Ustekinumab in the Treatment of Crohn’s Disease—A Narrative Review on Clinical Efficacy and Safety Profile
Abstract
1. Introduction
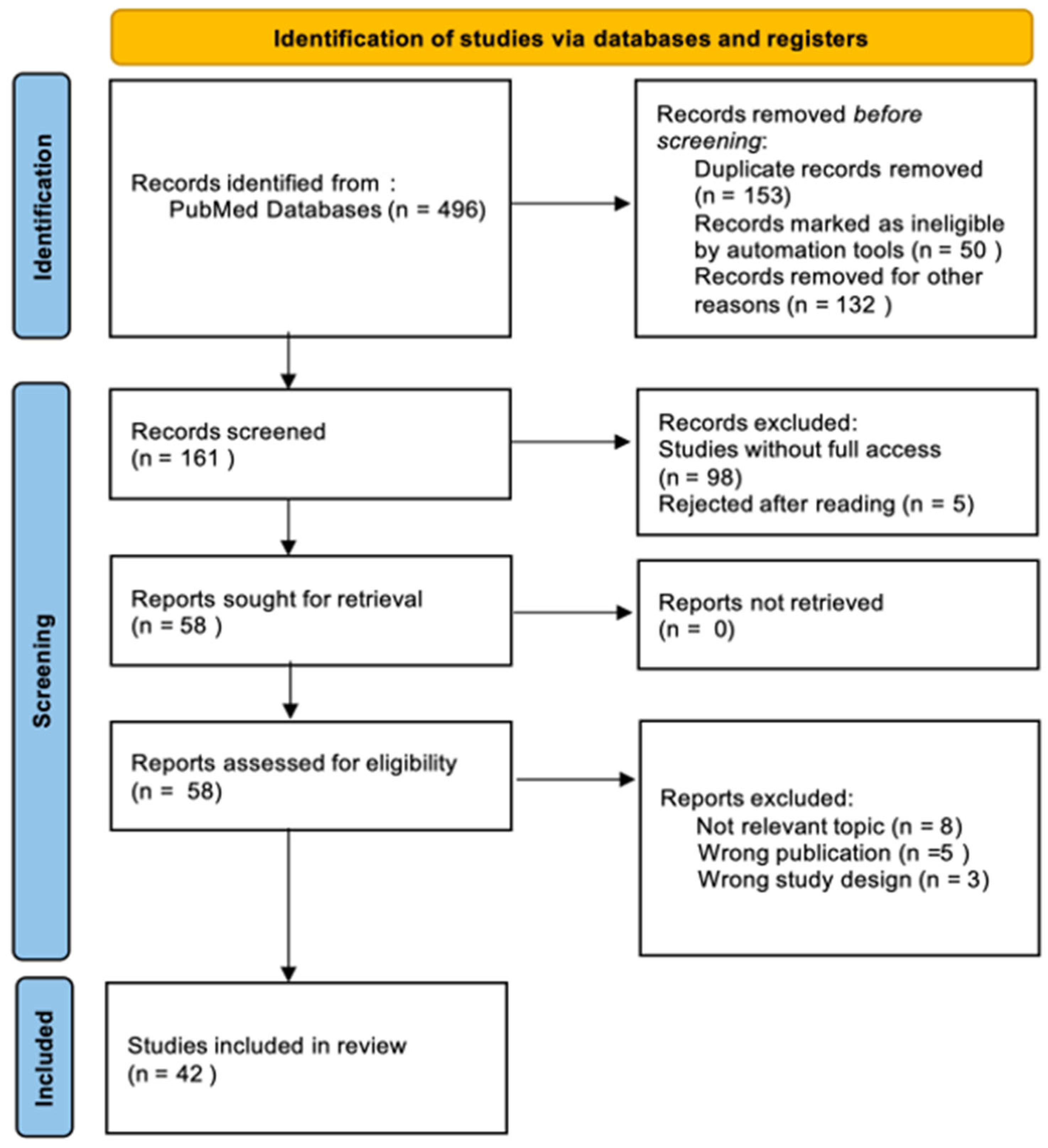
2. Materials and Methods
3. Results and Discussion
3.1. Efficacy of Ustekinumab in the Treatment of CD
3.2. Predictive Factors of Response to Therapy and Minimum UST Levels
3.3. Clinical Implications of UST Therapy
3.4. Quality of Life in Patients Undergoing UST Therapy
3.5. Comparison with Other Biologic Therapies
3.5.1. UST and Adalimumab
3.5.2. UST and Risankizumab
3.5.3. UST and Infliximab
3.5.4. UST and Biosimilars Substitutes
3.6. Ustekinumab in Pediatric Patients
4. Conclusions
5. Limitations of the Review
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UST | Ustekinumab |
| EN | Erythema nodosum |
| BMI | Body mass index |
| EIM | Extraintestinal manifestations |
| CD | Crohn’s disease |
| IBDs | Inflammatory bowel diseases |
| CR | Clinical remission |
| CRP | C-reactive protein |
| CDAIs | The Crohn’s Disease Activity Index |
| (STRIDE) II | Selecting Therapeutic Targets in Inflammatory Bowel Disease |
| RZB | Risankizumab |
| WPAI | Activity impairment |
| HRQoL | Health-related quality of life |
| IUS | non-invasive imaging methods—intestinal ultrasonography |
| SES-CD | Simple endoscopic score for Crohn’s disease |
| NGNA | N-glycolylneuraminic acid |
| ADA | Antidrug antibody |
| SoC | Standard of care |
| T2T | Treat-to-target |
| LTE | Long-term extension |
| MVL | Intestinal microvilli length |
| q12w | Every 12 weeks |
| q8w | Every 8 weeks |
| TNFα | Tumor necrosis factor alpha |
| GUS | Guselkumab |
| TIL | Tildrakizumab |
| AE | Adverse event |
References
- Ranasinghe, I.R.; Tian, C.; Hsu, R. Crohn Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Malik, T.F.; Aurelio, D.M. Extraintestinal Manifestations of Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Peyrin-Biroulet, L.; Chapman, J.C.; Colombel, J.F.; Caprioli, F.; D’Haens, G.; Ferrante, M.; Schreiber, S.; Atreya, R.; Danese, S.; Lindsay, J.O.; et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Crohn’s Disease. N. Engl. J. Med. 2024, 391, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, M.; Ma, C.; Griffith, J.; Crowell, M.; Neimark, E.; Kligys, K.; O’Connell, T. Matching-Adjusted Indirect Comparison Between Risankizumab and Ustekinumab for Induction and Maintenance Treatment of Moderately to Severely Active Crohn’s Disease. Adv. Ther. 2023, 40, 3896–3911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narula, N.; Aruljothy, A.; Wong, E.C.L.; Homenauth, R.; Alshahrani, A.A.; Marshall, J.K.; Reinisch, W. The impact of ustekinumab on extraintestinal manifestations of Crohn’s disease: A post hoc analysis of the UNITI studies. UEG J. 2021, 9, 581–589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumgart, D.C.; Stallmach, A.; Grunert, P.; Schubert, S.; Howaldt, S.; von Arnim, U.; Ochsenkühn, T.; Stein, J.; Lügering, A.; Schmidt, D.; et al. Induction and maintenance of mucosal healing in Crohn’s disease with ustekinumab in clinical practice across all care levels in Germany (MUCUS). Sci. Rep. 2024, 14, 20502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sands, B.E.; Irving, P.M.; Hoops, T.; Izanec, J.L.; Gao, L.L.; Gasink, C.; Greenspan, A.; Allez, M.; Danese, S.; Hanauer, S.B.; et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: A multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 2022, 399, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Wong, E.C.L.; Dulai, P.S.; Sengupta, N.K.; Marshall, J.K.; Colombel, J.F.; Reinisch, W. Comparative Efficacy and Rapidity of Action for Infliximab vs. Ustekinumab in Biologic Naïve Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 1579–1587.e2. