General Public Views, Attitudes, and Experiences toward Drug Safety in Dubai, United Arab Emirates: A Qualitative Approach
Abstract
1. Introduction
2. Methodology
2.1. Sampling and Recruitment
2.2. Data Collection
2.3. Data Analysis
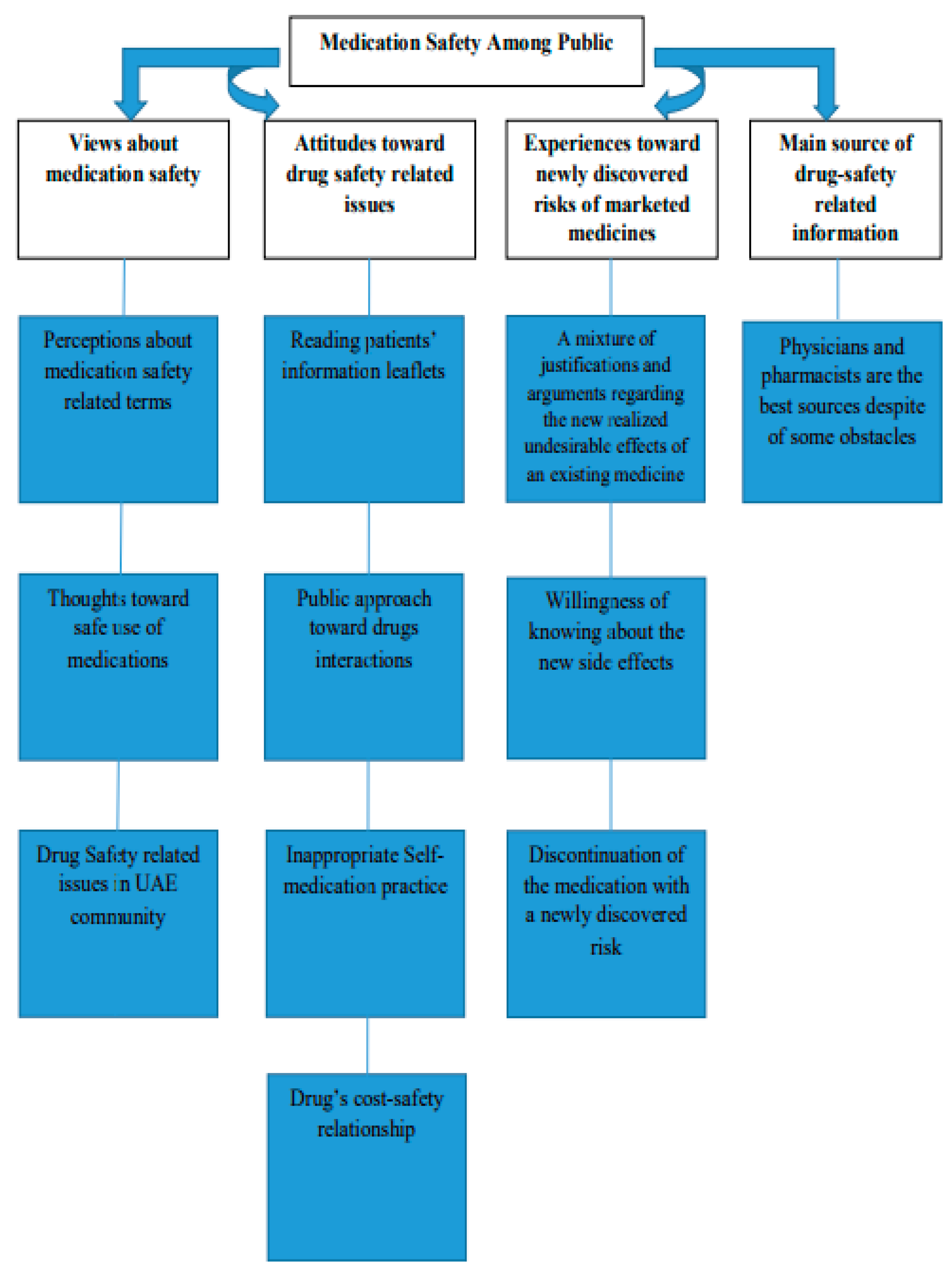
3. Results
3.1. Demographic Data
3.2. First Theme: Views about Medication Safety
3.2.1. Sub-Theme 1: Perceptions about Safety-Related Medication Terms
Effective Drug
- P13. The effective medicine works very fast with all of the patients with the same conditions.
