The Implementation of Pharmacy Competence Teaching in Estonia
Abstract
:1. Introduction
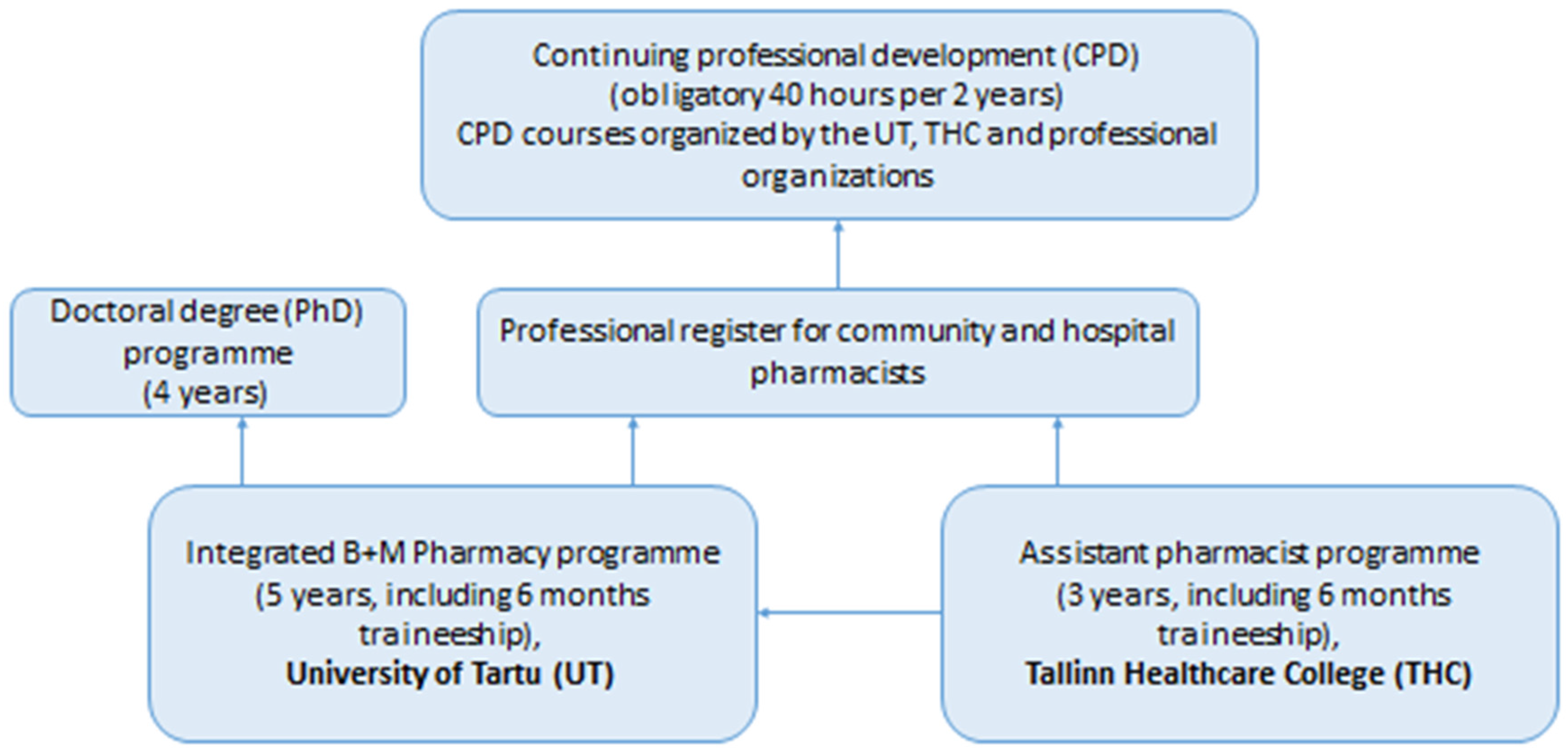
1.1. Pharmacy Education and Training in Estonia
1.2. How to Achieve Competency Based Pharmacy Education in Estonia?
- -
- to construct a curriculum mapping matrix;
- -
- to assess the pharmacy curriculum outcome based competency level, and
- -
- to identify the pharmacy curriculum gaps and evaluate the expediency of the EPCF as a curriculum mapping tool.
2. Materials and Methods
2.1. PHAR-QA Framework
2.2. Study Design and Sample
- -
- intended (existing) curriculum mapping: matrix construction of 50 EPCF competences and curriculum elements;
- -
- evaluation of expected competency level at the graduation of first-degree curriculum (MSc degree); and
- -
- identification of curriculum gaps.
