Teaching Pharmacovigilance to Healthcare Students: Identifying Gaps and Opportunities for Improvement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaire
2.3. Statistical Analysis
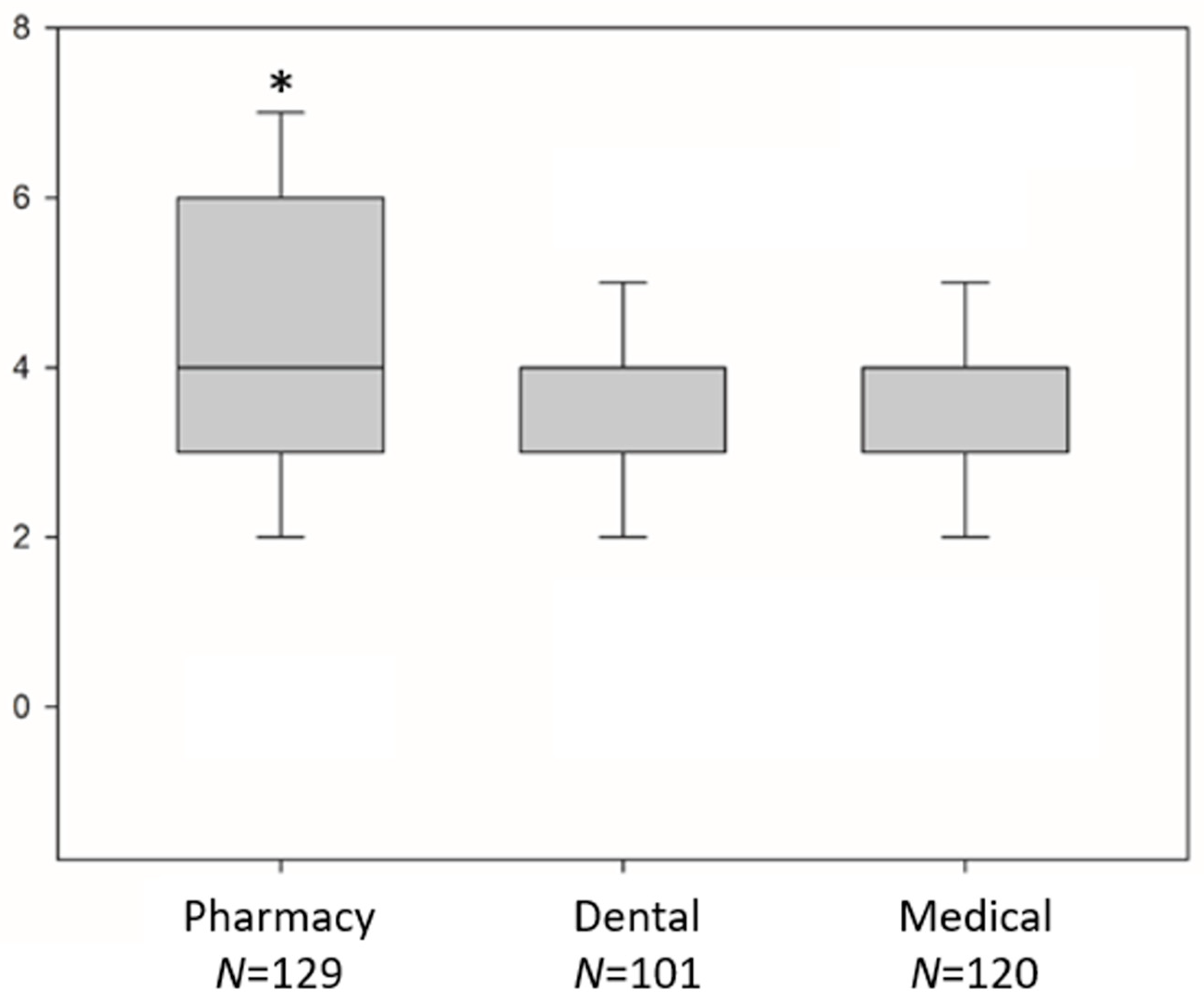
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/teams/regulation-prequalification/pharmacovigilance (accessed on 5 July 2021).
