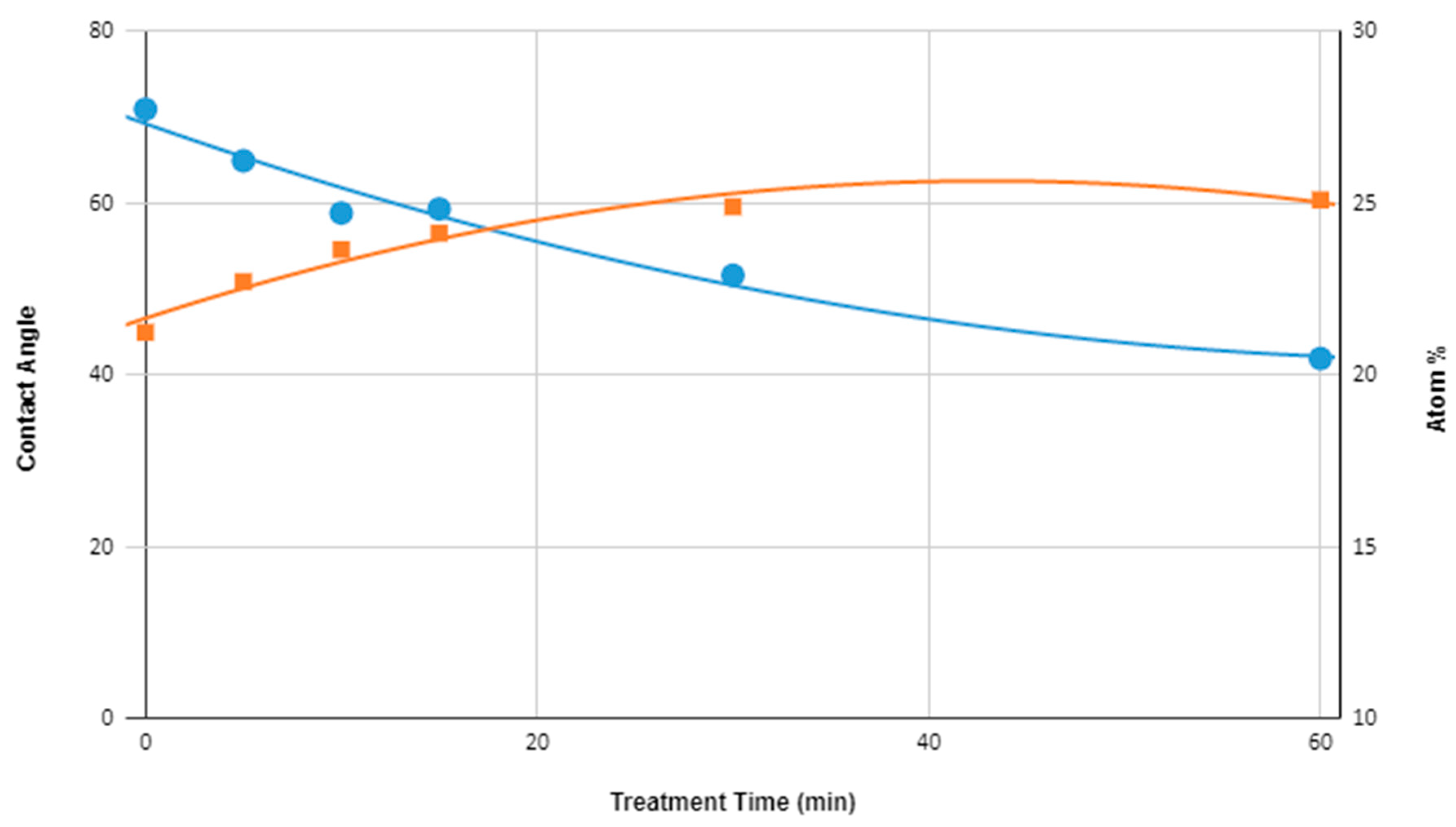
3.1. Quantitative XPS and Water Contact Angle
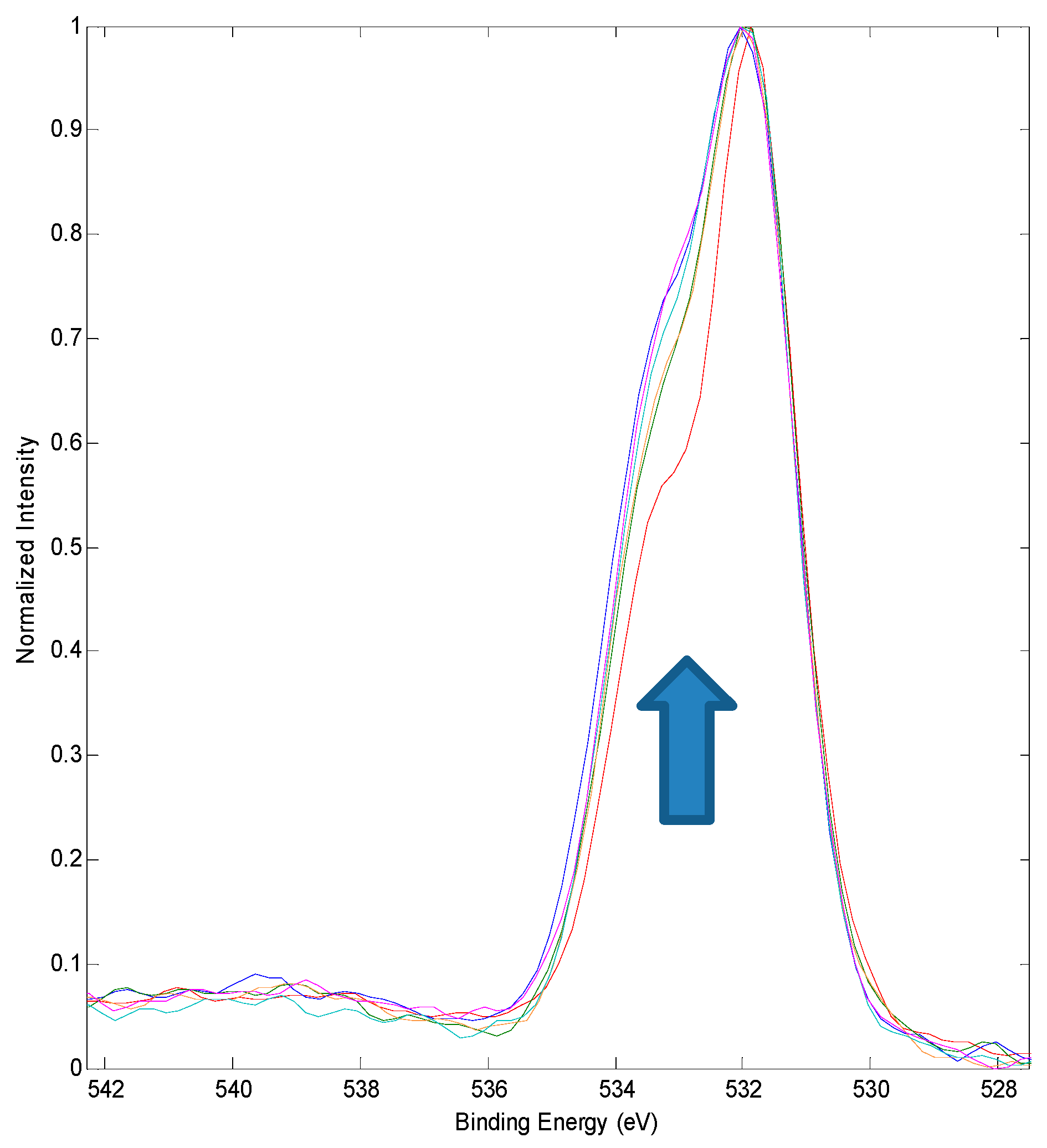
3.2. XPS Chemical State Analysis
3.3. Surface Topography for PES Treated with VUV Photo-Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Aguilar-Sanchez, A.; Jalvo, B.; Mautner, A.; Nameer, S.; Poehler, T.; Tammelin, T.; Mathew, A.P. Waterborne nanocellulose coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. J. Membr. Sci. 2021, 620, 118842–118851. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Dey, B.; Al Saidi, A.K.A.; Choudhury, A. Functionalized-graphene reinforced polyethersulfone nanocomposites with improved physical and mechanical properties. Polym. Compos. 2020, 41, 4104–4116. [Google Scholar] [CrossRef]
- Suhartono, J.; Pertiwi, D.S.; Noersalim, C.; Yulianingsih, D.; Sofianti, F.; Saptoro, A.; Chafidz, A. Characteristics and performances of blended polyethersulfone and carbon-based nanomaterial membranes: Effect of nanomaterial types and air exposure. Chem. Eng. Technol. 2020, 43, 1630–1637. [Google Scholar] [CrossRef]
