Prescribing-Assessment Tools for Long-Term Care Pharmacy Practice: Reaching Consensus through a Modified RAND/UCLA Appropriateness Method
Abstract
:1. Introduction
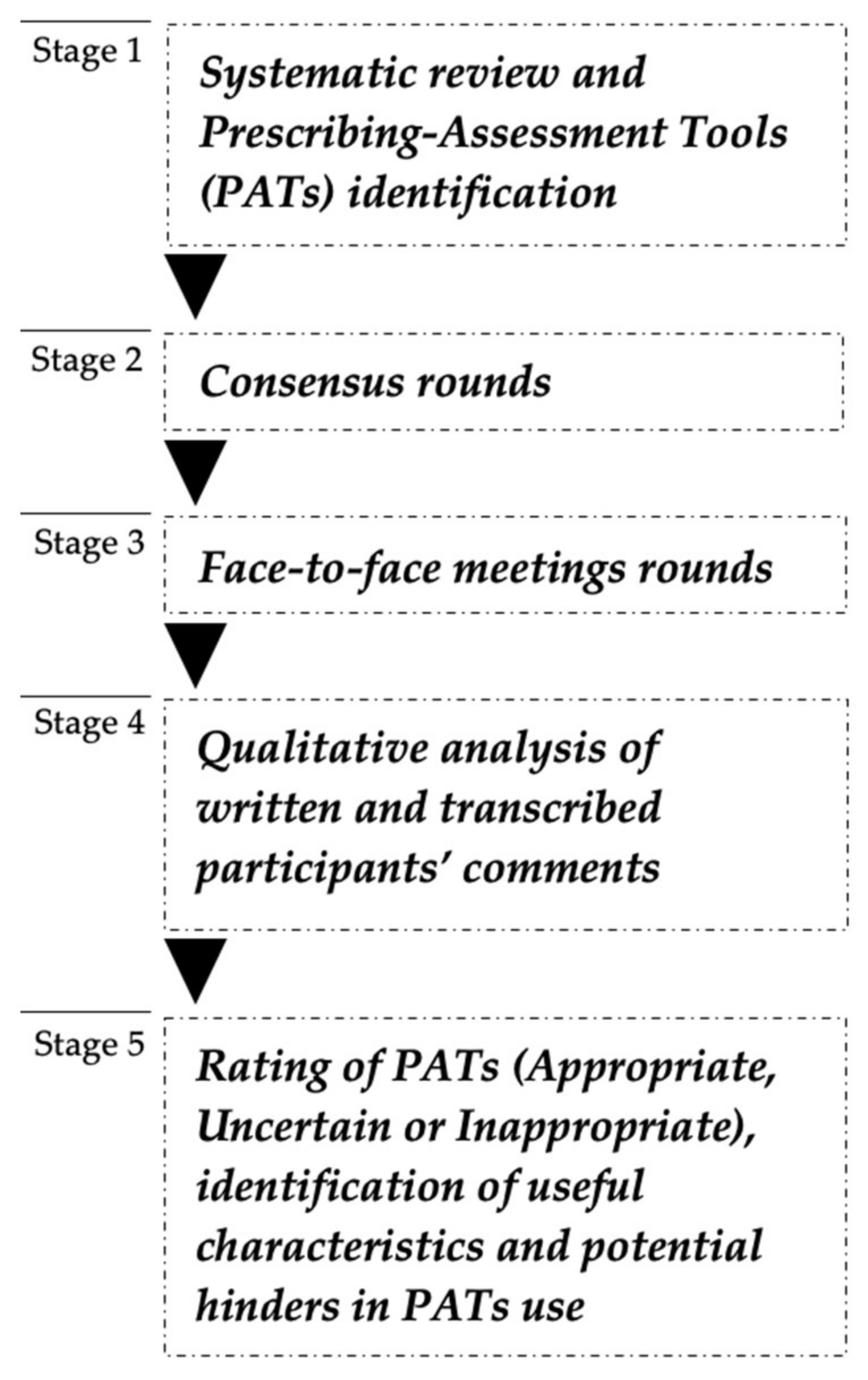
2. Materials and Methods
2.1. Literature Review and Prescribing-Assessment Tools Identification
2.2. Prescribing-Assessment Tools Independent Rating
2.3. Face-to-Face Meetings
2.4. Analysis of Panellists’ Accounts and Final Classification
3. Results
3.1. Literature Review
3.2. Panellists’ Characteristics
3.3. RAM Rounds, Face-to-Face Meetings and Final Consensus
3.4. Relevant Characteristics of PATs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Towards an International Consensus on Policy for Long-Term Care of the Ageing. WHO. 2000. Available online: https://apps.who.int/iris/handle/10665/66339 (accessed on 3 August 2021).
