EMDIA Case Series—Effective Medication Therapy Management (MTM) for Diabetes Type 2 Patients—A Proof of Concept Study
Abstract
:1. Introduction
Objectives
2. Materials and Methods
2.1. Trial Design and Participants
2.2. Intervention Design
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Summary-Relevant Patient Data
3.2. Results Primary Objective—Feasibility of Implementing Four Effective Pharmaceutical Care Components
3.3. Results Secondary Objective—Effects of the Four Pharmaceutical Care Components on Relevant Parameters
4. Discussion
4.1. Discussion Primary Objective—Feasibility of Implementing Four Effective Pharmaceutical Care Components
4.2. Discussion Secondary Objective—Effect of the Four Pharmaceutical Care Components on Relevant Parameters
4.2.1. Effect on the Number of Unsolved DRPs and MAI
4.2.2. Effect of the Four Pharmaceutical Care Components on HbA1c and FBG Values
4.2.3. Effect of the Four Pharmaceutical Care Components on the Well-Being Index
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sarwar, N.; Gao, P.; Kondapally Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, D.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Gregg, E.; Li, Y.; Wang, J.; Burrows, N.R.; Ali, M.K.; Rolka, D.; Williams, D.E.; Geiss, L. Changes in Diabetes-Related Complications in the United States, 1990–2010. N. Engl. J. Med. 2014, 370, 1514–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Schifano, F.; Robinson, P.; Philips, G.; Doherty, L.; Melnick, P.; Laming, L.; Sinclair, A.; Dhillon, S. Impact of community pharmacy diabetes monitoring and education programme on diabetes management: A randomized controlled study. Diabet. Med. 2012, 29, 326–333. [Google Scholar] [CrossRef]
- Castejon, A.M.; Calderón, J.L.; Perez, A.; Millar, C.; McLaughlin-Middlekauff, J.; Sangasubana, N.; Alvarez, G.; Arce, L.; Hardigan, P.; Rabionet, S.E. A Community-Based Pilot Study of a Diabetes Pharmacist Intervention in Latinos: Impact on Weight and Hemoglobin A1c. J. Heal. Care Poor Underserved 2014, 24, 48–60. [Google Scholar] [CrossRef]
- Doucette, W.R.; Witry, M.J.; Farris, K.B.; McDonough, R.P. Community Pharmacist–Provided Extended Diabetes Care. Ann. Pharmacother. 2009, 43, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Fornos, J.A.; Andrés, N.F.; Andrés, J.C.; Guerra, M.M.; Egea, B. A Pharmacotherapy Follow-Up Program in Patients with Type-2 Diabetes in Community Pharmacies in Spain. Pharm. World Sci. 2006, 28, 65–72. [Google Scholar] [CrossRef]
- Jahangard-Rafsanjani, Z.; Sarayani, A.; Nosrati, M.; Saadat, N.; Rashidian, A.; Hadjibabaie, M.; Ashouri, A.; Radfar, M.; Javadi, M.; Gholami, K. Effect of a community pharmacist-delivered diabetes support program for patients receiving specialty medical care: A randomized controlled trial. Diabetes Educ. 2015, 41, 127–135. [Google Scholar] [CrossRef]
- Jameson, J.P.; Baty, P.J. Pharmacist collaborative management of poorly controlled diabetes mellitus: A randomized controlled trial. Am. J. Manag. Care 2010, 16, 250–255. [Google Scholar]
- Kraemer, D.F.; Kradjan, W.A.; Bianco, T.M.; Low, J.A. A randomized study to assess the impact of pharmacist counseling of employer-based health plan beneficiaries with diabetes: The EMPOWER study. J. Pharm. Pract. 2012, 25, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Krass, I.; Armour, C.; Mitchell, B.; Brillant, M.; Dienaar, R.; Hughes, J.; Lau, P.; Peterson, G.; Stewart, K.; Taylor, S.; et al. The Pharmacy Diabetes Care Program: Assessment of a community pharmacy diabetes service model in Australia. Diabet. Med. 2007, 24, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Mehuys, E.; Van Bortel, L.; De Bolle, L.; Van Tongelen, I.; Annemans, L.; Remon, J.-P.; Giri, M. Effectiveness of a community pharmacist intervention in diabetes care: A randomized controlled trial. J. Clin. Pharm. Ther. 2010, 36, 602–613. [Google Scholar] [CrossRef]