- Takacs, G.A.; Miri, M.J.; Kovach, T. Vacuum UV surface photo-oxidation of polymeric and other materials for improving adhesion: A critical review. Rev. Adhes. Adhes. 2020, 8, 555–581. [Google Scholar]
- Samson, J.A.R. Techniques of Vacuum Ultraviolet Spectroscopy; John Wiley & Sons: New York, NY, USA, 1967. [Google Scholar]
- Li, X.; Toro, M.; Lu, F.; On, J.; Bailey, A.; Debies, T.; Mehan, M.; Gupta, S.K.; Takacs, G.A. Vacuum UV photo-oxidation of polystyrene. J. Adhes. Sci. Technol. 2016, 30, 2212–2223. [Google Scholar] [CrossRef]
- Kovach, T.; Boyd, S.; Garcia, A.; Fleischer, A.; Vega, K.; Hilfiker, R.; Shertok, J.; Mehan, M.; Gupta, S.K.; Takacs, G.A. Surface modification of polybenzimidazole (PBI) with microwave generated vacuum ultraviolet (VUV) photo-oxidation. Curr. Microw. Chem. 2021, 8. [Google Scholar] [CrossRef]
- Badey, P.; Urbaczewski-Espunche, E.; Jugnet, Y.; Sage, D.; Minh Duc, T.; Chabert, B. Surface modification of poly(tetrafluoroethylene) by microwave plasma downstream treatment. Polymer 1994, 35, 2472–2479. [Google Scholar] [CrossRef]