- OECD. A Good Life in Old Age? Monitoring and Improving Quality in Long-Term Care. OECD; June 2013 (OECD Health Policy Studies). Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/a-good-life-in-old-age_9789264194564-en (accessed on 5 August 2021).
- Lopes, H.; Mateus, C.; Hernández-Quevedo, C. Ten Years after the Creation of the Portuguese National Network for Long-Term Care in 2006: Achievements and Challenges. Health Policy 2018, 122, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Saúde, M.D. Monitorização da Rede Nacional de Cuidados Continuados Integrados (RNCCI)—2020. 2020. Available online: http://www.acss.min-saude.pt/wp-content/uploads/2021/08/Relatorio-de-Monitorização-da-RNCCI-Anual-2020-VF.pdf (accessed on 4 August 2021).
- Nobili, A.; Garattini, S.; Mannucci, P.M. Multiple Diseases and Polypharmacy in the Elderly: Challenges for the Internist of the Third Millennium. J. Comorbidity 2011, 1, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.P.; Flores, T.R.; Mielke, G.; Thumé, E.; Facchini, L.A. Multimorbidity and mortality in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2016, 67, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Khezrian, M.; McNeil, C.J.; Murray, A.; Myint, P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 2020, 11. [Google Scholar] [CrossRef]
- Gray, S.L.; Marcum, Z.A.; Schmader, K.E.; Hanlon, J.T. Update on Medication Use Quality and Safety in Older Adults, 2017. J. Am. Geriatr. Soc. 2018, 66, 2254–2258. [Google Scholar] [CrossRef]
- McLean, A.J.; le Couteur, D.G. Aging biology and geriatric clinical pharmacology. Pharmacol. Rev. 2004, 56, 163–184. [Google Scholar] [CrossRef] [Green Version]
- Guaraldo, L.; Cano, F.G.; Damasceno, G.S.; Rozenfeld, S. Inappropriate medication use among the elderly: A systematic review of administrative databases. BMC Geriatr. 2011, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Avorn, J.; Gurwitz, J.H. Drug Use in the Nursing Home. 1995. Available online: http://annals.org/ (accessed on 3 August 2021).
- Qassemi, S.; Pagès, A.; Rouch, L.; Bismuth, S.; Stillmunkes, A.; Lapeyre-Mestre, M.; McCambridge, C.; Cool, C.; Cestac, P. Potentially Inappropriate Drug Prescribing in French Nursing Home Residents: An Observational Study. Pharmacy 2020, 8, 133. [Google Scholar] [CrossRef]
- Storms, H.; Marquet, K.; Aertgeerts, B.; Claes, N. Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: A systematic review. Eur. J. Gen. Pract. 2017, 23, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007, 370, 173–184. [Google Scholar] [CrossRef]
- Cahir, C.; Bennett, K.; Teljeur, C.; Fahey, T. Potentially inappropriate prescribing and adverse health outcomes in community dwelling older patients. Br. J. Clin. Pharmacol. 2014, 77, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Rankin, A.; Cadogan, C.A.; Patterson, S.M.; Kerse, N.; Cardwell, C.R.; Bradley, M.C.; Ryan, C.; Hughes, C. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- O’connor, M.N.; Gallagher, P.; O’mahony, D. Inappropriate Prescribing Criteria, Detection and Prevention. Drugs Aging 2012, 29, 437–452. [Google Scholar] [CrossRef]
- O’Mahony, D.; Gallagher, P.F.; Lavan, A.H. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin. Interv. Aging 2016, 11, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Masnoon, N.; Shakib, S.; Ellett, L.K.; Caughey, G. Tools for Assessment of the Appropriateness of Prescribing and Association with Patient-Related Outcomes: A Systematic Review. Drugs Aging 2018, 35, 43–60. [Google Scholar] [CrossRef]
- Kaufmann, C.P.; Tremp, R.; Hersberger, K.E.; Lampert, M.L. Inappropriate prescribing: A systematic overview of published assessment tools. Eur. J. Clin. Pharmacol. 2013, 70, 1–11. [Google Scholar] [CrossRef]
- Scahill, S.; Atif, M.; Babar, Z. Defining pharmacy and its practice: A conceptual model for an international audience. Integr. Pharm. Res. Pract. 2017, 6, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Wiedenmayer, K.; Summers, R.S.; Mackie, C.A.; Gous, A.G.S.; Everard, M.; Tromp, D. Developing Pharmacy Practice: A Focus on Patient Care, 1st ed.; World Health Organization (International Pharmaceutical Federation): Hague, The Netherlands, 2006; Available online: https://apps.who.int/iris/bitstream/handle/10665/69399/WHO_PSM_PAR_2006.5_eng.pdf?sequence=1&isAllowed=y (accessed on 1 November 2021).
