Abstract
Medication errors (MEs) often prelude guilt and fear in health care professionals (HCPs), thereby resulting in under-reporting and further compromising patient safety. To improve patient safety, we conducted a study on the implementation of a voluntary medication error-reporting and monitoring programme. The ME reporting system was established using the principles based on prospective, voluntary, open, anonymous, and stand-alone surveillance in a tertiary care teaching hospital located in South India. A prospective observational study was carried out for three years and a voluntary Medication Error-reporting Form was developed to report medication errors MEs that had occurred in patients of either sex were included in the study, and the reporters were given the choice to remain anonymous. The analysis was carried out and discussed with HCPs to minimise the recurrence. A total of 1310 medication errors were reported among 20,256 hospitalised patients and the incidence was 6.4%. Common aetiologies were administration errors [501 (38.2%)], followed by prescribing and transcribing errors [363 (28%)]. Root-cause of these MEs were distractions, workload, and communications. Analgesics/antipyretics (19.4%) and antibiotics (15.7%) were the most commonly implicated classes of medications. A clinical pharmacist initiated non-punitive anonymous ME reporting system could improve patient safety.
1. Introduction
Patient safety is at the core of quality of health care as echoed in the Hippocratic Oath: “I will prescribe regimes for the good of my patient according to my ability and my judgment and never do harm to anyone … In every house whenever I come, I will enter only for the good of my patient” (Excerpt from the Hippocratic Oath c. 300–400 BCE.) The Hippocratic Oath guided most doctors to be non-maleficence and beneficence for a long time [1]. Unfortunately, these virtues in newer times are troubled by the aged predators called Medication Errors [2].
Despite of the growing attention to patient safety, the freedom from accidental injury due to medical care or from medical error, the realisation of this apophthegm is far from long shot [3,4,5]. Working on Patient safety has stuck a realisation that the power and complexity of modern medicine to cure and ameliorate ailments rendered hospitals as a ‘not so safe place’ for healing as perceived yet, those were places fraught with risk of patient harm [6].
Long before the words “medication error” and “patient safety” came into existence, the term Iatrogenesis had been widely known amongst the medical fraternity. This ancient word, iatrogenesis, is a combination of two Greek words, Iatros (healer) and Genesis (to bring forth). Iatrogenesis is defined as the undesirable side-effects of medical interventions [7]. Whereas, National Coordinating Council for Medication Error-reporting Programme (NCC MERP) defines medication error as “A medication error is any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care products, procedures, and systems, including prescribing, order communication, product labelling, packaging, and nomenclature, compounding, dispensing, distribution, administration, education, monitoring, and use. While near misses are defined as an event, situation, or error that took place but was captured before reaching the patient” [8,9].
Medication Errors, once known as a diseases of medical progress [10] in the 1950s and a ‘noxious episodes’ [11] in 1960s, evolved into a vast, profound, inevitable, and ineluctable reality of medication-based therapy. The cause of this evolution was the repercussions of current medicalisation of diseases and had dreadful effect on patients and medical staff alike [12]. Although hospitals and healthcare professionals (HCPs) avow and aim for providing the safest care possible, things may go wrong leading to extensive toll of inadvertent clinical iatrogenesis.
In 1984, the Harvard Medical Practice Study (HMPS) findings sparked the need for replication of HMPS in Australia, New Zealand, Denmark, the United Kingdom, and Canada, and altogether reported higher rates of adverse events (9–13%) and, roughly 50% of the adverse events were considered preventable [13,14,15,16,17]. The consecutive evidences of medication errors recorded in that decade transcended into the wake of sensational report by the Institute of Medicine (IOM), Building a Safer Health System, November 1999. This report has changed all the existing estimates of medication errors and claimed as many as 98,000 people die every year because of iatrogenesis in the United States of America (USA) alone. Followed by the United Kingdom (UK) Department of Health’s review of patient safety in the National Health Service (NHS), 2000, ‘An organisation with a memory’, extrapolated iatrogenic figures from the US studies to estimate that 250,000 patients might be iatrogenic suffering as a result of NHS care costing around £400 million per year, and the additional hospital bed days cost as much as £2 billion annually [18].
By 2013, various researchers who provided an estimate of 210,000 deaths per year were associated with preventable adverse events (PAEs) in hospitals using the Global Trigger Tool (GTT) [19]. These estimates were the lower limits of weighted averages of the various contemporary studies. With incomplete medical records these proposed values can be projected to an estimate of 400,000 per year premature deaths associated with preventable harm. Further, serious harm seems to range 10- to 20-fold more common than lethal [20].
On the other hand, these estimates of medication errors are inundated for not being absolute as it is pragmatically difficult to compute and arrive at prevalence rate of the errors due to the varying definitions of medication errors and classification systems. Usually, the reported rates vary depending on the denominator used (e.g., number of patients, number of prescriptions, number of doses or a specific medication etc.). The challenge of reaching a nearest possible estimate is compounded by differences in health care system organizations and the availability and use of medication error-reporting systems [21]. Whilst in the Indian scenario, the current workload on doctors (1:1800) and nursing staff (1.7 for every 1000 patients) has left breaches in patient safety [22]. Recently, blotched mass sterilisations and cataract surgeries in 2014 remain stark are reminders of inadequate accountability, limited infrastructure, and low-quality health services in India’s health care sector [23].