- Mourão, A.O.M.; Ferreira, W.R.; Martins, M.A.P.; Reis, A.M.M.; Carrillo, M.R.G.; Guimarães, A.G.; Ev, L.S. Pharmaceutical care program for type 2 diabetes patients in Brazil: A randomised controlled trial. Int. J. Clin. Pharm. 2012, 35, 79–86. [Google Scholar] [CrossRef]
- Obarcanin, E.; Krüger, M.; Müller, P.; Nemitz, V.; Schwender, H.; Hasanbegovic, S.; Kalajdzisalihovic, S.; Läer, S. Pharmaceutical care of adolescents with diabetes mellitus type 1: The DIADEMA study, a randomized controlled trial. Int. J. Clin. Pharm. 2015, 37, 790–798. [Google Scholar] [CrossRef]
- Park, C.; Guallar, E.; Linton, J.; Lee, D.C.; Jang, Y.; Son, D.K.; Han, E.-J.; Baek, S.J.; Yun, Y.D.; Jee, S.H.; et al. Fasting Glucose Level and the Risk of Incident Atherosclerotic Cardiovascular Diseases. Diabetes Care 2013, 36, 1988–1993. [Google Scholar] [CrossRef] [Green Version]
- Cameron, F.J.; Garvey, K.; Hood, K.K.; Acerini, C.L.; Codner, E. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetes in adolescence. Pediatr. Diabetes 2018, 19, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Deters, M.A.; Laven, A.; Castejon, A.; Doucette, W.R.; Ev, L.S.; Krass, I.; Mehuys, E.; Obarcanin, E.; Schwender, H.; Laeer, S. Effective Interventions for Diabetes Patients by Community Pharmacists: A Meta-analysis of Pharmaceutical Care Components. Ann. Pharmacother. 2017, 52, 198–211. [Google Scholar] [CrossRef]
- Saturni, S.; Bellini, F.; Braido, F.; Paggiaro, P.; Sanduzzi, A.; Scichilone, N.; Santus, P.; Morandi, L.; Papi, A. Randomized controlled trials and real life studies. Approaches and methodologies: A clinical point of view. Pulm. Pharmacol. Ther. 2014, 27, 129–138. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Associtation. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S66–S76. [Google Scholar] [CrossRef]
- BAK. Leitlinie der Bundesapothekerkammer zur Qualitätssicherung “Medikationsanalyse”. Available online: https://www.abda.de/fileadmin/user_upload/assets/Praktische_Hilfen/Leitlinien/Medikationsanalyse/LL_MedAnalyse.pdf (accessed on 8 May 2021).
- Griese-Mammen, N.; Hersberger, K.E.; Messerli, M.; Leikola, S.; Horvat, N.; Van Mil, J.W.F.; Kos, M. PCNE definition of medication review: Reaching agreement. Int. J. Clin. Pharm. 2018, 40, 1199–1208. [Google Scholar] [CrossRef]
- Wright, A.; Sittig, D.F.; McGowan, J.; Ash, J.S.; Weed, L.L. Bringing science to medicine: An interview with Larry Weed, inventor of the problem-oriented medical record. J. Am. Med. Inform. Assoc. 2014, 21, 964–968. [Google Scholar] [CrossRef] [Green Version]
- PCNE. PCNE Classification of Drug-Related Problems Version 8.03. 2019. Available online: https://www.pcne.org/upload/files/318_PCNE_classification_V8-03.pdf (accessed on 8 May 2021).
- Samsa, G.P.; Hanlon, J.T.; Schmader, K.E.; Weinberger, M.; Clipp, E.C.; Uttech, K.M. Lewis, I.K.; Landsman, P.B.; Cohen, H.J. A summated score for the medication appropriateness index: Development and assessment of clinimetric properties including content validity. J. Clin. Epidemiol. 1994, 47, 891–896. [Google Scholar] [CrossRef]
- Hanlon, J.T.; Schmader, K.E.; Samsa, G.P.; Weinberger, M.; Uttech, K.M.; Lewis, I.K.; Cohen, H.J.; Feussner, J.R. A method for assessing drug therapy appropriateness. J. Clin. Epidemiol. 1992, 45, 1045–1051. [Google Scholar] [CrossRef]
- Psykiatric Center North Zealand, Psychiatric Research Unit Dyrehavevej. WHO (Fünf)—FRAGEBOGEN ZUM WOHLBEFINDEN (WHO-Five Well-Being Index—German Translation). Available online: https://www.psykiatri-regionh.dk/who-5/who-5-questionnaires/Pages/default.aspx (accessed on 8 May 2021).