2.3. Data Analysis
- -
- 1: Theoretical education; LEVEL OF INDEPENDENCE 1;
- -
- 2: Theoretical education and practical skills; LEVEL OF INDEPENDENCE 2;
- -
- 3: Ability to use theoretical knowledge and practical skills in learning situations; LEVEL OF INDEPENDENCE 3;
- -
- 4: Ability to use theoretical knowledge and practical skills in authentic learning situations (classroom and during pharmacy internship); LEVEL OF INDEPENDENCE 4;
- -
- 5: Ability to use theoretical knowledge and practical skills in practice; LEVEL OF INDEPENDENCE.
3. Results
3.1. Construction of Curriculum Mapping Matrix
3.2. Competencies of Entry-Level Pharmacists
3.3. Curriculum Gaps
- -
- increase collaboration between different healthcare professions; more integrated training and common courses with medical students;
- -
- support more patient care competencies linking the pharmacy programme with practice on different fields of pharmacy starting already from the first years of studies;
- -
- support the reflection and practical implementation of theoretical knowledge, more broad use of problem-based learning in different subjects;
- -
- introduce business and entrepreneurship subjects to the pharmacy programme as from 2020, pharmacy ownership will be limited only by pharmacy profession in Estonia;
- -
- develop more detailed requirements for pharmacy students and for internship supervisors at community and hospital pharmacies as pharmacy internship plays very important role in implementing of professional competencies.
3.4. Usability of EPCF in Curriculum Mapping and Competency Level Evaluation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Pharmacy (300 ECTS) | ||||
| 1. Compulsory subjects (224 ECTS) | ||||
| 1.1 First course compulsory subjects (51 ECTS) | ||||
| ARFA.02.099 | Analytical Chemistry | 12 | ECTS | |
| ARAN.01.034 | Anatomy | 5 | ECTS | |
| ARFS.01.023 | Biophysics | 4 | ECTS | |
| LOKT.00.004 | General and Inorganic Chemistry | 6 | ECTS | |
| ARFS.01.063 | Human Physiology | 10 | ECTS | |
| ARFA.01.071 | Introduction and History of Pharmacy | 3 | ECTS | |
| FLKE.05.009 | Latin in Pharmacy | 3 | ECTS | |
| ARMB.00.001 | Medical Microbiology | 6 | ECTS | |
| ARFA.02.098 | Pharmaceutical Terminology | 3 | ECTS | |
| ARFA.01.072 | Pharmacognosy I | 3 | ECTS | |
| MTMS.01.085 | Statistical Analysis | 4 | ECTS | |
| 1.2 Second course compulsory subjects (52 ECTS) | ||||
| ARFA.02.099 | Analytical Chemistry | 12 | ECTS | |
| ARFA.02.108 | Bioethics | 4 | ECTS | |
| ARBK.01.042 | Bioorganic Chemistry | 5 | ECTS | |
| ARMB.01.053 | Clinical Microbiology | 2 | ECTS | |
| ARMP.01.030 | Genetics | 3 | ECTS | |
| ARFS.01.063 | Human Physiology | 10 | ECTS | |
| ARBK.01.043 | Medical Biochemistry | 5 | ECTS | |
| ARMP.03.031 | Pathophysiology | 6 | ECTS | |
| ARFA.02.109 | Pharmaceutical Chemistry I | 9 | ECTS | |
| ARFA.02.103 | Pharmaceutical Excipients | 5 | ECTS | |
| ARPO.00.020 | Primary Care Medicine | 5 | ECTS | |
| 1.3 Third course compulsory subjects (52 ECTS) | ||||
| ARMP.03.035 | Biotechnology | 3 | ECTS | |
| ARSK.03.017 | Introduction to the Laboratory Medicine | 2 | ECTS | |
| ARFA.02.112 | Pharmaceutical Chemistry II | 8 | ECTS | |
| ARFA.02.113 | Pharmaceutical Technology | 22 | ECTS | |
| ARFA.01.105 | Pharmacognosy II | 7 | ECTS | |
| ARFA.01.077 | Pharmacognosy III | 4 | ECTS | |
| ARFR.02.051 | Pharmacology | 11 | ECTS | |
| 1.4 Fourth course compulsory subjects (51 ECTS) | ||||
| ARFA.02.144 | Biopharmaceutics | 5 | ECTS | |
| ARFR.03.018 | Clinical Pharmacology | 2 | ECTS | |
| ARFA.01.104 | Clinical Pharmacy | 3 | ECTS | |
| ARFR.02.034 | Drug Toxicology | 3 | ECTS | |
| ARKI.01.004 | First Aid | 3 | ECTS | |
| ARMP.02.016 | Immunology | 2 | ECTS | |
| ARFA.02.123 | Metabolism of Active Substances | 3 | ECTS | |
| ARFA.02.113 | Pharmaceutical Technology | 22 | ECTS | |
| ARFA.01.059 | Pharmacoepidemiology and Pharmacoeconomics | 3 | ECTS | |
| ARFR.02.052 | Pharmacotherapy | 5 | ECTS | |
| ARFA.02.122 | Physical Pharmacy | 6 | ECTS | |