- Sanford, A.M.; Orrell, M.; Tolson, D.; Abbatecola, A.M.; Arai, H.; Bauer, J.M.; Cruz-Jentoft, A.J.; Dong, B.; Ga, H.; Goel, A.; et al. An International Definition for “Nursing Home”. J. Am. Med. Dir. Assoc. 2015, 16, 181–184. [Google Scholar] [CrossRef]
- World Health Organization—Regional Office for Europe. Integrated Care Models: An Overview Working Document. 2016. Available online: http://www.euro.who.int/pubrequest (accessed on 3 August 2021).
- Campbell, S.M.; Cantrill, J.A. Consensus methods in prescribing research. J. Clin. Pharm. Ther. 2001, 26, 5–14. [Google Scholar] [CrossRef]
- Humphrey-Murto, S.; Varpio, L.; Wood, T.J.; Gonsalves, C.; Ufholz, L.A.; Mascioli, K.; Wang, C.; Foth, T. The Use of the Delphi and Other Consensus Group Methods in Medical Education Research: A Review. Acad. Med. 2017, 92, 1491–1498. [Google Scholar] [CrossRef]
- Fitch, K.; Bernstein, S.; Aguilar, M.; Burnand, B. The Rand/UCLA Appropriateness Method User’s Manual; RAND: Santa Monica, CA, USA, 2001. [Google Scholar]
- Gonçalves, J.R.; Ramalhinho, I.; Sleath, B.L.; Lopes, M.J.; Cavaco, A.M. Probing pharmacists’ interventions in Long-Term Care: A systematic review. Eur. Geriatr. Med. 2021, 12, 673–693. [Google Scholar] [CrossRef]
- Din, S.; Kent, A.; Pollok, R.C.; Meade, S.; Kennedy, N.A.; Arnott, I.; Beattie, R.M.; Chua, F.; Cooney, R.; Dart, R.J.; et al. Adaptations to the British Society of Gastroenterology guidelines on the management of acute severe UC in the context of the COVID-19 pandemic: A RAND appropriateness panel. Gut 2020, 69, 1769–1777. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Salow, M.J.; Angelini, M.C.; McGlinchey, R.E. The Anticholinergic Risk Scale and Anticholinergic Adverse Effects in Older Persons. Arch. Intern. Med. 2008, 168, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Hilmer, S.N.; Mager, D.E.; Simonsick, E.M.; Cao, Y.; Ling, S.M.; Windham, B.G.; Harris, T.B.; Hanlon, J.T.; Rubin, S.M.; Shorr, R.I.; et al. A Drug Burden Index to Define the Functional Burden of Medications in Older People. Arch. Intern. Med. 2007, 167, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.C.; Go, A.S.; Chang, Y.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Singer, D.E. A New Risk Scheme to Predict Warfarin-Associated Hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J. Am. Coll. Cardiol. 2011, 58, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; De Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Gage, B.F.; Yan, Y.; Milligan, P.; Waterman, A.; Culverhouse, R.; Rich, M.W.; Radford, M. Clinical classification schemes for predicting hemorrhage: Results from the National Registry of Atrial Fibrillation (NRAF). Am. Heart J. 2006, 151, 713–719. [Google Scholar] [CrossRef]
- Loeb, M.; Bentley, D.W.; Bradley, S.; Crossley, K.; Garibaldi, R.; Gantz, N.; McGeer, A.; Muder, R.R.; Mylotte, J.; Nicolle, L.E.; et al. Development of Minimum Criteria for the Initiation of Antibiotics in Residents of Long-Term–Care Facilities: Results of a Consensus Conference. Infect. Control. Hosp. Epidemiol. 2001, 22, 120–124. [Google Scholar] [CrossRef]
- George, J.; Phun, Y.-T.; Bailey, M.J.; Kong, D.C.; Stewart, K. Development and Validation of the Medication Regimen Complexity Index. Ann. Pharmacother. 2004, 38, 1369–1376. [Google Scholar] [CrossRef]
- Melchiors, A.C.; Correr, C.J.; Fernández-Llimos, F. Translation and Validation into Portuguese Language of the Medication Regimen Complexity Index. Arq Bras Cardiol. 2007, 89, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Sluggett, J.; Ilomaki, J.; Hilmer, S.; Corlis, M.; Picton, L.J.; Dean, L.; Alderman, C.P.; Farinola, N.; Gailer, J.; et al. Development and validation of the Medication Regimen Simplification Guide for Residential Aged CarE (MRS GRACE). Clin. Interv. Aging 2018, 13, 975–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, J.T.; Schmader, K.E.; Samsa, G.P.; Weinberger, M.; Uttech, K.M.; Lewis, I.K.; Cohen, H.J.; Feussner, J.R. A method for assessing drug therapy appropriateness. J. Clin. Epidemiol. 1992, 45, 1045–1051. [Google Scholar] [CrossRef]