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, O.J.; Xu, Z.; Marano, C.; O’Brien, C.; Szapary, P.; Zhang, H.; Johanns, J.; Leong, R.W.; Hisamatsu, T.; Van Assche, G.; et al. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2244–2255.e9. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, M.; Kemp, A.K. Ustekinumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Afif, W.; Arasaradnam, R.P.; Abreu, M.T.; Danese, S.; Sandborn, W.J.; Miao, Y.; Zhang, H.; Panaccione, R.; Hisamatsu, T.; Scherl, E.J.; et al. Efficacy and Safety of Ustekinumab for Ulcerative Colitis Through 4 Years: Final Results of the UNIFI Long-Term Maintenance Study. Am. J. Gastroenterol. 2024, 119, 910–921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abreu, M.T.; Rowbotham, D.S.; Danese, S.; Sandborn, W.J.; Miao, Y.; Zhang, H.; Tikhonov, I.; Panaccione, R.; Hisamatsu, T.; Scherl, E.J.; et al. Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-term Extension. J. Crohns Colitis 2022, 16, 1222–1234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janssen Inc. Indication: Treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic or have medical contraindications to such therapies. In Pharmacoeconomic Review Report: Ustekinumab (Stelara/Stelara I.V.); Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar] [PubMed]
- Reuben, A. Ustekinumab. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar] [PubMed]
- Turner, D.; Rosh, J.R.; Cohen, S.A.; Griffiths, A.M.; Hyams, J.S.; Kierkuś, J.; Adedokun, O.J.; Strauss, R.; Kim, L.; Volger, S.; et al. Ustekinumab in paediatric patients with moderately to severely active Crohn’s disease: UniStar study long-term extension results. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Rosh, J.R.; Turner, D.; Griffiths, A.; Cohen, S.A.; Jacobstein, D.; Adedokun, O.J.; Padgett, L.; Terry, N.A.; O’Brien, C.; Hyams, J.S. Ustekinumab in Paediatric Patients with Moderately to Severely Active Crohn’s Disease: Pharmacokinetics, Safety, and Efficacy Results from UniStar, a Phase 1 Study. J. Crohns Colitis 2021, 15, 1931–1942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, J.Y.; Zhang, M.; Wang, W.; Peng, X.; Zhao, J.Z.; Liu, T.; Li, Z.W.; Sun, H.T.; Hu, P.; Zhi, M. Ustekinumab trough concentration affects clinical and endoscopic outcomes in patients with refractory Crohn’s disease: A Chinese real-world study. BMC Gastroenterol. 2021, 21, 380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almradi, A.; Hanzel, J.; Sedano, R.; Parker, C.E.; Feagan, B.G.; Ma, C.; Jairath, V. Clinical Trials of IL-12/IL-23 Inhibitors in Inflammatory Bowel Disease. BioDrugs 2020, 34, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, S.; Feagan, B.G.; Ott, E.; Gasink, C.; Godwin, B.; Marano, C.; Miao, Y.; Ma, T.; Loftus, E.V., Jr.; Sandborn, W.J.; et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis Through 5 Years in Crohn’s Disease and 4 Years in Ulcerative Colitis. J. Crohns Colitis 2024, 18, 1091–1101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peyrin-Biroulet, L.; Vermeire, S.; D’Haens, G.; Panés, J.; Dignass, A.; Magro, F.; Nazar, M.; Le Bars, M.; Lahaye, M.; Ni, L.; et al. Clinical trial: Clinical and endoscopic outcomes with ustekinumab in patients with Crohn’s disease: Results from the long-term extension period of STARDUST. Aliment. Pharmacol. Ther. 2024, 59, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sands, B.E.; Gasink, C.; Yeager, B.; Adedokun, O.J.; Izanec, J.; Ma, T.; Gao, L.L.; Lee, S.D.; Targan, S.R.; et al. Evolution of Symptoms After Ustekinumab Induction Therapy in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2024, 22, 144–153.e2. