Medical Cannabis Use and Healthcare Utilization Among Patients with Chronic Pain: A Causal Inference Analysis Using TMLE
Abstract
1. Introduction
- (1)
- Estimate the Average Treatment Effect of medical cannabis use on patient-reported quality of life (QoL).
- (2)
- Estimate the Average Treatment Effect of medical cannabis use on healthcare utilization, including urgent care, emergency department, and hospital visits.
- (3)
- Estimate the Relative Risk of healthcare utilization outcomes among medical cannabis-exposed verses cannabis-naïve patients.
2. Materials and Methods
2.1. Study Design and Population
2.2. Outcomes and Exposure Definition
2.3. Statistical Analysis
3. Results
3.1. Average Treatment Effects of Medical Cannabis
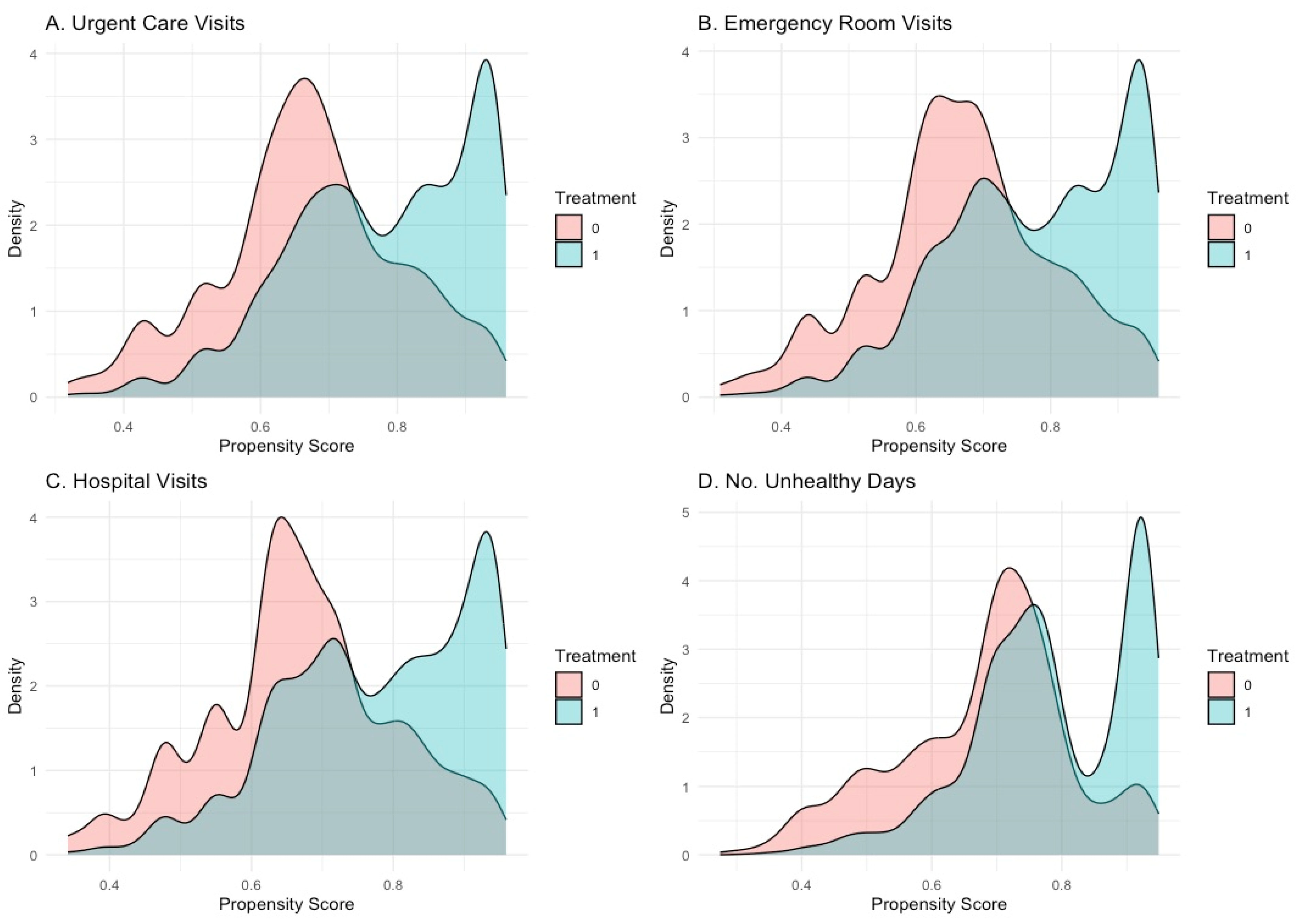
3.2. TMLE Model Performance and Covariate Balance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P. Chronic Pain Among Adults—United States, 2019–2021. Morb. Mortal. Wkly. Rep. 2023, 72, 379–385. [Google Scholar] [CrossRef]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of Chronic Pain among Adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Ramsden, C.E. The Silent Epidemic of Chronic Pain in Older Adults. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 284–290. [Google Scholar] [CrossRef]
- Marupuru, S.; Axon, D.R. Association of Multimorbidity on Healthcare Expenditures Among Older United States Adults With Pain. J. Aging Health 2021, 33, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Stubhaug, A.; Hansen, J.L.; Hallberg, S.; Gustavsson, A.; Eggen, A.E.; Nielsen, C.S. The Costs of Chronic Pain—Long-Term Estimates. Eur. J. Pain 2024, 28, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Foley, H.E.; Knight, J.C.; Ploughman, M.; Asghari, S.; Audas, R. Association of Chronic Pain with Comorbidities and Health Care Utilization: A Retrospective Cohort Study Using Health Administrative Data. Pain 2021, 162, 2737–2749. [Google Scholar] [CrossRef]
- Kirsch, E.P.; Yang, L.Z.; Lee, H.-J.; Parente, B.; Lad, S.P. Healthcare Resource Utilization for Chronic Low Back Pain among High-Utilizers. Spine J. 2024, 24, 601–616. [Google Scholar] [CrossRef]
- Park, J.-H.; Prochnow, T.; Smith, M.L.; Kim, S.J. Health Disparities of Healthcare Utilization and Opioid Use Disorders Among Chronic Pain Patients: Examination of a Representative National Inpatient Sample of US Hospitals from 2016–2020. Int. J. Ment. Health Addict. 2025, 1–16. [Google Scholar] [CrossRef]
- Choudry, E.; Rofé, K.L.; Konnyu, K.; Marshall, B.D.L.; Shireman, T.I.; Merlin, J.S.; Trivedi, A.N.; Schmidt, C.; Bhondoekhan, F.; Moyo, P. Treatment Patterns and Population Characteristics of Nonpharmacological Management of Chronic Pain in the United States’ Medicare Population: A Scoping Review. Innov. Aging 2023, 7, igad085. [Google Scholar] [CrossRef]