| ARFA.01.082 | Social Pharmacy and Drug Safety | 11 | ECTS | |
| 1.5 Fifth course (18 ECTS) | ||||
| ARFA.02.040 | Designing of Research Work | 1 | ECTS | |
| ARFA.02.044 | Pharmaceutical Commodities | 4 | ECTS | |
| ARFA.00.057 | Research Work | 6 | ECTS | |
| or | ARFS.01.076 | Diploma Work | 6 | ECTS |
| or | ARBK.01.048 | Research Work | 6 | ECTS |
| or | ARMB.01.059 | Diploma Work | 6 | ECTS |
| or | ARFR.02.061 | Diploma Work | 6 | ECTS |
| or | ARAN.00.040 | Research Project | 6 | ECTS |
| seminar of research (7 ECTS) or | ||||
| ARFA.02.117 | Research Seminar on Pharmaceutical Chemistry | 7 | ECTS | |
| ARFA.02.116 | Research Seminar on Pharmaceutical Technology | 7 | ECTS | |
| ARFA.01.078 | Research Seminar on Pharmacognosy | 7 | ECTS | |
| ARFA.01.080 | Research Seminar on Social Pharmacy | 7 | ECTS | |
| ARFA.01.096 | Research Seminars on Clinical Pharmacy | 7 | ECTS | |
| ARFA.02.118 | Research Seminars on History of Pharmacy | 7 | ECTS | |
| ARFS.01.075 | Research Workshop of Physiology | 7 | ECTS | |
| ARAN.02.027 | Seminar on Histology | 7 | ECTS | |
| ARBK.01.047 | Seminars of Research in Medical Biochemistry | 7 | ECTS | |
| ARMB.01.060 | Seminars of Research in Medical Microbiology | 7 | ECTS | |
| ARFR.02.060 | Seminars of Research in Pharmacology | 7 | ECTS | |
| 2. Elective courses (22 ECTS) | ||||
| 2.1 First course elective subjects (6 ECTS) | ||||
| FLKE.03.151 | Estonian for Students of Medicine, on the Basis of Russian, Level B1 >B2 | 3 | ECTS | |
| ARFA.02.080 | Exercises and Problems in Pharmaceutical Analysis | 1 | ECTS | |
| ARLA.01.034 | Foetal and Neonatal Physiology and Behaviour, Breastfeeding | 2 | ECTS | |
| ARSK.00.009 | Gerontology. Introduction (web-based) | 3 | ECTS | |
| FLFI.00.002 | Introduction to Philosophy | 2 | ECTS | |
| ARFA.01.106 | Pharmacist in pharmacy system | 2 | ECTS | |
| ARTH.04.023 | Prevention of HIV/AIDS and HIV Positive Patient | 2 | ECTS | |
| ARTH.04.022 | Principles of Sexuality Education | 3 | ECTS | |
| ARFA.02.145 | Seminars in Pharmaceutical Sciences I | 1 | ECTS | |
| ARTH.04.024 | Smoking and Health | 1 | ECTS | |
| LOKT.01.017 | Solutions | 2 | ECTS | |
| ARSI.01.015 | Spectacles, Contact Lenses and Refractive Surgery | 1 | ECTS | |
| ARLA.01.033 | Why and to Which Factors We are Allergic? | 2 | ECTS | |
| 2.2 Second course elective subjects (5 ECTS) | ||||
| ARBK.01.021 | Basic Knowledge for the Research in Medical Biochemistry (I) | 2 | ECTS | |
| ARMP.03.019 | Basic Research in Pathophysiology I | 6 | ECTS | |
| ARMP.03.020 | Basic Research in Pathophysiology II | 6 | ECTS | |
| ARFA.02.080 | Exercises and Problems in Pharmaceutical Analysis | 1 | ECTS | |
| ARLA.01.034 | Foetal and Neonatal Physiology and Behaviour, Breastfeeding | 2 | ECTS | |
| ARSK.00.009 | Gerontology. Introduction (web-based) | 3 | ECTS | |
| ARFA.02.138 | Interdisciplinary Seminars in Pharmacy | 2 | ECTS | |
| ARFS.01.005 | Neurophysiology of Pain | 2 | ECTS | |
| ARFA.01.106 | Pharmacist in pharmacy system | 2 | ECTS | |
| ARTH.04.023 | Prevention of HIV/AIDS and HIV Positive Patient | 2 | ECTS | |
| ARTH.04.022 | Principles of Sexuality Education | 3 | ECTS | |
| ARFA.02.019 | Propaedeutical Training | 3 | ECTS | |
| ARFA.02.081 | Radiopharmaceuticals | 1 | ECTS | |
| ARFA.02.145 | Seminars in Pharmaceutical Sciences I | 1 | ECTS | |
| ARTH.04.024 | Smoking and Health | 1 | ECTS | |
| ARSI.01.015 | Spectacles, Contact Lenses and Refractive Surgery | 1 | ECTS | |
| ARFA.02.128 | Student Research in Pharmacy I | 3 | ECTS | |
| ARFA.02.135 | Student Research in Pharmacy II | 6 | ECTS | |
| ARLA.01.033 | Why and to Which Factors We are Allergic? | 2 | ECTS | |
| 2.3 Third course elective subjects (5 ECTS) | ||||
| ARBK.01.035 | Basic Knowledge for the Research in Medical Biochemistry (II) | 5 | ECTS | |
| ARMP.03.019 | Basic Research in Pathophysiology I | 6 | ECTS | |
| ARMP.03.020 | Basic Research in Pathophysiology II | 6 | ECTS | |
| ARFA.02.124 | Chemistry of Vitamins | 2 | ECTS | |
| ARTH.01.080 | Environmental and Occupational Toxicology | 3 | ECTS | |
