Enhanced Air Stability of Perovskite Quantum Dots by Manganese Passivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Methylammonium Bromide (CH3NH2 HBr; MABr)
2.3. Synthesis of MAPbBr3 and Mn/MAPbBr3 QD Dispersion
2.4. Fabrication of MAPbBr3 and Mn/MAPbBr3 QD Thin Film
2.5. Characterization
3. Results
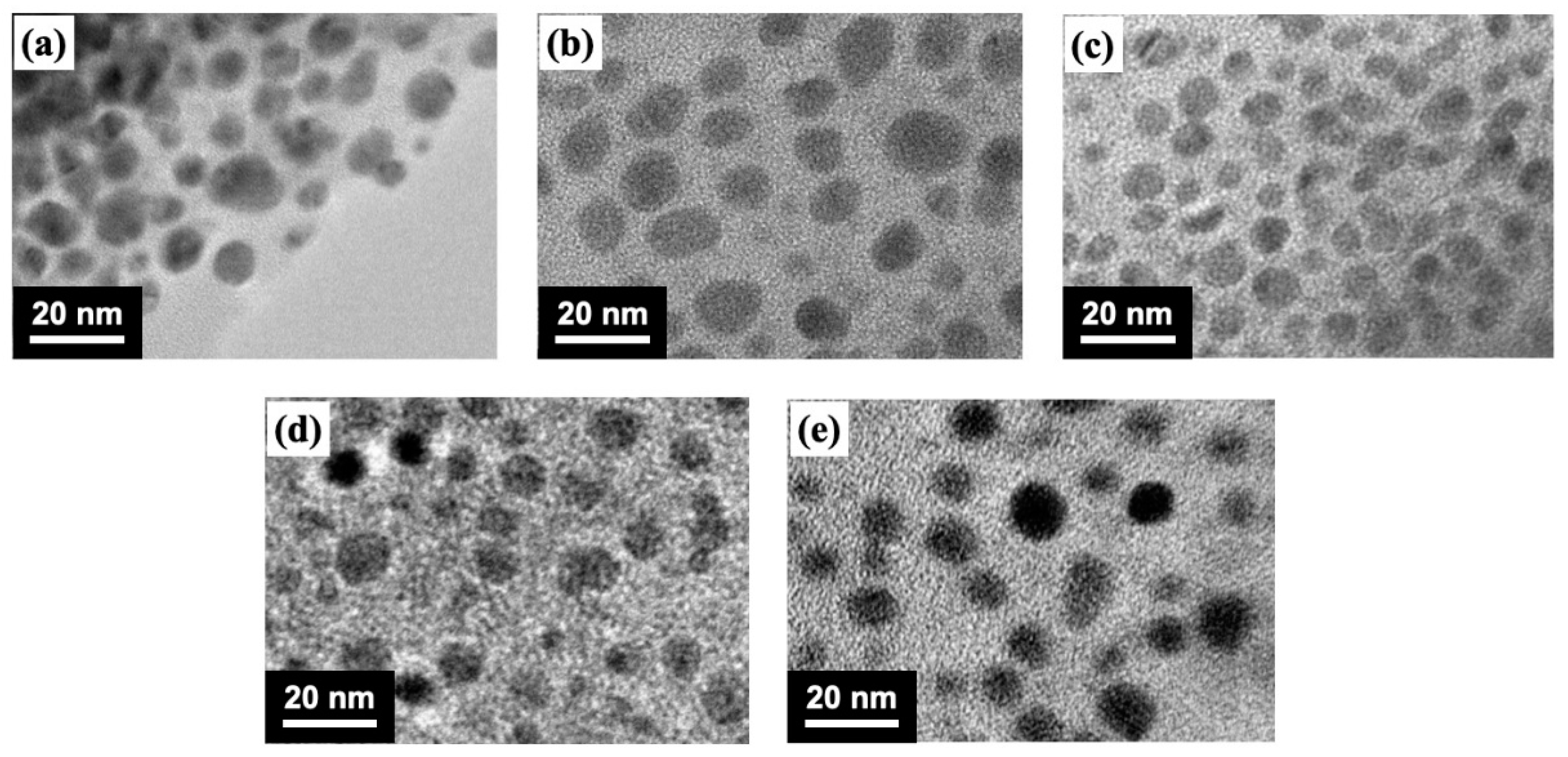
3.1. Morphologies of Prepared MAPbBr3 and Mn/MAPbBr3 QDs
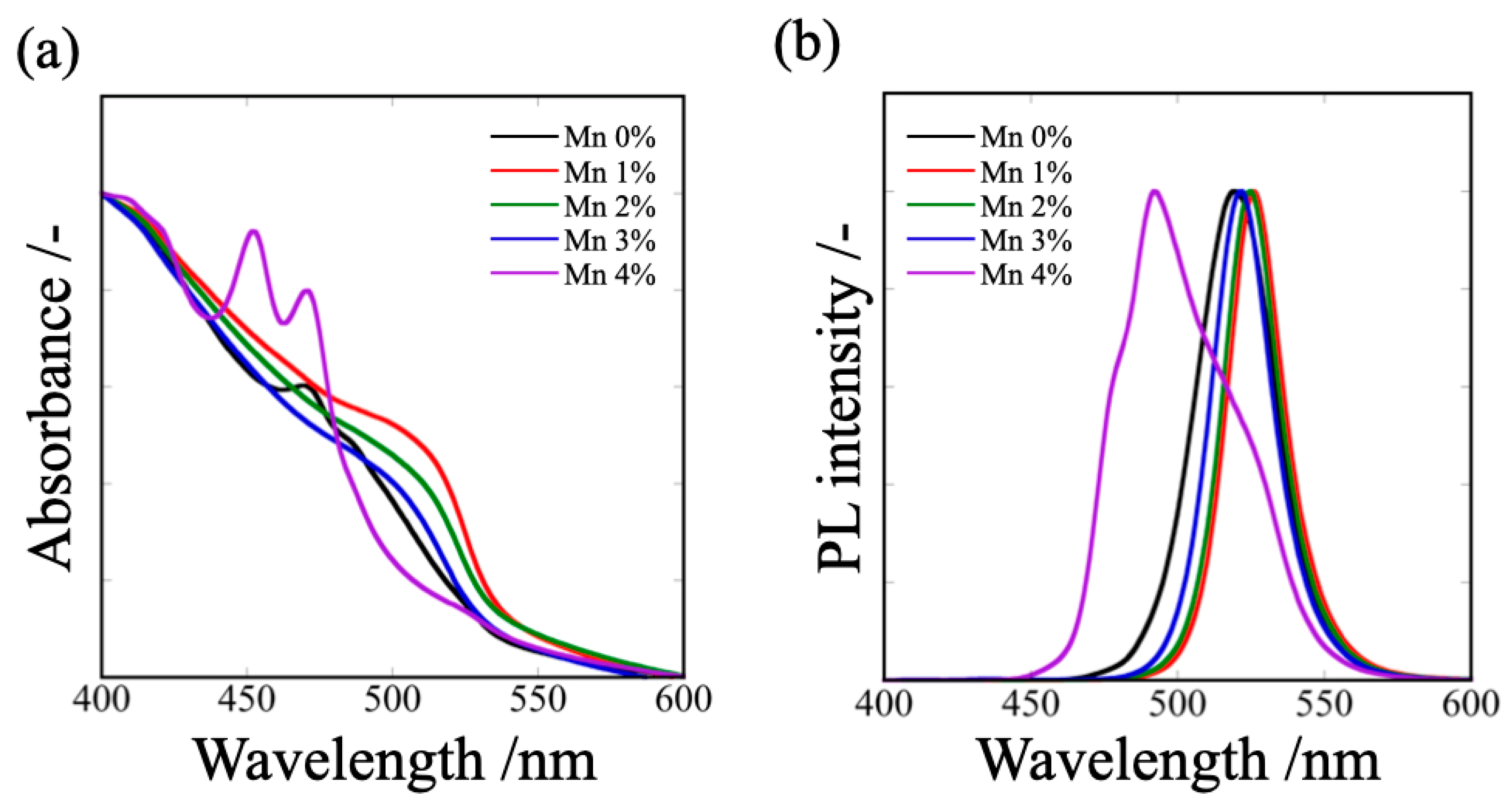
3.2. Optical Properties of MAPbBr3 and Mn/MAPbBr3 QDs
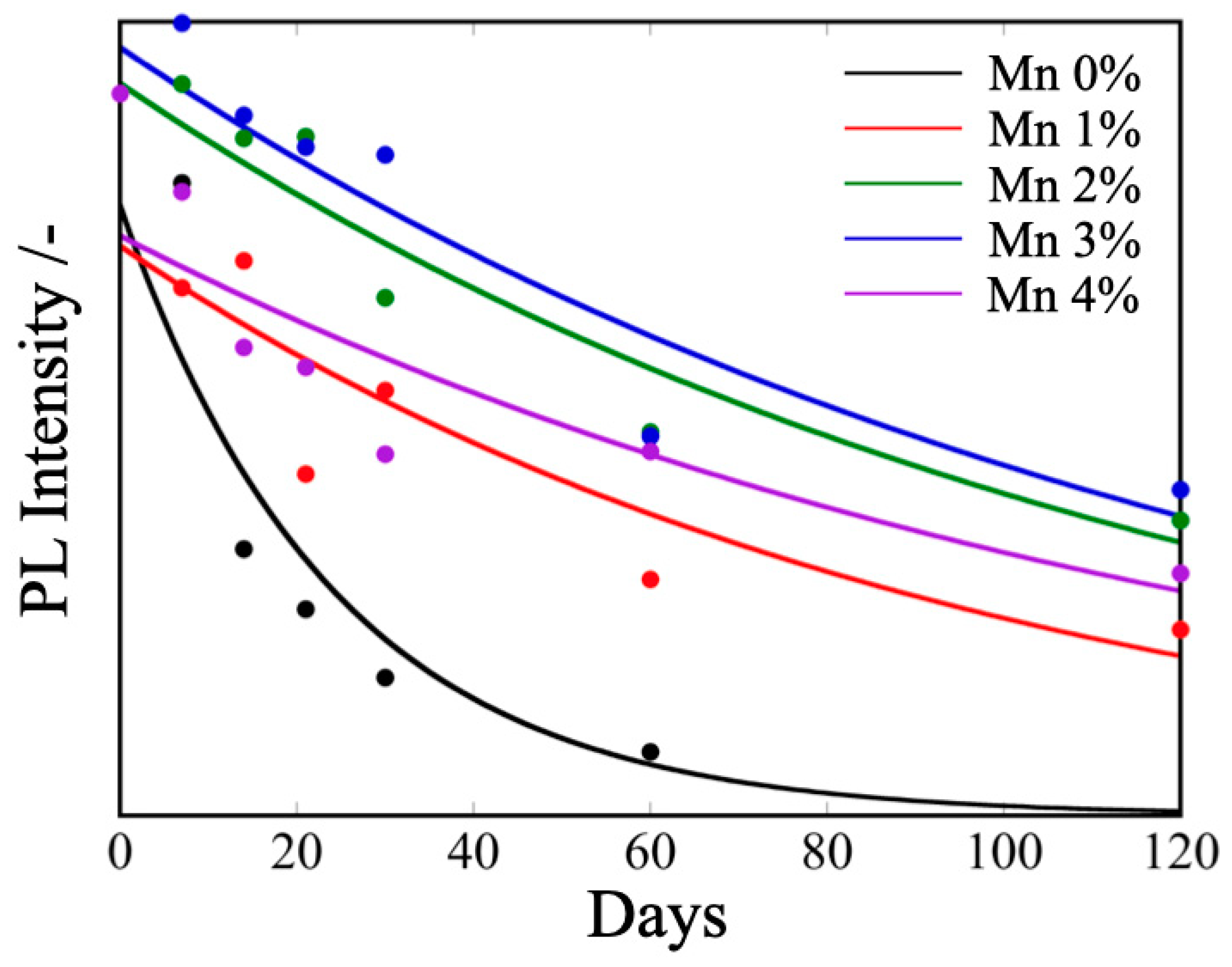
3.3. Air Stability of MAPbBr3 and Mn/MAPbBr3 QDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Zhou, X.; Wu, S.; Yan, Z.; Li, Y.; He, G. Dynamic Holographic Display Based on Perovskite Nanocrystal Doped Liquid Crystal Film. IEEE Photonics J. 2021, 13, 1–6. [Google Scholar] [CrossRef]
- Xie, C.; Liu, C.K.; Loi, H.L.; Yan, F. Perovskite-Based Phototransistors and Hybrid Photodetectors. Adv. Funct. Mater. 2019, 30, 1903907. [Google Scholar] [CrossRef]
- Koh, T.M.; Thirumal, K.; Soo, H.S.; Mathews, N. Multidimensional Perovskites: A Mixed Cation Approach Towards Ambient Stable and Tunable Perovskite Photovoltaics. ChemSusChem 2016, 9, 2541–2558. [Google Scholar] [CrossRef]
- Ryota, S.; Akito, M. Preparation and evaluation of perovskite quantum dots. Imaging Soc. Jpn. 2021, 60, 528–536. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Ji, K.; Anaya, M.; Abfalterer, A.; Stranks, S.D. Halide Perovskite Light-Emitting Diode Technologies. Adv. Opt. Mater. 2021, 9, 2002128. [Google Scholar] [CrossRef]
