Timing of Naldemedine Initiation and Occurrence of Diarrhea in Patients Receiving Strong Opioid Analgesics: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
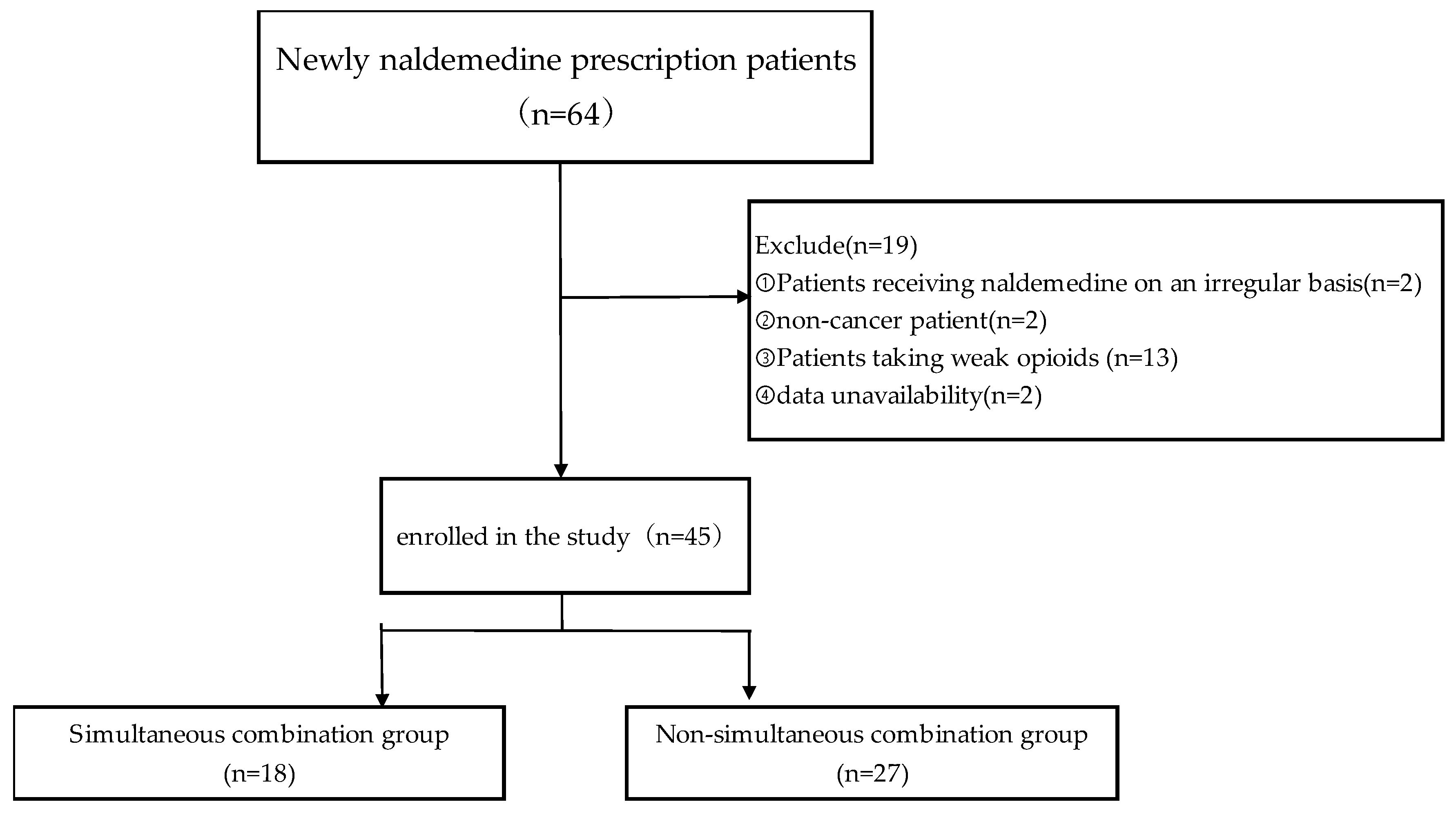
2.1. Patient Selection
2.2. Ethics
2.3. Evaluation of Diarrhea
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Incidence and Severity of Diarrhea
3.3. Factors Influencing the Development of Diarrhea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEs | adverse events |
| OIC | opioid-induced constipation |
| QOL | quality of life |
| AGA | American Gastroenterological Association |
| BSA | body surface area |
References
- World Health Organization. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Plante, G.E.; VanItallie, T.B. Opioids for cancer pain: The challenge of optimizing treatment. Metabolism 2010, 59 (Suppl. S1), S47–S52. [Google Scholar] [CrossRef]
- Lazzari, M.; Greco, M.T.; Marcassa, C.; Finocchi, S.; Caldarulo, C.; Corli, O. Efficacy and tolerability of oral oxycodone and oxycodone/naloxone combination in opioid-naïve cancer patients: A propensity analysis. Drug Des. Devel. Ther. 2015, 9, 5863–5872. [Google Scholar] [CrossRef]
- Bruera, E.; Paice, J.A. Cancer pain management: Safe and effective use of opioids. Am. Soc. Clin. Oncol. Educ. Book 2015, e593–e599. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, H.; Shinjo, T.; Morita, T.; Kohara, H.; Yomiya, K. Revision of pharmacological treatment recommendations for cancer pain: Clinical guidelines from the Japanese Society of Palliative Medicine. J. Palliat. Med. 2022, 25, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Drewes, A.M.; Chiarioni, G.; De Giorgio, R.; O’Brien, T.; Morlion, B.; Tack, J. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United Eur. Gastroenterol. J. 2019, 7, 7–20. [Google Scholar] [CrossRef]
- Drewes, A.M.; Munkholm, P.; Simrén, M.; Breivik, H.; Kongsgaard, U.E.; Hatlebakk, J.G.; Agreus, L.; Friedrichsen, M.; Christrup, L.L. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-Recommendations of the Nordic Working Group. Scand. J. Pain 2016, 11, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lissner, S.; Bassotti, G.; Coffin, B.; Drewes, A.M.; Breivik, H.; Eisenberg, E.; Emmanuel, A.; Laroche, F.; Meissner, W.; Morlion, B. Opioid-induced constipation and bowel dysfunction: A clinical guideline. Pain Med. 2017, 18, 1837–1863. [Google Scholar] [CrossRef]
- Hjalte, F.; Berggren, A.C.; Bergendahl, H.; Hjortsberg, C. The direct and indirect costs of opioid-induced constipation. J. Pain Symptom Manag. 2010, 40, 696–703. [Google Scholar] [CrossRef]
