Abstract
Naldemedine, a peripherally acting μ-opioid receptor antagonist, is used to treat opioid-induced constipation (OIC). However, it causes diarrhea as an adverse effect. This retrospective study aimed to investigate whether the occurrence of diarrhea was dependent on the timing of naldemedine treatment initiation. Inpatients who were initially treated with naldemedine at the Department of Respiratory Medicine, NHO Iwakuni Medical Center, Japan, between 1 December 2017 and 31 March 2021 were included in this study and divided into the simultaneous combination group, in which naldemedine was introduced at the same time as strong opioid analgesics, and the non-simultaneous combination group, in which naldemedine was introduced after the initiation of treatment with strong opioid analgesics. This study included 45 patients, 15 (33.3%) of whom developed diarrhea. Among the patients in the simultaneous combination group and non-simultaneous combination group, diarrhea occurred in 2 (11.1%) and 13 (48.1%) patients, respectively. Multivariate logistic regression analysis revealed that the delayed introduction of naldemedine was significantly associated with the development of diarrhea (odds ratio: 6.68, 95% confidence interval: 1.220–36.700, p = 0.028). Our analysis reveals that the simultaneous administration of naldemedine and oxycodone may prevent the development of diarrhea associated with naldemedine use for OIC.
1. Introduction
Opioid analgesics, which are widely used to relieve moderate-to-severe cancer-associated pain, act primarily by stimulating μ-opioid receptors in the central nervous system [1,2,3]. Typical adverse events (AEs) associated with opioid analgesic use include constipation, nausea, vomiting, and drowsiness [4]. Of these, nausea, vomiting, and drowsiness often occur when treatment is initiated or when the dose is increased; however, they diminish or resolve within days [5]. In contrast, opioid-induced constipation (OIC) occurs as a result of μ-opioid receptor stimulation in the intestinal tract, which inhibits intestinal peristalsis and causes persistent constipation [6,7]. OIC has been reported to occur in 39–85.7% of patients receiving long-term opioid treatment [8,9,10,11], and a survey of hospitalized patients with cancer in Japan revealed that approximately 40% of the patients experienced constipation [12]. OIC impacts the quality of life (QOL) of patients and can also lead to a reduction or discontinuation of opioid analgesic use, thus increasing pain levels [13,14,15].
The Japanese Society for Palliative Medicine and the American Gastroenterological Association (AGA) recommend conventional laxatives as first-line therapeutics for patients with OIC [5,16]. However, it has been reported that laxatives are ineffective in the treatment of OIC and instead increase AEs in patients, leading to a further reduction in QOL [17,18]. The AGA guidelines recommend the use of naldemedine as a second-line therapeutic if laxative treatment proves unsatisfactory.
Naldemedine is a peripherally acting μ-opioid receptor antagonist that minimizes the effects of exogenous opioids at peripheral μ-opioid receptors, including those in the gastrointestinal tract, without affecting receptors in the central nervous system responsible for the analgesic effects of opioids [19,20,21,22]. The most common AE associated with naldemedine is diarrhea, and 5% of patients who develop diarrhea discontinue naldemedine treatment [23]. Administration of naldemedine early after the initiation of treatment with opioid analgesics has been reported to decrease the incidence of diarrhea [24,25].
Although naldemedine is not currently recommended in Japan as a first-line treatment for OIC, Japanese prospective observational studies and randomized controlled trials have indicated its effectiveness as a prophylactic treatment for OIC [26,27]. However, few studies have reported the occurrence of diarrhea during prophylactic administration of naldemedine for OIC in clinical practice. Therefore, this study aimed to compare the incidence of diarrhea after concurrent and non-concurrent administration of naldemedine and opioid analgesics.
2. Materials and Methods
2.1. Patient Selection
This retrospective study included data collected from electronic medical records on 64 patients with lung cancer who were treated with naldemedine upon admission to the Department of Respiratory Medicine, NHO Iwakuni Medical Center, between 1 December 2017 and 31 March 2021. Patients who received naldemedine on an irregular basis, did not have cancer, were taking weak opioid analgesics, already exhibited diarrhea at the start of naldemedine administration, or had missing data were excluded from the study. (Figure 1). The patients were divided into two groups, depending on the timing of naldemedine administration: simultaneous combination, in which both agents were initiated at the same time, and non-simultaneous combination, in which naldemedine was introduced on or after the second day following the initiation of strong opioid analgesics.
