Water-Assisted Perovskite Quantum Dots with High Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CH3NH3Br (MABr)
2.3. Synthesis of Methylammonium Lead Halide (MAPbBr3) PeQDs
2.4. Characterization
3. Results
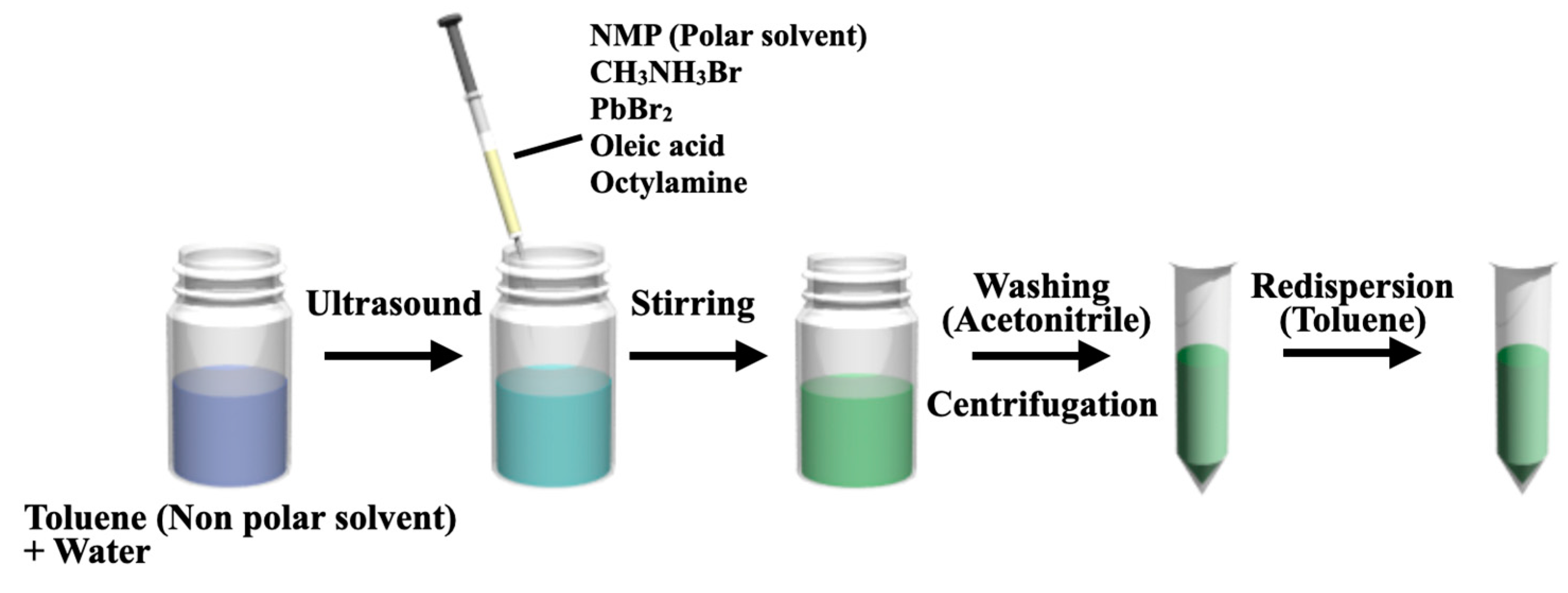
3.1. Water-Assisted LARP Method
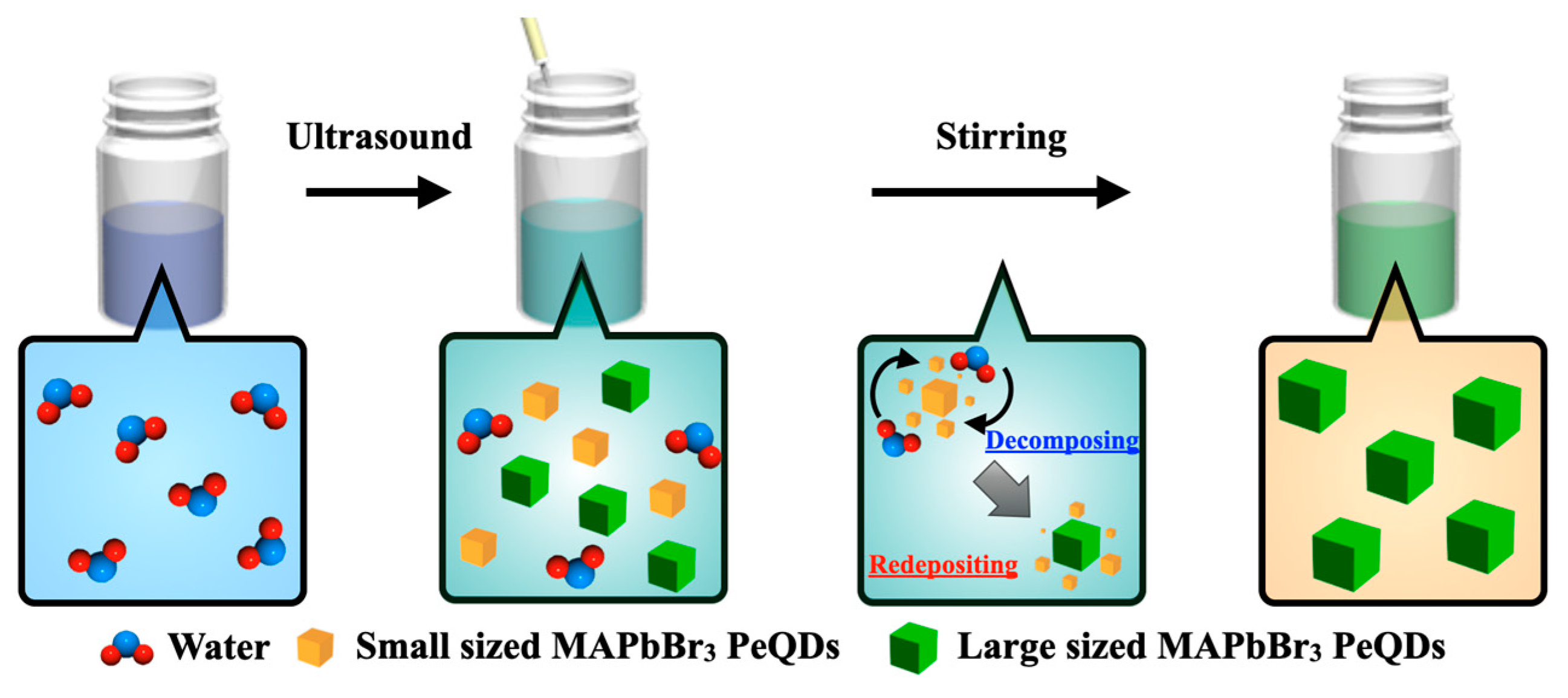
3.2. Ostwald Ripening of MAPbBr3 PeQDs by Assisting Water
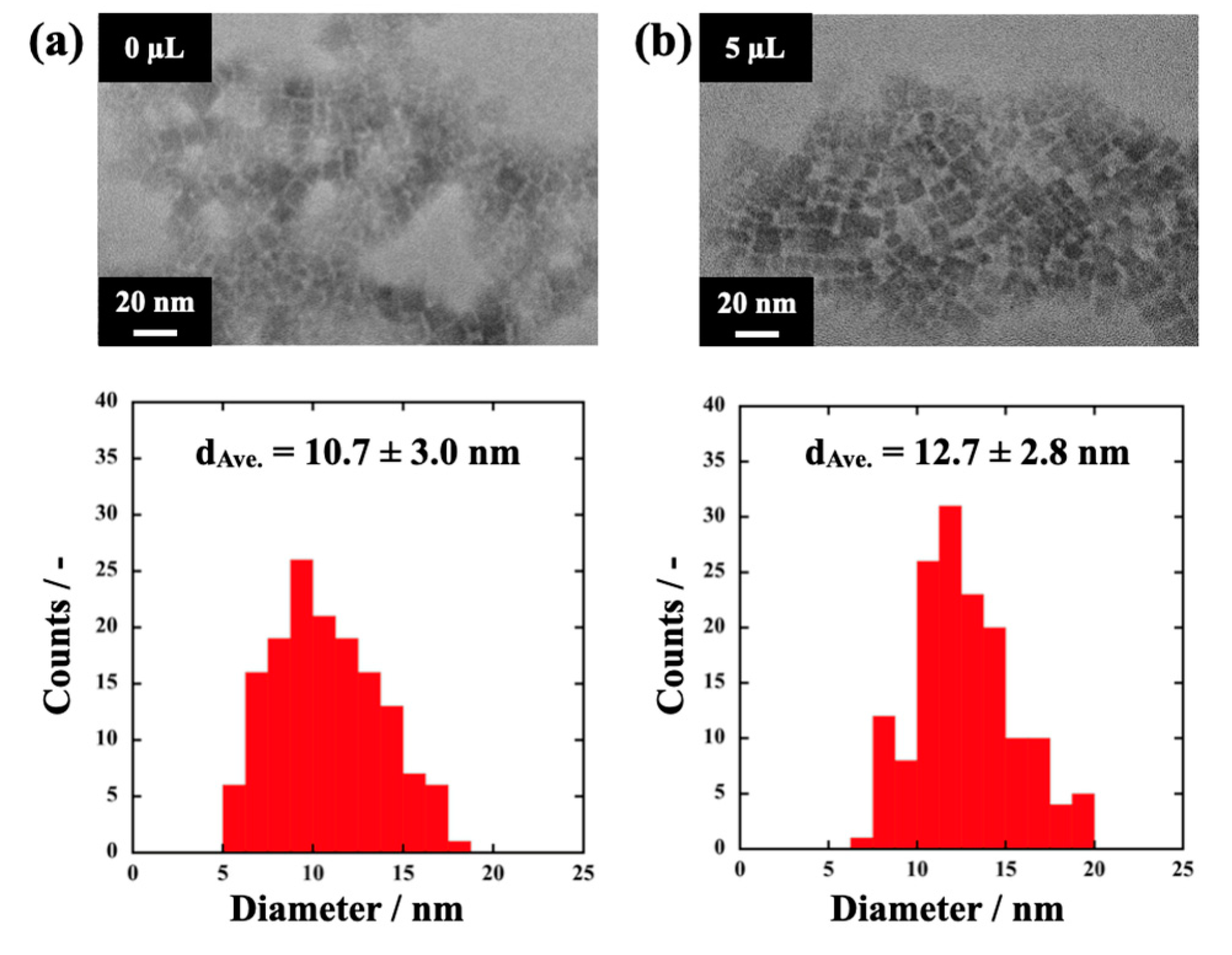
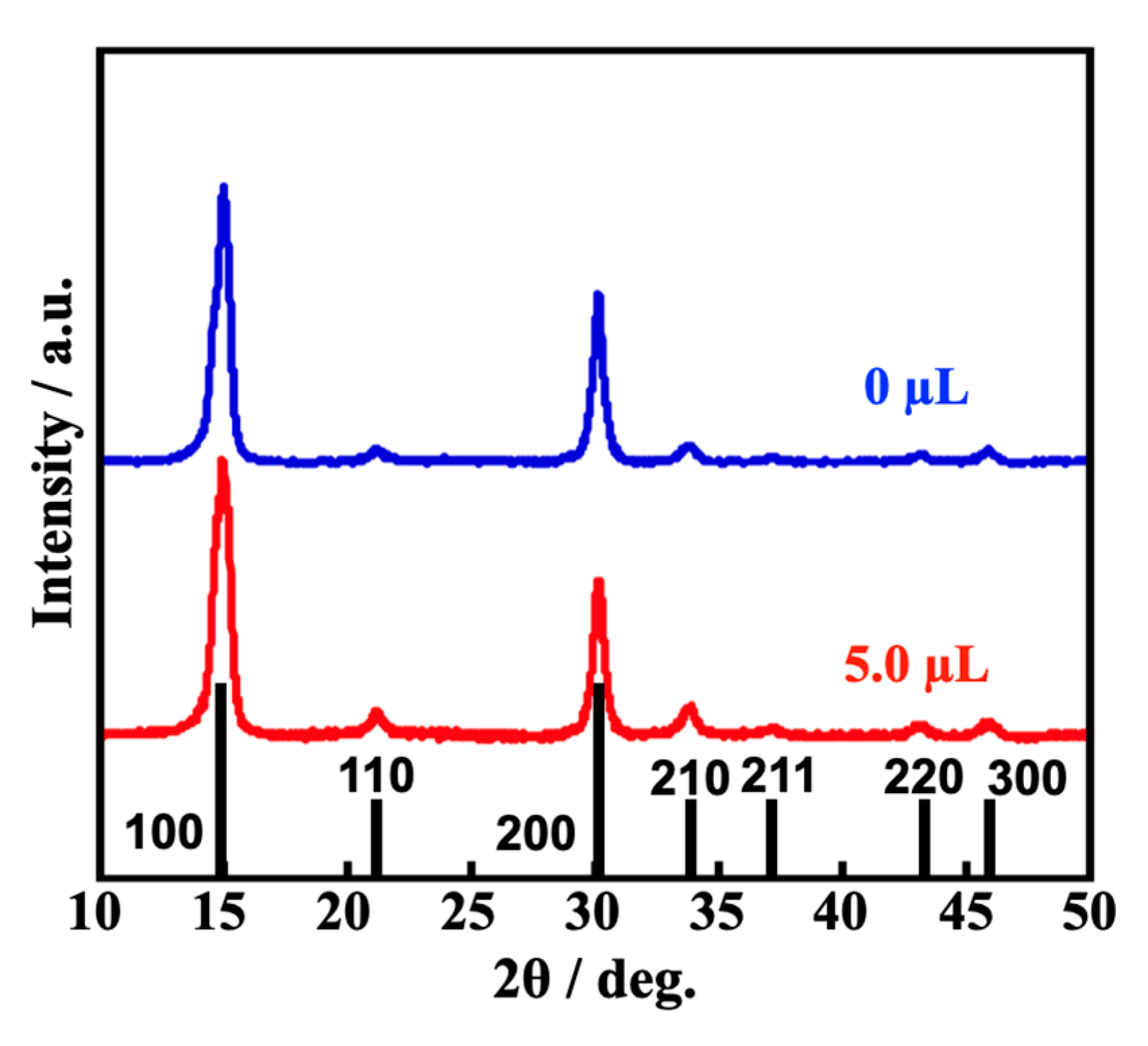
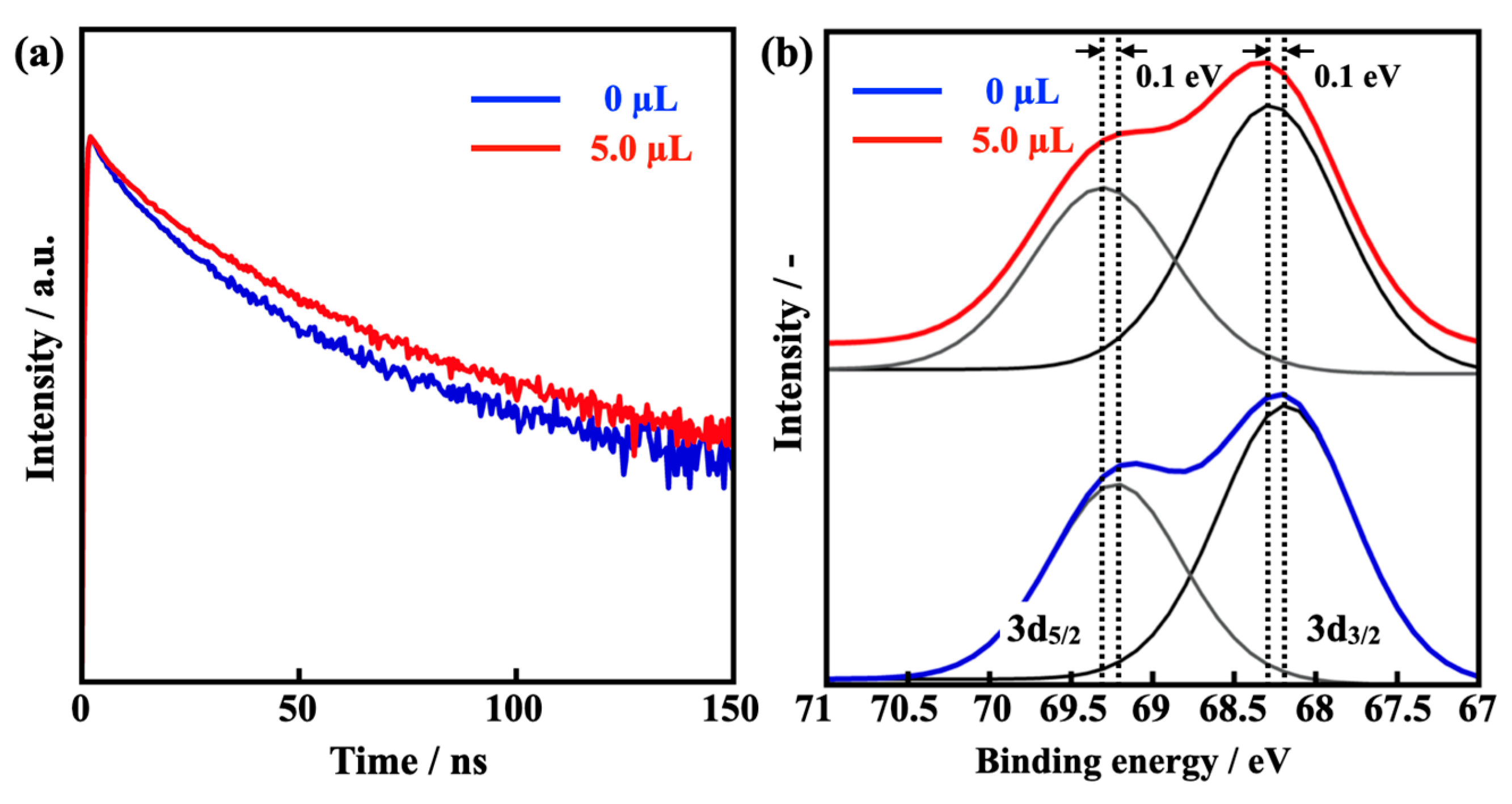
3.3. The Effect of the Assisting Water on the Prepared MAPbBr3 PeQDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, e1800764. [Google Scholar] [CrossRef]
- Chen, H.; Fan, L.; Zhang, R.; Bao, C.; Zhao, H.; Xiang, W.; Liu, W.; Niu, G.; Guo, R.; Zhang, L.; et al. High-Efficiency Formamidinium Lead Bromide Perovskite Nanocrystal-Based Light-Emitting Diodes Fabricated via a Surface Defect Self-Passivation Strategy. Adv. Opt. Mater. 2020, 8, 1901390. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX(3), X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.-G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, S.; Kakekhani, A.; Park, J.; Park, J.; Lee, Y.-H.; Xu, H.; Nagane, S.; Wexler, R.B.; Kim, D.-H.; et al. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics 2021, 15, 148–155. [Google Scholar] [CrossRef]