With this scenario, sustaining a well-established medication error-reporting system is an uphill battle in any developing economic state. Although there is a lack of medication error-reporting system in India, the few studies that have been conducted on medication errors and rate of errors ranged between 15.34% and 25.7%, respectively, in hospitalized patients [24,25]. The confidence in these numbers suffer due to severe under reporting and lack of uniform standards in gauzing the errors [26]. This scenario warrants that designing an indigenous error-reporting programme is essential to understand the nature and extent of medication errors to encourage patient safety.
2. Methodology
At the study site, the Dept. of Clinical Pharmacy has taken an initiative to design and implement medication error-reporting and a monitoring programme using the principles of prospective, voluntary, open, anonymous, and stand-alone surveillance in an 1800-bed tertiary care teaching hospital located in South India. Further to support the system a five-membered expert panel was created to oversee its functions. Members were drawn from various specialities within the hospital and were set to meet once every two months or, otherwise, on the basis of need to evaluate, monitor, and redress the medication errors and any other issues pertaining to the patient safety. NCC MERP’s definition of medication error was adapted for this study purpose and any deviation from the standard procedure involved in the prescribing, transcribing, dispensing, administration, and monitoring were considered as medication errors. The elements of the deviation are tabulated in Table 1 of results.

Table 1.
Detailed distribution of Medication errors across various stages.
For the purpose of the study, a prospective, observational study design was adapted and carried out for three years. MEs involving in-patients of either sex admitted to the wards of General Medicine, Emergency Medicine, Intensive care units, Surgery, and Obstetrics and Gynaecology were included in the study. The reporters were given the choice to remain anonymous and Institutional human ethics committee approval was obtained to carry out the study.
The functional aspects of the medication error-reporting system include:
- Voluntary: Health care professionals can report the medication errors without any compulsion.
- Open: Any health care professional of any specialisation of any status can report any medication error.
- Anonymous: Health care professionals can remain anonymous while reporting the medication error.
- Stand-alone: Thus, reported medication errors were pooled into a database and the data was secured and not shared outside the purview of the review committee.
- Exploratory: Reported medication errors were evaluated with the concept of “cause and effect”
- Paper and electronic based database: Reporter can use either of the available modes to report an error. HCPs were requested to avoid reporting the same medication error by using two different modes.
Medication Error reports were accepted from all the health care professionals of the different specialities irrespective of their status and types of services they provided. As mentioned earlier, the reporters were not obligated to disclose their identity while reporting. They were requested to report the error with a detailed description using any of the provided modes of reporting. For the study purpose, a system was created wherein a reporter can choose to report the error using paper-based system, electronic system and or telephone-based system.
2.1. Paper-Based System
The Voluntary Medication Error-reporting Form (vMERF) was designed by clinical pharmacists after observing the MedWatchTM, Institute for Safe Medication Practices (ISMP), ISMP-Canada, National Co-ordination Centre Medication Error-reporting Programme (NCC MERP), Joint Commission: Accreditation, Health Care, Certification (JCAHO) sentinel event reporting programs. Draft voluntary medication error-reporting form was circulated to all health care professionals for their feedback, and suggestions on utilisation of the vMERF were considered and incorporated as appropriate.
The final vMERF includes following elements:
- Medication Error’s date and time
- Patient’s age and gender
- Description of the medication error
- At which stage the medication error has occurred
- Whether the medication error has reached the patient, and if reached, did the error warranted for any intervention
- Personnel who identified and reported the medication error
- Place where the medication error has occurred
- NCC MERP’s medication error outcome category
- Contributing factors
- Details of the medication involved
- Optional details of the reporter.
2.2. Electronic and Telephonic Reporting System
To make reporting the ME simplified, an intranet system was developed by the clinical pharmacists and incorporated into the hospital information system (HIS) portal, wherein any HCP can log into their HIS portal account and can report a ME. The electronic form contains same elements as that the of paper form to report an error. The committee members can view and respond to these errors spontaneously. For instant reporting and immediate attention, a telephonic system was created. The speed dial telephone numbers of the expert panel and the clinical pharmacists were widely publicised in the hospital. Any HCP who wishes to use this system can dial the assigned numbers throughout the day and report an error. Use of either system could compromise the reporter’s anonymity and this was a limitation.
2.3. Implementation of the System
To create awareness on the importance of reporting the MEs and encourage spontaneous reporting by health care professionals, “Dear Health Care Professional” letters were drafted by the clinical pharmacists under the guidance of expert panel and distributed amongst all HCPs. Patient safety classes were conducted to the HCPs by the clinical pharmacists wherein the expert committee’s feedback letters were provided to the health care professionals. Further, these classes were liaised with the interdisciplinary members of the hospital and aimed at emphasising the need for reporting of medication errors without any fear.