- Mugosa, S.; Stankovic, M.; Turkovic, N.; Sahman-Zaimovic, M.; Besovic, Z.; Drljevic, M. Pharmacovigilance as an imperative of modern medicine - experience from Montenegro. Vojnosanitetski Pregled 2017, 74, 167–172. [Google Scholar] [CrossRef]
- Abu Farha, R.; Abu Hammour, K.; Rizik, M.; Aljanabi, R.; Alsakran, L. Effect of educational intervention on healthcare providers knowledge and perception towards pharmacovigilance: A tertiary teaching hospital experience. Saudi Pharm. J. 2018, 26, 611–616. [Google Scholar] [CrossRef]
- Aldryhim, A.Y.; Alomair, A.; Alqhtani, M.; Mahmoud, M.A.; Alshammari, T.M.; Pont, L.G.; Kamal, K.M.; Aljadhey, H.; Mekonnen, A.B.; Alwhaibi, M.; et al. Factors that facilitate reporting of adverse drug reactions by pharmacists in Saudi Arabia. Expert Opin. Drug. Saf. 2019, 18, 745–752. [Google Scholar] [CrossRef]
- Vigneshwaran, E.; Harichandana, V.; Sadiq, M.M.J.; Alavudeen, S.S.; Khan, N.A.; Ahmed, T. Knowledge, Attitude and Practice of Community Pharmacists towards Adverse Drug Reactions Reporting. J. Young Pharm. 2020, 12, 75–80. [Google Scholar] [CrossRef]
- Shrestha, S.; Sharma, S.; Bhasima, R.; Kunwor, P.; Adhikari, B.; Sapkota, B. Impact of an educational intervention on pharmacovigilance knowledge and attitudes among health professionals in a Nepal cancer hospital. BMC Med. Ed. 2020, 20, 179. [Google Scholar] [CrossRef]
- Ganesan, S.; Vikneswaran, G.; Reddy, K.C.; Subrahmanyam, D.K.; Adithan, C. A Survey on Knowledge, Attitude and Practice of Pharmacovigilance towards Adverse drug reactions reporting among Doctors and Nurses in a Tertiary Care Hospital in South India. J. Young Pharm. 2016, 8, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Januskiene, J.; Segec, A.; Slattery, J.; Genov, G.; Plueschke, K.; Kurz, X.; Arlett, K. What are the patients’ and healthcare professionals’ understanding and behaviors towards adverse drug reaction reporting and additional monitoring? Pharmacoepidemiol. Drug Saf. 2020, 30, 334–341. [Google Scholar] [CrossRef]
- Hartman, J.; Harmark, L.; van Puijenbroek, E. A global view of undergraduate education in pharmacovigilance. Eur. J. Clin. Pharmacol. 2017, 73, 891–899. [Google Scholar] [CrossRef]
- Herrera Comoglio, R. Undergraduate and postgraduate pharmacovigilance education: A proposal for appropriate curriculum content. Br. J. Clin. Pharmacol. 2020, 86, 779–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reumerman, M.; Tichelaar, J.; Piersma, B.; Richir, M.C.; van Agtmael, M.A. Urgent need to modernize pharmacovigilance education in healthcare curricula: Review of the literature. Eur. J. Clin. Pharmacol. 2018, 74, 1235–1248. [Google Scholar] [CrossRef] [Green Version]
- Steurbaut, S.; Hanssens, Y. Pharmacovigilance: Empowering healthcare professionals and patients. Int. J. Clin. Pharm. 2014, 36, 859–862. [Google Scholar] [CrossRef]
- Ali, M.D.; Ahmad, A.; Hassan, Y.A.M.; Ghosn, S.A.; Banu, N.; Alzahrani, M.G. Community Pharmacist’s Knowledge, Practice and Barrier towards Reporting of Adverse Drug Reactions in Dammam, Saudi Arabia: A Cross-Sectional Survey Based Study. J. Young Pharm. 2020, 12, 81–85. [Google Scholar] [CrossRef]