- Wang, Y.K.; Yuan, F.; Dong, Y.; Li, J.Y.; Johnston, A.; Chen, B.; Saidaminov, M.I.; Zhou, C.; Zheng, X.; Hou, Y.; et al. All-Inorganic Quantum-Dot LEDs Based on a Phase-Stabilized alpha-CsPbI3 Perovskite. Angew. Chem. Int. Ed. Engl. 2021, 60, 2–9. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, F.; Liu, L.; Zhang, F.; Wu, X.G.; Shi, L.; Zou, B.; Pei, Q.; Zhong, H. Emulsion Synthesis of Size-Tunable CH3NH3PbBr3 Quantum Dots: An Alternative Route toward Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 28128–28133. [Google Scholar] [CrossRef]
- Ling, Y.; Yuan, Z.; Tian, Y.; Wang, X.; Wang, J.C.; Xin, Y.; Hanson, K.; Ma, B.; Gao, H. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite Nanoplatelets. Adv. Mater. 2016, 28, 305–311. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.; Duan, H.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Jang, D.M.; Park, K.; Kim, D.H.; Park, J.; Shojaei, F.; Kang, H.S.; Ahn, J.P.; Lee, J.W.; Song, J.K. Reversible Halide Exchange Reaction of Organometal Trihalide Perovskite Colloidal Nanocrystals for Full-Range Band Gap Tuning. Nano Lett. 2015, 15, 5191–5199. [Google Scholar] [CrossRef]