- Staats, P.S.; Patel, K.; Gillen, E.M.; Bello, T.; Epple, T.; Bilder, S.M.; Wisor, D. Healthcare Utilization and Costs Associated with New-Onset Pain in a Medicare Population. Pain Physician 2022, 25, E1415–E1422. [Google Scholar] [PubMed]
- Mu, J.; Furlan, A.D.; Lam, W.Y.; Hsu, M.Y.; Ning, Z.; Lao, L. Acupuncture for Chronic Nonspecific Low Back Pain. Cochrane Database Syst. Rev. 2020, 12, CD013814. [Google Scholar] [CrossRef]
- Ebadi, S.; Henschke, N.; Forogh, B.; Nakhostin Ansari, N.; van Tulder, M.W.; Babaei-Ghazani, A.; Fallah, E. Therapeutic Ultrasound for Chronic Low Back Pain. Cochrane Database Syst. Rev. 2020, 7, CD009169. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Delanerolle, G.; Cavalini, H.; Deng, C.; Yang, X.; Boyd, A.; Fernandez, T.; Phiri, P.; Bhaskar, A.; Shi, J.Q. A Systematic Review and Network Meta-Analysis of Pharmaceutical Interventions Used to Manage Chronic Pain. Sci. Rep. 2024, 14, 1621. [Google Scholar] [CrossRef]
- Shetty, A.; Delanerolle, G.; Deng, C.; Thillainathan, A.; Cavalini, H.; Yang, X.; Bouchareb, Y.; Boyd, A.; Phiri, P.; Shi, J.Q.; et al. A Systematic Review and Bayesian Meta-Analysis of Medical Devices Used in Chronic Pain Management. Sci. Rep. 2024, 14, 13549. [Google Scholar] [CrossRef]
- Schmidt, H.; Pilat, C. Effects of Meditation on Pain Intensity, Physical Function, Quality of Life and Depression in Adults with Low Back Pain—A Systematic Review with Meta-Analysis. Complement. Ther. Med. 2023, 72, 102924. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Wang, J.; Zhang, X.; Cai, H.; Peng, F. Efficacy of Pilates on Pain, Functional Disorders and Quality of Life in Patients with Chronic Low Back Pain: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2850. [Google Scholar] [CrossRef]
- Martinez-Calderon, J.; García-Muñoz, C.; Rufo-Barbero, C.; Matias-Soto, J.; Cano-García, F.J. Acceptance and Commitment Therapy for Chronic Pain: An Overview of Systematic Reviews with Meta-Analysis of Randomized Clinical Trials. J. Pain 2024, 25, 595–617. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Fernández-de-las-Peñas, C.; Navarro-Santana, M.J.; Plaza-Manzano, G. Efficacy of Dry Needling and Acupuncture in Patients with Fibromyalgia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 9904. [Google Scholar] [CrossRef]
- Bartels, S.L.; Pelika, A.; Taygar, A.S.; Wicksell, R.K. Digital Approaches to Chronic Pain: A Brief Meta-Review of eHealth Interventions—Current Evidence and Future Directions. Curr. Opin. Psychol. 2025, 62, 101976. [Google Scholar] [CrossRef]
- Deshpande, V.; Simpson, E.; Caballero, J.; Haddad, C.; Smith, J.; Gardner, V. Cost-Utility of Lumbar Interbody Fusion Surgery: A Systematic Review. Spine J. 2025, 25, 1117–1138. [Google Scholar] [CrossRef] [PubMed]