- Tully, M.P.; Javed, N.; Cantrill, J.A. Development and Face Validity of Explicit indicators of Appropriateness of Long Term Prescribing. Pharm. World Sci. 2005, 27, 407–413. [Google Scholar] [CrossRef]
- Fick, D.M.; Semla, T.P.; Steinman, M.; Beizer, J.; Brandt, N.; Dombrowski, R.; DuBeau, C.E.; Pezzullo, L.; Epplin, J.J.; Flanagan, N. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar]
- Rognstad, S.; Brekke, M.; Fetveit, A.; Spigset, O.; Wyller, T.B.; Straand, J. The Norwegian General Practice (NORGEP) criteria for assessing potentially inappropriate prescriptions to elderly patients. Scand. J. Prim. Health Care 2009, 27, 153–159. [Google Scholar] [CrossRef]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Kuhn-Thiel, A.M.; Weiß, C.; Wehling, M. Consensus Validation of the FORTA (Fit fOR The Aged) List: A Clinical Tool for Increasing the Appropriateness of Pharmacotherapy in the Elderly. Drugs Aging 2014, 31, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Wehling, M. Arzneimitteltherapie im alter: Zu viel und zu wenig, was tun? Ein neues bewertungssystem: Fit for the aged (FORTA). Dtsch. Med. Wochenschr. 2008, 133, 2289–2291. [Google Scholar] [CrossRef]
- Winit-Watjana, W.; Sakulrat, P.; Kespichayawattana, J. Criteria for high-risk medication use in Thai older patients. Arch. Gerontol. Geriatr. 2008, 47, 35–51. [Google Scholar] [CrossRef]
- Rancourt, C.; Moisan, J.; Baillargeon, L.; Verreault, R.; Laurin, D.; Grégoire, J.-P. Potentially inappropriate prescriptions for older patients in long-term care. BMC Geriatr. 2004, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Poudel, A.; Ballokova, A.; Hubbard, R.; Gray, L.C.; Mitchell, C.A.; Nissen, L.M.; Scott, I. Algorithm of medication review in frail older people: Focus on minimizing the use of high-risk medications. Geriatr. Gerontol. Int. 2016, 16, 1002–1013. [Google Scholar] [CrossRef]
- McLeod, P.J.; Huang, A.R.; Tamblyn, R.M.; Gayton, D.C. Defining inappropriate practices in prescribing for elderly people: A national consensus panel. Can. Med. Assoc. J. 1997, 156, 385–391. [Google Scholar]
- Laroche, M.-L.; Charmes, J.-P.; Merle, L. Potentially inappropriate medications in the elderly: A French consensus panel list. Eur. J. Clin. Pharmacol. 2007, 63, 725–731. [Google Scholar] [CrossRef]
- Basger, B.J.; Chen, T.F.; Moles, R.J. Inappropriate Medication Use and Prescribing Indicators in Elderly Australians Development of a Prescribing Indicators Tool. Drugs Aging 2008, 25, 777–793. [Google Scholar] [CrossRef]
- Van der Spek, K.; Gerritsen, D.L.; Smalbrugge, M.; Nelissen-Vrancken, M.H.; Wetzels, R.B.; Smeets, C.H.; Zuidema, S.U.; Koopmans, R.T. A reliable and valid index was developed to measure appropriate psychotropic drug use in dementia. J. Clin. Epidemiol. 2015, 68, 903–912. [Google Scholar] [CrossRef]
- Holmes, H.M.; Sachs, G.A.; Shega, J.W.; Hougham, G.; Hayley, D.C.; Dale, W. Integrating Palliative Medicine into the Care of Persons with Advanced Dementia: Identifying Appropriate Medication Use. J. Am. Geriatr. Soc. 2008, 56, 1306–1311. [Google Scholar] [CrossRef]
- Kröger, E.; Wilchesky, M.; Marcotte, M.; Voyer, P.; Morin, M.; Champoux, N.; Monette, J.; Aubin, M.; Durand, P.J.; Verreault, R.; et al. Medication Use Among Nursing Home Residents With Severe Dementia: Identifying Categories of Appropriateness and Elements of a Successful Intervention. J. Am. Med. Dir. Assoc. 2015, 16, 629.e1–629.e17. [Google Scholar] [CrossRef]
- INFARMED. Deliberação 09/CD/2010. 2010. Available online: https://www.infarmed.pt/docu-428ments/15786/1219415/delib_09_CD_2010_Uni%25E3o_Miseric%25F3rdias%2520Portuguesas.pdf/4bb6580c-ec1a-4b6a-b2d4-4298cc1dddbb0a0 (accessed on 1 December 2021).
