Pharmacist-Led Interventions for Polypharmacy Management in Older Adults: A Systematic Review of Strategies and Outcomes in the United Kingdom and the Republic of Ireland
Abstract
1. Introduction
2. Methodology
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
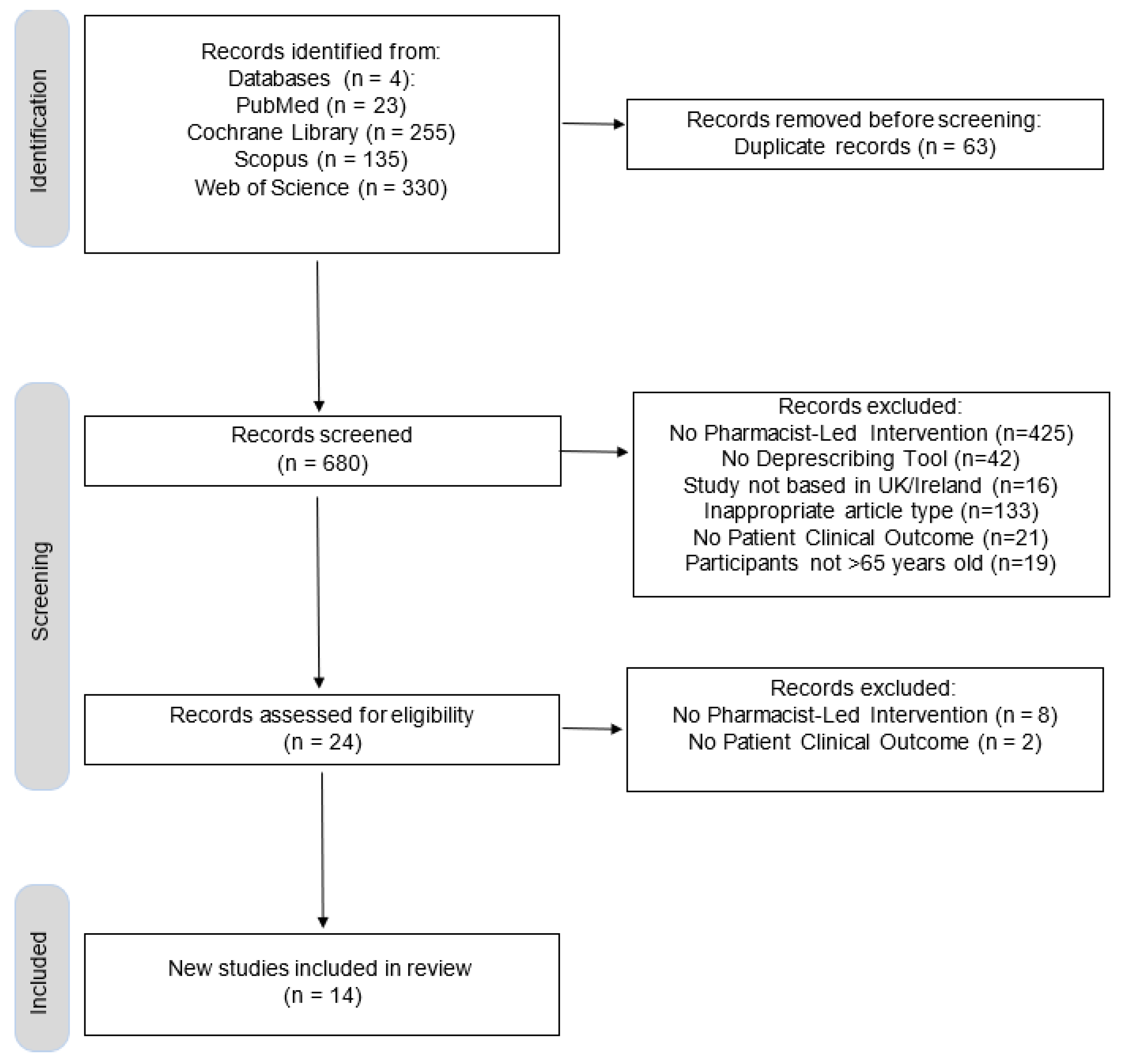
2.4. Study Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias Assessment
2.8. Synthesis Methods
2.9. Assessment of Reporting Biases and Certainty
3. Results
3.1. Study Characteristics
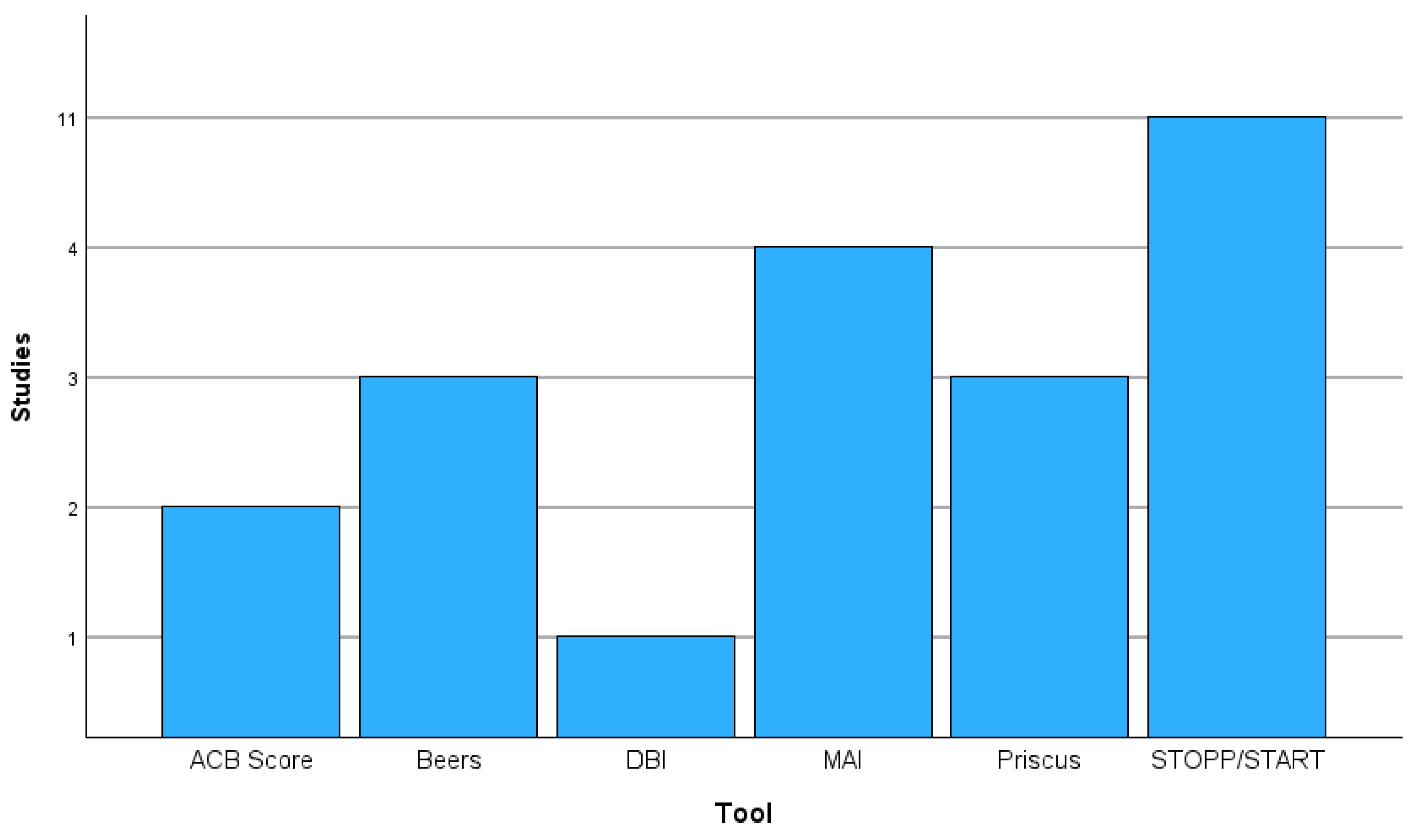
3.2. Screening Tools
3.3. Clinical Outcomes
3.4. Quality Analysis
3.5. Economic Impact Assessment
4. Discussion
4.1. Key Findings and Context
4.2. Tool-Specific Efficacy: Evidence for Contextual Adoption
4.3. Outcomes in Various Clinical Settings
4.4. Policy Implications: Evidence-Backed Recommendations
4.5. Limitations and Future Research Priorities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, R.A.; Camacho, E.; Jankovic, D.; Sculpher, M.J.; Faria, R. Economic Analysis of the Prevalence and Clinical and Economic Burden of Medication Error in England. BMJ Qual. Saf. 2021, 30, 96–105. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Medication Without Harm; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What Is Polypharmacy? A Systematic Review of Definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Bennie, M.; Santa-Ana-Tellez, Y.; Galistiani, G.F.; Trehony, J.; Despres, J.; Jouaville, L.S.; Poluzzi, E.; Morin, L.; Schubert, I.; MacBride-Stewart, S.; et al. The Prevalence of Polypharmacy in Older Europeans: A Multi-national Database Study of General Practitioner Prescribing. Br. J. Clin. Pharmacol. 2024, 90, 2124–2136. [Google Scholar] [CrossRef] [PubMed]
- Duerden, M.; Avery, T.; Payne, R. Polypharmacy and Medicines Optimisation; The King’s Fund: London, UK, 2013. [Google Scholar]
- Cooper, J.A.; Moriarty, F.; Ryan, C.; Smith, S.M.; Bennett, K.; Fahey, T.; Wallace, E.; Cahir, C.; Williams, D.; Teeling, M.; et al. Potentially Inappropriate Prescribing in Two Populations with Differing Socio-Economic Profiles: A Cross-Sectional Database Study Using the PROMPT Criteria. Eur. J. Clin. Pharmacol. 2016, 72, 583–591. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Flood, M.; Clyne, B.; Smith, S.M.; Wallace, E.; Boland, F.; Moriarty, F. Medication Changes and Potentially Inappropriate Prescribing in Older Patients with Significant Polypharmacy. Int. J. Clin. Pharm. 2023, 45, 191–200. [Google Scholar] [CrossRef]