- Abramowitz, L.; Béziaud, N.; Labreze, L.; Giardina, V.; Caussé, C.; Chuberre, B.; Allaert, F.A.; Perrot, S. Prevalence and impact of constipation and bowel dysfunction induced by strong opioids: A cross-sectional survey of 520 patients with cancer pain: DYONISOS study. J. Med. Econ. 2013, 16, 1423–1433. [Google Scholar] [CrossRef]
- Ducrotté, P.; Milce, J.; Soufflet, C.; Fabry, C. Prevalence and clinical features of opioid-induced constipation in the general population: A French study of 15,000 individuals. United Eur. Gastroenterol. J. 2017, 5, 588–600. [Google Scholar] [CrossRef]
- Ishihara, M.; Ikesue, H.; Matsunaga, H.; Suemaru, K.; Kitaichi, K.; Suetsugu, K.; Oishi, R.; Sendo, T.; Araki, H.; Itoh, Y.; et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin. J. Pain 2012, 28, 373–381. [Google Scholar] [CrossRef]
- Bell, T.J.; Panchal, S.J.; Miaskowski, C.; Bolge, S.C.; Milanova, T.; Williamson, R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med. 2009, 10, 35–42. [Google Scholar] [CrossRef]
- Varrassi, G.; Banerji, V.; Gianni, W.; Marinangeli, F.; Pinto, C. Impact and consequences of opioid-induced constipation: A survey of patients. Pain Ther. 2021, 10, 1139–1153. [Google Scholar] [CrossRef]
- Christensen, H.N.; Olsson, U.; From, J.; Breivik, H. Opioid-induced constipation, use of laxatives, and health-related quality of life. Scand. J. Pain 2016, 11, 104–110. [Google Scholar] [CrossRef]
- Crockett, S.D.; Greer, K.B.; Heidelbaugh, J.J.; Falck-Ytter, Y.; Hanson, B.J.; Sultan, S.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the medical management of opioid-induced constipation. Gastroenterology 2019, 156, 218–226. [Google Scholar] [CrossRef]
- Pappagallo, M. Incidence, prevalence, and management of opioid bowel dysfunction. Am. J. Surg. 2001, 182, 11S–18S. [Google Scholar] [CrossRef]
- Emmanuel, A.; Johnson, M.; McSkimming, P.; Dickerson, S. Laxatives Do Not Improve Symptoms of Opioid-Induced Constipation: Results of a Patient Survey. Pain Med. 2017, 18, 1932–1940. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Drewes, A.M.; Ayob, F.; Viswanath, O.; Urits, I.; Corli, O.; Pergolizzi, J., Jr.; Varrassi, G. Efficacy and safety of peripherally acting μ-opioid receptor antagonist (pamoras) for the management of patients with opioid-induced constipation: A systematic review. Cureus 2021, 13, e16201. [Google Scholar] [CrossRef]