- Thomson, M.S.; Gruneir, A.; Lee, M.; Baril, J.; Field, T.S.; Gurwitz, J.H.; Rochon, P.A. Nursing Time Devoted to Medication Administration in Long-Term Care: Clinical, Safety, and Resource Implications. J. Am. Geriatr. Soc. 2009, 57, 266–272. [Google Scholar] [CrossRef]
- Alves-Conceição, V.; da Silva, D.T.; de Santana, V.L.; dos Santos, E.G.; Santos, L.M.C.; de Lyra, D.P. Evaluation of pharmacotherapy complexity in residents of long-term care facilities: A cross-sectional descriptive study. BMC Pharmacol Toxicol. 2017, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Fagundes, D.F.; Costa, M.T.; Alves, B.B.D.S.; Benício, M.M.S.; Vieira, L.P.; Carneiro, L.S.F.; Nascimento, O.J.M.; Junior, R.S.M. Prevalence of dementia in long-term care institutions: A meta-analysis. J. Bras. Psiquiatr. 2021, 70, 59–67. [Google Scholar] [CrossRef]
- Helvik, A.S.; Engedal, K.; Benth, J.Š.; Selbæk, G. Prevalence and Severity of Dementia in Nursing Home Residents. Dement. Geriatr. Cogn. Disord. 2015, 40, 166–177. [Google Scholar] [CrossRef]
- Curtin, D.; Gallagher, P.F.; O’Mahony, D. Explicit criteria as clinical tools to minimize inappropriate medication use and its consequences. Ther. Adv. Drug Saf. 2019, 10. [Google Scholar] [CrossRef]
- Chang, C.-B.; Chan, D.-C. Comparison of Published Explicit Criteria for Potentially Inappropriate Medications in Older Adults. Drugs Aging 2010, 27, 947–957. [Google Scholar] [CrossRef] [PubMed]

| Participant | Gender | Setting of Professional Activity | Years of Practice |
|---|---|---|---|
| 1 | Female | Long-Term Care Facility | 10 |
| 2 | Female | Long-Term Care Facility | 15 |
| 3 | Female | Long-Term Care Facility | 5 |
| 4 | Female | Long-Term Care Facility | 15 |
| 5 | Female | Long-Term Care Facility | 11 |
| 6 | Female | Long-Term Care Facility | 1 |
| 7 | Female | Long-Term Care Facility | 3 |
| 8 | Female | Long-Term Care Facility | 1 |
| 9 | Female | Hospital | 3 |
| 10 | Female | Hospital | 4 |
| 11 | Male | Hospital | 5 |
| 12 | Male | Hospital | 4 |
| 13 | Male | Hospital | 24 |
| Prescribing-Assessment Tool | Round 1 Median Rating (13 Participants); Disagreement Index | Round 2 Median Rating (13 Participants); Disagreement Index | Face-to-Face Panels Appropriate (A), Uncertain (B) or Inappropriate (C) | Representative Quotations | Appropriate (Af), Uncertain (Bf) or Inappropriate (Cf) for LTC Pharmacy Practice | ||
|---|---|---|---|---|---|---|---|
| Panel 1 | Panel 2 | Panel 3 | |||||
| ARS | 7; 0.374 | 7; 0.164 | A | A | B | “It contains many drugs with anticholinergic potential, but in practice, there is not much alternative. We can even tell the physician that these drugs can cause an Adverse Drug Event (ADE), but what is the alternative? In theory, it is important, but in practice, not so much” P11 “I find it very useful because most patients take this medication” P7 “Can be useful to minimize common ADEs (e.g., falls in the elderly)” P13 | Af |
| DBI | 3; 0.748 | 2; 0.438 | C | C | B | “is inappropriate given my context“ P4 “I find it interesting, but in practical terms, it does not materialise into something I can use” P10 “I find it interesting because it takes into account the daily dose and defined daily dose and allows for better choices of dosages and promotion of non-pharmacological strategies; focuses on medicines widely used in our aged population” P13 | Cf |
| ATRIA | 6; 0.519 | 7; 0.164 | A | A | A | “At the level of daily professional practice, the scale is simple to use, all clinical criteria are easily accessed, unlike other similar scales in which personal history or other diagnoses are not always specified in the hospital discharge note” P5 “Very practical and straightforward, the existence of a “score”, that is, “a quantifiable value”, makes it much easier to argue with the physicians, when one intends to make medication reconciliation, for instance,” P12 | Af |
| CHA2DS2VASc | 6; 0.519 | 6; 0.519 | B | B | A | “Easy-to-apply algorithm in my daily practice” P6 “This scale may be more difficult to use, once we do not always have access to the personal background of patients” P5 | Bf |
| HAS-BLED | 6; 0.519 | 7; 0.217 | A | A | A | “Comprehensive, useful, very systematized” P4 | Af |