- Woodcock, T.; Lovett, D.; Ihenetu, G.; Novov, V.; Beaney, T.; Armani, K.; Quilley, A.; Majeed, A.; Aylin, P. Polypharmacy in Primary Care: A Population-Based Retrospective Cohort Study of Electronic Health Records. PLoS ONE 2024, 19, e0308624. [Google Scholar] [CrossRef]
- O’Mahony, D.; Cherubini, A.; Guiteras, A.R.; Denkinger, M.; Beuscart, J.B.; Onder, G.; Gudmundsson, A.; Cruz-Jentoft, A.J.; Knol, W.; Bahat, G.; et al. STOPP/START Criteria for Potentially Inappropriate Prescribing in Older People: Version 3. Eur. Geriatr. Med. 2023, 14, 625–632. [Google Scholar] [CrossRef]
- American Geriatrics Society Beers Criteria®. Update Expert Panel Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef]
- Hanlon, J.T.; Schmader, K.E.; Samsa, G.P.; Weinberger, M.; Uttech, K.M.; Lewis, I.K.; Jay Cohen, H.; Fjxjssner, J.R. A Method for Assessing Drug Therapy Appropriateness. J. Clin. Epidemiol. 1992, 45, 1045–1051. [Google Scholar] [CrossRef]
- O’Mahony, D.; Gudmundsson, A.; Soiza, R.L.; Petrovic, M.; Cruz-Jentoft, A.J.; Cherubini, A.; Fordham, R.; Byrne, S.; Dahly, D.; Gallagher, P.; et al. Prevention of Adverse Drug Reactions in Hospitalized Older Patients with Multi-Morbidity and Polypharmacy: The SENATOR* Randomized Controlled Clinical Trial. Age Ageing 2020, 49, 605–614. [Google Scholar] [CrossRef]
- Walsh, K.; O’riordan, D.; Kearney, P.M.; Timmons, S.; Byrne, S. Improving the Appropriateness of Prescribing in Older Patients: A Systematic Review and Meta-Analysis of Pharmacists’ Interventions in Secondary Care. Age Ageing 2016, 45, 201–209. [Google Scholar] [CrossRef]
- Thomas, R.; Huntley, A.L.; Mann, M.; Huws, D.; Elwyn, G.; Paranjothy, S.; Purdy, S. Pharmacist-Led Interventions to Reduce Unplanned Admissions for Older People: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Age Ageing 2014, 43, 174–187. [Google Scholar] [CrossRef]
- Ie, K.; Hirose, M.; Sakai, T.; Motohashi, I.; Aihara, M.; Otsuki, T.; Tsuboya, A.; Matsumoto, H.; Hashi, H.; Inoue, E.; et al. Medication Optimization Protocol Efficacy for Geriatric Inpatients: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2423544. [Google Scholar] [CrossRef]
- Desborough, J.A.; Clark, A.; Houghton, J.; Sach, T.; Shaw, V.; Kirthisingha, V.; Holland, R.C.; Wright, D.J.; James Desborough, C.A. Clinical and Cost Effectiveness of a Multi-Professional Medication Reviews in Care Homes (CAREMED). Int. J. Pharm. Pract. 2020, 28, 626–634. [Google Scholar] [CrossRef]
- Koay, A.; Devitt, C. What Obstructs Health Policy Implementation? A Multi-Method Qualitative Case Study of the Delayed Deployment of Community Pharmacies in Ireland’s National. Qual. Health Res. 2024, 1–17. [Google Scholar] [CrossRef]
