Deprescribing NSAIDs: The Potential Role of Community Pharmacists
Abstract
1. Introduction
1.1. Pain and Pain Management
1.2. Deprescription, Deprescribing Process, and a Potential Role of Pharmacists
2. Methods
2.1. Study Design and Target Group
2.2. Survey
2.2.1. Pilot Testing
2.2.2. Content of the Questionnaire
2.3. Ethics
2.4. Data Handling
2.5. Statistical Analysis
3. Results
3.1. Response Rate
3.2. Descriptive Statistics
3.2.1. Sociodemographic Characteristics of Study Participants
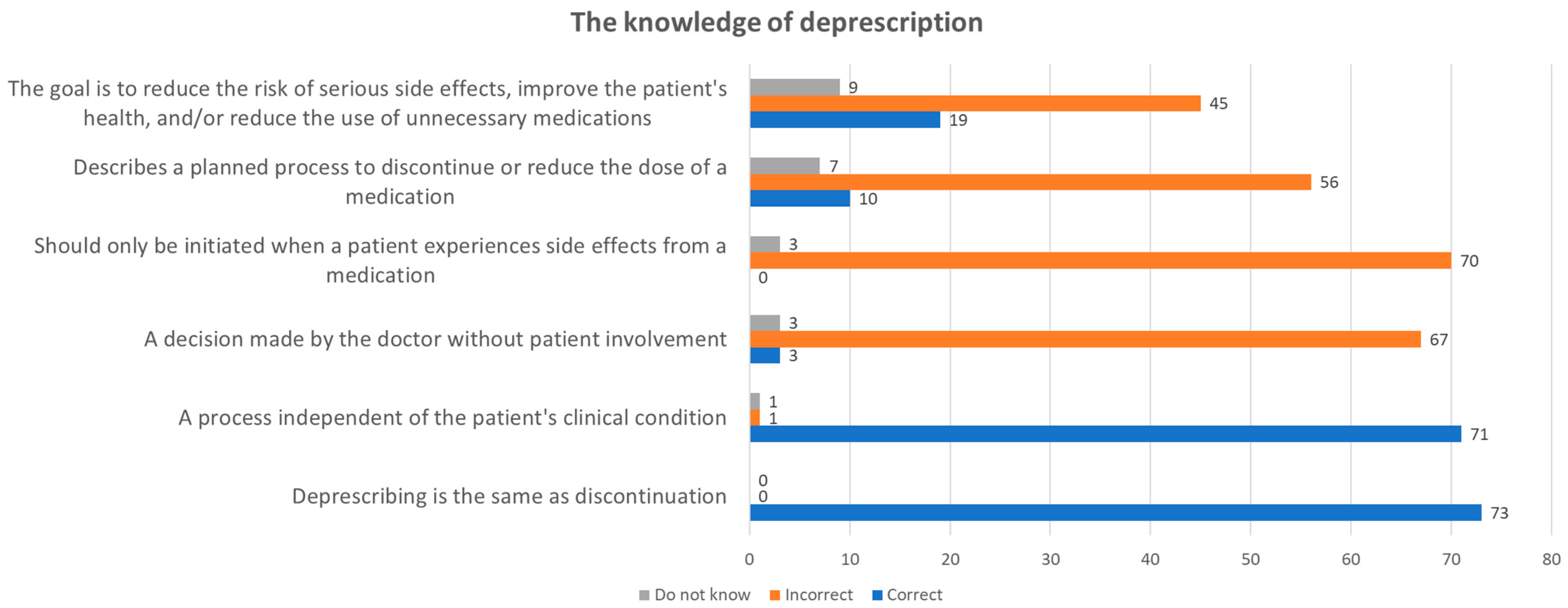
3.2.2. Knowledge about Deprescription
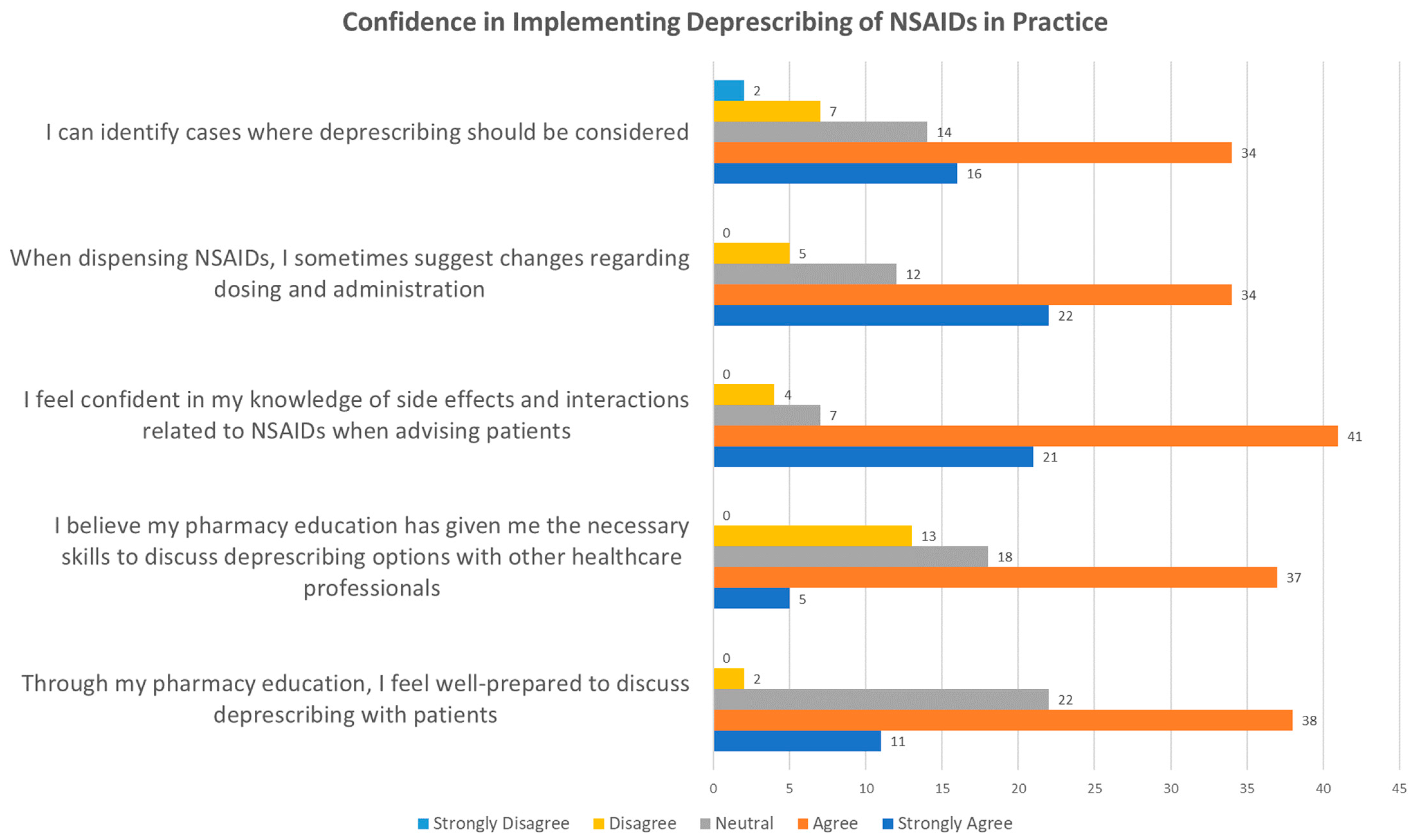
3.2.3. Confidence in Implementing Deprescription of NSAIDs in Practice
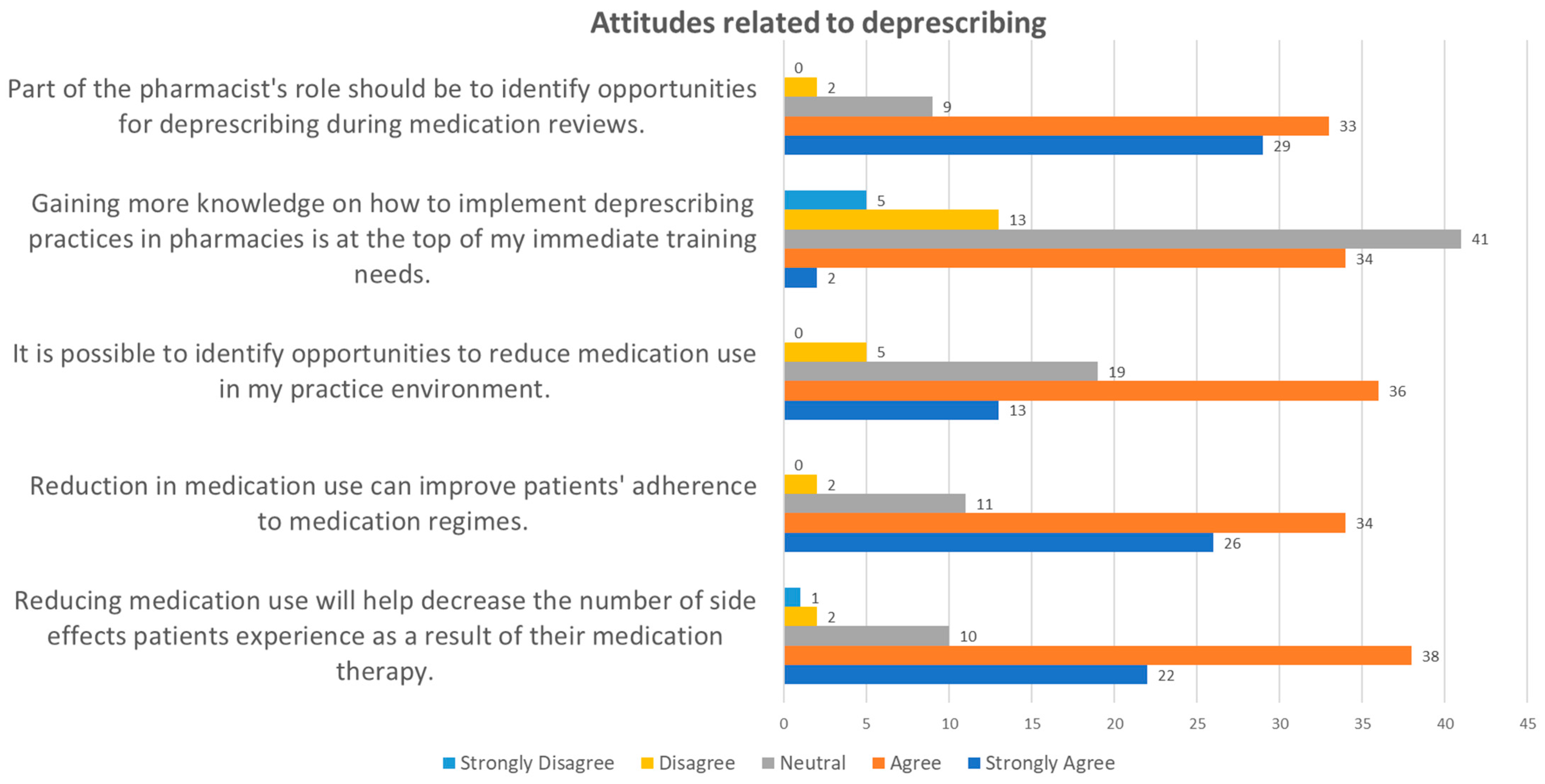
3.2.4. Attitudes Related to Deprescription
3.2.5. Challenges and Opportunities for Implementing Deprescription in Practice
3.3. Inferential Statistics
3.4. Existing Guidelines
4. Discussion
4.1. Discussion of Method
4.1.1. Choice of Method
4.1.2. Integration of Qualitative Data
4.2. Discussion of the Results
4.2.1. Response Rate
4.2.2. Results from Testing Hypotheses and Sociodemographic Characteristics
4.2.3. Knowledge about Deprescription
4.2.4. Pharmacists’ Confidence in Implementing Deprescription in Practice
4.2.5. Attitudes Related to Deprescription
4.2.6. Challenges and Opportunities for Implementing Deprescription in Practice
4.2.7. Guidelines for Deprescription Analgesics
4.3. Strengths and Weaknesses of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machado, G.C.; Abdel-Shaheed, C.; Underwood, M.; Day, R.O. Non-steroidal anti-inflammatory drugs (NSAIDs) for musculoskeletal pain. Bmj 2021, 372, n104. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Robson, J. The dangers of NSAIDs: Look both ways. Br. J. Gen. Pract. 2016, 66, 172. [Google Scholar] [CrossRef] [PubMed]