| ARFA.02.080 | Exercises and Problems in Pharmaceutical Analysis | 1 | ECTS | |
| ARLA.01.034 | Foetal and Neonatal Physiology and Behaviour, Breastfeeding | 2 | ECTS | |
| ARSK.00.009 | Gerontology. Introduction (web-based) | 3 | ECTS | |
| ARFA.02.126 | Granulation and Tableting Technology | 2 | ECTS | |
| ARTH.04.018 | Health Promotion | 2 | ECTS | |
| ARFA.02.138 | Interdisciplinary Seminars in Pharmacy | 2 | ECTS | |
| ARFS.01.005 | Neurophysiology of Pain | 2 | ECTS | |
| ARMP.03.025 | Pathophysiology of Stress | 1.5 | ECTS | |
| ARFA.01.106 | Pharmacist in pharmacy system | 2 | ECTS | |
| ARTH.04.023 | Prevention of HIV/AIDS and HIV Positive Patient | 2 | ECTS | |
| ARTH.04.022 | Principles of Sexuality Education | 3 | ECTS | |
| ARFA.02.081 | Radiopharmaceuticals | 1 | ECTS | |
| ARFA.02.145 | Seminars in Pharmaceutical Sciences I | 1 | ECTS | |
| ARTH.04.024 | Smoking and Health | 1 | ECTS | |
| ARSI.01.015 | Spectacles, Contact Lenses and Refractive Surgery | 1 | ECTS | |
| ARFA.02.128 | Student Research in Pharmacy I | 3 | ECTS | |
| ARFA.02.135 | Student Research in Pharmacy II | 6 | ECTS | |
| ARFA.02.130 | Student Research in Pharmacy III | 6 | ECTS | |
| ARFA.02.133 | Student Research in Pharmacy IV | 6 | ECTS | |
| ARFA.02.015 | Synthesis of Active Substances | 2 | ECTS | |
| ARFA.02.140 | Vibrational Spectroscopy- Different Applications in Pharmaceutical Drug Development | 1 | ECTS | |
| ARTH.01.014 | Water and Health | 2 | ECTS | |
| ARLA.01.033 | Why and to Which Factors We are Allergic? | 2 | ECTS | |
| ARTH.01.040 | Work Stress and Health | 2 | ECTS | |
| 2.4 Fourth course elective subjects (6 ECTS) | ||||
| AROT.00.030 | Andragogy and Higher Education | 3 | ECTS | |
| AROT.00.033 | Applications of Developmental Psychology in Nursing | 3 | ECTS | |
| ARMP.03.019 | Basic Research in Pathophysiology I | 6 | ECTS | |
| ARMP.03.020 | Basic Research in Pathophysiology II | 6 | ECTS | |
| ARTH.01.080 | Environmental and Occupational Toxicology | 3 | ECTS | |
| ARFA.02.080 | Exercises and Problems in Pharmaceutical Analysis | 1 | ECTS | |
| ARLA.01.034 | Foetal and Neonatal Physiology and Behaviour, Breastfeeding | 2 | ECTS | |
| ARSK.00.009 | Gerontology. Introduction (web-based) | 3 | ECTS | |
| ARFA.02.126 | Granulation and Tableting Technology | 2 | ECTS | |
| AROT.00.036 | Health Care System and Policy for Nursing Managers | 3 | ECTS | |
| ARTH.04.018 | Health Promotion | 2 | ECTS | |
| ARFA.02.138 | Interdisciplinary Seminars in Pharmacy | 2 | ECTS | |
| ARAR.00.001 | Medical Law | 2 | ECTS | |
| ARFA.02.134 | Minimally Invasive Medical Technology | 2 | ECTS | |
| ARFS.01.005 | Neurophysiology of Pain | 2 | ECTS | |
| ARFA.02.132 | Pharmaceutical Film and Nano-coatings | 2 | ECTS | |
| ARFA.02.141 | Pharmaceutical nanotechnology | 4 | ECTS | |
| ARFA.01.106 | Pharmacist in pharmacy system | 2 | ECTS | |
| AROT.01.013 | Planning and Designing of Developmental Projects in Nursing | 3 | ECTS | |
| ARTH.04.023 | Prevention of HIV/AIDS and HIV Positive Patient | 2 | ECTS | |
| ARTH.04.022 | Principles of Sexuality Education | 3 | ECTS | |
| ARFA.02.081 | Radiopharmaceuticals | 1 | ECTS | |
| ARFA.02.145 | Seminars in Pharmaceutical Sciences I | 1 | ECTS | |
| ARTH.04.024 | Smoking and Health | 1 | ECTS | |
| ARFA.01.060 | Social Pharmacy in the Professional Literature and Internet | 2 | ECTS | |
| ARSI.01.015 | Spectacles, Contact Lenses and Refractive Surgery | 1 | ECTS | |
| ARFA.02.135 | Student Research in Pharmacy II | 6 | ECTS | |
| ARFA.02.130 | Student Research in Pharmacy III | 6 | ECTS | |
| ARFA.02.133 | Student Research in Pharmacy IV | 6 | ECTS | |
| ARFA.02.137 | Student Research in Pharmacy V | 6 | ECTS | |
| ARFA.02.015 | Synthesis of Active Substances | 2 | ECTS | |
| ARSK.00.027 | Vaccine and Immunization Education | 3 | ECTS | |
| ARFA.02.140 | Vibrational Spectroscopy- Different Applications in Pharmaceutical Drug Development | 1 | ECTS | |
| ARTH.01.014 | Water and Health | 2 | ECTS | |