2.4. Collection of Reports
Drop boxes were made available at all nursing stations for easy access to paper vMERFs along with the facility to drop the filled forms. These forms were collected every 24 h. When a medication error report was dropped in one of the drop boxes, a clinical pharmacist would personally collect the form and follow up with the concerned HCPs to collect supportive information on what factors were involved in this error, how best the error could have been avoided, and any missing information in the report. Wherever possible, appropriate data pertaining to the reported errors was collected from the various data sources such as patient’s case file, interaction with other health care professionals, patients and their care providers etc. The reporters themselves were asked to mention the contributing factors involved in the error, such as work load, distractions, newer staff, ambiguous communications etc.; thus, the reported contributing factors of the respective medication errors were rather subjective but not quantified, which was another limitation.
2.5. Assessment of the Reports
During the analysis, a clinical pharmacists presented each of the reported error to the panel regularly twice a week to understand how an error has occurred. Their pattern, contributing factors, and root causes were closely monitored. NCC MERP’s Medication Error Index, which classifies an error according to the severity of the outcome was adopted to assess the severity of the reported errors. According to the index, the outcomes were classified from Category A to Category I (Figure 1). Based on these findings, medication error preventive strategies were developed accordingly, e.g., high risk medication tags to caution the end user, separation of look-alike and sound-alike drugs and train pharmacists etc. Data analysis was done using descriptive statistics.
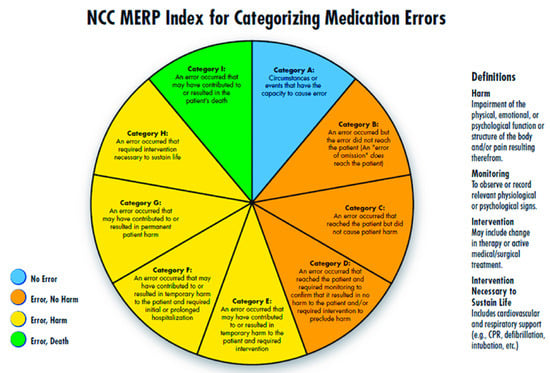
Figure 1.
National Coordination Centre Medication Error Reporting Programme (NCC MERP) Index for Categorising Medication Errors.
Wherever the patient identifiers were available, they were followed-up until discharge to ensure that the medication error has been addressed appropriately. Identifier details of the patient and other information related to the error were kept confidential.
3. Results
A total of 1310 errors were reported; the highest number of medication errors occurred during the medication administration stage [501 (38.2%)], followed by prescription errors [243 (18.5%)], dispensing errors [223 (17%)], procurement related errors [223 (17.02%)], and lastly, transcription errors [120 (1.29%)] were reported.
The majority of the medication errors were reported from the department of emergency medicine [458 (35%)] followed by Surgery and Obstetrics and Gynaecology specialities [304 (23%)]. Nonchalant operational conditions have resulted in the fewer medication errors [256 (19%)] in general medicine wards. During the study period, clinical pharmacists reported the highest medication errors [674 (51%)], followed by nursing staff and doctors [409 (31%)] and [227 (17%)] respectively.
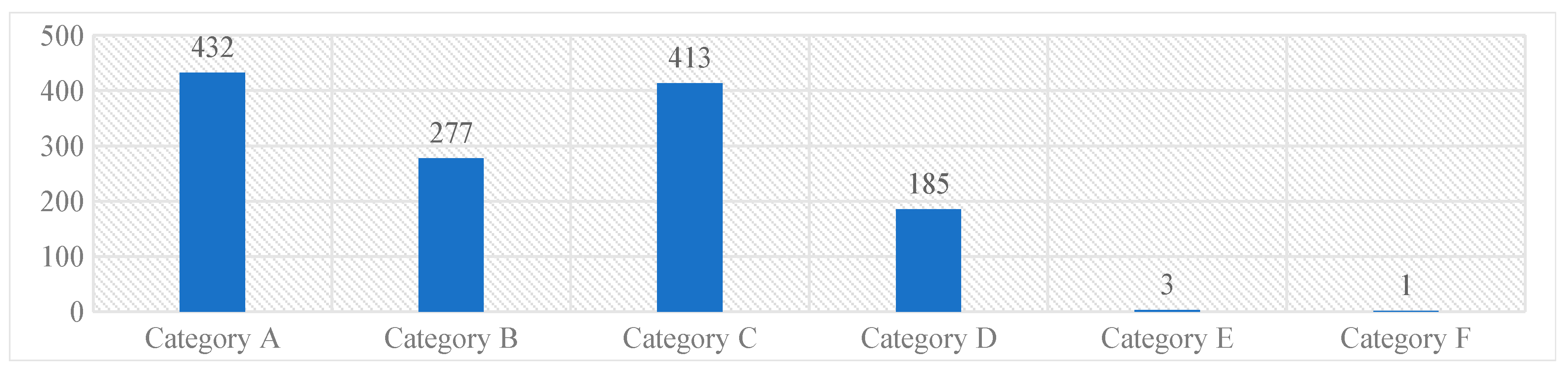
The majority of the reported medication errors belonged to Category A [432, (33%)], where the circumstances were potential to cause an error, while the least belonged to the Category F [1 (0.07%)]. None of the reported medication errors had outcomes corresponding to the Category G and above. These results were represented in Graph 1.