- R-Foundation. A Language and Environment for Statistical Computing. Available online: https://www.rstudio.com/products/rstudio/download/#download (accessed on 8 May 2021).
- Halliday, J.A.; Hendrieckx, C.; Busija, L.; Browne, J.L.; Nefs, G.; Pouwer, F.; Speight, J. Validation of the WHO-5 as a first-step screening instrument for depression in adults with diabetes: Results from Diabetes MILES – Australia. Diabetes Res. Clin. Pract. 2017, 132, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rauwerda, N.L.; Tovote, K.A.; Peeters, A.C.T.M.; Sanderman, R.; Emmelkamp, P.M.G.; Schroevers, M.J.; Fleer, J. WHO-5 and BDI-II are acceptable screening instruments for depression in people with diabetes. Diabet. Med. 2018, 35, 1678–1685. [Google Scholar] [CrossRef]
- Tichelaar, J.; Uil den, S.H.; Antonini, N.F.; van Agtmael, M.A.; de Veris, T.P.G.M.; Richir, M.C. A ‘SMART’ way to determine treatment goals in pharmacotherapy education. Br. J. Clin. Pharmacol. 2016, 82, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Podder, V.; Lew, V.; Ghassemzadeh, S. SOAP Notes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Feletto, E.; Lui, G.W.Y.; Armour, C.; Saini, B. Practice change in community pharmacy: Using change-management principles when implementing a pharmacy asthma management service in NSW, Australia. Int. J. Pharm. Pract. 2013, 21, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Moullin, J.C.; Sabater-Hernández, D.; Benrimoj, S.I. Qualitative study on the implementation of professional pharmacy services in Australian community pharmacies using framework analysis. BMC Health Serv. Res. 