- Lens, J.P.; Spaay, B.; Terlingen, J.G.A.; Engbers, G.H.M.; Feijen, J. Mechanism of the immobilization of surfactants on polymer surfaces by means of an argon plasma treatment: Influence of UV radiation. Plasmas Polym. 1999, 4, 159–182. [Google Scholar] [CrossRef]
- Calvert, J.G.; Pitts, J.N. Photochemistry; John Wiley & Sons: New York, NY, USA, 1966; pp. 206–447. [Google Scholar]
- Okabe, H. Photochemistry of Small Molecules; John Wiley & Sons: New York, NY, USA, 1978; pp. 106–179. [Google Scholar]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers; John Wiley & Sons: Chichester, UK, 1991. [Google Scholar]
- Weir, N.A. Photo and photooxidation reactions of polystyrene and of ring substituted polystyrenes. Dev. Polym. Degrad. 1982, 4, 143–188. [Google Scholar]
- Geuskens, G.; Baeyens-Volant, D.; Delaunois, G.; Lu-Vinh, Q.; Piret, W.; David, C. Photo-oxidation of polymers I. A quantitative study of the chemical reactions resulting from irradiation of polystyrene at 253.7 nm in the presence of oxygen. Euro. Polym. J. 1978, 14, 291–297. [Google Scholar] [CrossRef]
- Partridge, R.H. Vacuum-ultraviolet absorption spectrum of polystyrene. J. Chem. Phys. 1967, 47, 4223–4227. [Google Scholar] [CrossRef]