Graph 1. NCC MERP’s categorizing index of the reported medication errors.
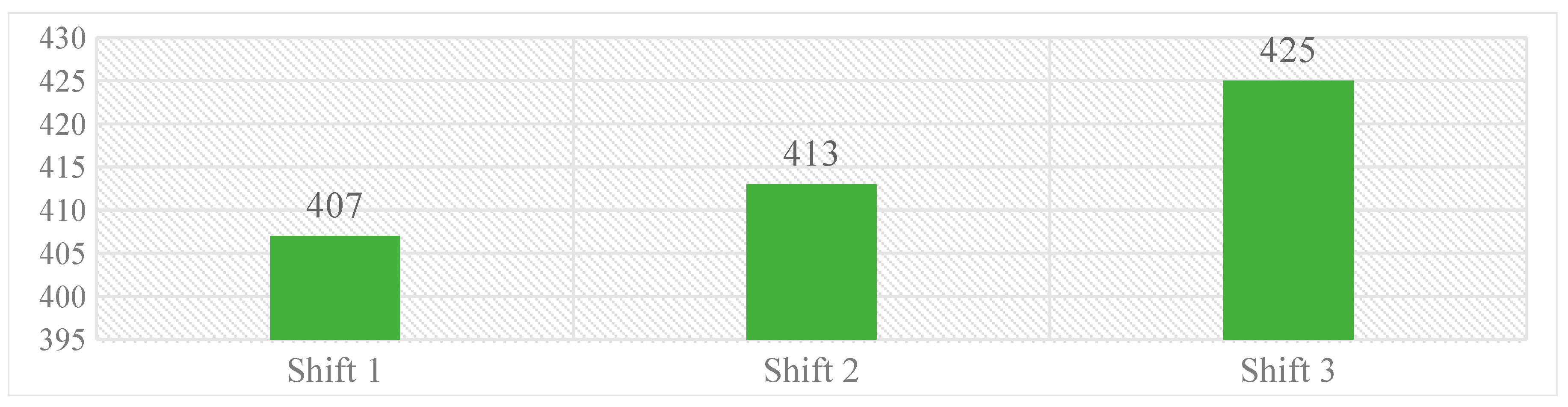
Work shifts were classified as work shift 1 (08:00–14:00 h), 2 (14:00 to 20:00 h), and 3 (20:00 h–06:00 h), wherein, majority of medication errors were reported during third work-shift [425 (32%)], while the fewer were reported during first work-shift [407 (31%)]. Over all, the difference in medication errors reported during three work shifts was limited. Graph 2 represents the distribution of errors over different work-shifts.
Graph 2. Distribution of medication errors occurred over different work-shifts.
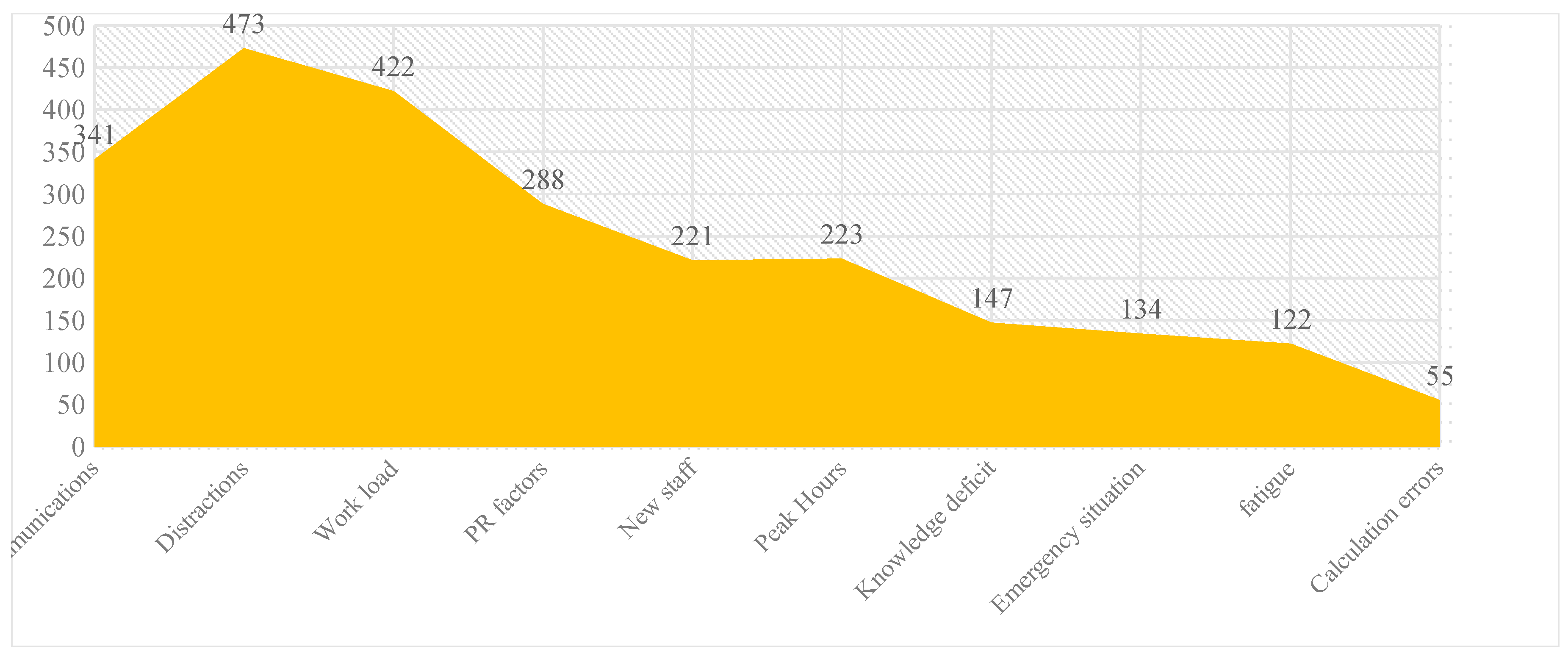
Our study reveals that the highest medication errors occurred due to distractions during work (473), while the lowest were due to calculation errors (55). Graph 3 reflects the distribution of various contribution factors causing the medication errors.