- Hale, M.; Wild, J.; Reddy, J.; Yamada, T.; Arjona Ferreira, J.C. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): Two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol. Hepatol. 2017, 2, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Oda, K.; Tauchi, K.; Nakata, K.; Shinozaki, K.; Yokota, T.; Suzuki, Y.; Narabayashi, M.; Boku, N. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J. Clin. Oncol. 2017, 35, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Kanemasa, T.; Koike, K.; Takase, K.; Arai, T.; Nakamura, A.; Morioka, Y.; Hasegawa, M. Pharmacological profile of naldemedine, a peripherally acting μ-opioid receptor antagonist: Comparison with naloxone and naloxegol. J. Pharmacol. Exp. Ther. 2020, 373, 438–444. [Google Scholar] [CrossRef]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized Phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J. Clin. Oncol. 2017, 35, 3859–3866. [Google Scholar] [CrossRef]
- Takagi, Y.; Osawa, G.; Kato, Y.; Ikezawa, E.; Kobayashi, C.; Aruga, E. Prevention and management of diarrhea associated with naldemedine among patients receiving opioids: A retrospective cohort study. BMC Gastroenterol. 2020, 20, 25. [Google Scholar] [CrossRef]
- Okamoto, A.; Ikemura, K.; Mizutani, E.; Wamoto, T.; Okuda, M. Opioid therapy duration before naldemedine treatment is a significant independent risk of diarrhea: A retrospective cohort study. J. Pharm. Health Care Sci. 2021, 7, 3. [Google Scholar] [CrossRef]
- Tokoro, A.; Imai, H.; Fumita, S.; Harada, T.; Noriyuki, T.; Gamoh, M.; Akashi, Y.; Sato, H.; Kizawa, Y. Incidence of opioid-induced constipation in Japanese patients with cancer pain: A prospective observational cohort study. Cancer Med. 2019, 8, 4883–4891. [Google Scholar] [CrossRef]
- Ozaki, A.; Kessoku, T.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Takeda, Y.; Kasai, Y.; Iwaki, M.; Kobayashi, T.; Yoshihara, T.; et al. Effectiveness of naldemedine compared with magnesium oxide in preventing opioid-induced constipation: A randomized controlled trial. Cancers 2022, 14, 2112. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311, discussion 312–313. [Google Scholar]
- Shionogi & Co. Ltd. Symproic® Tablets 0.2 mg, Interview Form, Revised in May 2023. 7th edn (6 May 2024). Available online: https://www.shionogi.com/jp/ja/ (accessed on 2 December 2024).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Fărcaş, R.A.; Grad, S.; Dumitraşcu, D.L. Microscopic colitis: An update. Med. Pharm. Rep. 2022, 95, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, J.; Shiojiri, K.; Ryu, E.; Kawauchi, Y.; Hasegawa, K.; Ezaki, N.; Yamashita, H.; Ishii, K.; Harasawa, H.; Nakamura, T.; et al. Analysis of predictive factors for diarrhea after the administration of naldemedine. Biol. Pharm. Bull. 2021, 44, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Jimaru, Y.; Torii, S.; Mitsuba, N.; Takahashi, K. A retrospective observational study of factors affecting the efficacy of concurrent prescription of naldemedine for opioid-induced constipation caused by oxycodone tablets. Biol. Pharm. Bull. 2023, 46, 1826–1831. [Google Scholar] [CrossRef]