- Lutondo, S.; Turo, M.; Sachdev, S.; Shertok, J.; Bailey, A.; Mehan, M.; Gupta, S.K.; Takacs, G.A. Surface modification of polyethersulfone (PES) with ozone. Ozone Sci. Eng. 2019, 41, 448–453. [Google Scholar] [CrossRef]
- Munro, H.S.; Clark, D.T. An ESCA investigation of the surface photo-oxidation of polyethersulphone. Polym. Degrad. Stab. 1985, 1, 225–231. [Google Scholar] [CrossRef]
- Kilduff, J.E.; Mattaraj, S.; Pieracci, J.P.; Belfort, G. Photochemical modification of poly(ethersulfone) and sulfonated poly(sulfone) nanofiltration membranes for control of fouling by natural organic matter. Desalination 2000, 132, 133–142. [Google Scholar] [CrossRef]
- Rivaton, A.; Gardette, J.L. Photodegradation of polyethersulfone and polysulfone. Polym. Degrad. Stab. 1999, 66, 385–403. [Google Scholar] [CrossRef]
- Vrlinic, T.; Vesel, A.; Cvelbar, U.; Krajnc, M.; Mozetic, M. Rapid surface functionalization of poly(ethersulphone) foils using a highly reactive oxygen-plasma treatment. Surf. Interface Anal. 2007, 39, 476–481. [Google Scholar] [CrossRef]