Safe Drug
- P2. Effectiveness of the medicine means that this medicine is giving excellent effect without causing any side effects.
- P5. Safe drug means it will not cause any negative effects upon exceeding the dose which is mentioned in the leaflet.
Side Effects and Adverse Drug Reactions:
- P1: The drug that has no side effects has no adverse drug reactions. There is no difference between them.
- P2: Adverse drug reactions mean that the drug has no side effects.
3.2.2. Sub-Theme 2: Thoughts toward Safe Use of Medications
- P5: Definitely I will ask about the side effects that this new medication will cause.
- P6: The most important thing to ask about is the possible side effects which may appear after having this newly prescribed medication.
- P10: I am sure that the main points I would ask about are: all the indications of this medicine, its dosage and side effects.
- P11: It is very necessary to ask about the dose of any newly prescribed medicine. Plus, I would like to reconfirm that it suits my condition since it is the first time for me to use it.
3.2.3. Sub-Theme 3: Drug-Safety-Related Issues in the UAE Community
- P11: Physicians usually prescribe antibiotics for all the patients who are suffering from high temperature.
- P12: One time a pharmacist had prescribed me an antibiotic when I complained of feeling dizziness.
- P11: Most of the time, when coming back home from the clinic and after referring to the medicine leaflet, I find that the prescribed dose is different.
- P4: Always the doses of the medicines which were prescribed for my children are lower than what has been mentioned in medicine’s brochure.
- P2: Without asking the physician or the pharmacist nobody is asking me whether I take other medications.
- P14: No healthcare professional is making sure that my prescribed medicine is contraindicated with my chronic medicines or not.
3.3. Second Theme: Attitudes toward Drug-Safety-Related Issues
3.3.1. Sub-Theme 1: Reading Patients’ Information Leaflets
- P9: I have the right to know all the side effects of the used medication; therefore, I refer to the product’s inserted leaflet.
- P7: I am not satisfied with the explanation of the healthcare personnel regarding the side effects of my medications. That’s why I usually read them in details from the leaflet.
- P8: I always like to read the medicine leaflet and ask the pharmacists about my suspicions before starting the medications.
- P4: I always find that the prescribed doses are incorrect for my child’s age and weight.
- P11: I don’t trust the dose mentioned by the physician or the pharmacist. I always check the dosage from the medicine’s leaflet.
- P1: I am a diabetic patient, and I must read before using any drug whether it will affect my blood sugar level or not.
- P2: I must ensure that the newly initiated medication is not contraindicated with my hypertension.
3.3.2. Sub-Theme 2: A Public Approach toward Drugs Interactions:
- P10: No healthcare provider gives me importance regarding information of drug interactions, therefore I think it something rare that happens and I don’t think about it.
- P5: I don’t think that drug interactions will cause severe harm or death to any patient.
- P6: I don’t care about it. If any drug interaction is found to be serious, definitely it will be mentioned by a physician.
3.3.3. Sub-Theme 3: Inappropriate Self-Medication Practice
- P12: I don’t ask the pharmacist or even the doctor if I decide to take vitamins and herbal products because they are safe.
- P5: Herbs are not harmful products even if the person takes them at high doses.
- P1: Definitely, I have to seek my doctor’s advice before taking any supplements as it may be contraindicated with my condition. For example, I take aspirin to increase the fluidity of blood, and I know that garlic containing products may increase the possibility of bleeding in such situation. Therefore, I have to ask before taking any product.
- P6: I have repeated the course of antibiotics based on what I have been prescribed in the past when I develop a similar condition.
- P4: As far as I remember, I had a cough and the pharmacist gave me medicine. Later, when I got a cough again, I have used that medicine without asking anybody.
- P10: Sometimes I read the leaflet and I find many uses for the medication that I take. In the future when I develop any of these conditions or diseases that are listed in the leaflet, I don’t mind to use the medication. But I don’t do this with my friends and relatives.
- P7: One of my friends recommended me a natural product. I took it, and a few days later I had very high and disturbing palpitations. I was scared and asked the pharmacist who clarified to me that it was a side effect of the product. I stopped it immediately and decided never to trust any friend’s suggestions.