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.B.; Fegadolli, C. Implementation of pharmaceutical care for older adults in the brazilian public health system: A case study and realistic evaluation. BMC Health Serv. Res. 2020, 20, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Köberlein-Neu, J.; Mennemann, H.; Hamacher, S.; Waltering, I.; Jaehde, U.; Schaffert, C.; Rose, O. Interprofessional Medication Management in Patients With Multiple Morbidities. Dtsch. Aerzteblatt Online 2016, 113, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Odegard, P.S.; Goo, A.; Hummel, J.; Williams, K.L.; Gray, S.L. Caring for Poorly Controlled Diabetes Mellitus: A Randomized Pharmacist Intervention. Ann. Pharmacother. 2005, 39, 433–440. [Google Scholar] [CrossRef]
- Van Mil, J.W.F.; Westerlund, L.O.T.; E Hersberger, K.; A Schaefer, M. Drug-Related Problem Classification Systems. Ann. Pharmacother. 2004, 38, 859–867. [Google Scholar] [CrossRef]
- Deters, M.A.; Läer, S.; Hasanbegović, S.; Nemitz, V.; Müller, P.; Krüger, M.; Schwender, H.; Obarcanin, E. Diabetes Stewardship – Pharmaceutical care of adolescents with type 1 diabetes mellitus provided by community pharmacists. Med. Mon. Pharm. 2016, 39, 477–482. [Google Scholar]
- Lee, G.; Kim, S.M.; Choi, S.; Kim, K.; Jeong, S.-M.; Son, J.S.; Yun, J.-M.; Park, S.M. The effect of change in fasting glucose on the risk of myocardial infarction, stroke, and all-cause mortality: A nationwide cohort study. Cardiovasc. Diabetol. 2018, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; Devries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Danne, T.; Kordonouri, O.; Biester, T.; Siegmund, T.; Kröger, J.; Bramlage, P.; Haak, T. Time in Range: Ein neuer Parameter - komplementär zum HbA 1c. Dtsch Arztebl Int. 2019, 116, 4. [Google Scholar]
- Sartore, G.; Chileli, N.C.; Burlina, S.; Di Stefano, P.; Piarulli, F.; Fedele, D.; Mosca, A.; Lapolla, A. The importance of HbA1c and glucose variability in patients with type 1 and type 2 diabetes: Outcome of continuous glucose monitoring (CGM). Acta Diabetol. 2012, 49, 153–160. [Google Scholar] [CrossRef]