- Gonzalez, E.; Barankin, M.D.; Guschl, P.C.; Hicks, R.F. Ring opening of aromatic polymers by remote atmospheric-pressure plasma. IEEE Trans. Plasma Sci. 2009, 37, 823–831. [Google Scholar] [CrossRef]
- Cisse, I.; Oakes, S.; Sachdev, S.; Toro, M.; Lutondo, S.; Shedden, D.; Atkinson, K.M.; Shertok, J.; Mehan, M.; Gupta, S.K.; et al. Surface modification of polyethersulfone (PES) with UV photo-oxidation. Technologies 2021, 9, 36. [Google Scholar] [CrossRef]
- Norrman, K.; Kingshott, P.; Kaeselev, B.; Ghanbari-Siahkali, A. Photodegradation of poly(ether sulfone) Part 1. A time-of-flight secondary ion mass spectrometry study. Surf. Interface Sci. 2004, 36, 1533–1541. [Google Scholar]
- Arikan, E.; Holtmannspotter, J.; Zimmer, F.; Hofmann, T. The role of chemical surface modification for structural adhesive bonding on polymers—Washability of chemical functionalization without reducing adhesion. Int. J. Adhes. Adhes. 2019, 95, 102409–102420. [Google Scholar] [CrossRef]
- Singh, N.L.; Pelagade, S.M.; Rane, R.S.; Mulkherjee, S.; Desphpande, U.P.; Ganeshan, V.; Shripathi, T. Influence of argon plasma treatment on polyethersulphone surface. Pramana 2013, 80, 133141. [Google Scholar] [CrossRef]