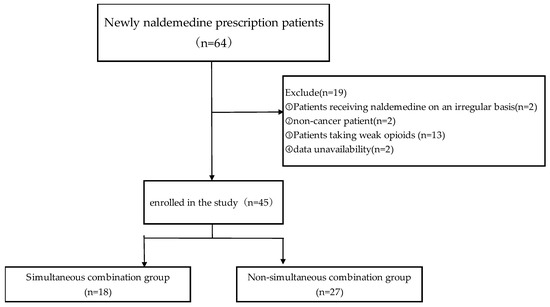
Figure 1.
Flow chart for patient selection.
Data regarding sex, age, height, weight, body surface area (BSA), performance status, suspected blood–brain barrier dysfunction, type of strong opioid, hematological parameters before naldemedine administration, and concomitant medications were collected. BSA was calculated using the Dubois formula: BSA [m2] = [body weight(kg)]0.425 × [height(cm)]0.725 × 0.007184 [28]. Blood–brain barrier dysfunction may be a result of brain tumors, dementia associated with acquired immunodeficiency syndrome, multiple sclerosis, or Alzheimer’s disease [29].
2.2. Ethics
This study was approved by the Ethics Committee of NHO Iwakuni Medical Center (Approval No. 0347) and conducted in accordance with the principles outlined in the Declaration of Helsinki. Data were collected from medical records in accordance with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects in Japan. Information regarding the conduct of this study was disclosed on the website, which also provided an opportunity for patients to opt out of the study.
2.3. Evaluation of Diarrhea
Diarrhea was defined as “diarrhea” written on the medical record within 2 weeks of the initiation of naldemedine administration, as previously described [23]. Diarrhea severity was assessed as grades 1–4, using a Japanese version of the Common Terminology Criteria for Adverse Events, version 5.0, as translated by the Japanese Clinical Oncology Group.
2.4. Statistical Analysis
Patient characteristics were analyzed using Fisher’s exact probability test and the Mann–Whitney U test for categorical and continuous variables, respectively. Univariate logistic regression analyses were performed to evaluate the odds ratio, 95% confidence intervals, and p value of each potential confounding factor for diarrhea induced by naldemedine. Multivariate logistic regression analysis was performed to exclude confounding factors. Multivariate logistic regression analysis was performed using sex and oxycodone as explanatory variables; these potential confounding factors were selected after reviewing the relevant literature. Results are expressed as odds ratios with 95% confidence intervals, and differences were considered statistically significant with a two-sided p value ≤ 0.05. Statistical analyses were performed using EZR software, version 1.66 [30].
3. Results
3.1. Patients
The patient selection process is summarized in Figure 1. Of the 64 inpatients screened, 19 patients were excluded from the study: 2 received naldemedine on an irregular basis, 2 did not have cancer, 2 had missing data, and 13 were administered weak opioid analgesics. Finally, 45 patients were included in the analysis, with 18 patients in the simultaneous combination group and 27 in the non-simultaneous combination group.
The baseline patient characteristics are summarized in Table 1. The median age of the patients was 71 years, and 33 patients (73%) were men. The performance status of 28 patients (62%) was 1. Concomitant medications included drugs that may be risk factors for diarrhea (proton pump inhibitor, histamine H2 receptor antagonist, non-steroidal anti-inflammatory drugs, selective serotonin reuptake inhibitors, aspirin, anticholinergics, digestive stimulant, and constipation medication) [31]; the CYP3A4 inducers, aprepitant and dexamethasone; and the P-glycoprotein inhibitors, suvorexant and tolvaptan. The median number of three consecutive days of spontaneous defecations prior to naldemedine administration was 1 in both the simultaneous and non-simultaneous combination groups. The median duration of opioid therapy prior to naldemedine administration was 3 days in the non-simultaneous combination group. The opioid analgesics administered were morphine or oxycodone in 35 (77.8%) of the 45 patients. The incidence of diarrhea was higher in the group that was administered oxycodone. Other baseline characteristics did not significantly differ between the simultaneous combination and the non-simultaneous combination groups.