- National Institute of Health and Care Excellence Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. Available online: https://pubmed.ncbi.nlm.nih.gov/26180890/ (accessed on 9 December 2022).
- O’Sullivan, D.; O’Mahony, D.; O’Connor, M.N.; Gallagher, P.; Gallagher, J.; Cullinan, S.; O’Sullivan, R.; Eustace, J.; Byrne, S. Prevention of Adverse Drug Reactions in Hospitalised Older Patients Using a Software-Supported Structured Pharmacist Intervention: A Cluster Randomised Controlled Trial. Drugs Aging 2016, 33, 63–73. [Google Scholar] [CrossRef]
- Wright, D.; Holland, R.; Alldred, D.P.; Bond, C.; Hughes, C.; Barton, G.; Poland, F.; Shepstone, L.; Arthur, A.; Birt, L.; et al. The Care Home Independent Pharmacist Prescriber Study (Chipps): Development and Implementation of an Rct to Estimate Safety, Effectiveness and Cost-Effectiveness. Programme Grants Appl. Res. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Aziz, V.; Hill, N.; Bulletin, S.K. Completed Audit Cycle to Explore the Use of the STOPP/START Toolkit to Optimise Medication in Psychiatric in-Patients with Dementia. BJPsych Bull 2018, 42, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.; Plumb, R.; Burns, P.; Flanagan, S.; Parsons, C. A Quantitative Study on the Impact of a Community Falls Pharmacist Role, on Medicines Optimisation in Older People at Risk of Falls. BMC Geriatr. 2024, 24, 604. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Jennings, E.; Daunt, R.; Curtin, S.; Randles, M.; Gallagher, P.; O’Mahony, D. Deprescribing in Older People Approaching End of Life: A Randomized Controlled Trial Using STOPPFrail Criteria. J. Am. Geriatr. Soc. 2020, 68, 762–769. [Google Scholar] [CrossRef]
- Dalton, K.; O’Mahony, D.; O’Sullivan, D.; O’Connor, M.N.; Byrne, S. Prescriber Implementation of STOPP/START Recommendations for Hospitalised Older Adults: A Comparison of a Pharmacist Approach and a Physician. Drugs Aging 2019, 36, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.S.; Adamson, G.; Mallett, J.; Darcy, C.; Friel, A.; Scott, M.G.; Miller, E.R. Minding the Gap-an Examination of a Pharmacist Case Management Medicines Optimisation Intervention for Older People in Intermediate Care Settings. Res. Soc. Adm. Pharm. 2022, 18, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.; Dalton, K.; Byrne, S.; Foley, T.; Walsh, E. Pharmacist-Led Deprescribing Using STOPPFrail for Frail Older Adults in Nursing Homes. J. Am. Med. Dir. Assoc. 2024, 25, 105122. [Google Scholar] [CrossRef] [PubMed]