- Gavaza, P.; Brown, C.M.; Lawson, K.A.; Rascati, K.L.; Steinhardt, M.; Wilson, J.P. Effect of social influences on pharmacists’ intention to report adverse drug events. J. Am. Pharm. Assoc. 2012, 52, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Hasford, J.; Goettler, A.; Munter, K.H.; Muller-Oerlinghausen, B. Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J. Clin. Epidemiol. 2002, 55, 945–950. [Google Scholar] [CrossRef]
- Guner, M.D.; Ekmekci, P.E. Healthcare professionals’ pharmacovigilance knowledge and adverse drug reaction reporting behavior and factors determining the reporting rates. J. Drug Assess. 2019, 8, 13–20. [Google Scholar] [CrossRef]
- Melo, J.R.R.; Duarte, E.C.; Ferreira, K.D.; Goncalves, Y.S.; de Moraes, M.V.; Arrais, P.S.D. Assessment of Knowledge, Attitude, and Practice of Pharmacovigilance among Healthcare Professionals in Brazil. J. Young Pharm. 2020, 12, 255–260. [Google Scholar] [CrossRef]
- Haines, H.M.; Meyer, J.C.; Summers, R.S.; Godman, B.B. Knowledge, attitudes and practices of healthcare professionals towards adverse drug reaction reporting in public sector primary healthcare facilities in a South African district. Eur. J. Clin. Pharmacol. 2020, 76, 991–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkayyal, N.; Cheema, E.; Hadi, M.A. Perspective of Saudi undergraduate pharmacy students on pharmacovigilance and adverse drug reaction reporting: A National Survey. Curr. Pharm. Teach. Learn. 2017, 9, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwhaibi, M.; Alhindi, G.; Alshamrani, M.; Essa, M.B.; Al Aloola, N.A.; Alhawassi, T.M. Pharmacovigilance in healthcare education: Students’ knowledge, attitude and perception: A cross-sectional study in Saudi Arabia. BMC Med. Educ. 2020, 20, 210. [Google Scholar] [CrossRef]
- Rajiah, K.; Maharajan, M.K.; Nair, S. Pharmacy students’ knowledge and perceptions about adverse drug reactions reporting and pharmacovigilance. Saudi Pharm. J. 2016, 24, 600–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bepari, A.; Assiri, R.A.; AlYahya, M.A.; AlGhamdi, S.J.; AlGhamdi, A.M.; AlOnazi, A.A. The comparative assessment of awareness, perspective, and basic practice skills about the Saudi pharmacovigilance system among students of different health-care professionals of a Saudi Female University. Saudi Pharm. J. 2020, 28, 828–836. [Google Scholar] [CrossRef]
- Yu, Y.M.; Kim, S.; Choi, K.H.; Jeong, K.H.; Lee, E. Impact of knowledge, attitude and preceptor behaviour in pharmacovigilance education. Basic Clin. Pharmacol. Toxicol. 2019, 124, 591–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegerius, A.; Caduff-Janosa, P.; Savage, R.; Ellenius, J. E-Learning in Pharmacovigilance: An Evaluation of Microlearning-Based Modules Developed by Uppsala Monitoring Centre. Drug Saf. 2020, 43, 1171–1180. [Google Scholar] [CrossRef]
- Seselja Perisin, A.; Mestrovic, A.; Bozic, J.; Kacic, J.; Bukic, J.; Leskur, D.; Rusic, D.; Zekan, L.; Stipic, M.; Modun, D. Interprofessional pharmacotherapy workshop: Intervention to improve health professionals’ and students’ attitudes towards collaboration between physicians and pharmacists. J. Interprof. Care 2019, 33, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Bukic, J.; Rusic, D.; Seselja Perisin, A.; Leskur, D.; Mestrovic, A.; Modun, D. Development and implementation of objective structured clinical examination (OSCE) at the Split School of Medicine Pharmacy studies. Farm. Glas. 2018, 74, 97–108. [Google Scholar]