Table 1.
Patient characteristics.
3.2. Incidence and Severity of Diarrhea
We summarized the incidence and severity of diarrhea in each group (Table 2). Diarrhea occurred in 15 (33.3%) of the 45 patients. Grade 1 diarrhea was experienced by 14 patients (93.3%), and grade 2 diarrhea was experienced by 1 (6.7%) patient; no cases of grade 3 or 4 diarrhea were observed. The incidence of diarrhea was significantly lower in the simultaneous combination group (n = 2; 11.1%) than in the non-simultaneous combination group (n = 13; 48.1%) (95% confidence interval: 1.269–75.679, p = 0.011).

Table 2.
Onset and severity of diarrhea according to the timing of naldemedine administration.
3.3. Factors Influencing the Development of Diarrhea
Univariate analysis suggested that the non-simultaneous administration of naldemedine was associated with the development of diarrhea (odds ratio: 7.43; 95% confidence interval: 1.42–38.80, p = 0.017) (Table 3). The results of the multivariate analysis identified the non-simultaneous introduction of naldemedine as an independent risk factor for the development of diarrhea (odds ratio: 6.68; 95% confidence interval: 1.220–36.700, p = 0.028).

Table 3.
Univariate and multivariate analysis of factors influencing the incidence of diarrhea.
4. Discussion
This retrospective study of the occurrence of diarrhea following naldemedine administration in patients with lung cancer assessed the impact of the timing of naldemedine treatment initiation. The results showed that diarrhea occurred in 11.1% of patients in whom naldemedine and strong opioid analgesics were introduced simultaneously. This is consistent with prior studies that reported the development of diarrhea in 8–10% of cases [27]. However, the present study also showed that diarrhea occurred in 48.14% of patients in whom the introduction of naldemedine was delayed. This is consistent with the findings of Japanese clinical trials in patients with cancer and OIC, which reported the development of diarrhea in 19.6–39.7% of patients [21,23]. Longer durations of opioid administration before initiating naldemedine treatment and initiating naldemedine treatment 8 days after the initiation of opioid analgesia have been reported as risk factors for diarrhea, suggesting the benefits of initiating naldemedine and opioid analgesics simultaneously [24,25,32]. However, it has also been reported that the prophylactic effect of naldemedine against OIC is low for oxycodone [33].
In this study, we also considered the concomitant use of constipation medications, sex, and intravenous anticancer drugs as potential confounding factors in the multivariate analysis [32]. Delayed naldemedine introduction was positively associated with the occurrence of diarrhea, even after adjusting for these factors. However, more patients in the non-simultaneous group were taking constipation medications. Although there was no significant difference, the patient population with abnormal bowel movements may be more likely to be included in the non-simultaneous group because they are taking constipation medications.
OIC is mainly caused by the binding of exogenous opioids and peripheral μ-opioid receptors in the submucosal plexus and mesenteric plexus of the enteric nervous system of the gastrointestinal tract. This causes changes in the neural output from the enteric nervous system, resulting in a decrease in intestinal motility, a decrease in intestinal fluid secretion, and an increase in intestinal fluid absorption. Naldemedine restores gastrointestinal function by inhibiting the action of opioids at peripheral μ-opioid receptors in the gastrointestinal tract, which may thereby promote the development of diarrhea [22,34]. Naldemedine is metabolized by CYP3A4 and is a substrate for P-glycoprotein. Concomitant use of CYP3A4 inhibitors or P-glycoprotein may therefore increase blood levels of naldemedine and the incidence of diarrhea, and is thus a potential confounding factor. This was particularly noticeable in the group being administered oxycodone.