- Marvin, V.; Ward, E.; Poots, A.J.; Heard, K.; Rajagopalan, A.; Jubraj, B. Deprescribing Medicines in the Acute Setting to Reduce the Risk of Falls. Eur. J. Hosp. Pharm. 2017, 24, 10–15. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.; O’Mahony, D.; O’Connor, M.N.; Gallagher, P.; Cullinan, S.; O’Sullivan, R.; Gallagher, J.; Eustace, J.; Byrne, S. The Impact of a Structured Pharmacist Intervention on the Appropriateness of Prescribing in Older Hospitalized Patients. Drugs Aging 2014, 31, 471–481. [Google Scholar] [CrossRef]
- Sallevelt, B.T.G.M.; Egberts, T.C.G.; Huibers, C.J.A.; Ietswaart, J.; Drenth-van Maanen, A.C.; Jennings, E.; O’Mahony, C.; Jungo, K.T.; Feller, M.; Rodondi, N.; et al. Detectability of Medication Errors With a STOPP/START-Based Medication Review in Older People Prior to a Potentially Preventable Drug-Related Hospital Admission. Drug Saf. 2022, 45, 1501–1516. [Google Scholar] [CrossRef]
- Tallon, M.; Barragry, J.; Allen, A.; Breslin, N.; Deasy, E.; Moloney, E.; Delaney, T.; Wall, C.; O’byrne, J.; Grimes, T. Impact of the Collaborative Pharmaceutical Care at Tallaght Hospital (PACT) Model on Medication Appropriateness of Older Patients. Eur. J. Hosp. Pharm. 2016, 23, 16–21. [Google Scholar] [CrossRef]
- Twigg, M.J.; Wright, D.; Barton, G.R.; Thornley, T.; Kerr, C. The Four or More Medicines (FOMM) Support Service: Results from an Evaluation of a New Community Pharmacy Service Aimed at over-65s. Int. J. Pharm. Pract. 2015, 23, 407–414. [Google Scholar] [CrossRef]
- Pellegrin, K.L.; Krenk, L.; Oakes, S.J.; Ciarleglio, A.; Lynn, J.; McInnis, T.; Bairos, A.W.; Gomez, L.; McCrary, M.B.; Hanlon, A.L.; et al. Reductions in Medication-Related Hospitalizations in Older Adults with Medication Management by Hospital and Community Pharmacists: A Quasi-Experimental Study. J. Am. Geriatr. Soc. 2017, 65, 212–219. [Google Scholar] [CrossRef]
- Miller, R.; Darcy, C.; Friel, A.; Scott, M.; Toner, S. Consultant Pharmacist Case Management of Older People in Intermediate Care: A New Innovative Model. Eur. J. Pers. Cent. Healthc. 2016, 4, 4652. [Google Scholar] [CrossRef]
| Author & Year | Setting | Region | Study Design | Sample Size | Screening Tool | Clinical Outcome | Conclusion |
|---|---|---|---|---|---|---|---|
| Aziz et al., 2018 [21] | Hospital | Wales | Observational Audit | n = 86 | STOPP/START | Comorbidities, Total Prescriptions | Significant reductions occurred in comorbidities and the number of prescriptions. No clinically significant differences were observed for the reduction of polypharmacy or the number of patients in a specialist dementia ward. |