- Davin, S.; Lapin, B.; Udeh, B.; Rispinto, S.; Thompson, N.R.; Honomichl, R.; Machado, A.; Katzan, I.L. Health Care Utilization Patterns of Patients Enrolled in an Interdisciplinary Program for Back Pain. N. Am. Spine Soc. J. 2025, 21, 100584. [Google Scholar] [CrossRef] [PubMed]
- Doucette, M.L.; Casarett, D.J.; Hemraj, D.; Grelotti, D.J.; Macfarlan, D.L.; Fisher, E. Towards a Comprehensive Understanding of Medical Conditions among Medical Cannabis Patients in a Large Database: A Descriptive Analysis. Popul. Med. 2024, 6, 26. [Google Scholar] [CrossRef]
- Doucette, M.L.; Hemraj, D.; Fisher, E.; Macfarlan, D.L. Measuring the Impact of Medical Cannabis Law Adoption on Employer-Sponsored Health Insurance Costs: A Difference-in-Difference Analysis, 2003-2022. Appl. Health Econ. Health Policy 2024, 23, 119–129. [Google Scholar] [CrossRef]
- Fairman, B.J. Trends in Registered Medical Marijuana Participation across 13 US States and District of Columbia. Drug Alcohol Depend. 2016, 159, 72–79. [Google Scholar] [CrossRef]
- Boehnke, K.F.; Gangopadhyay, S.; Clauw, D.J.; Haffajee, R.L. Qualifying Conditions of Medical Cannabis License Holders in the United States. Health Aff. 2019, 38, 295–302. [Google Scholar] [CrossRef]
- Mahabir, V.K.; Merchant, J.J.; Smith, C.; Garibaldi, A. Medical Cannabis Use in the United States: A Retrospective Database Study. J. Cannabis Res. 2020, 2, 32. [Google Scholar] [CrossRef]
- Mahabir, V.K.; Smith, C.S.; Vannabouathong, C.; Merchant, J.J.; Garibaldi, A.L. Comparing Medical Cannabis Use in 5 US States: A Retrospective Database Study. J. Cannabis Res. 2021, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Dean, O.; Haffajee, R.; Hosanagar, A. US Trends in Registration for Medical Cannabis and Reasons for Use from 2016–2020: An Observational Study. Ann. Intern. Med. 2022, 175, 945–951. [Google Scholar] [CrossRef]
- Doucette, M.L.; Hemraj, D.; Bruce, D.; Fisher, E.; Macfarlan, D.L. Medical Cannabis Patients Under the Age of 21 in the United States: Description of Demographics and Conditions from a Large Patient Database, 2019-2023. Adolesc. Heal. Med. Ther. 2024, 15, 63–72. [Google Scholar] [CrossRef]
- Boehnke, K.F.; Sinclair, R.; Gordon, F.; Hosanagar, A.; Roehler, D.R.; Smith, T.; Hoots, B. Trends in U.S. Medical Cannabis Registrations, Authorizing Clinicians, and Reasons for Use From 2020 to 2022. Ann. Intern. Med. 2024, 177, 458–466. [Google Scholar] [CrossRef]