Although this study showed that the incidence of diarrhea was reduced when naldemedine and strong opioid analgesics were introduced simultaneously, careful consideration is required before adopting this strategy. Naldemedine is not currently recommended as a first-line treatment for OIC, and the economic burden of administering naldemedine to patients without OIC should also be taken into account [35,36].
However, clinical practice does not always adhere to guidelines, and it has previously been reported that 12.2% of patients were simultaneously administered laxatives and naldemedine [37]. Considering the high prevalence of OIC and its impact on patient QOL, early administration of naldemedine may be a valid treatment option [8,9,10,11,12,17,18].
This study exhibited several limitations. First, this was a retrospective study involving a small number of patients conducted at a single institution, based on data obtained from medical records, from which some information may have been missing or recorded inconsistently. Second, owing to the retrospective nature of the study, we cannot exclude the potential influence of other unknown confounding factors, such as physical activity and dietary intake, on the development of diarrhea. Third, because this study focused on AEs, we did not examine treatment efficacy. Further prospective studies are therefore needed to confirm our findings and address the clinical questions raised by this study.
5. Conclusions
In conclusion, although the results are from a retrospective study with a small sample size, they indicate that the simultaneous administration of naldemedine and oxycodone may prevent the development of diarrhea.
Author Contributions
T.M. (Takuma Matsumoto) designed the protocol, carried out the study, and drafted the manuscript. T.M. (Takuma Matsumoto) collected, analyzed, and parsed data from clinical records. T.M. (Takuya Mura), S.W., T.W., Y.T., N.M., T.H., S.H. and Y.S. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethics Committee of NHO Iwakuni Medical Center (Approval No. 0347) and was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon reasonable request, following the institutional applicable guidelines and approvals.
Acknowledgments
We would like to express our sincere gratitude to Natsumi Yamashita of the NHO Shikoku Cancer Center for her advice on statistical analysis. We also express our sincere gratitude to all those who cooperated with us in conducting this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AEs | adverse events |
| OIC | opioid-induced constipation |
| QOL | quality of life |
| AGA | American Gastroenterological Association |
| BSA | body surface area |
References
- World Health Organization. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Plante, G.E.; VanItallie, T.B. Opioids for cancer pain: The challenge of optimizing treatment. Metabolism 2010, 59 (Suppl. S1), S47–S52. [Google Scholar] [CrossRef]
- Lazzari, M.; Greco, M.T.; Marcassa, C.; Finocchi, S.; Caldarulo, C.; Corli, O. Efficacy and tolerability of oral oxycodone and oxycodone/naloxone combination in opioid-naïve cancer patients: A propensity analysis. Drug Des. Devel. Ther. 2015, 9, 5863–5872. [Google Scholar] [CrossRef]
- Bruera, E.; Paice, J.A. Cancer pain management: Safe and effective use of opioids. Am. Soc. Clin. Oncol. Educ. Book 2015, e593–e599. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, H.; Shinjo, T.; Morita, T.; Kohara, H.; Yomiya, K. Revision of pharmacological treatment recommendations for cancer pain: Clinical guidelines from the Japanese Society of Palliative Medicine. J. Palliat. Med. 2022, 25, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Drewes, A.M.; Chiarioni, G.; De Giorgio, R.; O’Brien, T.; Morlion, B.; Tack, J. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United Eur. Gastroenterol. J. 2019, 7, 7–20. [Google Scholar] [CrossRef]