| HEMORR2HAGES | 5; 0.968 | 5; 0.519 | B | B | B | “it may be important because it includes the CYP2C9 polymorphisms, although this information is rarely available” P13 “Presents pertinent parameters such as genetic polymorphisms and alcohol abuse” P7 “I find it a useful algorithm, but it requires data that is not always accessible in my daily professional practice” P6 | Bf |
| Loeb criteria | 6; 1.04 | 6; 0.652 | A | A | C | “I consider an algorithm very adapted to the LTC reality in order to assess the prescription of antibiotics for the most common infections” P5 “It addresses the three main types of infection that we face daily and allows you to screen the appropriateness of antibiotic prescription in a very quick and simple way ” P1 “Not useful in a practical context, since clinical conditions are much more complex than the algorithm reflects” P12 | Bf |
| MRCI | 5; 1.70 | 5; 0.702 | B | B | B | “Although time consuming, very interesting” P2 “It is not feasible for a regular use due to its length” P9 “Can be useful in the post-discharge moment; however, it is not practical” P5 “It would be very useful in my professional practice, since reducing the complexity of the regimens will reduce potential medication errors, increase adherence and reduce costs” P6 | Bf |
| Mrs. Grace | 5; 0.997 | 5; 0.997 | B | B | B | “Complete and with objective instructions for action” P10 “Very interesting; however, I believe its implementation is hindered due to the limited human resources available in LTC” P4 “I find it very useful to use it in the planned discharges as a way to adapt the therapeutic regimens individually to the patient and caregivers and as a way to promote adherence to the therapeutic regimen” P3 “the algorithm has little practical application since during patient staying it is not always possible to “simplify” therapy due to having a pre-defined drug formulary” P5 | Bf |
| MAI | 7; 0.219 | 8; 0.219 | A | B | A | “Very adapted to pharmacists. I find it particularly useful for some patients and not for everyone. It allows our participation in multidisciplinary meetings to become more useful. It can be important in deprescribing activities” P1 “I find this algorithm very useful and easy to query. However, it is very time-consuming as it is necessary to review each drug at ten different points for each patient” P6 “Interesting for therapeutic reconciliation, although these aspects are already taken into account. It can serve more as a guide than for use in practice” P4 | Af |
| PAI | 5; 0.000 | 5; 0.000 | B | C | B | “There are many questions that we already ask in daily practice” P5 “I find it very easy to use, clear, simple; I think it could be a complement to MAI” P10 | Bf |
| Australian Prescribing Indicators Tool | 3; 0.561 | 3; 0.519 | C | C | B | “Time-consuming, and its use may not always be feasible. Contains very useful information for my technical-scientific development” P13 “It is a too lengthy PAT” P9 “I consider this PAT impractical to consult due to its organization by statements and not by medications, physiological systems or therapeutic classes” P6 | Cf |
| Beers criteria | 7; 0.292 | 7; 0.292 | A | A | A | “Quite adequate to the reality of LTC. Although it is the best known among health professionals and this implies that the prescriptions are very much in line with this criterion, I believe its use is fundamental” P1 “Very useful, just missing the suggestion of alternative” P4 | Af |
| FORTA | 7; 0.000 | 7; 0.09 | A | A | A | “Good instrument for medication review in the elderly and based on a diagnosis. Facilitator of doctor-pharmacist and nurse-pharmacist interactions” P13 “Very organized and quick and easy to consult” P2 | Af |
| Laroche criteria | 7; 0.000 | 7; 0.000 | A | A | A | “Very useful and easy to apply in my daily professional practice. Its main advantage is the fact that it presents the reasons for non-suitability and what are the safest alternatives” P6 “It has a good compromise between extension, reasons and alternatives” P3 | Af |
| McLeod criteria | 6; 0.217 | 6; 0.217 | A | B | B | “I appreciate this PAT because it offers alternatives, the risk associated to PIMs and the statistics about the consensus” P9 “It is organized by incorrect practice and not by medication; it is not so direct, it is less practical” P10 “It can be difficult to consult, but it is useful to suggest therapeutic alternatives to prescribers” P2 | Bf |