- Ajrawat, P.; Yang, Y.; Wasilewski, E.; Leroux, T.; Ladha, K.S.; Bhatia, A.; Singh, M.; Thaker, S.; Kapoor, M.; Furlan, A.D.; et al. Medical Cannabis Use and Inflammatory Cytokines and Chemokines Among Adult Chronic Pain Patients. Cannabis Cannabinoid Res. 2024, 9, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, K.; Lasek, P.; Trąbka, N.; Binkowska, P.; Demidowicz, G.; Lasota, N.; Smerdzyński, M.; Łach, K.; Ściurka, K.; Panuciak, K. Medical Cannabis: Mechanisms of Action and Therapeutic Targets. J. Educ. Health Sport 2024, 58, 176–190. [Google Scholar] [CrossRef]
- Matos, C.; Pereira, A.T.; Dias, M.J.; Sousa, C.; Vinha, A.F.; Moutinho, C.; Carvalho, M. Cannabis for Chronic Pain: Mechanistic Insights and Therapeutic Challenges. Stresses 2025, 5, 7. [Google Scholar] [CrossRef]
- Mick, G.; Douek, P. Clinical Benefits and Safety of Medical Cannabis Products: A Narrative Review on Natural Extracts. Pain Ther. 2024, 13, 1063–1094. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.; D’Souza, R.S.; Karri, J.; Kalia, H.; Weisbein, J.; Kassa, B.J.; Hussain, N.; Chitneni, A.; Budwany, R.R.; Hagedorn, J.; et al. Medical Cannabis: A Review from the American Society of Pain and Neuroscience. J. Pain Res. 2023, 16, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017; p. 24625. ISBN 978-0-309-45304-2. [Google Scholar]
- Capano, A.; Weaver, R.; Burkman, E. Evaluation of the Effects of CBD Hemp Extract on Opioid Use and Quality of Life Indicators in Chronic Pain Patients: A Prospective Cohort Study. Postgrad. Med. 2020, 132, 56–61. [Google Scholar] [CrossRef]
- Safakish, R.; Ko, G.; Salimpour, V.; Hendin, B.; Sohanpal, I.; Loheswaran, G.; Yoon, S.Y.R. Medical Cannabis for the Management of Pain and Quality of Life in Chronic Pain Patients: A Prospective Observational Study. Pain Med. 2020, 21, 3073–3086. [Google Scholar] [CrossRef]
- Harris, M.; Erridge, S.; Ergisi, M.; Nimalan, D.; Kawka, M.; Salazar, O.; Ali, R.; Loupasaki, K.; Holvey, C.; Coomber, R.; et al. UK Medical Cannabis Registry: An Analysis of Clinical Outcomes of Medicinal Cannabis Therapy for Chronic Pain Conditions. Expert Rev. Clin. Pharmacol. 2022, 15, 473–485. [Google Scholar] [CrossRef]
- Kelley, M.D.; Obaid, M.; Miller, E.M.; Bowie, M.; Heeter, Z.S. Observational Analysis of the Influence of Medical Marijuana Use on Quality of Life in Patients. Med. Cannabis Cannabinoids 2024, 7, 44–50. [Google Scholar] [CrossRef]
- Wang, L.; Hong, P.J.; May, C.; Rehman, Y.; Oparin, Y.; Hong, C.J.; Hong, B.Y.; AminiLari, M.; Gallo, L.; Kaushal, A.; et al. Medical Cannabis or Cannabinoids for Chronic Non-Cancer and Cancer Related Pain: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. BMJ 2021, 374, n1034. [Google Scholar] [CrossRef] [PubMed]
- Bapir, L.; Erridge, S.; Nicholas, M.; Pillai, M.; Dalavaye, N.; Holvey, C.; Coomber, R.; Hoare, J.; Khan, S.; Weatherall, M.W.; et al. Comparing the Effects of Medical Cannabis for Chronic Pain Patients with and without Co-Morbid Anxiety: A Cohort Study. Expert Rev. Neurother. 2023, 23, 281–295. [Google Scholar] [CrossRef]