- Umemoto, K.; Ebe, H.; Sato, R.; Enomoto, J.; Oshita, N.; Kimura, T.; Inose, T.; Nakamura, T.; Chiba, T.; Asakura, S.; et al. Simple Production of Highly Luminescent Organometal Halide Perovskite Nanocrystals Using Ultrasound-Assisted Bead Milling. ACS Sustain. Chem. Eng. 2020, 8, 16469–16476. [Google Scholar] [CrossRef]
- Chiba, T.; Hayashi, Y.; Ebe, H.; Hoshi, K.; Sato, J.; Sato, S.; Pu, Y.-J.; Ohisa, S.; Kido, J. Anion-exchange red perovskite quantum dots with ammonium iodine salts for highly efficient light-emitting devices. Nat. Photonics 2018, 12, 681–687. [Google Scholar] [CrossRef]
- Dong, Y.; Qiao, T.; Kim, D.; Parobek, D.; Rossi, D.; Son, D.H. Precise Control of Quantum Confinement in Cesium Lead Halide Perovskite Quantum Dots via Thermodynamic Equilibrium. Nano Lett. 2018, 18, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Han, D.; Imran, M.; Zhang, M.; Chang, S.; Wu, X.G.; Zhang, X.; Tang, J.; Wang, M.; Ali, S.; Li, X.; et al. Efficient Light-Emitting Diodes Based on in Situ Fabricated FAPbBr3 Nanocrystals: The Enhancing Role of the Ligand-Assisted Reprecipitation Process. ACS Nano 2018, 12, 8808–8816. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Bai, Y.; Zeiske, S.; Ren, L.; Liu, J.; Yuan, Y.; Zarrabi, N.; Cheng, N.; Ghasemi, M.; Chen, P.; et al. Ligand-assisted cation-exchange engineering for high-efficiency colloidal Cs1−xFAxPbI3 quantum dot solar cells with reduced phase segregation. Nat. Energy 2020, 5, 79–88. [Google Scholar] [CrossRef]