| Crawford et al., 2024 [22] | Community | Northern Ireland | Intervention Study | n = 92 | ACBCalc®, MAI | Medication Appropriateness, Falls | Post-intervention analysis revealed a reduction in polypharmacy and an improvement in the ACB score. There was an improvement in medication appropriateness, and a clinically significant reduction in exposure to fall-related medications occurred. |
| Curtin et al., 2020 [23] | Hospital | Ireland | RCT | n = 130 | STOPPFrail | PIMs, Falls, Hospitalisations, Fractures, Mortality and QOL | This trial significantly reduced polypharmacy; however, there were no differences in terms of other health outcomes, such as hospitalisations, falls, fractures, QOL, and mortality. |
| Dalton et al., 2019 [24] | Hospital | Ireland | RCT | n = 285 | STOPP/ START, Beers Criteria, and Priscus List | ADRs, PPOs, Hospital LOS, Mortality Rate | Pharmacist-led interventions resulted in a significant reduction in ADRs and significant implementation of the START criteria. There were no significant differences in mortality rates or hospital LOS. |
| Desborough et al., 2020 [16] | Care Home | England | RCT | n = 826 | STOPP | Falls, Emergency Visits, Mortality | There were significant reductions in PIMs after 12 months; however, the difference was borderline significant after 6 months. There was no significant reduction in falls, emergency visits, or survival. |
| Doherty et al., 2022 [25] | Intermediate Care | Northern Ireland | Observational Study | n = 532 | MAI | Medication Appropriateness, Hospital Readmissions | There were significant reductions in the MAI from admission to discharge. There were no significant differences in hospital readmissions; however, those who received educational intervention were less likely to be readmitted to acute care. |
| Hurley et al., 2024 [26] | Care Home | Ireland | Intervention Study | n = 99 | STOPPFrail, DBI Score, and ACB Score | Medication Burden, MAI, Falls, Hospitalisation, Emergency Visits, HRQOL, and Mortality Rates | This study found a significant reduction in the medication burden. There were no significant falls, hospitalisations, or mortality increases, highlighting the safe implementation of deprescribing. However, there were no significant improvements in falls, emergency visits or QOL. DBI and ACB scores significantly decreased post-review, suggesting reduced medicine-related sedation and frailty and increased medication appropriateness. |
| Marvin et al., 2017 [27] | Hospital | England | Observational Study | n = 100 | STOPP and STOPIT | Fall Risk | Reduction of fall risk medications. |