- Drewes, A.M.; Munkholm, P.; Simrén, M.; Breivik, H.; Kongsgaard, U.E.; Hatlebakk, J.G.; Agreus, L.; Friedrichsen, M.; Christrup, L.L. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-Recommendations of the Nordic Working Group. Scand. J. Pain 2016, 11, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lissner, S.; Bassotti, G.; Coffin, B.; Drewes, A.M.; Breivik, H.; Eisenberg, E.; Emmanuel, A.; Laroche, F.; Meissner, W.; Morlion, B. Opioid-induced constipation and bowel dysfunction: A clinical guideline. Pain Med. 2017, 18, 1837–1863. [Google Scholar] [CrossRef]
- Hjalte, F.; Berggren, A.C.; Bergendahl, H.; Hjortsberg, C. The direct and indirect costs of opioid-induced constipation. J. Pain Symptom Manag. 2010, 40, 696–703. [Google Scholar] [CrossRef]
- Abramowitz, L.; Béziaud, N.; Labreze, L.; Giardina, V.; Caussé, C.; Chuberre, B.; Allaert, F.A.; Perrot, S. Prevalence and impact of constipation and bowel dysfunction induced by strong opioids: A cross-sectional survey of 520 patients with cancer pain: DYONISOS study. J. Med. Econ. 2013, 16, 1423–1433. [Google Scholar] [CrossRef]
- Ducrotté, P.; Milce, J.; Soufflet, C.; Fabry, C. Prevalence and clinical features of opioid-induced constipation in the general population: A French study of 15,000 individuals. United Eur. Gastroenterol. J. 2017, 5, 588–600. [Google Scholar] [CrossRef]
- Ishihara, M.; Ikesue, H.; Matsunaga, H.; Suemaru, K.; Kitaichi, K.; Suetsugu, K.; Oishi, R.; Sendo, T.; Araki, H.; Itoh, Y.; et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin. J. Pain 2012, 28, 373–381. [Google Scholar] [CrossRef]
- Bell, T.J.; Panchal, S.J.; Miaskowski, C.; Bolge, S.C.; Milanova, T.; Williamson, R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med. 2009, 10, 35–42. [Google Scholar] [CrossRef]
- Varrassi, G.; Banerji, V.; Gianni, W.; Marinangeli, F.; Pinto, C. Impact and consequences of opioid-induced constipation: A survey of patients. Pain Ther. 2021, 10, 1139–1153. [Google Scholar] [CrossRef]
- Christensen, H.N.; Olsson, U.; From, J.; Breivik, H. Opioid-induced constipation, use of laxatives, and health-related quality of life. Scand. J. Pain 2016, 11, 104–110. [Google Scholar] [CrossRef]
- Crockett, S.D.; Greer, K.B.; Heidelbaugh, J.J.; Falck-Ytter, Y.; Hanson, B.J.; Sultan, S.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the medical management of opioid-induced constipation. Gastroenterology 2019, 156, 218–226. [Google Scholar] [CrossRef]
- Pappagallo, M. Incidence, prevalence, and management of opioid bowel dysfunction. Am. J. Surg. 2001, 182, 11S–18S. [Google Scholar] [CrossRef]
- Emmanuel, A.; Johnson, M.; McSkimming, P.; Dickerson, S. Laxatives Do Not Improve Symptoms of Opioid-Induced Constipation: Results of a Patient Survey. Pain Med. 2017, 18, 1932–1940. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Drewes, A.M.; Ayob, F.; Viswanath, O.; Urits, I.; Corli, O.; Pergolizzi, J., Jr.; Varrassi, G. Efficacy and safety of peripherally acting μ-opioid receptor antagonist (pamoras) for the management of patients with opioid-induced constipation: A systematic review. Cureus 2021, 13, e16201. [Google Scholar] [CrossRef]
- Hale, M.; Wild, J.; Reddy, J.; Yamada, T.; Arjona Ferreira, J.C. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): Two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol. Hepatol. 2017, 2, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Oda, K.; Tauchi, K.; Nakata, K.; Shinozaki, K.; Yokota, T.; Suzuki, Y.; Narabayashi, M.; Boku, N. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J. Clin. Oncol. 2017, 35, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Kanemasa, T.; Koike, K.; Takase, K.; Arai, T.; Nakamura, A.; Morioka, Y.; Hasegawa, M. Pharmacological profile of naldemedine, a peripherally acting μ-opioid receptor antagonist: Comparison with naloxone and naloxegol. J. Pharmacol. Exp. Ther. 2020, 373, 438–444. [Google Scholar] [CrossRef]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized Phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J. Clin. Oncol. 2017, 35, 3859–3866. [Google Scholar] [CrossRef]