- Smith-Palmer, J.; Brändle, M.; Trevisan, R.; Federici, M.O.; Liabat, S.; Valentine, W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Šoupal, J.; Škrha, J.; Fajmon, M.; Horová, E.; Mráz, M.; Prázný, M. Glycemic Variability Is Higher in Type 1 Diabetes Patients with Microvascular Complications Irrespective of Glycemic Control. Diabetes Technol. Ther. 2014, 16, 198–203. [Google Scholar] [CrossRef]
- Heinemann, L.; Deiss, D.; Siegmund, T.; Schlüter, S.; Naudorf, M.; von Sengbusch, S.; Lange, K.; Freckmann, G. Glukosemessung und-kontrolle bei Patienten mit Typ-1-oder Typ-2-Diabetes. Diabetol. Stoffwechs. 2020, 15, 18–39. [Google Scholar]
- Wright, E.E.; Morgan, K.; Fu, D.K.; Wilkins, N.; Guffey, W.J. Time in Range: How to Measure It, How to Report It, and Its Practical Application in Clinical Decision-Making. Clin. Diabetes 2020, 38, 439–448. [Google Scholar] [CrossRef] [PubMed]
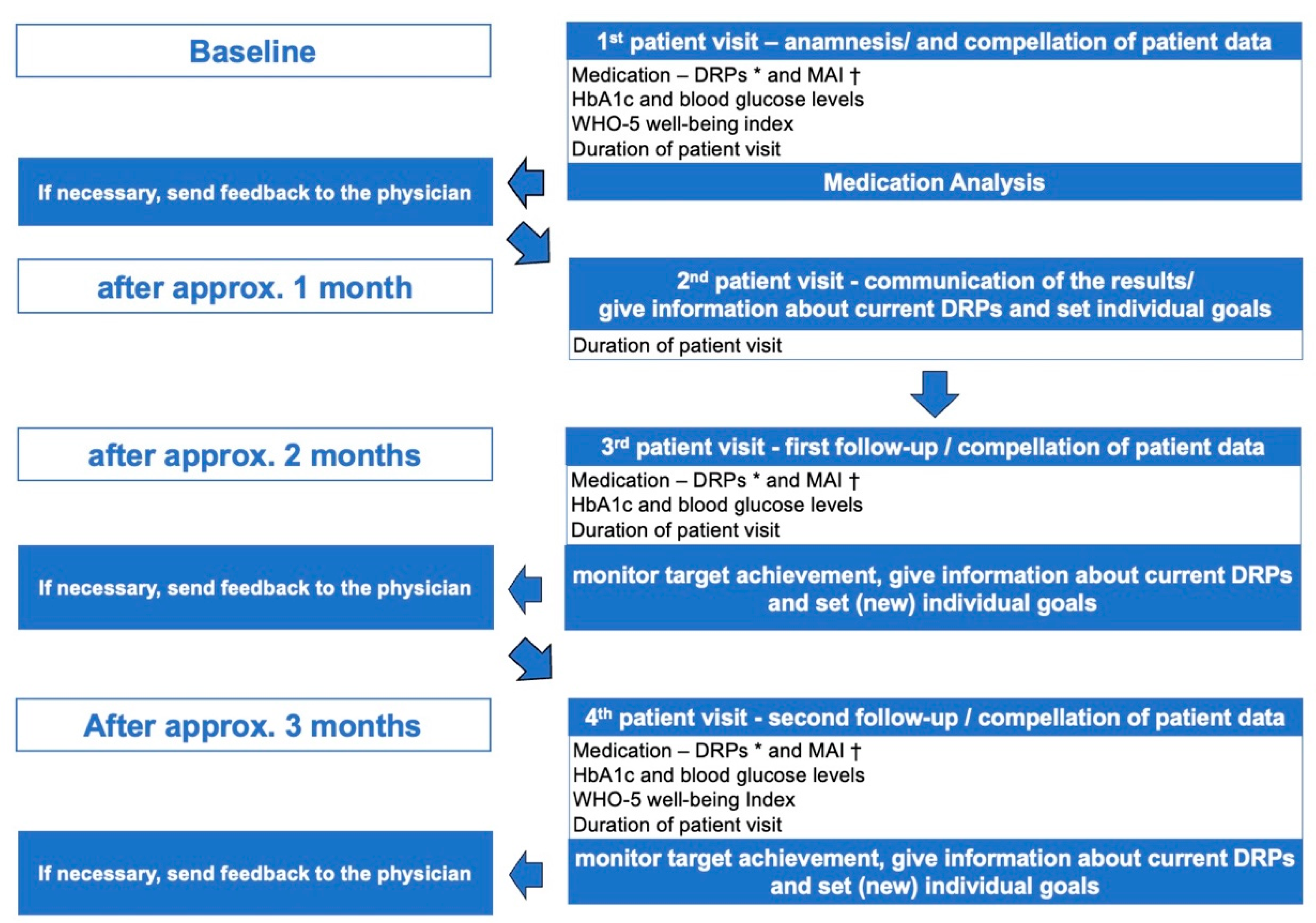
| Relevant Data and Study Endpoints | 1st Patient Visit: Anamnesis | 3rd Patient Visit: First Follow-Up | 4th Patient Visit: Second Follow-Up |
|---|---|---|---|
| Medication | |||
| Number of medications per patient | 10.50 ± 3.75 n = 10 | 11.00 ± 4.37 n = 10 | 10.90 ± 4.07 n = 10 |
| Unsolved DRPs 1 per patient | 6.90 ± 2.60 n = 10 | 2.30 ± 2.11 p-value: <0.003 * n = 10 | 1.89 ± 1.90 p-value: <0.003 * n = 10 |
| Solved DRPs 1 per patient | none | 3.20 ± 1.99 n = 10 | 3.70 ± 2.45 n = 10 |
| Partially solved DRPs 1 per patient | none | 1.45 ± 2.16 n = 10 | 1.80 ± 2.30 n = 10 |
| DRPs 1 per patient with unknown result | none | 0.90 ± 1.28 n = 10 | 1.00 ± 1.41 n = 10 |
| Average MAI 2 | 19.10 ± 13.24 n = 10 | 10.20 ± 9.80 p-value: 0.007 * n = 10 | 6.40 ± 8.88 p-value: 0.007 * n = 10 |
| Glycemic control | |||
| HbA1c value [%] | 7.04 ± 0.90 n = 9 | 6.90 ± 0.56 p-value: 0.50 n = 9 | 7.00 ± 0.61 p-value: 0.78 n = 9 |