- Lucas, P.; Walsh, Z. Medical Cannabis Access, Use, and Substitution for Prescription Opioids and Other Substances: A Survey of Authorized Medical Cannabis Patients. Int. J. Drug Policy 2017, 42, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bradford, A.C.; Bradford, W.D. The Impact of Medical Cannabis Legalization on Prescription Medication Use and Costs under Medicare Part D. J. Law Econ. 2018, 61, 461–487. [Google Scholar] [CrossRef]
- Bradford, A.C.; Bradford, W.D. Medical Marijuana Laws May Be Associated With A Decline In The Number Of Prescriptions For Medicaid Enrollees. Health Aff. 2017, 36, 945–951. [Google Scholar] [CrossRef]
- Bradford, A.C.; Bradford, W.D. Medical Marijuana Laws Reduce Prescription Medication Use In Medicare Part D. Health Aff. 2016, 35, 1230–1236. [Google Scholar] [CrossRef]
- Jeddi, H.M.; Busse, J.W.; Sadeghirad, B.; Levine, M.; Zoratti, M.J.; Wang, L.; Noori, A.; Couban, R.J.; Tarride, J.-E. Cannabis for Medical Use versus Opioids for Chronic Non-Cancer Pain: A Systematic Review and Network Meta-Analysis of Randomised Clinical Trials. BMJ Open 2024, 14, e068182. [Google Scholar] [CrossRef]
- Benedict, G.; Sabbagh, A.; Conermann, T. Medical Cannabis Used as an Alternative Treatment for Chronic Pain Demonstrates Reduction in Chronic Opioid Use—A Prospective Study. Pain Physician 2022, 25, E113–E119. [Google Scholar]
- Nguyen, T.; Li, Y.; Greene, D.; Stancliff, S.; Quackenbush, N. Changes in Prescribed Opioid Dosages Among Patients Receiving Medical Cannabis for Chronic Pain, New York State, 2017-2019. JAMA Netw. Open 2023, 6, e2254573. [Google Scholar] [CrossRef]
- Takakuwa, K.M.; Sulak, D. A Survey on the Effect That Medical Cannabis Has on Prescription Opioid Medication Usage for the Treatment of Chronic Pain at Three Medical Cannabis Practice Sites. Cureus 2020, 12, e11848. [Google Scholar] [CrossRef]
- Takakuwa, K.M.; Hergenrather, J.Y.; Shofer, F.S.; Schears, R.M. The Impact of Medical Cannabis on Intermittent and Chronic Opioid Users with Back Pain: How Cannabis Diminished Prescription Opioid Usage. Cannabis Cannabinoid Res. 2020, 5, 263–270. [Google Scholar] [CrossRef]
- Tyree, G.A.; Sarkar, R.; Bellows, B.K.; Ellis, R.J.; Atkinson, J.H.; Marcotte, T.D.; Wallace, M.S.; Grant, I.; Shi, Y.; Murphy, J.D.; et al. A Cost-Effectiveness Model for Adjunctive Smoked Cannabis in the Treatment of Chronic Neuropathic Pain. Cannabis Cannabinoid Res. 2019, 4, 62–72. [Google Scholar] [CrossRef]
- Vannabouathong, C.; Zhu, M.; Chang, Y.; Bhandari, M. Can Medical Cannabis Therapies Be Cost-Effective in the Non-Surgical Management of Chronic Knee Pain? Clin. Med. Insights 2021, 14, 11795441211002492. [Google Scholar] [CrossRef]