Graph 3. Distribution of contributing factors of medication errors.
Of the medicines implicated in the medication error reports, antipyretics/analgesics [255 (19.46%)] were the main ones, followed by antibiotics [206 (15.72)]. The detailed distribution of the medicines implicated are tabulated in Table 2.

Table 2.
Classification of medicines involved in reported medication errors.
4. Discussion
The incidence of MEs at the study site was 6.4%. While, in India, the incidence of medication errors ranged between 3% and 33.4% [27,28]. Such a broad range in the ME rates may be attributable to the disparities in the methodologies adopted in identifying and reporting the errors and its associated variables such as definition of medication error adapted, type of the medication error-reporting implemented, nature of the hospital, i.e., workforce and work load, occupancy ratio, etc., duration of the study and type of the patients followed [29,30,31,32].
Enforcing a mandatory reporting system may lead to coercing the covert of reporting medication errors within the HCPs.
On the other side, voluntary reporting may not be absolute as HCP’s intellectual domains on overall awareness on MEs and what, how and where to report, and the ability to handle the repercussions of reported errors without resorting to the blame culture influences the overall reporting trends. Hence, many errors remain under-reported. In Barach et al.’s study, the under-reporting of MEs is estimated to vary from 50% to 60% annually and reporting is usually done in an informal manner. Although, errors are usually discussed verbally at morbidity or mortality review meetings without formal written reports [33]. Implementation of medication safety practices and improvement of patient safety domains remains a mirage san written reports.
Across the globe, the incidence of medication errors is almost certainly low and not often reliable due to under-reporting tendencies. Reasons for underreporting may span across apprehension of reprisal, lawsuit concerns, time constraints, the uncertainty of which errors to report, concerns of implicating other HCPs, peer pressures, and lack of feedback on reported MEs [34,35]. Further, the information on incidences of medication errors that are reported in selected clinical specialisations with focus on certain medications were well documented; however, the overall data pertaining to the incidence of medication errors occurring in a complete hospital setup like ours is limited. Despite detecting and reporting the medication errors, quantifying these errors’ rates is not an easy task. Anonymous reporting may encourage the reporters to report an error, but addressing patient safety under the anonymous tag may get compromised by repetitive and preventable errors [36]. Further, enormous diversity in the taxonomy used in reporting medication errors, and expressing the error rates using various denominators such as patient days, for 100 prescription, for 100 doses, etc., is an arduous task in harmonising the overall error rate [37].
4.1. Overall Trend in Voluntary Reporting of Medication Errors
All medication errors were reported using a paper-based system as it may relatively protect the anonymity of the reporter compared to electronic and telephonic systems. The doctors, nursing staff, and clinical pharmacists reported 227 (17.3%), 409 (31%) and 674 (51.4%) errors, respectively, in five selected different study units. Of these errors, doctors reported medication administration errors [72 (31%)], majorly followed by prescription errors [49 (22%)], and dispensing errors [31 (14%)] and so on. Amongst the errors reported by the nursing staff, the majority were procurement errors [89 (22%)] followed by transcription and administration errors [70 (17%)] each. Lastly, clinical pharmacists reported administration errors [211 [n = 674 (31%)] followed by dispensing 138 (21%) and prescription errors [129 (19%)]. In our study setting, clinical pharmacists reported the majority of the medication errors compared to other health care professionals for two reasons. Firstly, the amount of time CPs spend with their patients in the wards and secondly, the reporting system is driven by CPs themselves.