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.L.; Dulai, P.S.; Marshall, J.K.; Jairath, V.; Reinisch, W.; Narula, N. Predictors of Clinical Remission to Placebo in Clinical Trials of Crohn’s Disease. Inflamm. Bowel Dis. 2023, 29, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.L.; Dulai, P.S.; Marshall, J.K.; Jairath, V.; Reinisch, W.; Narula, N. Improvement in serum eosinophilia is observed in clinical responders to ustekinumab but not adalimumab in inflammatory bowel disease. J. Crohns Colitis 2025, 19, jjaf006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, E.C.L.; Marshall, J.K.; Reinisch, W.; Narula, N. Body Mass Index Does Not Impact Clinical Efficacy of Ustekinumab in Crohn’s Disease: A Post Hoc Analysis of the IM-UNITI Trial. Inflamm. Bowel Dis. 2021, 27, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.T.; VanDussen, K.L.; Gordon, I.O.; Davis, E.M.; Li, K.; Simpson, K.; Ciorba, M.; Glover, S.C.; Abraham, B.; Guo, X.; et al. Epithelial Cell Biomarkers Are Predictive of Response to Biologic Agents in Crohn’s Disease. Inflamm. Bowel Dis. 2021, 27, 677–685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tursi, A.; Mocci, G.; Cuomo, A.; Allegretta, L.; Aragona, G.; Colucci, R.; Della Valle, N.; Ferronato, A.; Forti, G.; Gaiani, F.; et al. Real-life efficacy and safety of Ustekinumab as second- or third-line therapy in Crohn’s disease: Results from a large Italian cohort study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Danese, S.; O’Brien, C.D.; Ott, E.; Marano, C.; Baker, T.; Zhou, Y.; Volger, S.; Tikhonov, I.; et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies. Inflamm. Bowel Dis. 2021, 27, 994–1007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, V.; Mytych, D.T.; Das, S.; Franklin, J. Pharmacokinetic Similarity of ABP 654, An Ustekinumab Biosimilar Candidate: Results from a Randomized, Double-blind Study in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2023, 12, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Wilkens, R.; D’Agostino, M.A.; Maconi, G.; Le Bars, M.; Lahaye, M.; Bravatà, I.; Nazar, M.; Ni, L.; Ercole, E.; et al. Early Ultrasound Response and Progressive Transmural Remission After Treatment with Ustekinumab in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 153–163.e12. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vermeire, S.; D’Haens, G.; Panés, J.; Dignass, A.; Magro, F.; Nazar, M.; Le Bars, M.; Lahaye, M.; Ni, L.; et al. Treat to target versus standard of care for patients with Crohn’s disease treated with ustekinumab (STARDUST): An open-label, multicentre, randomised phase 3b trial. Lancet Gastroenterol. Hepatol. 2022, 7, 294–306, Erratum in Lancet Gastroenterol. Hepatol. 2022, 7, e8. [Google Scholar] [CrossRef] [PubMed]
- Panés, J.; Vermeire, S.; D’Haens, G.R.; Danese, S.; Magro, F.; Nazar, M.; Le Bars, M.; Lahaye, M.; Ni, L.; Bravatà, I.; et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe Crohn’s disease: Results up to Week 104 of the STARDUST trial. United Eur. Gastroenterol. J. 2023, 11, 410–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, E.C.L.; Dulai, P.S.; Marshall, J.K.; Jairath, V.; Reinisch, W.; Narula, N. Comparative Efficacy of Infliximab vs Ustekinumab for Maintenance of Clinical Response in Biologic Naïve Crohn’s Disease. Inflamm. Bowel Dis. 2023, 29, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Balser, S.; Nopora, K.; Körner, J.; Wedemeyer, R.S.; Anschütz, M.; Schug, B. New Ustekinumab Biosimilar Candidate FYB202: Pharmacokinetic Equivalence Demonstrated in a Randomized, Double-Blind, Parallel-Group, Single-Dose Trial in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2024, 13, 1308–1316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narula, N.; Wong, E.C.L.; Dulai, P.S.; Marshall, J.K.; Jairath, V.; Reinisch, W. Delayed Ustekinumab and Adalimumab Responders Have Similar Outcomes as Early Responders in Biologic-Naïve Crohn’s Disease. Am. J. Gastroenterol. 