- Doucette, M.L.; Macfarlan, D.L.; Kasabuski, M.; Chin, J.; Fisher, E. Impact of Medical Cannabis Treatment on Healthcare Utilization in Patients with Post-Traumatic Stress Disorder: A Retrospective Cohort Study. MedRxiv 2024. [Google Scholar] [CrossRef]
- Mielenz, T.; Jackson, E.; Currey, S.; DeVellis, R.; Callahan, L.F. Psychometric Properties of the Centers for Disease Control and Prevention Health-Related Quality of Life (CDC HRQOL) Items in Adults with Arthritis. Health Qual. Life Outcomes 2006, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.S.; Kobau, R.; Moriarty, D.G.; Zack, M.M.; Holt, J.; Donehoo, R.; Centers for Disease Control and Prevention (CDC). Health-Related Quality of Life Surveillance—United States, 1993–2002. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2005, 54, 1–35. [Google Scholar]
- Korff, M.V.; DeBar, L.L.; Krebs, E.E.; Kerns, R.D.; Deyo, R.A.; Keefe, F.J. Graded Chronic Pain Scale Revised: Mild, Bothersome, and High Impact Chronic Pain. Pain 2020, 161, 651–661. [Google Scholar] [CrossRef]
- van der Laan, M.J.; Rubin, D. Targeted Maximum Likelihood Learning. Int. J. Biostat. 2006, 2, 11. [Google Scholar] [CrossRef]
- Schuler, M.S.; Rose, S. Targeted Maximum Likelihood Estimation for Causal Inference in Observational Studies. Am. J. Epidemiol. 2017, 185, 65–73. [Google Scholar] [CrossRef]
- Smith, M.J.; Phillips, R.V.; Luque-Fernandez, M.A.; Maringe, C. Application of Targeted Maximum Likelihood Estimation in Public Health and Epidemiological Studies: A Systematic Review. Ann. Epidemiol. 2023, 86, 34–48.e28. [Google Scholar] [CrossRef]
- Luque-Fernandez, M.A.; Schomaker, M.; Rachet, B.; Schnitzer, M.E. Targeted Maximum Likelihood Estimation for a Binary Treatment: A Tutorial. Stat. Med. 2018, 37, 2530–2546. [Google Scholar] [CrossRef] [PubMed]
- Polley, E.C.; Rose, S.; van der Laan, M.J. Super Learning. In Targeted Learning: Causal Inference for Observational and Experimental Data; van der Laan, M.J., Rose, S., Eds.; Springer: New York, NY, USA, 2011; pp. 43–66. ISBN 978-1-4419-9782-1. [Google Scholar]
- Phillips, R.V.; van der Laan, M.J.; Lee, H.; Gruber, S. Practical Considerations for Specifying a Super Learner. Int. J. Epidemiol. 2023, 52, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.5.1; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Gruber, S.; van der Laan, M. Tmle: An R Package for Targeted Maximum Likelihood Estimation. J. Stat. Softw. 2012, 51, 1–35. [Google Scholar] [CrossRef]
- Polley, E.; LeDell, E.; Kennedy, C.; Van Der Laan, M. SuperLearner: Super Learner Prediction, Version 2.0-29. 2025. Available online: https://CRAN.R-project.org/package=SuperLearner (accessed on 2 July 2025).