Further, this reporting system is the first of its kind in our setting, and factors such as availability of the time for HCPs for reporting an error, their workload, fear of legal liability, misconceptions on job threat, adverse economic effects, concerns regarding protecting the professional status at the workplace, and other negative consequences of reporting might have played an important role on why there were fewer errors reported by the doctors and nursing staff [38]. In order to understand and overcome these factors, one-to-one and group discussions were conducted periodically by the expert committee, reinforcing the non-punitive and anonymous principles of the reporting system.
Another reason for lower medication error-reporting rate compared to the earlier mentioned studies, which was we identified during casual discussions with health care professionals, was “preference” of doctors and nursing staff, to keep medication errors in-house. Wherein, believing loyalty to colleagues, and “whistle-blowing” were both unsupportive and unethical. Similar opinions were quoted in age old Webb et al.’s study [38]. Another reason for lower reporting of Medication errors is that, they often instigate remorse in the implicated personnel. Adapting a mandatory reporting system will drastically affect the overall reporting trends and having a voluntary reporting system is by no means an antidote for the complacency attitude amongst HCPs. We presume, the extent of such barriers may influence the reporting rates across the developing countries.
One of the strategies to reduce the impact of such preferences on overall reporting rate is utilisation of clinical phatrmacists in medication error-reporting systems. They can support the HCPs by maintaining the confidentiality of errors, obtaining detailed medication history from the patients and their care takers and provide efficient medication reconciliation, and thus reducing the medication errors [39,40]. The utilisation of the clinical pharmacists in the current study set-up has been the reason for reporting the majority of the errors and, thus, reducing associated implications.
4.2. Outcomes of the Reported Errors
Most of the medication errors were reported to have the potential to cause error [432, (32.9%)]. Followed by errors that occurred and did not reach the patient [413 (31.5%)]. These errors were intercepted by the clinical pharmacist during the ward rounds and intensive follow-up of the patients and prevented them from manifesting into actual errors. Category A errors are the errors which have the potential to cause an error, and Category B errors are actual errors which were identified and prevented before reaching the patient. Distractions while filling the prescriptions and drawing the medicine into syringes, illegible hand writing in prescriptions etc., have potential to cause an error if not rectified immediately. Further, when the Category A errors are left unattended, they may evolve into Category B level errors, that are just might reach the patient if not stopped by the HCPs. From there onwards, the progress of the category of these errors depends on to what level the patient was affected. The range of such errors is confined between Category C and I, wherein category C medication errors are those that have reached the patient but did not affect safety, and category I errors might have caused patient death.
Employing double checks on medication usage at every step by those whoever are responsible in handling the drugs can reduce medication errors effectively than any sheer luck interceptions. As clinical pharmacists are bestowed with more patient bed-side time, they intercepted the potential errors and reported. Implementation of the Computerised Physician Order Entry systems (CPOE) could reduce the progression of errors from Category A to Category I. However, use of such systems is modest in the resource restricted countries. Towards inculcation of patient safety culture amongst all HCPs, we encouraged them to report voluntarily whatever they deemed an error irrespective of their severity. This has encouraged the HCPs to report Medication Errors. Giving incentives to the HCPs could improve the overall rate of ME reporting, but it may not be viable model in the developing countries.
4.3. Contributing Factors
Distractions were one of the major contributing factors for reported medication errors followed by increased workload and incoherent communications. Being a 1800-bed hospital with a constant 80% occupancy rate and housing hundreds of HCPs, the contributing factors such as distractions and work load are likely to be more. A total of 2560 contributing factors were associated with reported 1310 medication errors (a medication error can have multiple contributing factors).
Further, we have identified that the majority of the medication errors were reported during night shifts 425, [(32.4%) n = 1310] (20:00 to 08:00 h) followed by 407, [(31.06%) n = 1310] during the first shift (08:00 to 14:00 h). The reporting rate of medication errors during night seems to be lesser compared to other shifts. HCPs regularly sacrifice their breaks to provide patient care, therefore during the night shifts bright lights, rest breaks, power naps, and exercises, can be used to provide relief from the symptoms of fatigue. To reduce distractions and improve interpersonal communications, CPOE systems can be employed and thus improving patient safety [41].
5. Conclusions
As a considerable number of MEs were preventable, it is important to develop and implement strategies that are unique to the work environment to try to overcome such MEs in the future. Intense monitoring of patients for potential MEs and its early detection and reporting by all HCPs may result in improved therapeutic outcomes and decreased unnecessary healthcare related mortality.
Medication errors, such as the ones reported here, not only harm the patients, but also wreck the confidence of HCPs involved. Reporting systems should be designed to encourage and instil the confidence in reporting the errors openly by HCPs. Our study laid foundations for successful establishment of medication error-reporting and monitoring programme in a tertiary care teaching hospital and is contributing significantly towards the patient safety involving all stakeholders. With greater emphasis on a non-punitive course of corrective measures and anonymity of the HCPs on handling medication errors, improves the overall reporting in terms of quality and quantity. Fault finding attitudes should be replaced with open, inclusive, and common improvement agendas involving all HCPs alike for fortifying patient safety. Clinical pharmacists, working in the close liaison with other HCPs can provide excellent support in promoting and sustaining patient safety.