- Mori, T.; Shibasaki, Y.; Matsumoto, K.; Shibasaki, M.; Hasegawa, M.; Wang, E.; Masukawa, D.; Yoshizawa, K.; Horie, S.; Suzuki, T. Mechanisms that underlie μ-opioid receptor agonist-induced constipation: Differential involvement of μ-opioid receptor sites and responsible regions. J. Pharmacol. Exp. Ther. 2013, 347, 91–99. [Google Scholar] [CrossRef]
- Ozaki, A.; Kessoku, T.; Iwaki, M.; Kobayashi, T.; Yoshihara, T.; Kato, T.; Honda, Y.; Ogawa, Y.; Imajo, K.; Higurashi, T.; et al. Comparing the effectiveness of magnesium oxide and naldemedine in preventing opioid-induced constipation: A proof of concept, single institutional, two arm, open-label, phase II, randomized controlled trial: The MAGNET study. Trials 2020, 21, 453. [Google Scholar] [CrossRef]
- Kistemaker, K.R.J.; Sijani, F.; Brinkman, D.J.; de Graeff, A.; Burchell, G.L.; Steegers, M.A.H.; van Zuylen, L. Pharmacological prevention and treatment of opioid-induced constipation in cancer patients: A systematic review and meta-analysis. Cancer Treat. Rev. 2024, 125, 102704. [Google Scholar] [CrossRef]
- Hiruta, E.; Fujita, Y.; Imai, H.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Ito, M.; Takei, S.; Matsuura, M.; et al. Real-world patient characteristics and treatment patterns of naldemedine for the treatment of opioid-induced constipation in patients with cancer: A multicenter retrospective chart review study. Medicina 2021, 57, 1233. [Google Scholar] [CrossRef]
| Characteristic | All Patients (n = 45) | Simultaneous Combination (n = 18) | Non-Simultaneous Combination (n = 27) | p Value |
|---|---|---|---|---|
| Sex (female) | 12 (26.7%) | 7 (38.8%) | 5 (18.5%) | 0.175 (a) |
| Age (years) | 71 [65–80] | 71 [63–77] | 72 [66–81] | 0.215 (b) |
| Height (cm) | 162.2 [155.5–168.5] | 162 [154.5–166.6] | 164.4 [156.9–169.3] | 0.424 (b) |
| Weight (kg) | 53.9 [44.2–64] | 55.1 [45.3–64.1] | 51.7 [45.1–59.8] | 0.516 (b) |
| BSA (m2) | 1.569 [1.402–1.688] | 1.599 [1.415–1.683] | 1.535 [1.419–1.694] | 0.853 (b) |
| Performance status (2–3) | 17 (37.8%) | 5 (27.8%) | 12 (44.4%) | 0.315 (a) |
| Serum creatinine (mg/dL) | 0.75 [0.64–0.95] | 0.79 [0.72–0.95] | 0.71 [0.61–0.93] | 0.190 (b) |