- Austin, P.C. Balance Diagnostics for Comparing the Distribution of Baseline Covariates between Treatment Groups in Propensity-Score Matched Samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.C.; Sirmans, E.T.; Stype, A. Medical Cannabis Laws Lower Individual Market Health Insurance Premiums. Int. J. Drug Policy 2023, 119, 104143. [Google Scholar] [CrossRef]
| Cannabis-Exposed * | Unexposed * | Total | Test Statistic | Missing | |||
| N (%) | 3943 (75.2%) | 1299 (24.8%) | 5242 | ||||
| Demographics | |||||||
| Sex | 0.597 | 25 | |||||
| Female | 1693 (43.1%) | 547 (42.3%) | 2240 (42.9%) | ||||
| Male | 2231 (56.9%) | 746 (57.7%) | 2977 (57.1%) | ||||
| Race/ethnicity | <0.001 | ||||||
| All other race/ethnicities | 903 (22.9%) | 398 (30.6%) | 1301 (24.8%) | ||||
| White non-Hispanic | 3040 (77.1%) | 901 (69.4%) | 3941 (75.2%) | ||||
| Current Smoking Status | 0.121 | --- | |||||
| No | 3149 (79.9%) | 1063 (81.8%) | 4212 (80.4%) | ||||
| Yes | 794 (20.1%) | 236 (18.2%) | 1030 (19.6%) | ||||
| No Alcoholic Drinks in the past 7 days? | <0.001 | --- | |||||
| No (Current drinker) | 2587 (65.6%) | 717 (55.2%) | 3304 (63.0%) | ||||
| Yes (Nondrinker) | 1356 (34.4%) | 582 (44.8%) | 1938 (37.0%) | ||||
| Age, Quintiles | <0.001 | --- | |||||
| Quintile 1 | 705 (17.9%) | 367 (28.3%) | 1072 (20.5%) | ||||
| Quintile 2 | 873 (22.1%) | 235 (18.1%) | 1108 (21.1%) | ||||
| Quintile 3 | 816 (20.7%) | 236 (18.2%) | 1052 (20.1%) | ||||
| Quintile 4 | 775 (19.7%) | 210 (16.2%) | 985 (18.8%) | ||||
| Quintile 5 | 774 (19.6%) | 251 (19.3%) | 1025 (19.6%) | ||||
| Health Status | |||||||
| Quality of Life, in number of unhealthy weeks | <0.001 | --- | |||||
| Two or less unhealthy weeks per month | 1890 (47.9%) | 309 (23.8%) | 2199 (41.9%) | ||||
| Three or more weeks unhealthy weeks per month | 2053 (52.1%) | 990 (76.2%) | 3043 (58.1%) | ||||
| Chronic Pain Severity | <0.001 | 91 | |||||
| Mild Chronic Pain | 1606 (41.4%) | 187 (14.7%) | 1793 (34.8%) | ||||
| Bothersome or High-impact Chronic Pain | 2270 (58.6%) | 1088 (85.3%) | 3358 (65.2%) | ||||
| Health Insurance? | 0.006 | --- | |||||
| No | 490 (12.4%) | 200 (15.4%) | 690 (13.2%) | ||||
| Yes | 3453 (87.6%) | 1099 (84.6%) | 4552 (86.8%) | ||||
| Healthcare Utilization | |||||||
| Received urgent care at least one time in the past 6 months | <0.001 | --- | |||||
| No | 3751 (95.1%) | 1169 (90.0%) | 4920 (93.9%) | ||||
| Yes | 192 (4.9%) | 130 (10.0%) | 322 (6.1%) | ||||
| Received emergency room care at least one time in the past 6 months | <0.001 | --- | |||||
| No | 3719 (94.3%) | 1153 (88.8%) | 4872 (92.9%) | ||||
| Yes | 224 (5.7%) | 146 (11.2%) | 370 (7.1%) | ||||
| Was hospitalized at least one time in the past 6 months | <0.001 | --- | |||||
| No | 3791 (96.1%) | 1213 (93.4%) | 5004 (95.5%) | ||||
| Yes | 152 (3.9%) | 86 (6.6%) | 238 (4.5%) | ||||
| Outcome | ATE * | ATT * | ATC * | RR * | ||||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |||||
| Urgent Care | −0.020 | −0.036 | −0.004 | −0.017 | −0.033 | −0.001 | −0.029 | −0.049 | −0.009 | 0.732 | 0.577 | 0.928 |