- Michaljanicova, I.; Sleepicka, P.; Vesely, M.; Svorcik, V. Efficient nanostructure construction of polymer substrates by plasma treatment for tissue engineering. In Proceedings of the 2016 IEEE 16th International Conference on Nanotechnology (IEEE-NANO), Sendai, Japan, 22–25 August 2016; pp. 149–152. [Google Scholar]
- Hopkins, J.; Badyal, J.P.S. Plasma modification of poly(ether sulfone). Macromolecules 1994, 27, 5498–5503. [Google Scholar] [CrossRef]
- Saxena, N.; Prabhavathy, C.; Sirshendu, D.; DasGupta, S. Flux enhancement by argon-oxygen plasma treatment of polyethersulfone membranes. Sep. Purif. Technol. 2009, 70, 160–165. [Google Scholar] [CrossRef]


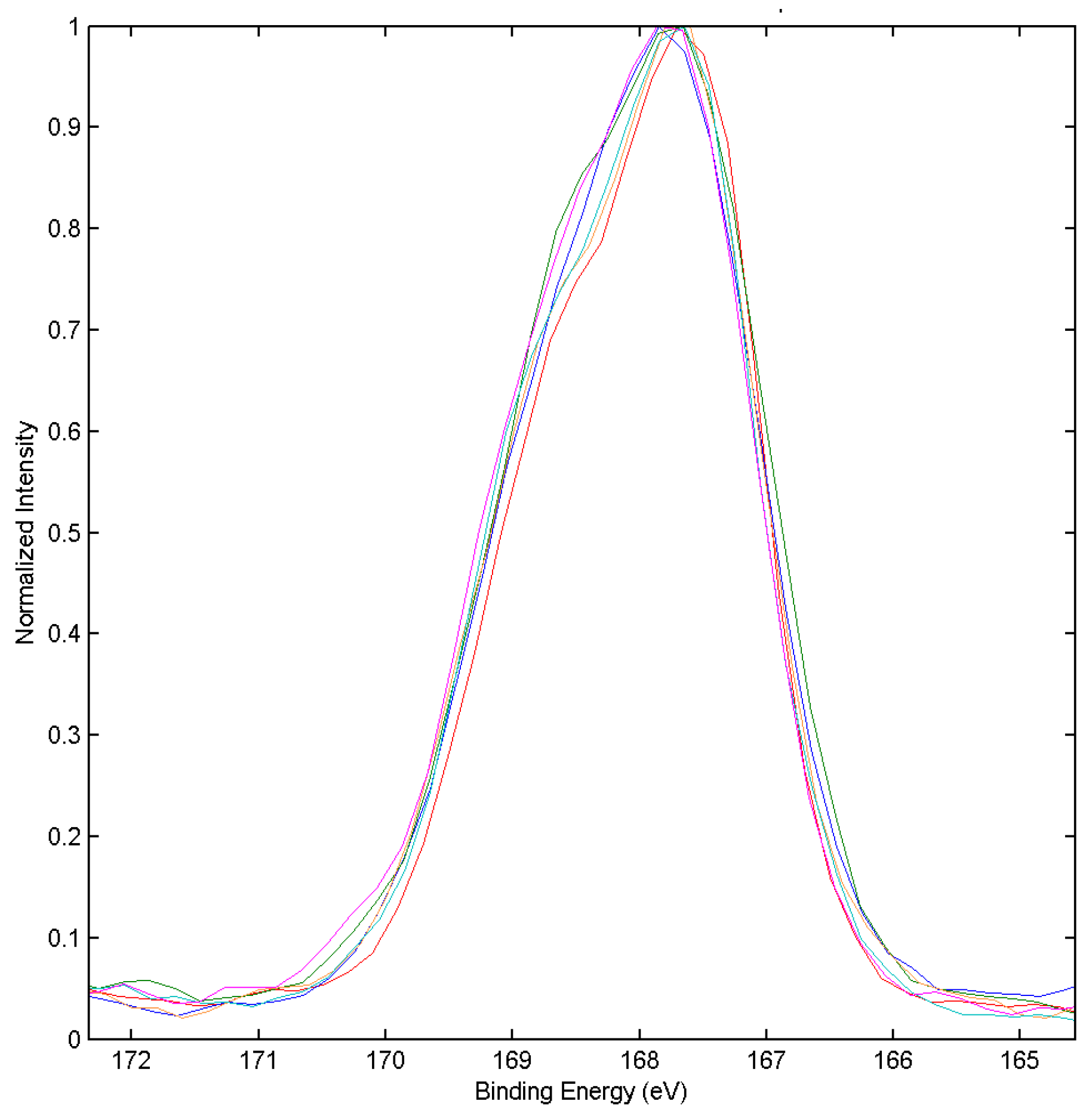
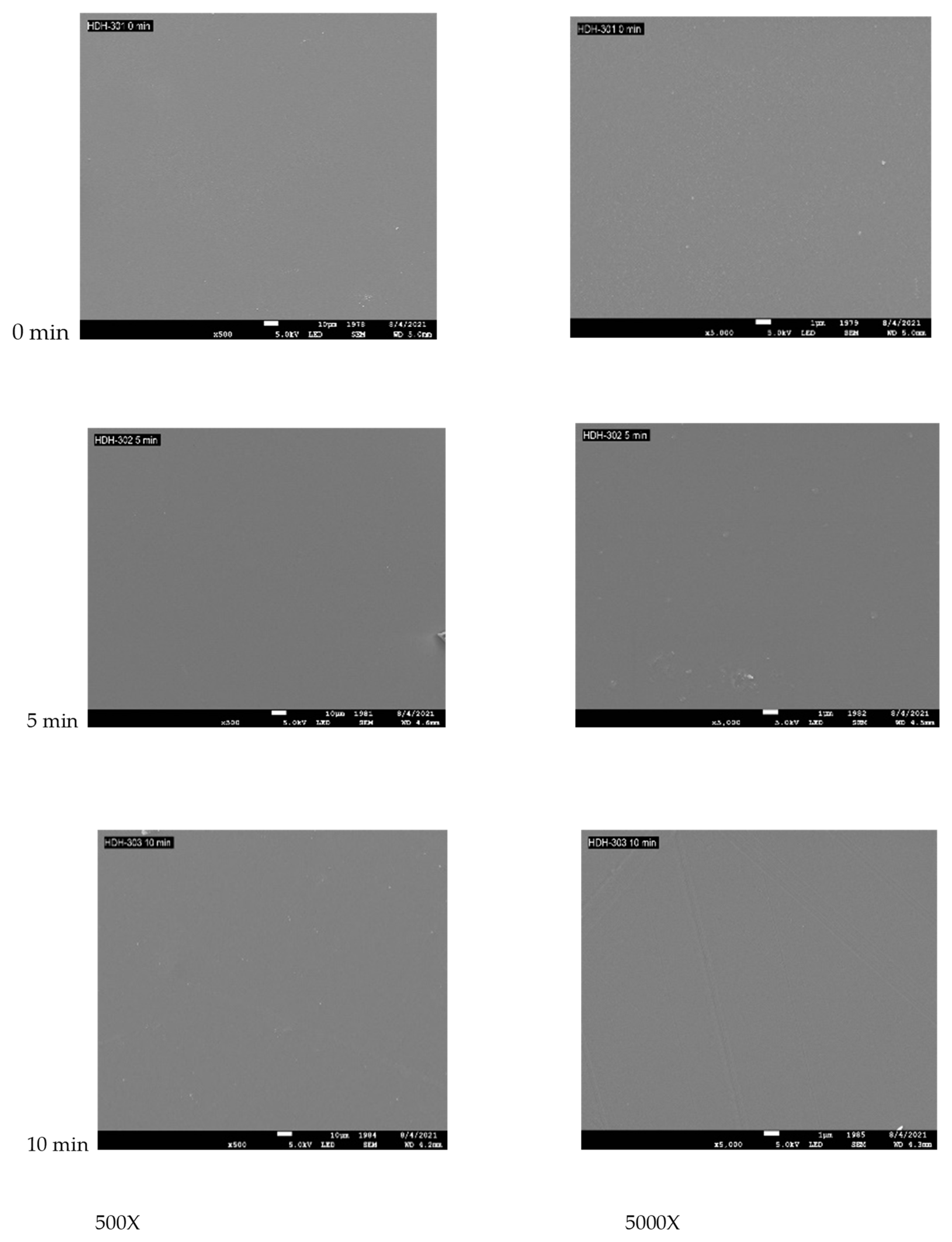
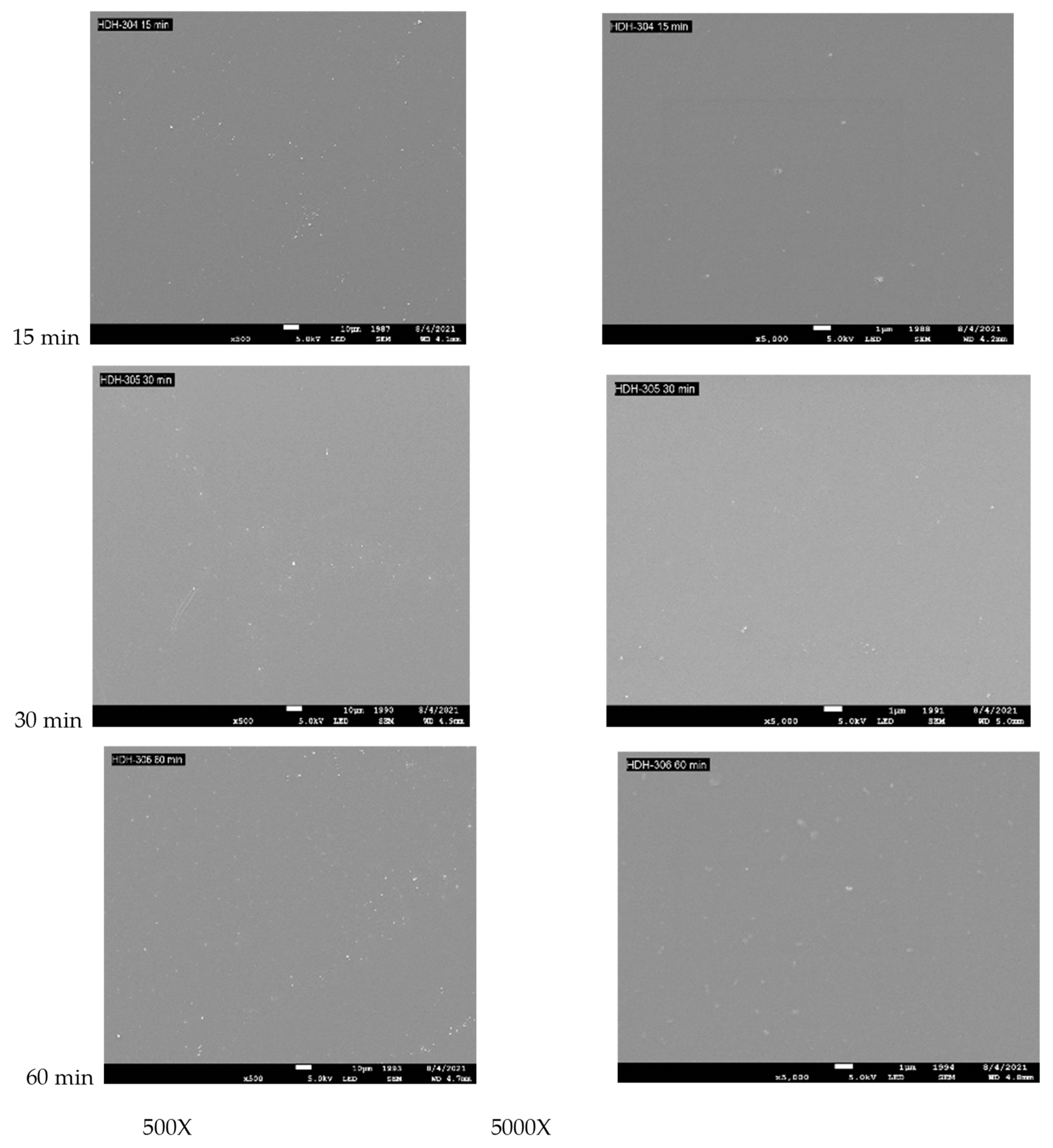
| Binding | Species | Treatment Time (min) | |||||
|---|---|---|---|---|---|---|---|
| Energy (eV) | 0 | 5 | 10 | 15 | 30 | 60 | |
| 284.7 | C-C aromatic ring (1) | 65.0 | 63.0 | 63.3 | 64.0 | 62.7 | 62.5 |
| 285.3 | C-S (2) | 15.6 | 15.3 | 16.6 | 16.2 | 16.8 | 16.1 |