- Matos, C.; Harmark, L.; van Hunsel, F. Patient Reporting of Adverse Drug Reactions: An International Survey of National Competent Authorities’ Views and Needs. Drug Saf. 2016, 39, 1105–1116. [Google Scholar] [CrossRef]
- Banovac, M.; Candore, G.; Slattery, J.; Houyez, F.; Haerry, D.; Genov, G.; Arlett, P. Patient Reporting in the EU: Analysis of EudraVigilance Data. Drug Saf. 2017, 40, 629–645. [Google Scholar] [CrossRef]
- Inacio, P.; Cavaco, A.; Airaksinen, M. Current trends in pharmacovigilance: Value and gaps of patient reporting. Int. J. Clin. Pharm. 2018, 40, 754–757. [Google Scholar] [CrossRef]
- Riera-Arnau, J.; Alvarado Aguirre, L.A.; Garcia Dolade, N.; Vidal Guitart, X.; Figueras, A.; Cereza Garcia, G. Patients’ contribution to drug safety in Catalonia: The interest of personal feelings on adverse drug reactions. Eur. J. Clin. Pharmacol. 2020, 77, 637–642. [Google Scholar] [CrossRef]
- Al Dweik, R.; Yaya, S.; Stacey, D.; Kohen, D. Patients’ experiences on adverse drug reactions reporting: A qualitative study. Eur. J. Clin. Pharmacol. 2020, 76, 1723–1730. [Google Scholar] [CrossRef]
- Pierce, C.E.; de Vries, S.T.; Bodin-Parssinen, S.; Harmark, L.; Tregunno, P.; Lewis, D.J.; Maskell, S.; Van Eemeren, R.; Ptaszynska-Neophytou, A.; Newbould, V.; et al. Recommendations on the Use of Mobile Applications for the Collection and Communication of Pharmaceutical Product Safety Information: Lessons from IMI WEB-RADR. Drug Saf. 2019, 42, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Bukic, J.; Rusic, D.; Mas, P.; Karabatic, D.; Bozic, J.; Seselja Perisin, A.; Leskur, D.; Krnic, D.; Tomic, S.; Modun, D. Analysis of spontaneous reporting of suspected adverse drug reactions for non-analgesic over-the-counter drugs from 2008 to 2017. BMC Pharmacol. Toxicol. 2019, 20, 60. [Google Scholar] [CrossRef] [Green Version]
- Tsuyuki, R.T.; Beahm, N.P.; Okada, H.; Al Hamarneh, Y.N. Pharmacists as accessible primary healthcare providers: Review of the evidence. Can. Pharm. J. 2018, 151, 4–5. [Google Scholar] [CrossRef] [Green Version]
| Participants | Pharmacy Students N (%) | Dental Students N (%) | Medical Students N (%) | Total N (%) | P Value * |
|---|---|---|---|---|---|
| Number of students | 129 (36.9) | 101 (28.9) | 120 (34.3) | 350 (100) | |
| Female gender | 112 (86.8) | 79 (78.2) | 81 (67.5) | 272 (77.7) | 0.001 |
| Family members who are healthcare professionals | 40 (31.0) | 38 (37.6) | 45 (37.6) | 123 (35.1) | 0.465 |
| Use of medication (if ever) | 115 (89.1) | 93 (92.1) | 105 (87.5) | 313 (89.4) | 0.540 |
| Adverse drug reaction experienced during use | 43 (33.3) | 36 (35.6) | 42 (35.0) | 121 (34.6) | 0.928 |
| Adverse drug reaction reported | 22 (17.1) | 3 (3.0) | 14 (11.7) | 39 (11.1) | 0.003 |
| Knowledge of mobile application reporting route | 85 (65.9) | 9 (8.9) | 61 (50.8) | 155 (44.3) | 0.001 |
| Knowledge of patients reporting | 119 (92.2) | 22 (21.8) | 85 (70.8) | 226 (64.6) | 0.001 |
| Attitude Item | Pharmacy (N = 129) | Dental (N = 101) | Medical (N = 120) | P Value * |