| ED Visits | −0.032 | −0.051 | −0.012 | −0.034 | −0.055 | −0.013 | −0.026 | −0.048 | −0.004 | 0.671 | 0.533 | 0.844 |
| Hospital Visits | −0.010 | −0.023 | 0.003 | −0.008 | −0.021 | 0.004 | −0.015 | −0.032 | 0.002 | 0.812 | 0.621 | 1.062 |
| Unhealthy Days | −3.517 | −4.280 | −2.755 | −3.591 | −4.416 | −2.766 | −3.311 | −3.970 | −2.652 | NA | NA | NA |
| Treatment model SuperLearner weights | ||||
| Urgent Care | ED Visits | Hospital Visits | Unhealthy Days | |
| Extreme Gradient Boosting | 0.0413 | 0.1538 | 0.1371 | 0.1777 |
| Random Forest | 0.000 | 0.000 | 0.000 | 0.0304 |
| Generalized Additive Models | 0.7472 | 0.7784 | 0.8629 | 0.7655 |
| Multivariate Adaptive Regression Splines | 0.2115 | 0.0678 | 0.000 | 0.0265 |
| Outcome model SuperLearner weights | ||||
| Urgent Care | ED Visits | Hospital Visits | Unhealthy Days | |
| Extreme Gradient Boosting | 0.111 | 0.0177 | 0.000 | 0.0153 |
| Random Forest | 0.000 | 0.000 | 0.1049 | 0.1831 |
| Generalized Additive Models | 0.8294 | 0.9311 | 0.664 | 0.612 |
| Multivariate Adaptive Regression Splines | 0.0596 | 0.0512 | 0.231 | 0.1896 |
| Post-TMLE Standardized Mean Difference (SMD) | |||||
| Outcome Models | |||||
| Covariates | Baseline SMD | Urgent Care | ED Visits | Hospital Visits | Unhealthy Days |
| Sex | 0.015 | 0.0076 | 0.0004 | 0.1038 | 0.0037 |
| Race/ethnicity | 0.178 | 0.01 | 0.0081 | 0.0066 | 0.0019 |
| Current smoking status | 0.053 | 0.0097 | 0.0053 | 0.0064 | 0.0053 |
| Alcohol consumption status | 0.211 | 0.0312 | 0.0341 | 0.0378 | 0.0378 |
| Health Insurance status | 0.081 | 0.0244 | 0.0108 | 0.005 | 0.0116 |
| Quality of life, in number of unhealthy weeks | 0.519 | 0.0163 | 0.0155 | 0.0195 | NA |
| Chronic pain status | 0.626 | 0.0199 | 0.0204 | 0.0192 | 0.03 |
| Age, Quintile 1 | 0.25 | 0.0076 | 0.0002 | 0.0033 | 0.0029 |
| Age Quintile 2 | 0.103 | 0.0376 | 0.0304 | 0.0407 | 0.0312 |
| Age Quintile 3 | 0.06 | 0.0336 | 0.0532 | 0.0257 | 0.0479 |
| Age Quintile 4 | 0.094 | 0.0106 | 0.0308 | 0.0022 | 0.0285 |
| Age Quintile 5 | 0.009 | 0.0071 | 0.0081 | 0.0139 | 0.0147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doucette, M.L.; Fisher, E.; Chin, J.; Kitsantas, P. Medical Cannabis Use and Healthcare Utilization Among Patients with Chronic Pain: A Causal Inference Analysis Using TMLE. Pharmacy 2025, 13, 96. https://doi.org/10.3390/pharmacy13040096
Doucette ML, Fisher E, Chin J, Kitsantas P. Medical Cannabis Use and Healthcare Utilization Among Patients with Chronic Pain: A Causal Inference Analysis Using TMLE. Pharmacy. 2025; 13(4):96. https://doi.org/10.3390/pharmacy13040096
Chicago/Turabian StyleDoucette, Mitchell L., Emily Fisher, Junella Chin, and Panagiota Kitsantas. 2025. "Medical Cannabis Use and Healthcare Utilization Among Patients with Chronic Pain: A Causal Inference Analysis Using TMLE" Pharmacy 13, no. 4: 96. https://doi.org/10.3390/pharmacy13040096
APA StyleDoucette, M. L., Fisher, E., Chin, J., & Kitsantas, P. (2025). Medical Cannabis Use and Healthcare Utilization Among Patients with Chronic Pain: A Causal Inference Analysis Using TMLE. Pharmacy, 13(4), 96. https://doi.org/10.3390/pharmacy13040096