| Fasting blood glucose [mg/dL] | 141.86 ± 32.03 n = 7 | 147.29 ± 31.76 p-value: 0.85 n = 7 | 119.63 ± 18.95 p-value: 0.05 n = 8 |
| Other relevant measurements | |||
| Average WHO-5 Well-Being Index | 17.10 ± 6.62 n = 10 | no measurement | 20.40 ± 5.83 p-value: 0.02 * n = 10 |
| Duration of patient visits | 48.70 ± 8.83 n = 10 | 30.50 ± 10.19 n = 10 | 27.90 ± 9.57 n = 10 |
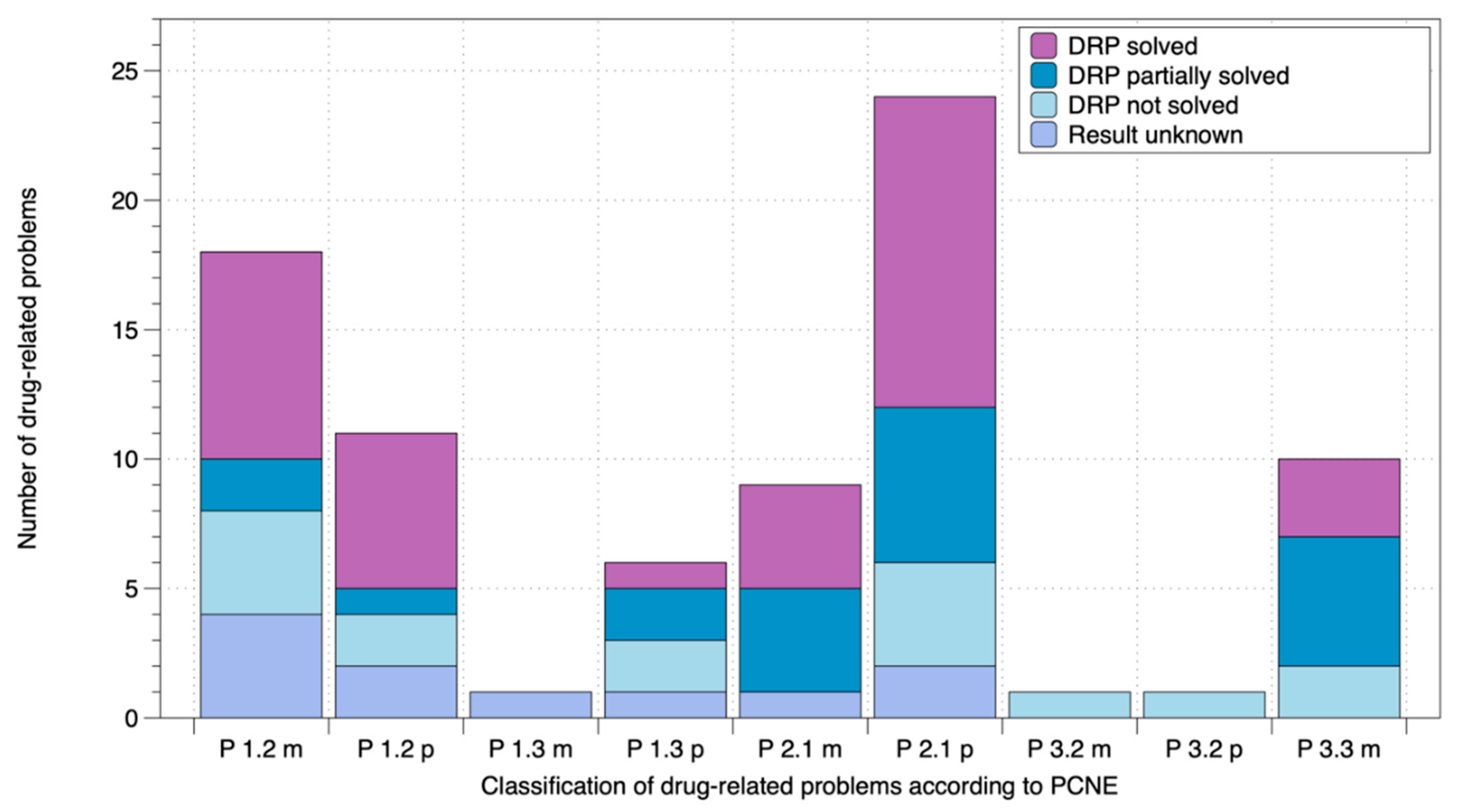
| Solved DRPs 1 | Partially Solved DRPs 1 | Unsolved DRPs 1 | DRPs 1 With Unknown Result | |
|---|---|---|---|---|
| Oral antidiabetics | 0.5 ± 0.7 (62.5%) | 0.1 ± 0.3 (12.5%) | 0.1 ± 0.3 (12.5%) | 0.1 ± 0.3 (12.5%) |
| Insulin | 0.1 ± 0.3 (10%) | 0.6 ± 1.1 (60%) | 0.1 ± 0.3 (10%) | 0.2 ± 0.6 (20%) |
| Residual medication | 3.1 ± 1.9 (48.4%) | 1.1 ± 1.7 (17.2%) | 1.5 ± 2.0 (23.4%) | 0.7 ± 1.1 (10.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deters, M.A.; Obarcanin, E.; Schwender, H.; Läer, S. EMDIA Case Series—Effective Medication Therapy Management (MTM) for Diabetes Type 2 Patients—A Proof of Concept Study. Pharmacy 2021, 9, 137. https://doi.org/10.3390/pharmacy9030137
Deters MA, Obarcanin E, Schwender H, Läer S. EMDIA Case Series—Effective Medication Therapy Management (MTM) for Diabetes Type 2 Patients—A Proof of Concept Study. Pharmacy. 2021; 9(3):137. https://doi.org/10.3390/pharmacy9030137
Chicago/Turabian StyleDeters, Maira Anna, Emina Obarcanin, Holger Schwender, and Stephanie Läer. 2021. "EMDIA Case Series—Effective Medication Therapy Management (MTM) for Diabetes Type 2 Patients—A Proof of Concept Study" Pharmacy 9, no. 3: 137. https://doi.org/10.3390/pharmacy9030137
APA StyleDeters, M. A., Obarcanin, E., Schwender, H., & Läer, S. (2021). EMDIA Case Series—Effective Medication Therapy Management (MTM) for Diabetes Type 2 Patients—A Proof of Concept Study. Pharmacy, 9(3), 137. https://doi.org/10.3390/pharmacy9030137