| NORGEP | 3; 0.652 | 3; 0.652 | C | C | C | “It didn’t seem very useful to me due to its sparse coverage” P4 “Little information compared to other criteria” P11 | Cf |
| Poudel criteria | 8; 0.292 | 8; 0.292 | A | A | A | “This PAT is very interesting because it has indications for withdrawal regimens, in addition to therapeutic alternatives” P2 “It complements the Beers criteria by presenting suggestions of drug tapering. Moreover, it presents therapeutic alternatives, similar to Laroche’s criteria” P12 | Af |
| Rancourt criteria | 5; 0.851 | 5; 0.851 | B | B | B | “I find it interesting in terms of posology–duration of treatment and dosage–as a way to sensitize the doctor” P13 “It is not very interesting, as it did not suggest alternatives or give guidance, compared to other PATs” P4 “It is not totally incomplete because it considers interactions and dosages” P6 | Bf |
| STOPP/START | 7; 0.164 | 7; 0.164 | A | A | A | “Easy to consult since it is organized by physiological systems. It also has the advantage of allowing the identification of potential prescribing omissions” P6 “Complete and with clear indications of actions” P10 | Af |
| Winit-Watjana criteria | 5; 0.968 | 4; 0.519 | B | B | C | “It summarizes the most significant interactions and adverse reactions, being useful for a consultation” P5 “Nowadays listing interactions doesn’t make much sense because we have search engines for interactions” P10 “Does not propose alternatives. Just mention MPIs and some interactions” P13 | Bf |
| APID | 5; 0.968 | 5; 0.968 | B | B | B | “We often do not have data on admission about the patient to answer these questions” P6 “It should include situations of exacerbation (e.g., delirium)” P12 | Bf |
| Holmes criteria | 5; 0.851 | 5; 0.851 | B | B | B | “Informative and user-friendly” P10 “Very little information […] depends on each case” P4 | Bf |
| Kroger criteria | 6; 0.519 | 6; 0.376 | B | B | A | “It is an adequate PAT because it offers some justification and rationale for the use of medication” P9 “More interesting for technical development than for practical application” P4 | Bf |
| Prescribing-Assessment Tools’ Useful Characteristic | Representative Quotations |
|---|---|
| Levels of evidence | [FORTA] because it is organized by level of evidence (P7) […] having the advantage of presenting the level of evidence (P8, on Beers’) |
| Dose and duration of treatment | I consider it a very complete and valuable criterion as it considers factors such as dose and duration of treatment (P8 on Rancourt’s) I find it very interesting because it considers daily dose and allows to choose better posology (P13, on DBI’s) Useful because it gives a maximum of days, and many times physicians want to use antibiotics more days than those preconized (P3, on Loeb’s) |
| Scoring system | […] a “score”, i.e., a quantifiable value, greatly facilitates discussion with physicians when delivering therapeutic reconciliation, for example (P12, on ATRIA’s) |
| Reasons for PIM classification | Contains very useful information such as reasons for PIM classification (P13, on Laroche’s) Presents the reasons why the medicine is potentially inappropriate (P8, on Beers’) |
| Inclusion of alternatives to PIMs | Very useful, only the suggestion of alternatives is missing (P4, on Beers’) suggests therapeutic alternatives (P1, on Laroche’s) It is a helpful PAT because it suggests therapeutical alternatives (P9, on Laroche’s) […] suggests therapeutical alternative […] (P5, on McLeod’s) […] it offers therapeutical alternatives […] (P9, on McLeod’s) […] presents therapeutical alternatives, similarly to Laroche’s (P12, on Poudel’s) It does not suggest alternatives; just mention PIMs and interactions (P13, on Winit-Watjana’s) […] a helpful PAT should include therapeutical alternatives (P3, McLeod) Contains valuable information such as alternative medicines (P13, on Laroche’s) It suggests therapeutical alternatives (P13, on Poudel’s) It does not have alternatives; I consider it a “lower version” (P12, on Winit-Watjana’s) |