- P9: I usually suffer from insomnia. I was suggested to take Phenergan tablets 2 hours before my sleeping time. The next day I fainted down in the street and when the first aid people arrived, they informed me that I suffered from severe hypotension. Therefore, I stopped believing in the friends’ suggestions for medications.
3.3.4. Sub-Theme 4: Drug’s Cost–Safety Relationship
- P1: I always prefer to get an expensive form of the medication because I think it is stronger and causes fewer side effects.
- P8: The expensive medicine is much better than the cheap one. Because I think it is highly pure medicine, therefore, it will not cause serious side effects.
3.4. Third Theme: Experiences toward Newly Discovered Risks of Marketed Medicines
3.4.1. Sub-Theme 1: A Mixture of Justifications and Arguments Regarding the Newly Realized Undesirable Effects of an Existing Medicine.
- Some participants have justified the newly discovered risks due to the difficulty of gathering the entire drug-related risks (by the pharmaceutical companies) before the marketing stage.
- On the other hand, some other participants argued and related such newly discovered risks of already marketed products due to the shortage of the clinical trial period of the concerned drug. They said that this was the main cause of newly appearing side effects post-marketing.
3.4.2. Sub-Theme 2: Willingness of Knowing about the New Side Effects
3.4.3. Sub-Theme 3: Discontinuation of the Medication with a Newly Discovered Risk
- P7: I will stop it immediately and consult the doctor for the alternative one.
- P8: I am not going to continue a medication with side effects even if I don’t develop those side effects.
- P2: I will not trust this medication anymore; I will not use it at all. I will consult the physician to find the alternative.
3.5. Fourth Theme: Main Source of Drug-Safety-Related Information
Sub-Theme: Physicians and Pharmacists Are the Best Sources Despite Some Obstacles
- P9: I feel myself confused when there is no communication between the pharmacist and the physician that I consulted, especially when I visit more than one physician.
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bapna, J.S.; Tripathi, C.D.; Tekur, U. Drug utilization patterns in the Third World. Pharmacoeconomics 1996, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.A.; Mackey, T.K. Prevalence and global health implications of social media in direct-to-consumer drug advertising. J. Med. Internet Res. 2011, 13, e63. [Google Scholar] [CrossRef] [PubMed]
- Van Grootheest, K.; de Jong-van den Berg, L. Patients’ role in reporting adverse drug reactions. Expert Opin. Drug Saf. 2004, 3, 363–368. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Alliance for Patient Safety: WHO Draft Guidelines for Adverse Event Reporting and Learning Systems: From Information to Action; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Dawood, O.T.; Hassali, M.A.; Saleem, F. A qualitative study exploring medicines use pattern and practice among general public in Malaysia. Pharm. Pract. (Granada) 2016, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.S.; Pradhan, H.S.; Mohanta, G.P. Concept of essential medicines and rational use in public health. Indian J. Community Med. 2010, 35, 10–13. [Google Scholar] [CrossRef]
- Said, A.S.; Hussain, N. Adverse Drug Reaction Reporting Practices Among United Arab Emirates Pharmacists and Prescribers. Hosp. Pharm. 2017, 52, 361–366. [Google Scholar] [CrossRef]
- Alshammari, T.M. Drug safety: The concept, inception and its importance in patients’ health. Saudi Pharm. J. 2016, 24, 405–412. [Google Scholar] [CrossRef]
- Wilbur, K. Pharmacovigilance in the middle east. Drug Saf. 2013, 36, 25–30. [Google Scholar] [CrossRef]