2024, 119, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Allez, M.; Sands, B.E.; Feagan, B.G.; D’Haens, G.; De Hertogh, G.; Randall, C.W.; Zou, B.; Johanns, J.; O’Brien, C.; Curran, M.; et al. A Phase 2b, Randomised, Double-blind, Placebo-controlled, Parallel-arm, Multicenter Study Evaluating the Safety and Efficacy of Tesnatilimab in Patients with Moderately to Severely Active Crohn’s Disease. J. Crohns Colitis 2023, 17, 1235–1251. [Google Scholar] [CrossRef] [PubMed]
- Biemans, V.B.C.; van der Meulen-de Jong, A.E.; van der Woude, C.J.; Löwenberg, M.; Dijkstra, G.; Oldenburg, B.; de Boer, N.K.H.; van der Marel, S.; Bodelier, A.G.L.; Jansen, J.M.; et al. Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J. Crohns Colitis 2020, 14, 33–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forss, A.; Clements, M.; Myrelid, P.; Strid, H.; Söderman, C.; Wagner, A.; Andersson, D.; Hjelm, F.; PROSE SWIBREG study group; Olén, O.; et al. Ustekinumab Is Associated with Real-World Long-Term Effectiveness and Improved Health-Related Quality of Life in Crohn’s Disease. Dig Dis Sci. 2023, 68, 65–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Straatmijer, T.; Biemans, V.B.C.; Hoentjen, F.; de Boer, N.K.H.; Bodelier, A.G.L.; Dijkstra, G.; van Dop, W.A.; Haans, J.J.L.; Jansen, J.M.; Maljaars, P.W.J.; et al. Ustekinuma b for Crohn’s Disease: Two-Year Results of the Initiative on Crohn and Colitis (ICC) Registry, a Nationwide Prospective Observational Cohort Study. J. Crohns Colitis 2021, 15, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Baston-Rey, I.; Fernández-Salgado, E.; González García, J.; Ramos, L.; Diz-Lois Palomares, M.T.; Argüelles-Arias, F.; Iglesias Flores, E.; Cabello, M.; Rubio Iturria, S.; et al. Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn’s Disease Patients: The SUSTAIN Study. Inflamm. Bowel Dis. 2022, 28, 1725–1736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iborra, M.; Beltrán, B.; Fernández-Clotet, A.; Iglesias-Flores, E.; Navarro, P.; Rivero, M.; Gutiérrez, A.; Sierra-Ausin, M.; Mesonero, F.; Ferreiro-Iglesias, R.; et al. Real-world long-term effectiveness of ustekinumab in Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharmacol. Ther. 2020, 52, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
| Reference | Citation | Year | n | Short Description of the Study | Summary of Results |
|---|---|---|---|---|---|
| Ahmed Almradi | [19] | 2020 | 334 | Clinical trials of IL-12/IL-23 inhibitors | UST has demonstrated efficacy and safety in treating moderate-to-severe CD. Significant improvements were observed in clinical response, clinical remission, endoscopic, and histologic outcomes, with the 8-week dosing regimen being particularly effective in patients previously treated with biologics. |
| Laurent Peyrin-Biroulet | [4] | 2024 | 527 | Risankizumab versus ustekinumab for moderate-to-severe Crohn’s disease | Risankizumab was non-inferior to ustekinumab in achieving clinical remission at week 24 and superior in endoscopic remission at week 48, with a comparable safety profile between both groups. |
| Marla Dubinsky | [5] | 2023 | - | Matching-adjusted indirect comparison between risankizumab and ustekinumab for induction and maintenance treatment | The indirect analysis showed that risankizumab achieved better clinical and endoscopic outcomes than ustekinumab during the induction phase, while CDAI remission in the maintenance phase was comparable. |