| 286.3 | C-O (3), C-O (286.1) | 15.0 | 16.8 | 16.7 | 16.1 | 16.5 | 17.1 |
| 287.0 | C=O | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 288.6 | O-C=O | 0 | 0 | 0 | 0 | 0 | 0 |
| 289.1 | O=C-OH | 0 | 0 | 0 | 0 | 0.4 | 0.5 |
| 289.8 | O-(C=O)-O | 0 | 0 | 0 | 0 | 0 | 0 |
| 291.5 | Energy loss peak | 4.0 | 4.9 | 3.4 | 3.7 | 3.6 | 3.7 |
| Binding | Species | Treatment Time (min) | |||||
|---|---|---|---|---|---|---|---|
| Energy (eV) | 0 | 5 | 10 | 15 | 30 | 60 | |
| 531.6 | O=S (1) | 69.4 | 45.8 | 47.3 | 47.2 | 46.2 | 44.3 |
| 532.2 | O*=C-OH | 0 | 19.0 | 18.3 | 17.8 | 19.5 | 19.9 |
| 533.3 | C-O-C (ring) (2) (533.3), C-O (533.1) too close to separate, O-C-O*H (533.5) | 30.6 | 35.2 | 34.4 | 35.0 | 34.4 | 35.8 |
| Binding | Species | Treatment Time (min) | |||||
|---|---|---|---|---|---|---|---|
| Energy (eV) | 0 | 5 | 10 | 15 | 30 | 60 | |
| 167.6 | C-S=O S 2p3/2 | 66.1 | 65.6 | 65.8 | 66.7 | 64.8 | 63.5 |
| 168.8 | C-S=O S 2p1/2 | 33.9 | 31.8 | 30.9 | 31.1 | 32.3 | 33.5 |
| 168.6 | Sulphonate or Sulphate S 2p3/2 | 0 | 1.7 | 2.1 | 1.5 | 1.9 | 2.0 |