- Dameh, M. United Arab Emirates pharmacists’ practices and views on adverse drug reaction and medication error reporting and health professional expectations. IDSR-JPBS 2015, 18, 86–96. [Google Scholar]
- Albraiki, F. Pharmacovigilance overview and activities. In Proceedings of the UAE. Second national pharmacovigilance conference, Dubai, UAE, 21–22 April 2012. [Google Scholar]
- Patsuree, A.; Krska, J.; Jarernsiripornkul, N. Experiences relating to adverse drug reactions in the community: A cross-sectional survey among patients and the general public in Thailand. Expert Opin. Drug Saf. 2016, 15, 287–295. [Google Scholar] [CrossRef]
- Narumol, J.; Arunrot, P.; Krska, J. Survey of patients’ experiences and their certainty of suspected adverse drug reactions. Int. J. Clin. Pharm. 2015, 37, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Krska, J.; Jones, L.; McKinney, J.; Wilson, C. Medicine safety: Experiences and perceptions of the general public in Liverpool. Pharmacoepidemiol. Drug Saf. 2011, 20, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Van Dole, K.B.; West, S.L.; Mease, M.; Olsen, A. Perception of drug safety and knowledge influences drug selection. Arch. Intern. Med. 2011, 171, 90–91. [Google Scholar] [PubMed]
- Elkalmi, R.; Hassali, M.A.; Al-Lela, O.Q.; Awadh, A.I.J.; Al-Shami, A.K.; Jamshed, S.Q. Adverse drug reactions are reporting: Knowledge and opinion of the general public in Penang, Malaysia. J. Pharm. Bioallied Sci. 2013, 5, 224–228. [Google Scholar] [PubMed]
- Brounéus, F.; Macleod, G.; Maclennan, K.; Parkin, L.; Paul, C. Drug safety awareness in New Zealand: Public knowledge and preferred sources for information. J. Prim. Health Care 2012, 4, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Smith, F. Conducting Your Pharmacy Practice Research Project: A Step-by-Step Approach, 2nd Revised ed.; Pharmaceutical Press: London, UK, 2010; pp. 75–89. [Google Scholar]
- Azhar, S.; Latif, U.; Murtaza, G.; Khan, S.A.; Hussain, I. Mixed methodology approach in pharmacy practice research. Acta Pol. Pharm. 2013, 70, 1123–1130. [Google Scholar] [PubMed]
- Holloway, I. Qualitative Research in Health Care, 3rd ed.; Open University Press: Milton Keynes, UK, 2005. [Google Scholar]
- Austin, Z.; Sutton, J. Qualitative research: Getting started. Can. J. Hosp. Pharm. 2014, 67, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Merriam, S.B. Qualitative Research in Practice: Examples for Discussion and Analysis, 1st ed.; Jossey-Bass Inc. Pub.: San Francisco, CA, USA, 2002; ISBN 978-0-787-95895-4. [Google Scholar]
- Polkinghorne, D.E. Language and meaning: Data collection in qualitative research. J. Couns. Psychol. 2005, 52, 137–145. [Google Scholar] [CrossRef]
- Morse, J.M. Qualitative Health Research: Creating a New Discipline, 1st ed.; Routledge publications: Abingdon, UK, 2016; pp. 51–63. [Google Scholar]
- Punch, K.F. Introduction to Social Research: Quantitative and Qualitative Approaches, 3rd ed.; SAGE Publisher: New York, NY, USA, 2013; pp. 143–163. [Google Scholar]
- Sutton, J.; Austin, Z. Qualitative research: Data collection, analysis, and management. Can. J. Hosp. Pharm. 2015, 68, 226–231. [Google Scholar] [CrossRef]
- Hassali, M.A.; Shafie, A.A.; Saleem, F.; Al-Qazaz, H.; Masood, I.; Atif, M.; Aljadhey, H. A pilot study exploring awareness among general public toward issues related to medication safety in the state of Penang, Malaysia. Chron. Young Sci. 2012, 3, 156–159. [Google Scholar] [CrossRef]
- Hsiao, F.-Y.; Lee, J.-A.; Huang, W.-F.; Chen, S.-M.; Chen, H.-Y. Survey of medication knowledge and behaviors among college students in Taiwan. Am. J. Pharm. Educ. 2006, 70, 30. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.; Aronson, J. Communicating information about drug safety. BMJ 2006, 333, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Hamoudi, N.M.; Al-Obaidi, N.Y.; Khan, F.M. Drug interaction awareness among public attending GMCH Ajman/UAE. Asian J. Biomed. Pharm. 2013, 3, 17–30. [Google Scholar]