- Wehling, M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: Management and mitigation of risks and adverse effects. Eur. J. Clin. Pharmacol. 2014, 70, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Reeve, E.; Gnjidic, D.; Long, J.; Hilmer, S. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: Implications for future research and clinical practice. Br. J. Clin. Pharmacol. 2015, 80, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Phueanpinit, P.; Pongwecharak, J.; Krska, J.; Jarernsiripornkul, N. Evaluation of community pharmacists’ roles in screening and communication of risks about non-steroidal anti-inflammatory drugs in Thailand. Prim. Health Care Res. Dev. 2018, 19, 598–604. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Fulda, K.G.; Blythe, R.; Chui, M.A.; Reeve, E.; Young, R.; Espinoza, A.; Hendrix, N.; Xiao, Y. Defining and enhancing collaboration between community pharmacists and primary care providers to improve medication safety. Expert. Opin. Drug Saf. 2022, 21, 1357–1364. [Google Scholar] [CrossRef]
- Cernasev, A.; Barenie, R.E.; Metzmeier, S.; Axon, D.R.; Springer, S.P.; Scott, D. Student Perspectives on the Pharmacist’s Role in Deprescribing Opioids: A Qualitative Study. Pharmacy 2023, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Ashkanani, F.Z.; Rathbone, A.P.; Lindsey, L. The role of pharmacists in deprescribing benzodiazepines: A scoping review. Explor. Res. Clin. Soc. Pharm. 2023, 12, 100328. [Google Scholar] [CrossRef]
- Jaber, D.; Al Shihab, A.; Tamimi, L.N. Efficacy and Safety of Pharmacist-Managed NSAIDs Deprescribing: A Jordanian Outpatient Study. J. Clin. Pharm. Ther. 2024, 2024, 5874686. [Google Scholar] [CrossRef]
- Alorfi, N.M.; Ashour, A.M.; Algarni, A.S.; Alsolami, F.A.; Alansari, A.M.; Tobaiqy, M. Assessment of the Community Pharmacists’ Knowledge and Attitudes Toward Pain and Pain Management in Saudi Arabia. Int. J. Gen. Med. 2022, 15, 8527–8537. [Google Scholar] [CrossRef]