- Jang, D.M.; Kim, D.H.; Park, K.; Park, J.; Lee, J.W.; Song, J.K. Ultrasound synthesis of lead halide perovskite nanocrystals. J. Mater. Chem. C 2016, 4, 10625–10629. [Google Scholar] [CrossRef]
- Zheng, W.; Wan, Q.; Zhang, Q.; Liu, M.; Zhang, C.; Wang, B.; Kong, L.; Li, L. High-efficiency perovskite nanocrystal light-emitting diodes via decorating NiOx on the nanocrystal surface. Nanoscale 2020, 12, 8711–8719. [Google Scholar] [CrossRef]
- Yan, F.; Xing, J.; Xing, G.; Quan, L.; Tan, S.T.; Zhao, J.; Su, R.; Zhang, L.; Chen, S.; Zhao, Y.; et al. Highly Efficient Visible Colloidal Lead-Halide Perovskite Nanocrystal Light-Emitting Diodes. Nano Lett. 2018, 18, 3157–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Chin, X.Y.; Perumal, A.; Bruno, A.; Yantara, N.; Veldhuis, S.A.; Martínez-Sarti, L.; Chandran, B.; Chirvony, V.; Lo, A.S.-Z.; So, J.; et al. Self-assembled hierarchical nanostructured perovskites enable highly efficient LEDs via an energy cascade. Energy Environ. Sci. 2018, 11, 1770–1778. [Google Scholar] [CrossRef]
- Zirak, M.; Moyen, E.; Alehdaghi, H.; Kanwat, A.; Choi, W.-C.; Jang, J. Anion- and Cation-Codoped All-Inorganic Blue-Emitting Perovskite Quantum Dots for Light-Emitting Diodes. ACS Appl. Nano Mater. 