- Tezuka, Y.; Umemoto, K.; Takeda, M.; Takahashi, Y.; Ebe, H.; Enomoto, J.; Rodbuntum, S.; Nohara, T.; Fontecha, D.; Asakura, S.; et al. Effects of alkylamine chain length on perovskite nanocrystals after washing and perovskite light-emitting diodes. Jpn. J. Appl. Phys. 2020, 59, SDDC04. [Google Scholar] [CrossRef]
- Yang, F.; Chen, H.; Zhang, R.; Liu, X.; Zhang, W.; Zhang, J.; Gao, F.; Wang, L. Efficient and Spectrally Stable Blue Perovskite Light-Emitting Diodes Based on Potassium Passivated Nanocrystals. Adv. Funct. Mater. 2020, 30, 1908760. [Google Scholar] [CrossRef]
- Hoshi, K.; Chiba, T.; Sato, J.; Hayashi, Y.; Takahashi, Y.; Ebe, H.; Ohisa, S.; Kido, J. Purification of Perovskite Quantum Dots Using Low-Dielectric-Constant Washing Solvent “Diglyme” for Highly Efficient Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 24607–24612. [Google Scholar] [CrossRef] [PubMed]
- Woo Choi, J.; Woo, H.C.; Huang, X.; Jung, W.G.; Kim, B.J.; Jeon, S.W.; Yim, S.Y.; Lee, J.S.; Lee, C.L. Organic-inorganic hybrid perovskite quantum dots with high PLQY and enhanced carrier mobility through crystallinity control by solvent engineering and solid-state ligand exchange. Nanoscale 2018, 10, 13356–13367. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, S.; Kakekhani, A.; Park, J.; Park, J.; Lee, Y.-H.; Xu, H.; Nagane, S.; Wexler, R.B.; Kim, D.-H.; et al. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics 2021, 15, 148–155. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef]