- Korenvain, C.; MacKeigan, L.; Dainty, K.; Guilcher, S.J.T.; McCarthy, L. Exploring deprescribing opportunities for community pharmacists: Protocol for a qualitative study. Can Pharm. J. 2018, 151, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, K. The Role of the Community Pharmacists in the Management of Acute Pain in Adults: A Scoping Review. OSF 2022. [Google Scholar] [CrossRef]
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public. Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Sá, K.N.; Moreira, L.; Baptista, A.F.; Yeng, L.T.; Teixeira, M.J.; Galhardoni, R.; de Andrade, D.C. Prevalence of chronic pain in developing countries: Systematic review and meta-analysis. Pain Rep. 2019, 4, e779. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Eisenberg, E.; O’Brien, T. The individual and societal burden of chronic pain in Europe: The case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.A.; Campos, M.M. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef] [PubMed]
- Racine, M. Chronic pain and suicide risk: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 269–280. [Google Scholar] [CrossRef]
- Hadi, M.A.; McHugh, G.A.; Closs, S.J. Impact of Chronic Pain on Patients’ Quality of Life: A Comparative Mixed-Methods Study. J. Patient Exp. 2019, 6, 133–141. [Google Scholar] [CrossRef]
- Pandelani, F.F.; Nyalunga, S.L.N.; Mogotsi, M.M.; Mkhatshwa, V.B. Chronic pain: Its impact on the quality of life and gender. Front. Pain Res. 2023, 4, 1253460. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Graham, P.L.; Schofield, D.; Costa, D.S.J.; Nicholas, M. Productivity outcomes from chronic pain management interventions in the working age population; a systematic review. Pain 2024, 165, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.K. The biopsychosocial model of