- Takagi, Y.; Osawa, G.; Kato, Y.; Ikezawa, E.; Kobayashi, C.; Aruga, E. Prevention and management of diarrhea associated with naldemedine among patients receiving opioids: A retrospective cohort study. BMC Gastroenterol. 2020, 20, 25. [Google Scholar] [CrossRef]
- Okamoto, A.; Ikemura, K.; Mizutani, E.; Wamoto, T.; Okuda, M. Opioid therapy duration before naldemedine treatment is a significant independent risk of diarrhea: A retrospective cohort study. J. Pharm. Health Care Sci. 2021, 7, 3. [Google Scholar] [CrossRef]
- Tokoro, A.; Imai, H.; Fumita, S.; Harada, T.; Noriyuki, T.; Gamoh, M.; Akashi, Y.; Sato, H.; Kizawa, Y. Incidence of opioid-induced constipation in Japanese patients with cancer pain: A prospective observational cohort study. Cancer Med. 2019, 8, 4883–4891. [Google Scholar] [CrossRef]
- Ozaki, A.; Kessoku, T.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Takeda, Y.; Kasai, Y.; Iwaki, M.; Kobayashi, T.; Yoshihara, T.; et al. Effectiveness of naldemedine compared with magnesium oxide in preventing opioid-induced constipation: A randomized controlled trial. Cancers 2022, 14, 2112. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311, discussion 312–313. [Google Scholar]
- Shionogi & Co. Ltd. Symproic® Tablets 0.2 mg, Interview Form, Revised in May 2023. 7th edn (6 May 2024). Available online: https://www.shionogi.com/jp/ja/ (accessed on 2 December 2024).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Fărcaş, R.A.; Grad, S.; Dumitraşcu, D.L. Microscopic colitis: An update. Med. Pharm. Rep. 2022, 95, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, J.; Shiojiri, K.; Ryu, E.; Kawauchi, Y.; Hasegawa, K.; Ezaki, N.; Yamashita, H.; Ishii, K.; Harasawa, H.; Nakamura, T.; et al. Analysis of predictive factors for diarrhea after the administration of naldemedine. Biol. Pharm. Bull. 2021, 44, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Jimaru, Y.; Torii, S.; Mitsuba, N.; Takahashi, K. A retrospective observational study of factors affecting the efficacy of concurrent prescription of naldemedine for opioid-induced constipation caused by oxycodone tablets. Biol. Pharm. Bull. 2023, 46, 1826–1831. [Google Scholar] [CrossRef]
- Mori, T.; Shibasaki, Y.; Matsumoto, K.; Shibasaki, M.; Hasegawa, M.; Wang, E.; Masukawa, D.; Yoshizawa, K.; Horie, S.; Suzuki, T. Mechanisms that underlie μ-opioid receptor agonist-induced constipation: Differential involvement of μ-opioid receptor sites and responsible regions. J. Pharmacol. Exp. Ther. 2013, 347, 91–99. [Google Scholar] [CrossRef]
- Ozaki, A.; Kessoku, T.; Iwaki, M.; Kobayashi, T.; Yoshihara, T.; Kato, T.; Honda, Y.; Ogawa, Y.; Imajo, K.; Higurashi, T.; et al. Comparing the effectiveness of magnesium oxide and naldemedine in preventing opioid-induced constipation: A proof of concept, single institutional, two arm, open-label, phase II, randomized controlled trial: The MAGNET study. Trials 2020, 21, 453. [Google Scholar] [CrossRef]
- Kistemaker, K.R.J.; Sijani, F.; Brinkman, D.J.; de Graeff, A.; Burchell, G.L.; Steegers, M.A.H.; van Zuylen, L. Pharmacological prevention and treatment of opioid-induced constipation in cancer patients: A systematic review and meta-analysis. Cancer Treat. Rev. 2024, 125, 102704. [Google Scholar] [CrossRef]
- Hiruta, E.; Fujita, Y.; Imai, H.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Ito, M.; Takei, S.; Matsuura, M.; et al. Real-world patient characteristics and treatment patterns of naldemedine for the treatment of opioid-induced constipation in patients with cancer: A multicenter retrospective chart review study. Medicina 2021, 57, 1233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).