| William J. Sandborn | [20] | 2022 | - | Five-year efficacy and safety of ustekinumab treatment in Crohn’s disease | UST 90 mg subcutaneously every 12 weeks and every 8 weeks safely maintained clinical response and remission in patients with CD. |
| Torsten Kucharzik | [31] | 2022 | 77 | Early ultrasound response and progressive transmural remission after treatment with ustekinumab | IUS showed that ustekinumab-treated CD patients achieved progressive IUS response (46.3%) and transmural remission (24.1%) through week 48, with a more robust response in the colon and biologic-naive patients. |
| Sylwio Danese | [32] | 2022 | 498 | Assessment that included early endoscopy, regular monitoring of biomarkers and clinical symptoms, and dose intensification | Intensification of ustekinumab treatment based on early endoscopic response, clinical symptoms, and biomarkers did not result in a significant improvement in endoscopic outcomes after 48 weeks compared to standard symptom-based treatment. |
| Subrata Ghosh | [21] | 2024 | 2575 | Safety of ustekinumab in inflammatory bowel disease | The long-term analysis confirms the favorable safety profile of ustekinumab in treating Crohn’s disease with a low incidence of serious adverse events. These findings support the continued use of ustekinumab as a safe therapeutic option for patients with IBD. |
| Dan Turner | [16] | 2024 | 34 | Ustekinumab in pediatric patients-UniStar study long-term extension results | The results confirm the effectiveness and safety of ustekinumab in children with CD, and further studies aim to refine the dosing for children with lower body weight. |
| Joel R. Rosh | [17] | 2021 | 44 | Ustekinumab in pediatric patients with moderately to severely active Crohn’s disease | UST treatment in children improved clinical and endoscopic disease markers as well as inflammatory biomarkers. Pharmacokinetics and safety were consistent with data from adults, and the dosing is appropriate for children weighing ≥40 kg. |
| Jean-Frédéric Colombel | [23] | 2024 | 915 | Evolution of symptoms after ustekinumab induction therapy | Ustekinumab provides rapid symptom relief as early as the first day after infusion, with its efficacy increasing through weeks 16 and 44. |
| Julian Panes | [33] | 2023 | 440 | Ustekinumab improves health-related quality-of-life results up to Week 104 | Ustekinumab improved HRQoL, reduced fatigue, and increased patient productivity over 2 years. |
| Laurent Peyrin-Biroulet | [22] | 2024 | 440 | Clinical and endoscopic outcomes with ustekinuma—results from the long-term extension period | The two-year LTE study confirmed the effectiveness of ustekinumab in treating moderate-to-severe Crohn’s disease. |
| Neeraj Narula | [9] | 2022 | 420 | Comparative efficacy and rapidity of action for Infliximab vs. ustekinumab in biologic naïve Crohn’s disease | Infliximab and ustekinumab show comparable efficacy and rate of action |
| Emily C.L. Wong | [34] | 2023 | 220 | Comparative efficacy of Infliximab vs. ustekinumab for maintenance of clinical response | Infliximab may give better results in terms of endoscopic outcomes. |
| Neeraj Narula | [6] | 2021 | 941 | The effect of ustekinumab on extraintestinal symptoms of Crohn’s disease | UST does not reduce the risk of new EIMs in the short term but improves their course in the long term, especially in arthritis and erythema nodosum. |
| Emily C.L. Wong | [26] | 2021 | 254 | Randomized controlled trial about BMI and clinical efficacy of UST | After 308 days of treatment, UST levels were significantly lower in obese individuals, but no correlation was found between BMI and treatment effectiveness. |