| ALB (g/L) | 2.8 [2.2–3.4] | 3.25 [2.5–3.75] | 2.7 [2.15–3.1] | 0.053 (b) |
| AST (IU/L) | 24 [18–35] | 21.5 [18–38.8] | 24 [18.5–33] | 0.954 (b) |
| ALT (IU/L) | 16 [12–32] | 18 [10–52.3] | 15 [13–21.5] | 0.618 (b) |
| eGFR (mL/min/1.73 m2) | 75 [55–90] | 65 [48.5–76.8] | 79 [60–96] | 0.088 (b) |
| Suspected blood–brain barrier dysfunction | 0 | 0 | 0 | |
| Duration of opioid therapy before naldemedine administration (days) | 1 [0–5] | 0 | 3 [1–8] | <0.001 (b) |
| Opioid analgesics (regular dosing) | ||||
| Morphine | 15 (33.3%) | 7 (38.9%) | 8 (29.6%) | 0.538 (a) |
| Oxycodone | 20 (44.4%) | 6 (33.3%) | 14 (51.9%) | 0.359 (a) |
| Fentanyl | 1 (2.2%) | 0 | 1 (3.7%) | 1.000 (a) |
| Hydromorphone | 9 (20.0%) | 5 (27.8%) | 4 (14.8%) | 0.192 (a) |
| Onset of diarrhea | ||||
| Morphine | 4 (8.9%) | 2 (11.1%) | 2 (7.4%) | 1.000 (a) |
| Oxycodone | 10 (22.2%) | 0 | 10 (37.0%) | 0.011 (a) |
| Fentanyl | 0 | 0 | 0 | |
| Hydromorphone | 1 (2.2%) | 0 | 1 (3.7%) | 0.444 (a) |
| Median number of 3-day defecations prior to naldemedine (−3 to −1) | 3 [1–5.5] | 1 [0.25–2] | 1 [1–3] | 0.192 (a) |
| Co-administrated drugs | ||||
| Drug with risk of constipation | 38 (84.4%) | 14 (77.8%) | 24 (88.9%) | 0.412 (a) |
| PPI | 28 (62.2%) | 12 (66.7%) | 16 (59.3%) | 0.757 (a) |
| H2-blocker | 3 (6.7%) | 0 | 3 (11.1%) | 0.264 (a) |
| NSAIDs | 30 (66.7%) | 10 (55.6%) | 20 (74.1%) | 0.218 (a) |
| SSRI | 1 (2.2%) | 0 | 1 (3.7%) | 1.000 (a) |
| Aspirin | 2 (4.4%) | 0 | 2 (7.4%) | 0.509 (a) |
| Anticholinergics | 0 | 0 | 0 | |
| Digestive stimulant | 7 (15.6%) | 1 (5.6%) | 6 (22.2%) | 0.215 (a) |
| Concomitant use of constipation medication (regular dosing) | 30 (66.7%) | 13 (72.2%) | 17 (63.0%) | 0.748 (a) |
| Magnesium oxide | 28 (62.2%) | 13 (72.2%) | 15 (55.6%) | 0.351 (a) |
| Lubiprostone | 5 (11.1%) | 3 (16.7%) | 2 (7.4%) | 0.375 (a) |
| Linaclotide | 1 (2.2%) | 1 (5.6%) | 0 | 0.400 (a) |
| CYP3A4 inhibitor | 0 | 0 | 0 | |
| CYP3A4 inducer | 22 (48.9%) | 8 (44.4%) | 14 (51.9%) | 0.763 (a) |
| P-glycoprotein inhibitor | 4 (8.9%) | 2 (11.1%) | 2 (7.4%) | 0.375 (a) |
| Intravenous anticancer drug | 28 (62.2%) | 13 (72.2%) | 15 (55.6%) | 0.351 (a) |
| Amrubicin | 2 (4.4%) | 2 (11.1%) | 0 | 0.155 (a) |
| Atezolizumab | 2 (4.4%) | 1 (5.6%) | 1 (3.7%) | 1.000 (a) |
| Bevacizumab | 2 (4.4%) | 0 | 2 (7.4%) | 0.509 (a) |
| Carboplatin | 3 (6.7%) | 1 (5.6%) | 2 (7.4%) | 1.000 (a) |
| Cisplatin | 2 (4.4%) | 0 | 2 (7.4%) | 0.509 (a) |
| Dacomitinib | 1 (2.2%) | 0 | 1 (3.7%) | 1.000 (a) |
| Docetaxel | 1 (2.2%) | 1 (5.6%) | 0 | 0.400 (a) |
| Gemcitabine | 2 (4.4%) | 0 | 2 (7.4%) | 0.509 (a) |