| ARLA.01.033 | Why and to Which Factors We are Allergic? | 2 | ECTS | |
| ARTH.01.040 | Work Stress and Health | 2 | ECTS | |
| 3. Pharmacy Practice (37 ECTS) | ||||
| ARFA.02.071 | Pharmacy Practice | 37 | ECTS | |
| 4. Final Exam (5 ECTS) | ||||
| ARFA.02.060 | Graduation Examination | 5 | ECTS | |
| 5. Optional courses (12 ECTS) | ||||
Appendix B
| Domains and compenetnes | |
|---|---|
| Domain 1: Personal competences—learning and knowledge | |
| 1 | Ability to identify learning needs and to learn independently (including continuous professional development, CPD). |
| 2 | Ability to apply logic to problem solving. |
| 3 | Ability to critically appraise relevant knowledge and to summarise the key points. |
| 4 | Ability to evaluate scientific data in line with current scientific and technological knowledge. |
| 5 | Ability to apply preclinical and clinical evidence-based medical science to pharmaceutical practice. |
| 6 | Ability to apply current knowledge of relevant legislation and codes of pharmacy practice. |
| Domain 2: Personal competences—values | |
| 7 | A professional approach to tasks and human relations. |
| 8 | Ability to maintain confidentiality. |
| 9 | Ability to take full responsibility for patient care. |
| 10 | Ability to inspire the confidence of others in one’s actions and advise. |
| 11 | Knowledge of appropriate legislation and of ethics. |
| Domain 3: Personal competences—communication and organisational skills | |
| 12 | Ability to communicate effectively—both oral and written—in the locally relevant language. |
| 13 | Ability to effectively use information technology. |
| 14 | Ability to work effectively as part of a team. |
| 15 | Ability to implement general legal requirements that impact upon the practice of pharmacy (e.g., health and safety legislation, employment law). |
| 16 | Ability to contribute to the training of staff. |
| 17 | Ability to manage risk and quality of service issues. |
| 18 | Ability to identify the need for new services. |
| 19 | Ability to understand a business environment and develop entrepreneurship. |
| Domain 4: Personal competences—research and industrial pharmacy | |
| 20 | Knowledge of design, synthesis, isolation, characterisation and biological evaluation of active substances. |
| 21 | Knowledge of good manufacturing practice and of good laboratory practice. |
| 22 | Knowledge of European directives on qualified persons. |
| 23 | Knowledge of drug registration, licensing and marketing. |
| 24 | Knowledge of the importance of research in pharmaceutical development and practice. |
| Domain 5: Patient care competences—patient consultation and assessment | |
| 25 | Ability to interpret basic medical laboratory tests. |
| 26 | Ability to perform appropriate diagnostic tests e.g., measurement of blood pressure or blood sugar. |
| 27 | Ability to recognise when referral to another member of the healthcare team is needed. |
| Domain 6: Patient care competences—need for drug treatment | |
| 28 | Ability to retrieve and interpret information on the patient’s clinical background. |
| 29 | Ability to compile and interpret a comprehensive drug history for an individual patient. |
| 30 | Ability to identify non-adherence to medicine therapy and make an appropriate intervention. |
| 31 | Ability to advise to physicians on the appropriateness of prescribed medicines and—in some cases—to prescribe medication. |
| Domain 7: Patient care competences—drug interactions | |
| 32 | Ability to identify and prioritise drug-drug interactions and advise appropriate changes to medication. |
| 33 | Ability to identify and prioritise drug-patient interactions, including those that prevent or require the use of a specific drug, based on pharmaco-genetics, and advise on appropriate changes to medication. |
| 34 | Ability to identify and prioritise drug-disease interactions (e.g., NSAIDs in heart failure) and advise on appropriate changes to medication. |
| Domain 8: Patient care competences—drug dose and formulation | |