This study is indeed a pilot study to understand the implications of Medication Errors at our study site. The three years have given us deeper insights into how HCPs react to the varying levels of MEs. We promptly discussed the MEs with concerned HCPs on the ethical basis to alert them regarding such happenings. HCPs intervened on time to minimise the impact of these errors. This learning experience has helped all HCPs to be cautious of such happenings in the future, and they emphasised the importance of prevention of errors to the associated team members. In this way, this study has contributed to the early steps towards patient safety. Overall, this is a first of its kind study in our region and we are trying to balance the interests of all stakeholders, most importantly, the patient’s.
Author Contributions
Conceptualization, M.R. and G.P.; Methodology, M.R.; Software, C.S.H.; Validation, M.R., G.P. and C.S.H.; Formal Analysis, C.S.H.; Investigation, C.S.H.; Resources, C.S.H.; Data Curation, C.S.H. and M.R.; Writing-Original Draft Preparation, S.H.C.; Writing-Review & Editing, C.S.H., M.R. and G.P.; Supervision, M.R. and G.P.; Project Administration, M.R. and G.P.; Funding Acquisition, C.S.H.
Funding
This research was funded by Ministry of Science and Technology’s Department of Science and Technology’s INSPIRE fellowship component, Govt. of India—grant number IF140214.
Acknowledgments
Authors thank the Principal, JSS College of Pharmacy and the Staff at JSS Hospital for their constant support endowed for carrying out this project. We extend our earnest gratitude to the JSS Academy of Higher Education and Research and INSPIRE Fellowship Component—Department of Science and Technology, Ministry of Science and Technology, Government of India for supporting this project. We thank Dr Ann Vazhayil Kuruvilla for proof reading the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Riddick, F.A. The Code of Medical Ethics of the American Medical Association. Ochsner J. 2003, 5, 6–10. [Google Scholar] [PubMed]
- Inman, T. Foundation for a New Theory and Practice of Medicine; John Churchill: London, UK, 1860. [Google Scholar]
- Stelfox, H.T.; Palmisani, S.; Scurlock, C.; Orav, E.J.; Bates, D.W. The “To Err is Human” report and the patient safety literature. Qual. Saf. Health Care 2006, 15, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Leape, L.L.; Berwick, D.M. Five years after To Err Is Human: What have we learned? JAMA 2005, 293, 4–90. [Google Scholar] [CrossRef] [PubMed]
- Leape, L.L. Scope of problem and history of patient safety. Obstet. Gynecol. Clin. N. Am. 2008, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, L.; Berwick, D.; Conway, J.; Combes, J.; Hatlie, M.; Leape, L.; Reason, J.; Schyve, P.; Vincent, C.; Walton, M. What Exactly Is Patient Safety? Advances in Patient Safety: New Directions and Alternative Approaches; Henriksen, K., Battles, J.B., Keyes, M.A., Grady, M.L., Eds.; Volume 1 Assessment; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. [Google Scholar]
- Furrow, B.R. Iatrogenesis and medical error: The case for medical malpractice litigation. Law Med. Health Care 1981, 9, 1–4. [Google Scholar] [CrossRef]
- National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). Definition. Available online: http://www.nccmerp.org/about-medication-errors (accessed on 3 September 2013).
- Institute for Safe Medication Practices. Definition of a Close Call (Near Miss). Available online: https://www.ismp.org/newsletters/acutecare/articles/20090924.asp (accessed on 3 January 2016).
- Illich, I. Clinical damage, medical monopoly, the expropriation of health: Three dimensions of iatrogenic tort. J. Med. Ethics 1975, 1, 78–80. [Google Scholar] [CrossRef]
- Schimmel, E.M. The Hazards of Hospitalization. Ann. Intern. Med. 1964, 60, 100–110. [Google Scholar] [CrossRef]
- Kalantri, S.P. Medical errors and ethics. Indian J. Anaesth. 2003, 47, 174–175. [Google Scholar]
- Barker, K.N.; Flynn, E.A.; Pepper, G.A.; Bates, D.W.; Mikeal, R.L. Medication errors observed in 36 health care facilities. Arch. Intern. Med. 2002, 162, 1897–1903. [Google Scholar] [CrossRef]
- Bates, D.W.; Cullen, D.; Laird, N.; Petersen, L.A.; Small, S.D.; Servi, D.; Laffel, G.; Sweitzer, B.J.; Shea, B.F.; Hallisey, R.; et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA 1995, 274, 29–34. [Google Scholar] [CrossRef]
- Barker, K.; Allan, E. Research on drug-use system errors. Am. J. Health Syst. Pharm. 1995, 52, 400–403. [Google Scholar] [PubMed]
- Lesar, T.; Briceland, L.; Delcoure, K.; Parmalee, J.C.; Masta-Gornic, V.; Pohl, H. Medication prescribing errors in a teaching hospital. JAMA 1990, 263, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.; Thornton, J.; Kecskes, S.; Perry, M.; Feldman, S. Medication errors in neonatal and paediatric intensive-care units. Lancet 1989, 3, 374–379. [Google Scholar] [CrossRef]
- Donaldson, L. An organisation with a memory. Clin. Med. 2002, 2, 452–457. [Google Scholar] [CrossRef]
- James, J.T. A new, evidence-based estimate of patient harms associated with hospital care. J. Patient Saf. 2013, 9, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, C.P.; Parry, G.J.; Bones, C.B.; Hackbarth, A.D.; Goldmann, D.A.; Sharek, P.J. Temporal trends in rates of patient harm resulting from medical care. N. Engl. J. Med. 2010, 363, 2124–2134. [Google Scholar] [CrossRef] [PubMed]
- Inch, J.; Watson, M.C.; Anakwe-Umeh, S. Patient versus healthcare professional spontaneous adverse drug reaction reporting: A systematic review. Drug Saf. 2012, 35, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, F.; Abbasi, M.N.; Abrishami, R.; Sistanizad, M. Transcription errors observed in a teaching hospital. Arch. Iran. Med. 2009, 12, 173–175. [Google Scholar] [PubMed]
- Indicators Data [Internet]. Available online: http://data.worldbank.org/indicator/SH.MED.PHYS.ZS (accessed on 7 January 2016).