- Lv, W.; Li, L.; Xu, M.; Hong, J.; Tang, X.; Xu, L.; Wu, Y.; Zhu, R.; Chen, R.; Huang, W. Improving the Stability of Metal Halide Perovskite Quantum Dots by Encapsulation. Adv. Mater. 2019, 31, e1900682. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, H.; Lin, W.; Wang, J.; Chi, Y. Enhancing air-stability of CH3NH3PbBr3 perovskite quantum dots by in-situ growth in metal-organic frameworks and their applications in light emitting diodes. J. Solid State Chem. 2019, 272, 221–226. [Google Scholar] [CrossRef]
- Shi, Z.; Li, S.; Li, Y.; Ji, H.; Li, X.; Wu, D.; Xu, T.; Chen, Y.; Tian, Y.; Zhang, Y.; et al. Strategy of Solution-Processed All-Inorganic Heterostructure for Humidity/Temperature-Stable Perovskite Quantum Dot Light-Emitting Diodes. ACS Nano 2018, 12, 1462–1472. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, G.; Wang, G.; Wang, J.; Li, X.; Zhang, C. Novel perovskite quantum dots and hydroxyapatite nanocomposites: Enhanced thermal stability, improved emission intensity, and color-tunable luminescence. J. Alloys Compd. 2021, 861, 157989. [Google Scholar] [CrossRef]
- Chakraborty, R.; Maiti, A.; Ghorai, U.K.; Pal, A.J. Defect Passivation of Mn2+-Doped CsPbCl3 Perovskite Nanocrystals as Probed by Scanning Tunneling Spectroscopy: Toward Boosting Emission Efficiencies. ACS Appl. Nano Mater. 2021, 4, 10155–10163. [Google Scholar] [CrossRef]
- Parobek, D.; Roman, B.J.; Dong, Y.; Jin, H.; Lee, E.; Sheldon, M.; Son, D.H. Exciton-to-Dopant Energy Transfer in Mn-Doped Cesium Lead Halide Perovskite Nanocrystals. Nano Lett. 2016, 16, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Yokoyama, M.; Umemoto, K.; Lyu, B.; Takahashi, Y.; Rodbuntum, S.; Enomoto, J.; Tozawa, K.; Nohara, T.; Tabata, K.; et al. Synthesis of highly luminescent CH3NH3PbBr3 perovskite nanocrystals via a forced thin film reactor. Jpn. J. Appl. Phys. 2020, 59, SIIG02. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, W.; Tong, C.J.; Chen, X.; Cao, T.; Chen, M. Strain induced electronic structure variation in methyl-ammonium lead iodide perovskite. Sci. Rep. 2018, 8, 7760. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]


| Mn 0% | Mn 1% | Mn 2% | Mn 3% | Mn 4% | |
|---|---|---|---|---|---|
| Room light |  |  |  |  |  |
| UV irradiation |  |  |  |  |  |
| Mn 0% | Mn 1% | Mn 2% | Mn 3% | Mn 4% | |
|---|---|---|---|---|---|
| UV irradiation |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, R.; Umemoto, K.; Asakura, S.; Masuhara, A. Enhanced Air Stability of Perovskite Quantum Dots by Manganese Passivation. Technologies 2022, 10, 10. https://doi.org/10.3390/technologies10010010
Sato R, Umemoto K, Asakura S, Masuhara A. Enhanced Air Stability of Perovskite Quantum Dots by Manganese Passivation. Technologies. 2022; 10(1):10. https://doi.org/10.3390/technologies10010010
Chicago/Turabian StyleSato, Ryota, Kazuki Umemoto, Satoshi Asakura, and Akito Masuhara. 2022. "Enhanced Air Stability of Perovskite Quantum Dots by Manganese Passivation" Technologies 10, no. 1: 10. https://doi.org/10.3390/technologies10010010
APA StyleSato, R., Umemoto, K., Asakura, S., & Masuhara, A. (2022). Enhanced Air Stability of Perovskite Quantum Dots by Manganese Passivation. Technologies, 10(1), 10. https://doi.org/10.3390/technologies10010010