|---|---|---|---|---|
| Reporting of known adverse drug reactions makes no significant contribution to the reporting system. | 2 (1–3) | 2 (2–3) | 2 (2–3) | 0.192 |
| I believe a pharmacist is one of the most important healthcare professionals to report the adverse drug reactions of OTC drugs. | 5 (4–5) ab | 4 (3–4) | 4 (3–4) | <0.001 |
| I believe a pharmacist is one of the most important healthcare professionals to report the adverse drug reactions of prescription drugs. | 4 (4–5) ab | 3 (3–4) b | 3 (2–4) a | <0.001 |
| I believe a physician is one of the most important healthcare professionals to report the adverse drug reactions of prescription drugs. | 4 (4–4) | 4 (3–4) | 4 (3.5–4) | 0.482 |
| I believe a dentist is one of the most important healthcare professionals to report the adverse drug reactions of prescription drugs. | 4 (3–4) | 4 (2.75–4) | 4 (3–4) | 0.631 |
| I believe serious and unexpected reactions that are not fatal or life-threatening during clinical trials must not be reported. | 1 (1–2) ab | 2 (1–3) | 2 (1–4) | <0.001 |
| I’m willing to report any adverse drug reaction in my future practice. | 5 (4–5) ab | 4 (4–5) b | 4 (4–5) a | <0.001 |
| Adverse drug reaction reporting should be made compulsory for healthcare professionals. | 5 (4–5) ab | 4 (4–5) | 4 (4–5) | 0.002 |
| Attitude Item | Pharmacy (N = 129) | Dental (N = 101) | Medical (N = 120) | P Value * |
|---|---|---|---|---|
| I believe that the topic of pharmacovigilance is not well-covered in my school curriculum. | 3 (3–3.25) ab | 3 (3–4) b | 4 (3–4) a | <0.001 |
| Pharmacovigilance should be included as a core topic in formal education. | 4 (4-5) ab | 4 (3–4) | 4 (3–4) | <0.001 |
| Students can perform adverse drug reactions reporting during their clerkship. | 4 (4-5) ab | 4 (3–4) | 4 (3–4) | <0.001 |
| Information on how to report adverse drug reactions should be taught to senior students. | 5 (4-5) ab | 4 (4–4) | 4 (3–4) | <0.001 |
| With my present knowledge, I am very well prepared to report any adverse drug reactions in my future practice. | 3 (2–4) b | 2 (2–3) b | 3 (2–4) a | <0.001 |
| I do not have any idea of how to report adverse drug reactions to the relevant authorities. | 2 (1–4) a | 4 (3–4) b | 3 (2–4) a | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seselja Perisin, A.; Bukic, J.; Rusic, D.; Leskur, D.; Bozic, J.; Mihanovic, A.; Vilovic, M.; Cohadzic, T.; Modun, D. Teaching Pharmacovigilance to Healthcare Students: Identifying Gaps and Opportunities for Improvement. Pharmacy 2021, 9, 147. https://doi.org/10.3390/pharmacy9030147
Seselja Perisin A, Bukic J, Rusic D, Leskur D, Bozic J, Mihanovic A, Vilovic M, Cohadzic T, Modun D. Teaching Pharmacovigilance to Healthcare Students: Identifying Gaps and Opportunities for Improvement. Pharmacy. 2021; 9(3):147. https://doi.org/10.3390/pharmacy9030147
Chicago/Turabian StyleSeselja Perisin, Ana, Josipa Bukic, Doris Rusic, Dario Leskur, Josko Bozic, Ante Mihanovic, Marino Vilovic, Tin Cohadzic, and Darko Modun. 2021. "Teaching Pharmacovigilance to Healthcare Students: Identifying Gaps and Opportunities for Improvement" Pharmacy 9, no. 3: 147. https://doi.org/10.3390/pharmacy9030147