- Rasool, B.K.A.; Fahmy, S.A.; Abu-Gharbieh, E.F.; Ali, H.S. Professional practices and perception towards rational use of medicines according to WHO methodology in United Arab Emirates. Pharm. Pract. (Granada) 2010, 8, 70–76. [Google Scholar] [PubMed]
- Senok, A.C.; Ismaeel, A.Y.; Al-Qashar, F.A.; Agab, W.A. Pattern of upper respiratory tract infections and physicians’ antibiotic prescribing practices in Bahrain. Med. Princ. Pract. 2009, 18, 170–174. [Google Scholar] [CrossRef]
- Otoom, S.; Sequeira, R. Health care providers’ perceptions of the problems and causes of irrational use of drugs in two Middle East countries. Int. J. Clin. Pract. 2006, 60, 565–570. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, E.C.; Philbert, D.; van Boheemen, C.; van Dijk, L.; Bos, M.B.; Bouvy, M.L. Patients’ satisfaction with information and experiences with counseling on cardiovascular medication received at the pharmacy. Patient Educ. Couns. 2011, 83, 303–309. [Google Scholar] [CrossRef]
- Serper, M.; McCarthy, D.M.; Patzer, R.E.; King, J.P.; Bailey, S.C.; Smith, S.G.; Parker, R.M.; Davis, T.C.; Ladner, D.P.; Wolf, M.S. What patients think doctors know: Beliefs about provider knowledge as barriers to safe medication use. Patient Educ. Couns. 2013, 93, 306–311. [Google Scholar] [CrossRef]
- Nair, K.; Dolovich, L.; Cassels, A.; McCormack, J.; Levine, M.; Gray, J.; Mann, K.; Burns, S. What patients want to know about their medications. Focus group study of patient and clinician perspectives. Can. Fam. Physician 2002, 48, 104–110. [Google Scholar]
- Jose, J.; Jimmy, B.; Al-Mamari, M.N.; Al-Hadrami, T.S.; Al-Zadjali, H.M. Knowledge, Beliefs, and Behaviours Regarding the Adverse Effects of Medicines in an Omani Population: Cross-sectional survey. Sultan Qaboos Univ. Med. J. 2015, 15, e250–e256. [Google Scholar]
- Orleans-Lindsay, J. Pharmacovigilance Medical Writing: A Good Practice Guide, 1st ed.; Wiley Publisher: London, UK, 2012; pp. 75–114. [Google Scholar]
- Kullberg, A.; Sharp, L.; Johansson, H.; Bergenmar, M. Information exchange in oncological inpatient care–Patient satisfaction, participation, and safety. Eur. J. Oncol. Nurs. 2015, 19, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Delaney, L.J. Patient-centred care as an approach to improving health care in Australia. Collegian 2018, 25, 119–123. [Google Scholar] [CrossRef]
- Frøkjær, B.; Bolvig, T.; Griese, N.; Herborg, H.; Rossing, C. Prevalence of drug-related problems in self-medication in Danish community pharmacies. Inov. Pharm. 2012, 3. [Google Scholar] [CrossRef]
- Ruiz, M.E. Risks of self-medication practices. Curr. Drug Saf. 2010, 5, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.; Zollman, C. ABC of complementary medicine: Herbal medicine. BMJ 1999, 319, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Abasaeed, A.; Vlcek, J.; Abuelkhair, M.; Kubena, A. Self-medication with antibiotics by the community of Abu Dhabi Emirate, United Arab Emirates. J. Infect. Dev. Ctries 2009, 3, 491–497. [Google Scholar] [CrossRef]
- Shehnaz, S.I.; Khan, N.; Sreedharan, J.; Issa, K.J.; Arifulla, M. Self-medication and related health complaints among expatriate high school students in the United Arab Emirates. Pharm. Pract. (Granada) 2013, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Staff Report. UAE to Issue law Against Buying Antibiotics without Prescription. Khaleej Times, 19 November 2017. [Google Scholar]
- Alhaddad, M.S.; Abdallah, Q.M.; Alshakhsheer, S.M.; Alosaimi, S.B.; Althmali, A.R.; Alahmari, S.A. General public knowledge, preferred dosage forms, and beliefs toward medicines in western Saudi Arabia. Saudi Med. J. 2014, 35, 578–584. [Google Scholar]