| O’Mahoney et al., 2020 [12] | Hospital | Ireland & Scotland | RCT | n = 1537 | STOPP/START | ADRs, All-Cause Mortality Rates, Hospital Readmission Rates, HRQOL | The intervention did not significantly improve clinical outcomes, possibly due to a 15% adherence to recommendations. No impact was found regarding the reduction of ADRs, mortality, readmission, or QOL. |
| O’Sullivan et al., 2014 [28] | Hospital | Ireland | Intervention Study | n = 361 | STOPP/ START, Beers Criteria, and Priscus List, MAI | Medication Appropriateness | Statistically significant improvement in MAI scores after the intervention, along with a significant reduction in PIP by STOPP criteria. Whilst Beers and Priscus showed slight improvements in PIP, they were not statistically significant. |
| O’Sullivan et al., 2016 [19] | Hospital | Ireland | RCT | n = 737 | STOPP/ START, Beers Criteria, and Priscus List | ADRs, Hospital LOS, All-Cause Mortality | Significant reduction in hospital-acquired ADRs. No effect on hospital LOS or all-cause mortality. |
| Sallevelt et al., 2022 [29] | Hospital | Ireland (Multicentre) | Observational Study | n = 963 | STOPP/START | Drug-Related Admissions | STOPP/START medication reviews did not significantly reduce the occurrence of drug-related hospital admissions. |
| Tallon et al., 2016 [30] | Hospital | Ireland | Observational study | n = 108 | MAI | Medication Appropriateness | The application of the MAI significantly improved medication appropriateness and reduced the number of inappropriate prescriptions at discharge. |
| Twigg et al., 2015 [31] | Community | England | Evaluation Study | n = 620 | STOPP/START | Fall Risk, Pain Management, Medication Adherence, HRQOL | Significant reduction in falls, increase in medication adherence and QOL (EQ-5D-5L scores). No significant changes were observed in pain scores. |
| Clinical Setting | Studies, N (%) |
|---|---|
| Hospital | 9 (65%) |
| Care Home | 2 (14%) |
| Community | 2 (14%) |
| Intermediate Care | 1 (7%) |
| Study Design | |
| RCT | 5 (36%) |
| Observational Study | 5 (36%) |
| Intervention Study | 3 (21%) |
| Evaluation Study | 1 (7%) |
| Study Location | |
| England | 3 (20%) |
| Scotland | 1 (7%) |
| Wales | 1 (7%) |
| Northern Ireland | 2 (13%) |
| Republic of Ireland | 8 (53%) |
| Clinical Outcome | Positive Outcome Observed, N (%) | No Positive Outcome, N (%) | Outcome Assessed, N (%) | Outcome Not Assessed, N (%) |
|---|---|---|---|---|
| Improved Medication Appropriateness | 5 (35%) | 0 (0%) | 5 (35%) | 9 (65%) |
| Reduction of Polypharmacy | 2 (14%) | 2 (14%) | 4 (28%) | 10 (72%) |