| 35 | Knowledge of the bio-pharmaceutical, pharmacodynamic and pharmacokinetic activity of a substance in the body. |
| 36 | Ability to recommend interchangeability of drugs based on in-depth understanding and knowledge of bioequivalence, bio-similarity and therapeutic equivalence of drugs. |
| 37 | Ability to undertake a critical evaluation of a prescription ensuring that it is clinically appropriate and legally valid. |
| 38 | Knowledge of the supply chain of medicines thus ensuring timely flow of quality drug products to the patient. |
| 39 | Ability to manufacture medicinal products that are not commercially available. |
| Domain 9: Patient care competences—patient education | |
| 40 | Ability to promote public health in collaboration with other professionals within the healthcare system. |
| 41 | Ability to provide appropriate lifestyle advice to improve patient outcomes (e.g., advice on smoking, obesity, etc.). |
| 42 | Ability to use pharmaceutical knowledge and provide evidence-based advice on public health issues involving medicines. |
| Domain 10: Patient care competences—provision of information and service | |
| 43 | Ability to use effective consultations to identify the patient’s need for information. |
| 44 | Ability to provide accurate and appropriate information on prescription medicines. |
| 45 | Ability to provide evidence-based support for patients in selection and use of non- prescription medicines. |
| Domain 11: Patient care competences—monitoring of drug therapy | |
| 46 | Ability to identify and prioritise problems in the management of medicines in a timely and effective manner and so ensure patient safety. |
| 47 | Ability to monitor and report Adverse Drug Events and Adverse Drug Reactions (ADEs and ADRs) to all concerned, in a timely manner, and in accordance with current regulatory guidelines on Good Pharmacovigilance Practices (GVPs). |
| 48 | Ability to undertake a critical evaluation of prescribed medicines to confirm that current clinical guidelines are appropriately applied. |
| 49 | Ability to monitor patient care outcomes to optimise treatment in collaboration with the prescriber. |
| 50 | Ability to contribute to the cost effectiveness of treatment by collection and analysis of data on medicines use. |
References
- Medical Faculty, University of Tartu, Estonia. Available online: http://meditsiiniteadused.ut.ee/en (accessed on 19 December 2016).
- Sepp, K.; Lenbaum, K.; Laius, O.; Viidalepp, A.; Volmer, D. The “Quality guidelines for community pharmacy services”—A tool for harminozation of community pharmacy practice in Estonia. Int. J. Clin. Pharm. 2015, 10 (Suppl. 1), 33. [Google Scholar]
- Tallinn Healthcare College. Available online: http://www.ttk.ee/en/assistant-pharmacist-0 (accessed on 27 December 2016).
- Medicinal Products Act. Available online: https://www.riigiteataja.ee/en/eli/ee/525112013005/consolide/current (accessed on 27 December 2016).
- Directive 2005/36/EC of the European Parliament and of the Council on the recognition of professional qualifications. Off. J. Eur. Union 2005, L255, 22–142. Available online: http://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32005L0036 (accessed on 28 March 2017).
- Directive 2013/55/EU of the European Parliament and of the Council amending Directive 2005/36/EC on the recognition of professional qualifications. Off. J. Eur. Union 2013, 354, 132–170. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32013L0055 (accessed on 28 March 2017).
- Medicine Study Programme Group. Self-Evaluation Report; Department of Pharmacy, University of Tartu: Tartu, Estonia, 2014. [Google Scholar]
- Atkinson, J.; Rombaut, B. The 2011 PHARMINE report on pharmacy and pharmacy education in the European Union. Pharm. Pract. (Internet) 2011, 9, 169–187. [Google Scholar] [CrossRef]
- Quality Guidelines for Community Pharmacy Services. Available online: http://www.ravimiamet.ee/sites/default/files/documents/publications/apteegiteenuse_kvaliteedijuhis_2016/apteegiteenuse_kvaliteedijuhis_2016.html#/0 (accessed on 10 December 2016).