| Daniel C. Baumgart | [7] | 2024 | 52 | Clinical trial about UST and mucosal healing | Among the patients, 52% achieved a combined clinical and endoscopic response, and 15 patients achieved an endoscopic response. Adverse events occurred in 69.2% of patients, among which 15.4% were classified as severe, but no serious events or deaths were observed. |
| Neeraj Narula | [36] | 2024 | 373 | Ustekinumab and adalimumab | The clinical response after one year of treatment with both drugs was similar in the group of patients who responded early and those with a delayed response. |
| Bruce E. Sands | [8] | 2022 | 386 | Ustekinumab versus adalimumab | In this group of patients who had not been previously treated with biologics, both ustekinumab and adalimumab in monotherapy demonstrated high efficacy, with no differences observed in the primary treatment outcome between these drugs. |
| Jia-yin Yao | [18] | 2021 | 20 | Ustekinumab trough concentration affects treatment | The optimal minimum concentration of UST was determined to be 1.12 μg/mL. Patients with UST levels exceeding 1.12 μg/mL demonstrated better clinical response outcomes. |
| Vincent Chow | [30] | 2023 | 238 | Randomized controlled trial about ABP 654, an ustekinumab biosimilar candidate | Study may contribute to the globalization of CD therapy with ABP 65. |
| Sigrid Balser | [35] | 2024 | 491 | FYB202, an ustekinumab biosimilar candidate | FYB202 has pharmacokinetic parameters comparable to EU/US USTs. |
| Emily C.L. Wong | [24] | 2023 | 683 | Predictors of CD clinical remission | Inclusion of people with shorter disease duration may increase the effectiveness of therapy and improve interpretation of results. |
| Emily C.L. Wong | [25] | 2025 | 683 | The role of eosinophils as a potential biomarker of response to ustekinumab | Those responding positively to therapy had significantly higher initial eosinophil counts. |
| Tursi | [28] | 2021 | 194 | Ustekinumab as second- or third-line therapy in Crohn’s disease | UST is regarded as an effective and safe second-line therapy for CD patients refractory to other biologic treatments. |
| Mark T. Osterman | [27] | 2021 | 106 | Epithelial cell biomarkers | MVL may be an important biomarker for predicting the response to UST treatment. |
| William J. Sandborn | [29] | 2021 | 2574 | Safety of ustekinumab | UST has a favorable safety profile in the treatment of inflammatory bowel disease. |
| Matthieu Allez | [37] | 2023 | 243 | Safety of ustekinumab | UST was associated with a safe action profile compared to Tesnatilimab. |
| Vince B. C. Biemans | [38] | 2020 | 221 | Observational study about UST and CD | Ustekinumab is a safe and effective treatment option for CD patients who did not respond to prior TNF and integrin inhibitor therapies. |
| Anders Forss | [39] | 2023 | 114 | UST long-term effectiveness and improved health-related quality of life | The study confirms the long-term effectiveness and safety of ustekinumab in treating CD. |
| Tessa Straatmijer | [40] | 2021 | 252 | Safety of ustekinumab | Approximately one in three patients with CD achieved clinical remission. |
| Maria Chaparro | [41] | 2022 | 463 | Long-term real-world effectiveness and safety | UST safe as short- and long-term treatment. |
| Marisa Iborra | [42] | 2020 | 407 | Long-term safety of UST | UST proved effective in achieving both clinical and endoscopic remission. |
| Subsection | Main Findings and Conclusions with Citations |
|---|---|