| Reduction of Falls or Fall Risk Medicine | 3 (21%) | 3 (21%) | 6 (43%) | 8 (57%) |
| Reduction of Inappropriate Prescribing | 1 (7%) | 0 (0%) | 1 (7%) | 13 (93%) |
| Reduction of Adverse Drug Reactions | 2 (14%) | 2 (14%) | 4 (28) | 10 (72%) |
| Reduction of Medication Burden | 2 (14%) | 0 (0%) | 2 (14%) | 12 (86%) |
| Reduction in Comorbidities | 1 (7%) | 0 (0%) | 1 (7%) | 13 (93%) |
| Improved Medication Adherence | 1 (7%) | 0 (0%) | 1 (7%) | 13 (93%) |
| Improved Quality of Life | 1 (7%) | 3 (21%) | 4 (28%) | 10 (72%) |
| Reduction in Hospitalisation | 0 (0%) | 6 (43%) | 6 (43%) | 8 (57%) |
| Improved Mortality Rates | 0 (0%) | 6 (43%) | 6 (43%) | 8 (57%) |
| Reduction in Hospital LOS | 0 (0%) | 3 (21%) | 3 (21%) | 11 (79%) |
| Selection/Topic | Item | Curtin et al. [23] | Dalton et al. [24] | Desborough et al. [16] | O’Mahoney et al. [12] | O’Sullivan et al. [19] |
|---|---|---|---|---|---|---|
| Research Question | Q1 | Yes | Yes | Yes | Yes | Yes |
| Randomisation | Q2 | Yes | Yes | Yes | Yes | Yes |
| Patients Accounted for at Conclusion | Q3 | Yes | Yes | No | Yes | Yes |
| Blinding of Participants | Q4a | No | No | No | No | No |
| Blinding of Investigators | Q4b | No | No | No | No | No |
| Blinding of Assessors | Q4c | Yes | No | Yes | Yes | Yes |
| Similarity of Study Groups | Q5 | Yes | Yes | No | Yes | Yes |
| Equal Care within Study Groups | Q6 | Yes | Yes | Yes | Yes | Yes |
| Comprehensive Reporting of Effects | Q7 | Yes | Yes | Yes | Yes | Yes |
| Reporting of Precision of Effects | Q8 | Yes | Yes | Yes | Yes | Yes |
| Benefits Outweigh the Risks | Q9 | Yes | Yes | No | No | Yes |
| Applicability to Context/Locality | Q10 | Yes | Yes | Yes | Can’t Tell | Yes |
| Value of Intervention Versus Existing Interventions | Q11 | Yes | Yes | No | No | Yes |
| Selection/Topic | Aziz et al. [21] | Crawford et al. [22] | Doherty et al. [25] | Hurley et al. [26] | Marvin et al. [27] | O’Sullivan et al. [28] | Sallevelt et al. [29] | Tallon et al. [30] | Twigg et al. [31] |
|---|---|---|---|---|---|---|---|---|---|
| Clear Focused Issue | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Acceptable Recruitment | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Accurate Exposure Measurement | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Accurate Outcome Measurement | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Identification of Confounding Factors | Yes | Yes | Yes | No | Cannot Tell | Yes | Yes | Cannot Tell | No |
| Account of Confounding Factors in Study Design | Yes | Yes | Yes | No | Cannot Tell | Yes | Yes | No | No |