- Good Hospital Pharmacy Practice. Available online: http://media.voog.com/0000/0003/1027/files/16.02.18%20-%20Eesti%20haiglafarmaatsia%20head%20tavad_Kodulehele.pdf (accessed on 10 December 2016).
- CoDEG (Competency Development and Evaluation Group). General Level Practice. Available online: http://www.codeg.org/frameworks/general-level-practice (accessed on 16 December 2016).
- Accreditation Council for Pharmacy Education. ACPE Open Forum: Accreditation Standards 2016. Available online: http://www.aacp.org/governance/SIGS/assessment/Assessment%20Docs/AACP%202014%20Interim%20Meeting%20%20presentation%20-%20ACPE%20Standards%202016.pdf (accessed on 16 December 2016).
- Stupans, I.; McAllister, S.; Clifford, R.; Hughes, J.; Krass, I.; March, G.; Owen, S.; Woulfeg, J. Nationwide collaborative development of learning outcomes and exemplar standards for Australian pharmacy programmes. Int. J. Pharm. Pract. 2015, 23, 283–291. [Google Scholar] [CrossRef] [PubMed]
- FIP/WHO. International Pharmaceutical Federation—World Health Organisation: Standards for Quality of Pharmacy Services. Available online: https://www.fip.org (accessed on 16 December 2016).
- Antoniou, S.; Webb, D.G.; Mcrobbie, D.; Davies, J.G.; Bates, I.P. A controlled study of the general level framework: Results of the South of England Competency Study. Pharm. Educ. 2005, 5, 201–207. [Google Scholar] [CrossRef]
- Coombes, I.; Avent, M.; Cardiff, L.; Bettenay, K.; Coombes, J.; Whitfield, K.; Stokes, J.; Davies, G.; Bates, I. Improvement in pharmacist’s performance facilitated by an adapted competency-based general level framework. J. Pharm. Pract. Res. 2010, 40, 111–118. [Google Scholar] [CrossRef]
- AFPC. Educational Outcomes for First Professional Degree Programs in Pharmacy (Entry to Practice Pharmacy Programs) in Canada. Association of Faculties of Pharmacy of Canada (AFPC): Vancouver, 2010. Available online: www.afpc.info/sites/default/files/AFPC%20Educational%20Outcomes.pdf (accessed on 16 December 16 2016).
- GPhC. Standards for the Initial Education and Training of Pharmacists. General Pharmaceutical Council (GPhC), 2011. Available online: www.pharmacyregulation. org/sites/default/files/GPhC_Future_Pharmacists.pdf (accessed on 16 December 2016).
- NCSF. National Competency Standards Framework for Pharmacists in Australia, Pharmaceutical Society for Australia (PSA), 2010. Available online: www.psa.org.au/ downloads/standards/competency-standards-complete.pdf (accessed on 16 December 2016).
- Atkinson, J.; Rombaut, B.; Sánchez Pozo, A.; Rekkas, D.; Veski, P.; Hirvonen, J.; Bozic, B.; Skowron, A.; Mircioiu, C.; Marcincal, A.; Wilson, K. Systems for Quality Assurance in Pharmacy Education and Training in the European Union. Pharmacy 2014, 2, 17–26. [Google Scholar] [CrossRef]
- Atkinson, J.; De Paepe, K.; Sánchez Pozo, A.; Rekkas, D.; Volmer, D.; Hirvonen, J.; Bozic, B.; Skowron, A.; Mircioiu, C.; Marcincal, A.; et al. The PHAR-QA Project: Competency Framework for Pharmacy Practice—First Steps, the Results of the European Network Delphi Round 1. Pharmacy 2015, 3, 307–329. [Google Scholar] [CrossRef]
- Atkinson, J.; De Paepe, K.; Sánchez Pozo, A.; Rekkas, D.; Volmer, D.; Hirvonen, J.; Bozic, B.; Skowron, A.; Mircioiu, C.; Marcincal, A.; et al. The Second Round of the PHAR-QA Survey of Competences for Pharmacy Practice. Pharmacy 2016, 4, 27. [Google Scholar] [CrossRef]
- Pharmacist Competency Framework & Domain-specific Frame of Reference for the Netherlands. Available online: https://www.knmp.nl/downloads/pharmacist-competency-frameworkandDSFR-Netherlands.pdf (accessed on 19 February 2017).
- Quality Assessment of the Study Programme Group Medicine at the University of Tartu, Estonia. Available online: http://ekka.archimedes.ee/wp-content/uploads/TU-meditsiin-assessment-report.pdf (accessed on 15 December 2016).