| 3.1 Efficacy of ustekinumab in the treatment of CD | Clinical/endoscopic response was achieved in 2%; Significant predictors: age. Fistula healing, intestinal manifestations dropped 20.2% at 52 weeks [7]. UST induces endoscopic remission, especially after multiple biologics [7]. UST levels ≥ 1.12 μg/mL linked to better outcomes [18]. EIMs reduced 35% (week 6) and 72% (week 52) [6]. Higher remission rates in UST groups vs. placebo (UNITI trials) [19]. Long-term remission with UST (IM-UNITI study): clinical remission 28.7% (q12w), 34.4% (q8w) after 5 years [20]. STARDUST: 50.2% clinical remission, 39.3% endoscopic response at 104 weeks [22]. Colombel JF: Higher remission in early-phase UST therapy [23]. |
| 3.2 Predictive factors of response to therapy | Shorter disease duration (<5 years for remission, <1 year for clinical response) and higher eosinophil counts predict positive response [24]. UST levels ≥ 1.12 μg/mL improve endoscopic remission [18]. BMI impacts UST drug levels, but not treatment success [26]. Ileal microvessel length > 1.7 µm correlates with higher efficacy [27]. |
| 3.3 Clinical implications of UST therapy | Symptoms improved in 75% at 4 months and 70% at 6 months. Endoscopic symptom improvement in 50%. CRP and fecal calprotectin decreased, FC > 200 µg/g correlated with lack of remission [28]. Safety profile: TEAEs in 69.2%, 15.4% severe but no deaths [7]. Common TEAEs: infections, headache, fatigue, nasopharyngitis [29]. UST response and trans-wall remission observed (STARDUST) [31]. HRQoL and productivity improved with UST, no significant difference between T2T and SoC [33]. At week 104, 50.2% clinical remission, 39.3% endoscopic response. Flexible dosing maintained efficacy [22]. |
| 3.4 Quality of life in patients undergoing UST therapy | Ustekinumab significantly improves quality of life in Crohn’s disease patients, as shown in adult (STARDUST) and pediatric (UniStar) studies, through symptom reduction, decreased fatigue, and better daily and social functioning. |
| 3.5.1 UST and adalimumab | Clinical remission was 4% greater in UST vs. adalimumab. Endoscopic lesion improvement similar in both groups [8]. Early and late responders had comparable outcomes [25]. |
| 3.5.2 UST and risankizumab | UST no worse than RZB at 24 weeks; RZB superior at 48 weeks for endoscopic remission [4]. RZB superior in inducing endoscopic remission after prior biologic failure [5]. |
| 3.5.3 UST and infliximab | No significant differences in clinical remission between UST and infliximab [9]. Infliximab better for endoscopic response and remission [34]. |
| 3.5.4 UST and biosimilars substitutes | ABP 65 shows fewer ADA antibodies compared to UST, with a similar safety profile [30]. FYB202 had comparable safety and pharmacokinetics to UST [30,35]. |
| 3.6 Ustekinumab in pediatric patients | UniStar study: UST improved efficacy and serum levels in children [16,17]. Long-term UST treatment improved clinical remission, quality of life, and biomarkers [16]. |
| Adverse Event | Study | n | n% |
|---|---|---|---|
| Discontinuation of treatment in the study due to adverse events | [4] Laurent Peyrin-Biroulet [16] Dan Turner [17] Joel R. Rosh [22] Laurent Peyrin-Biroulet [8] Bruce E. Sands [28] Tursi | 13 5 2 13 5 4 | 4.9 14.7 5 4 6 2 |
| Serious adverse events | [4] Laurent Peyrin-Biroulet [16] Dan Turner [8] Bruce E. Sands [30] Vincent Chow [29] William J. Sandborn | 46 11 25 1 70 | 17.4 32.4 13 1.3 4.4 |
| Infections | [8] Bruce E. Sands [16] Dan Turner [17] Joel R. Rosh [22] Laurent Peyrin-Biroulet [29] William J. Sandborn [41] Maria Chaparro | 65 25 17 45 305 5 | 34 73.5 39 13.9 19.3 1.1 |
| Serious infections | [4] Laurent Peyrin-Biroulet [16] Dan Turner [17] Joel R. Rosh [8] Bruce E. Sands [30] Vincent Chow [29] William J. Sandborn | 11 0 0 4 2 18 | 4.2 0 0 2 2.5 1.1 |
| Nasopharyngitis | [32] Sylwio Danese [22] Laurent Peyrin-Biroulet [8] Bruce E. Sands [30] Vincent Chow [35] Sigrid Balser [29] William J. Sandborn | 29 28 14 2 101 78 | 13 8.7 7 2.5 20.6 4.9 |