| Subject Follow-Up Complete | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Appropriate Follow- Up Time | Yes | Yes | Yes | Cannot Tell | No | Yes | Yes | Yes | No |
| Results | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Precision of Results | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Belief of Results | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Applicability to Local Population | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Results Fit Available Evidence | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Implications for Practice | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Study/(Tool) | Economic Outcome Assessment | Conclusion |
|---|---|---|
| Crawford et al. [22] (ACBCalc, MAI) | Annual cost avoidance and drug cost savings of GBP 40–80 K. | Cost-effective |
| Curtin et al. [23] (STOPPFrail) | Monthly medication cost Reduction of USD 60 ± USD 25 (p = 0.02) after 3 months. | Cost-effective |
| Desborough et al. [16] (STOPP) | Intervention mean cost per resident was GBP 375 higher than control. | Not cost-effective |
| Hurley et al. [26] (STOPPFrail, DBI, ACBCalc) | No reduction in mean monthly costs after a 6- month follow-up. | Not cost-effective |
| Twigg et al. [31] (STOPP/START) | Cost per quality-adjusted life year estimates from GBP 11–32 K. | Potential cost-effectiveness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGrory, F.; Elnaem, M.H. Pharmacist-Led Interventions for Polypharmacy Management in Older Adults: A Systematic Review of Strategies and Outcomes in the United Kingdom and the Republic of Ireland. Pharmacy 2025, 13, 109. https://doi.org/10.3390/pharmacy13040109
McGrory F, Elnaem MH. Pharmacist-Led Interventions for Polypharmacy Management in Older Adults: A Systematic Review of Strategies and Outcomes in the United Kingdom and the Republic of Ireland. Pharmacy. 2025; 13(4):109. https://doi.org/10.3390/pharmacy13040109
Chicago/Turabian StyleMcGrory, Fionnuala, and Mohamed Hassan Elnaem. 2025. "Pharmacist-Led Interventions for Polypharmacy Management in Older Adults: A Systematic Review of Strategies and Outcomes in the United Kingdom and the Republic of Ireland" Pharmacy 13, no. 4: 109. https://doi.org/10.3390/pharmacy13040109
APA StyleMcGrory, F., & Elnaem, M. H. (2025). Pharmacist-Led Interventions for Polypharmacy Management in Older Adults: A Systematic Review of Strategies and Outcomes in the United Kingdom and the Republic of Ireland. Pharmacy, 13(4), 109. https://doi.org/10.3390/pharmacy13040109