2019, 2, 5655–5662. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, H.; Bai, S.; Kuang, C.; Luo, X.; Teng, P.; Yin, C.; Zeng, P.; Hou, L.; Yang, Y.; et al. High-Brightness Perovskite Light-Emitting Diodes Based on FAPbBr3 Nanocrystals with Rationally Designed Aromatic Ligands. ACS Energy Lett. 2021, 6, 2395–2403. [Google Scholar] [CrossRef]
- Zhang, L.; Ju, M.G.; Liang, W. The effect of moisture on the structures and properties of lead halide perovskites: A first-principles theoretical investigation. Phys. Chem. Chem. Phys. 2016, 18, 23174–23183. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Liu, Q.; Xia, Z. Synergetic Effect of Postsynthetic Water Treatment on the Enhanced Photoluminescence and Stability of CsPbX3 (X = Cl, Br, I) Perovskite Nanocrystals. Chem. Mater. 2018, 30, 6922–6929. [Google Scholar] [CrossRef]
- Umemoto, K.; Takeda, M.; Tezuka, Y.; Chiba, T.; White, M.S.; Inose, T.; Yoshida, T.; Asakura, S.; Toyouchi, S.; Uji-i, H.; et al. Separation of mono-dispersed CH3NH3PbBr3 perovskite quantum dots via dissolution of nanocrystals. CrystEngComm 2018, 20, 7053–7057. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.S.; Noh, S.H.; Suh, E.H.; Jung, J.; Oh, J.G.; Lee, K.H.; Jang, J. Enhanced Stabilities and Production Yields of MAPbBr3 Quantum Dots and Their Applications as Stretchable and Self-Healable Color Filters. ACS Appl. Mater. Interfaces 2021, 13, 4374–4384. [Google Scholar] [CrossRef]
- De Geyter, B.; Hens, Z. The absorption coefficient of PbSe/CdSe core/shell colloidal quantum dots. Appl. Phys. Lett. 2010, 97, 161908. [Google Scholar] [CrossRef] [Green Version]
- Woo Choi, J.; Woo, H.C.; Huang, X.; Jung, W.G.; Kim, B.J.; Jeon, S.W.; Yim, S.Y.; Lee, J.S.; Lee, C.L. Organic-inorganic hybrid perovskite quantum dots with high PLQY and enhanced carrier mobility through crystallinity control by solvent engineering and solid-state ligand exchange. Nanoscale 2018, 10, 13356–13367. [Google Scholar] [CrossRef]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.Z.; Garcia Cortadella, R.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.K.; et al. Quantum Size Effect in Organometal Halide Perovskite Nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef]
- Shao, H.; Bai, X.; Pan, G.; Cui, H.; Zhu, J.; Zhai, Y.; Liu, J.; Dong, B.; Xu, L.; Song, H. Highly efficient and stable blue-emitting CsPbBr3@SiO2 nanospheres through low temperature synthesis for nanoprinting and WLED. Nanotechnology 2018, 29, 285706. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, X.; Yuan, M.; Turyanska, L.; Yang, X. Core/Shell Perovskite Nanocrystals: Synthesis of Highly Efficient and Environmentally Stable FAPbBr3/CsPbBr3 for LED Applications. Adv. Funct. Mater. 2020, 30, 1910582. [Google Scholar] [CrossRef]
- Lee, H.B.; Kumar, N.; Tyagi, B.; Ko, K.-J.; Kang, J.-W. Dimensionality and Defect Engineering Using Fluoroaromatic Cations for Efficiency and Stability Enhancement in 3D/2D Perovskite Photovoltaics. Solar RRL 2020, 5, 2000589. [Google Scholar] [CrossRef]
- He, S.; Kumar, N.; Beng Lee, H.; Ko, K.-J.; Jung, Y.-J.; Kim, J.-I.; Bae, S.; Lee, J.-H.; Kang, J.-W. Tailoring the refractive index and surface defects of CsPbBr3 quantum dots via alkyl cation-engineering for efficient perovskite light-emitting diodes. Chem. Eng. J. 2021, 425, 130678. [Google Scholar] [CrossRef]

| Water-Assisted | λPL/nm | FWHM/nm | PLQY/% |
|---|---|---|---|
| 0 µL | 522 | 24.9 | 82.7 |
| 2.5 µL | 524 | 23.3 | 91.6 |
| 5.0 µL | 526 | 21.8 | 95.6 |
| 7.5 µL | 524 | 23.0 | 90.4 |
| A1/% | A2/% | A3/% | τ1/ns | τ2/ns | τ3/ns | τave./ns | |
|---|---|---|---|---|---|---|---|
| 0 µL | 50.2 | 44.8 | 5.0 | 3.3 | 10.9 | 29.1 | 12.6 |
| 2.5 µL | 32.3 | 49.3 | 18.4 | 1.8 | 9.6 | 22.5 | 14.8 |