- Gaur, S.; Sinha, A.; Srivastava, B. Medication errors in medicine wards in a tertiary care teaching hospital of a hill state in India. Asian J. Pharm. Life Sci. 2012, 2, 56–63. [Google Scholar]
- Kumar, K.S.; Venkateswarlu, K.; Ramesh, A. A study of medication administration errors in a tertiary care hospital. Indian J. Pharm. Pract. 2011, 4, 37–42. [Google Scholar]
- Twerski, R.J. Medical Errors: Focusing more on what and why less on Who. J. Oncol. Pract. JOP 2007, 3, 66–70. [Google Scholar]
- Alsulami, Z.; Conroy, S.; Choonara, I. Medication errors in the Middle East countries: A systematic review of the literature. Eur. J. Clin. Pharmacol. 2013, 69, 995–1008. [Google Scholar] [CrossRef]
- Morimoto, T.; Sakuma, M.; Matsui, K.; Kuramoto, N.; Toshiro, J.; Murakami, J.; Fukui, T.; Saito, M.; Hiraide, A.; Bates, D.W. Incidence of Adverse Drug Events and Medication Incidents in Japan: The JADE Study. J. Gen. Intern. Med. 2011, 26, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M. The epidemiology of medication errors: How many, how serious? Br. J. Clin. Pharmacol. 2009, 67, 621–623. [Google Scholar] [CrossRef]
- Harding, L.; Petrick, T. Nursing student medication errors: A retrospective review. J. Nurs. Educ. 2008, 47, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Ciarkowski, S.L.; Stalburg, C.M. Medication Safety in Obstetrics and Gynecology. Clin. Obstet. Gynecol. 2010, 58, 482–499. [Google Scholar] [CrossRef]
- WHO. Reporting and Learning Systems for Medication Errors: The Role of Pharmacovgilence Centers. Available online: http://apps.who.int/medicinedocs/documents/s21625en/s21625en.pdf (accessed on 7 January 2016).
- Barach, P.; Small, S.D. Reporting and preventing medical mishaps: Lessons from non-medical near miss reporting systems. Br. Med. J. 2000, 320, 759–763. [Google Scholar] [CrossRef]
- Dean Franklin, B.; Vincent, C.; Schachter, M.; Barber, N. The incidence of prescribing errors in hospital inpatients: An overview of the research methods. Drug Saf. 2005, 28, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Travaglia, J.F.; Westbrook, M.T.; Braithwaite, J. Implementation of a patient safety error management system as viewed by doctors, nurses and allied health professionals. Health (London) 2009, 13, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Triandis, H. Values, attitudes and interpersonal behaviour. Nebr. Symp. Motiv. 1980, 27, 195–259. [Google Scholar] [PubMed]
- Rothschild, J.M.; Churchill, W.; Erickson, A.; Munz, K.; Schuur, J.D.; Salzberg, C.A.; Lewinski, D.; Shane, R.; Aazami, R.; Patka, J.; et al. Medication errors recovered by emergency department pharmacists. Ann. Emerg. Med. 2010, 55, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Webb, W.B.; Agnew, J.H.W. Sleep efficiency for sleep-wake cycles of varying lengths. Psychophysiology 1975, 12, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Bayazidi, S.K. Medication Error Reporting Rate and Its Barriers and Facilitators among Nurses. J. Caring Sci. 2012, 1, 231–236. [Google Scholar] [PubMed]
- Mrayyan, M.T.; Shishani, K.; Al-Faouri, I. Rate, causes and reporting of medication errors in Jordan: Nurses’ perspectives. J. Nurs. Manag. 2007, 15, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, S.H.; Ramesh, M. Towards patient safety: Assessment of medication errors in the intensive care unit in a developing country’s tertiary care teaching hospital. Eur. J. Hosp. Pharm. 2017, 24, 361–365. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).