- Babar, Z.U.; Stewart, J.; Reddy, S.; Alzaher, W.; Vareed, P.; Yacoub, N.; Dhroptee, B.; Rew, A. An evaluation of consumers’ knowledge, perceptions and attitudes regarding generic medicines in Auckland. Pharm. World Sci. 2010, 32, 440–448. [Google Scholar] [CrossRef]
- Shrank, W.H.; Cox, E.R.; Fischer, M.A.; Mehta, J.; Choudhry, N.K. Patients’ perceptions of generic medications. Health Aff. (Millwood) 2009, 28, 546–556. [Google Scholar] [CrossRef]
- Hassali, M.A.; Shafie, A.A.; Jamshed, S.; Ibrahim, M.I.; Awaisu, A. Consumers’ views on generic medicines: A review of the literature. Int. J. Pharm. Pract. 2009, 17, 79–88. [Google Scholar] [PubMed]
- Matos, C.; van Hunsel, F.; Joaquim, J. Are consumers ready to take part in the Pharmacovigilance System?—A Portuguese preliminary study concerning ADR reporting. Eur. J. Clin. Pharmacol. 2015, 71, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Krska, J.; Murphy, E.; Avery, A.; Collaboration, Y.C.S. The importance of direct patient reporting of suspected adverse drug reactions: A patient perspective. Br. J. Clin. Pharmacol. 2011, 72, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Coulter, A.; Ellins, J. Effectiveness of strategies for informing, educating, and involving patients. BMJ 2007, 335, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Longtin, Y.; Sax, H.; Leape, L.L.; Sheridan, S.E.; Donaldson, L.; Pittet, D. Patient participation: Current knowledge and applicability to patient safety. Mayo Clin. Proc. 2010, 85, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.E.; Zillich, A.J.; Primack, B.A.; Rice, K.R.; Somma McGivney, M.A.; Pringle, J.L.; Smith, R.B. Exploring successful community pharmacist-physician collaborative working relationships using mixed methods. Res. Soc. Adm. Pharm. 2010, 6, 307–323. [Google Scholar] [CrossRef]
- Makowsky, M.J.; Schindel, T.J.; Rosenthal, M.; Campbell, K.; Tsuyuki, R.T.; Madill, H.M. Collaboration between pharmacists, physicians and nurse practitioners: A qualitative investigation of working relationships in the inpatient medical setting. J. Interprof. Care 2009, 23, 169–184. [Google Scholar] [CrossRef]
- Brock, K.A.; Doucette, W.R. Collaborative working relationships between pharmacists and physicians: An exploratory study. J. Am. Pharm. Assoc. (2003) 2004, 44, 358–365. [Google Scholar] [CrossRef]
| Total Number of Participants | 14 (P1–P14) |
|---|---|
| Age | 22–64 years old |
| Gender | |
| Male | 6 |
| Female | 8 |
| Nationality | |
| Local | 2 |
| Non-Local | 12 |
| Education Level | |
| Secondary School | 5 |
| University | 9 |
| Health Status | |
| Healthy | 7 |
| Chronic Diseases | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhalidi, D.; Jamshed, S.Q.; Elkalmi, R.M.; Baig, M.R.; Aslam, A.; Hassali, M.A. General Public Views, Attitudes, and Experiences toward Drug Safety in Dubai, United Arab Emirates: A Qualitative Approach. Pharmacy 2019, 7, 19. https://doi.org/10.3390/pharmacy7010019
Alkhalidi D, Jamshed SQ, Elkalmi RM, Baig MR, Aslam A, Hassali MA. General Public Views, Attitudes, and Experiences toward Drug Safety in Dubai, United Arab Emirates: A Qualitative Approach. Pharmacy. 2019; 7(1):19. https://doi.org/10.3390/pharmacy7010019
Chicago/Turabian StyleAlkhalidi, Doaa, Shazia Qasim Jamshed, Ramadan Mohamed Elkalmi, Mirza Rafi Baig, Adeel Aslam, and Mohamed Azmi Hassali. 2019. "General Public Views, Attitudes, and Experiences toward Drug Safety in Dubai, United Arab Emirates: A Qualitative Approach" Pharmacy 7, no. 1: 19. https://doi.org/10.3390/pharmacy7010019
APA StyleAlkhalidi, D., Jamshed, S. Q., Elkalmi, R. M., Baig, M. R., Aslam, A., & Hassali, M. A. (2019). General Public Views, Attitudes, and Experiences toward Drug Safety in Dubai, United Arab Emirates: A Qualitative Approach. Pharmacy, 7(1), 19. https://doi.org/10.3390/pharmacy7010019