- Occupational Qualification Standards for Pharmacists and Assistant Pharmacists were Approved in Estonia. Available online: http://www.kutsekoda.ee/et/kutseregister/kutsestandardid/10622084 (accessed on 18 December 2016).
- Good Practice of Learning. Available online: http://www.tyye.ee/en/good-practice-learning (accessed on 27 December 2016).
- Good Practice of Teaching. Available online: http://www.ut.ee/en/search/google/good%20practice%20of%20teaching (accessed on 27 December 2016).
| Subject Area | Proportion of Subject Areas in Pharmacy Programmes EU Main % | Proportion of Subject Areas in the Pharmacy Programme (UT), Estonia % |
|---|---|---|
| Chemical sciences | 26 | 21 |
| Physics, mathematics | 6 | 4 |
| Biological sciences | 11 | 2 |
| Pharmaceutical technology | 16 | 21 |
| Medical sciences | 28 | 39 |
| Law and social sciences | 7 | 10 |
| Generic subjects, traineeship | 6 | 3 |
| Study Year | Drug Analysis | Pharmaceutical Technology | Medical Sciences and Patient Care Subjects | Social Sciences |
|---|---|---|---|---|
| First | Inorganic and analytical chemistry | - | Anatomy, physiology, medical microbiology | Latin, pharmaceutical terminology, history of pharmacy |
| Second | Analytical, bioorganic and pharmaceutical chemistry | Pharmaceutical excipients | Physiology, pathophysiology, clinical microbiology, medical biochemistry, genetics, primary care medicine | Bioethics |
| Third | Pharmacognosy, pharmaceutical chemistry | Pharmaceutical technology, biotechnology | Laboratory medicine, pharmacology | - |
| Fourth | Metabolism of active substances | Physical pharmacy, biopharmacy | Immunology, pharmacotherapy, first aid, drug toxicology, clinical pharmacology, clinical pharmacy, | Pharmaco- epidemiology and pharmaco-economics,social pharmacy, and drug safety |
| Competence Domains | Specific Subjects | Subject Area/-s | Full Programme | Mean Competency Level *, Academia | Mean Competency Level *, Other Pharmacy Stakeholders |
|---|---|---|---|---|---|
| Personal competences | |||||
| 1. Learning and knowledge | NA ** | Medical and social sciences, pharmacy internship | Yes | 4.0 | 3.4 |
| 2. Values | NA | Social sciences, pharmacy internship | Yes | 4.4 | 3.7 |
| 3. Communication and organizational skills | NA | Social sciences, pharmacy internship | Yes | 3.8 | 3.1 |
| 4. Research and industrial pharmacy | Yes | Pharmaceutical technology, drug analysis | Yes, only one competence | 4.0 | 2.5 |
| Personal competence domains 1–4 mean value | 4.1 | 3.2 | |||
| Patient care competences | |||||
| 5. Patient consultation and assessment | Yes | Medical sciences | NA | 3.3 | 3.2 |
| 6. Need for drug treatment | Yes | Medical sciences | NA | 3.0 | 3.0 |
| 7. Drug interactions | Yes | Medical sciences, pharmaceutical technology | NA | 3.0 | 3.2 |
| 8. Drug dose and formulation | Yes | Pharmaceutical technology, medical sciences | NA | 4.2 | 3.0 |
| 9. Patient education | Yes | No | NA | 4.0 | 3.7 |
| 10. Provision of information and service | Yes | Medical and social sciences, pharmaceutical technology, pharmacy internship | NA | 4.7 | 3.5 |
| 11. Monitoring of drug therapy | Yes | Medical and social sciences, pharmaceutical technology, pharmacy internship | NA | 3.2 | 2.4 |
| Patient care competence domains 5–11 mean value | 3.6 | 3.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volmer, D.; Sepp, K.; Veski, P.; Raal, A. The Implementation of Pharmacy Competence Teaching in Estonia. Pharmacy 2017, 5, 18. https://doi.org/10.3390/pharmacy5020018
Volmer D, Sepp K, Veski P, Raal A. The Implementation of Pharmacy Competence Teaching in Estonia. Pharmacy. 2017; 5(2):18. https://doi.org/10.3390/pharmacy5020018
Chicago/Turabian StyleVolmer, Daisy, Kristiina Sepp, Peep Veski, and Ain Raal. 2017. "The Implementation of Pharmacy Competence Teaching in Estonia" Pharmacy 5, no. 2: 18. https://doi.org/10.3390/pharmacy5020018
APA StyleVolmer, D., Sepp, K., Veski, P., & Raal, A. (2017). The Implementation of Pharmacy Competence Teaching in Estonia. Pharmacy, 5(2), 18. https://doi.org/10.3390/pharmacy5020018