| Organised by medicines groups | I consider this criterion more complex to consult because it is organized by inappropriate practice and not by medicine, therapeutic indication, or physiological system (P8, on McLeod’s) I prefer a tool that evaluates by pharmacotherapeutic group and not so much by pathology because when I’m evaluating a prescription, I evaluate medicine by medicine and, if a question arises, it will help me to have a PAT by the pharmacotherapeutic group to consult and preferably, with alternatives in case you find something wrong (P10) A PAT organized by pharmacotherapeutic groups helps more; it makes it simpler (P13) It seemed to be the most organized as it is divided into therapeutic groups (P3, on McLeod’s) Easy to consult as it is organized by therapeutic groups (P2, on Poudel’s) |
| Inclusion of risk associated with PIM | I find this PAT interesting because it mentions the risk to the patient (P5, on McLeod’s) It includes the risk to the patient […] (P9, on McLeod’s) It has the risk associated with the patient (P3, McLeod’s) |
| Organised by disease/syndrome | I prefer a disease-oriented PAT (P11) It includes the most prevalent diseases on the LTC national network (P1, on FORTA’s) |
| Consensus on PIM/alternative | It includes the panel agreement on the PIM alternative (P5, on McLeod’s) It includes statistics about the consensus on the use of a particular medicine (P9, on McLeod’s) |
| Withdrawal regimens | It has indications on how to withdraw the PIM, being a differentiating factor from the other PATs (P5, on Poudel’s) In a way, it complements the Beers’ criteria, as it suggests withdrawal regimens (P12, on Poudel’s) It suggests withdrawal regimens (P13, on Poudel’s) |
| Potential PATs Use Hinders | Representative Quotations |
|---|---|
| Lack of time / Lack of pharmacists | Challenging to use considering the low human resources ratio in LTC (P4, on DBI’s) I think this PAT would be very useful […] The main limitation is related to the time spent in its application (P8, on Mrs. Grace’s) I consider it very useful in my daily professional practice as it is complete […] however, it is a very time-consuming PAT to apply as it is necessary to review each medicine of each patient at ten different points (P8, on MAI’s) The amount and turnover of patients does not allow us to look detailed at every prescription using some of these PATs (P1) |
| Assistance to several units | It is not easy to apply in my daily professional practice because it involves discussing these situations with the clinical team when prescribing, and I am not present full time at the facilities (P8, on Loeb’s) It is not possible to use it for all prescriptions since there are daily therapeutic changes in the various facilities that I assist, and most of the time, I’m not at the facilities when new prescriptions occur (P3, on PAI’s) |
| Communication barriers with the healthcare team | Despite the quality and usefulness of PATs, the difficulty can come from the physicians because they hardly listen to the pharmacist’s opinion (P2) |
| Limited access or availability of information | We often do not have information to use this PAT because the physician usually does not register this data, and we do not have access to the entire clinical file (P13, on Loeb’s) We often don’t have access to patients’ background or is not even available from the transition of care (P5, on HAS-BLED’s/ HEMORR2HAGES’) Sometimes I don’t have access to some clinical data (e.g., liver function tests) (P8, on HAS-BLED’s/HEMORR2HAGES’) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, J.R.; Sleath, B.L.; Lopes, M.J.; Cavaco, A.M. Prescribing-Assessment Tools for Long-Term Care Pharmacy Practice: Reaching Consensus through a Modified RAND/UCLA Appropriateness Method. Pharmacy 2021, 9, 194. https://doi.org/10.3390/pharmacy9040194
Gonçalves JR, Sleath BL, Lopes MJ, Cavaco AM. Prescribing-Assessment Tools for Long-Term Care Pharmacy Practice: Reaching Consensus through a Modified RAND/UCLA Appropriateness Method. Pharmacy. 2021; 9(4):194. https://doi.org/10.3390/pharmacy9040194
Chicago/Turabian StyleGonçalves, João R., Betsy L. Sleath, Manuel J. Lopes, and Afonso M. Cavaco. 2021. "Prescribing-Assessment Tools for Long-Term Care Pharmacy Practice: Reaching Consensus through a Modified RAND/UCLA Appropriateness Method" Pharmacy 9, no. 4: 194. https://doi.org/10.3390/pharmacy9040194
APA StyleGonçalves, J. R., Sleath, B. L., Lopes, M. J., & Cavaco, A. M. (2021). Prescribing-Assessment Tools for Long-Term Care Pharmacy Practice: Reaching Consensus through a Modified RAND/UCLA Appropriateness Method. Pharmacy, 9(4), 194. https://doi.org/10.3390/pharmacy9040194