| 169.8 | Sulphonate or Sulphate S 2p1/2 | 0 | 0.9 | 1.2 | 0.7 | 1.0 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oakes, S.; Keeley, R.; Heineman, H.; Allston, T.; Shertok, J.; Mehan, M.; Thompson, G.K.; Takacs, G.A. Vacuum UV (VUV) Photo-Oxidation of Polyethersulfone (PES). Technologies 2022, 10, 49. https://doi.org/10.3390/technologies10020049
Oakes S, Keeley R, Heineman H, Allston T, Shertok J, Mehan M, Thompson GK, Takacs GA. Vacuum UV (VUV) Photo-Oxidation of Polyethersulfone (PES). Technologies. 2022; 10(2):49. https://doi.org/10.3390/technologies10020049
Chicago/Turabian StyleOakes, Sarah, Ryan Keeley, Hunter Heineman, Tom Allston, Joel Shertok, Michael Mehan, Gregory K. Thompson, and Gerald A. Takacs. 2022. "Vacuum UV (VUV) Photo-Oxidation of Polyethersulfone (PES)" Technologies 10, no. 2: 49. https://doi.org/10.3390/technologies10020049
APA StyleOakes, S., Keeley, R., Heineman, H., Allston, T., Shertok, J., Mehan, M., Thompson, G. K., & Takacs, G. A. (2022). Vacuum UV (VUV) Photo-Oxidation of Polyethersulfone (PES). Technologies, 10(2), 49. https://doi.org/10.3390/technologies10020049