| 5.0 µL | 50.2 | 44.1 | 5.7 | 4.1 | 13.3 | 33.4 | 15.4 |
| 7.5 µL | 36.5 | 54.1 | 9.4 | 3.1 | 10.5 | 25.7 | 13.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoyama, M.; Sato, R.; Enomoto, J.; Oshita, N.; Kimura, T.; Kikuchi, K.; Asakura, S.; Umemoto, K.; Masuhara, A. Water-Assisted Perovskite Quantum Dots with High Optical Properties. Technologies 2022, 10, 11. https://doi.org/10.3390/technologies10010011
Yokoyama M, Sato R, Enomoto J, Oshita N, Kimura T, Kikuchi K, Asakura S, Umemoto K, Masuhara A. Water-Assisted Perovskite Quantum Dots with High Optical Properties. Technologies. 2022; 10(1):11. https://doi.org/10.3390/technologies10010011
Chicago/Turabian StyleYokoyama, Masaaki, Ryota Sato, Junya Enomoto, Naoaki Oshita, Taisei Kimura, Keisuke Kikuchi, Satoshi Asakura, Kazuki Umemoto, and Akito Masuhara. 2022. "Water-Assisted Perovskite Quantum Dots with High Optical Properties" Technologies 10, no. 1: 11. https://doi.org/10.3390/technologies10010011
APA StyleYokoyama, M., Sato, R., Enomoto, J., Oshita, N., Kimura, T., Kikuchi, K., Asakura, S., Umemoto, K., & Masuhara, A. (2022). Water-Assisted Perovskite Quantum Dots with High Optical Properties. Technologies, 10(1), 11. https://doi.org/10.3390/technologies10010011