| Nivolumab | 2 (4.4%) | 1 (5.6%) | 1 (3.7%) | 1.000 (a) |
| Osimertinib | 1 (2.2%) | 0 | 1 (3.7%) | 1.000 (a) |
| Paclitaxel | 3 (6.7%) | 2 (11.1%) | 1 (3.7%) | 0.555 (a) |
| Pembrolizumab | 5 (11.1%) | 3 (16.7%) | 2 (7.4%) | 0.375 (a) |
| Pemetrexed | 6 (13.3%) | 2 (11.1%) | 4 (14.8%) | 1.000 (a) |
| Tegafur/Gimeracil/Oteracil potassium | 4 (8.9%) | 2 (11.1%) | 2 (7.4%) | 1.000 (a) |
| Vinorelbine | 1 (2.2%) | 0 | 1 (3.7%) | 1.000 (a) |
| Diarrhea | Simultaneous Combination (n = 18) | Non-Simultaneous Combination (n = 27) | 95% CI | p Value |
|---|---|---|---|---|
| No diarrhea | 16 (88.9%) | 14 (51.9%) | ||
| Diarrhea | 2 (11.1%) | 13 (48.1%) | 1.269–75.679 | 0.011 |
| Grade 1 | 2 (11.1%) | 12 (44.4%) | ||
| Grade 2 | 0 (0%) | 1 (3.7%) | ||
| Grade 3 | 0 (0%) | 0 (0%) | ||
| Grage 4 | 0 (0%) | 0 (0%) |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | |
| Non-simultaneous naldemedine administration | 7.430 | 1.420–38.800 | 0.017 | 6.680 | 1.220–36.700 | 0.028 |
| Sex | 1.000 | 0.289–3.460 | 1.000 | 0.574 | 0.104–3.180 | 0.525 |
| Oxycodone | 4.000 | 1.070–14.900 | 0.038 | 3.530 | 0.857–14.500 | 0.080 |
| Intravenous anticancer drug | 0.868 | 0.243–3.100 | 0.828 | |||
| Concomitant use of constipation medication | 1.000 | 0.269–3.720 | 1.000 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Mura, T.; Wada, T.; Tsugo, Y.; Mukai, N.; Hamaoka, T.; Horita, S.; Semba, Y.; Watanabe, S. Timing of Naldemedine Initiation and Occurrence of Diarrhea in Patients Receiving Strong Opioid Analgesics: A Retrospective Study. Pharmacy 2025, 13, 47. https://doi.org/10.3390/pharmacy13020047
Matsumoto T, Mura T, Wada T, Tsugo Y, Mukai N, Hamaoka T, Horita S, Semba Y, Watanabe S. Timing of Naldemedine Initiation and Occurrence of Diarrhea in Patients Receiving Strong Opioid Analgesics: A Retrospective Study. Pharmacy. 2025; 13(2):47. https://doi.org/10.3390/pharmacy13020047
Chicago/Turabian StyleMatsumoto, Takuma, Takuya Mura, Tsubasa Wada, Yuki Tsugo, Naoko Mukai, Terutaka Hamaoka, Shuji Horita, Yasushi Semba, and Shinichi Watanabe. 2025. "Timing of Naldemedine Initiation and Occurrence of Diarrhea in Patients Receiving Strong Opioid Analgesics: A Retrospective Study" Pharmacy 13, no. 2: 47. https://doi.org/10.3390/pharmacy13020047
APA StyleMatsumoto, T., Mura, T., Wada, T., Tsugo, Y., Mukai, N., Hamaoka, T., Horita, S., Semba, Y., & Watanabe, S. (2025). Timing of Naldemedine Initiation and Occurrence of Diarrhea in Patients Receiving Strong Opioid Analgesics: A Retrospective Study. Pharmacy, 13(2), 47. https://doi.org/10.3390/pharmacy13020047