| Abdominal pain | [32] Sylwio Danese [22] Laurent Peyrin-Biroulet [8] Bruce E. Sands [30] Vincent Chow | 23 22 24 1 | 11 6.8 13 1.3 |
| Joint pain | [22] Laurent Peyrin-Biroulet [8] Bruce E. Sands [29] William J. Sandborn | 20 12 69 | 6.2 6 4.4 |
| Upper respiratory tract infection | [8] Bruce E. Sands [29] William J. Sandborn | 12 44 | 6 2.8 |
| Rash at the injection site | [8] Bruce E. Sands | 3 | 2 |
| Vomiting | [8] Bruce E. Sands [30] Vincent Chow [29] William J. Sandborn | 10 0 41 | 5 0 2.6 |
| Diarrhea | [8] Bruce E. Sands [35] Sigrid Balser | 11 17 | 6 3.5 |
| Hypersensitivity | [4] Laurent Peyrin-Biroulet [41] Maria Chaparro | 24 9 | 9.1 1.9 |
| Liver events | [4] Laurent Peyrin-Biroulet | 14 | 5.3 |
| Headache | [32] Sylwio Danese [17] Joel R. Rosh [22] Laurent Peyrin-Biroulet [35] Sigrid Balser [29] William J. Sandborn [41] Maria Chaparro | 24 8 24 142 106 7 | 11 18 7.4 28.9 6.7 1.5 |
| Anemia | [17] Joel R. Rosh | 7 | 16 |
| COVID-19 | [35] Sigrid Balser | 36 | 7.3 |
| Fever | [29] William J. Sandborn [41] Maria Chaparro | 58 1 | 3.7 0.2 |
| Category | Adults | Pediatric Patients |
|---|---|---|
| Induction regimen | Intravenous: standard dose of 6 mg/kg body weight. | Intravenous: 130 mg or 390 mg for ≥40 kg; 3 mg/kg or 9 mg/kg for <40 kg (UniStar study). |
| Maintenance regimen | Subcutaneous: 90 mg every 8 or 12 weeks, depending on clinical response. | Subcutaneous: 90 mg (≥40 kg) or 2 mg/kg (<40 kg), starting from week 8. |
| Efficacy | Confirmed in several studies (e.g., UNITI-1, UNITI-2, IM-UNITI); clinical remission and biomarker improvement achieved. | In the UniStar study: clinical improvement and reduction in inflammatory biomarkers by week 8; remission achieved in 41.2% of patients at week 48. |
| Long-term efficacy | Maintained efficacy over years with regular treatment, supported by observational data. | Long-term treatment (up to 240 weeks) resulted in sustained remission and improved quality of life (Turner D. et al.). |
| Drug levels | Stable, predictable pharmacokinetics. | Children < 40 kg had lower serum drug levels—suggesting the need for dose adjustment. |
| Safety | Favorable safety profile; rare serious adverse events; low immunogenicity. | Good safety profile. Most common serious adverse event: CD exacerbation (n = 6); only one case of anti-UST antibodies. |
| Immunogenicity (antibodies) | Low incidence of anti-UST antibodies in adult population. | No significant anti-UST antibodies detected, except in one case. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piecuch, D.; Hańczyk, E.; Kopciał, S.; Pawelec, N.; Mazur, W.; Kornatowska, K. Ustekinumab in the Treatment of Crohn’s Disease—A Narrative Review on Clinical Efficacy and Safety Profile. Pharmacy 2025, 13, 73. https://doi.org/10.3390/pharmacy13030073
Piecuch D, Hańczyk E, Kopciał S, Pawelec N, Mazur W, Kornatowska K. Ustekinumab in the Treatment of Crohn’s Disease—A Narrative Review on Clinical Efficacy and Safety Profile. Pharmacy. 2025; 13(3):73. https://doi.org/10.3390/pharmacy13030073
Chicago/Turabian StylePiecuch, Dawid, Edyta Hańczyk, Szymon Kopciał, Natalia Pawelec, Weronika Mazur, and Karolina Kornatowska. 2025. "Ustekinumab in the Treatment of Crohn’s Disease—A Narrative Review on Clinical Efficacy and Safety Profile" Pharmacy 13, no. 3: 73. https://doi.org/10.3390/pharmacy13030073
APA StylePiecuch, D., Hańczyk, E., Kopciał, S., Pawelec, N., Mazur, W., & Kornatowska, K. (2025). Ustekinumab in the Treatment of Crohn’s Disease—A Narrative Review on Clinical Efficacy and Safety Profile. Pharmacy, 13(3), 73. https://doi.org/10.3390/pharmacy13030073