pain 40 years on: Time for a reappraisal? Pain 2022, 163, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Bannon, S.; Greenberg, J.; Mace, R.A.; Locascio, J.J.; Vranceanu, A.M. The role of social isolation in physical and emotional outcomes among patients with chronic pain. Gen. Hosp. Psychiatry 2021, 69, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, K.R.; Simpson, P.M.; Raff, H.; Grayson, M.H.; Zhang, L.; Weisman, S.J. Circulating inflammatory biomarkers in adolescents: Evidence of interactions between chronic pain and obesity. Pain Rep. 2021, 6, e916. [Google Scholar] [CrossRef] [PubMed]
- Foley, H.E.; Knight, J.C.; Ploughman, M.; Asghari, S.; Audas, R. Association of chronic pain with comorbidities and health care utilization: A retrospective cohort study using health administrative data. Pain 2021, 162, 2737–2749. [Google Scholar] [CrossRef] [PubMed]
- Tick, H.; Nielsen, A.; Pelletier, K.R.; Bonakdar, R.; Simmons, S.; Glick, R.; Ratner, E.; Lemmon, R.L.; Wayne, P.; Zador, V. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. Explore 2018, 14, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Malpus, Z. Pain as a Biopsychosocial Experience. In Pain; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Berger, S.E.; Baria, A.T. Assessing Pain Research: A Narrative Review of Emerging Pain Methods, Their Technosocial Implications, and Opportunities for Multidisciplinary Approaches. Front Pain Res. 2022, 3, 896276. [Google Scholar] [CrossRef]
- Finnerup, N.B. Nonnarcotic Methods of Pain Management. N. Engl. J. Med. 2019, 380, 2440–2448. [Google Scholar] [CrossRef]
- Alorfi, N.M. Pharmacological Methods of Pain Management: Narrative Review of Medication Used. Int. J. Gen. Med. 2023, 16, 3247–3256. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.E.; Rizzolo, D. An update on the pharmacologic management and treatment of neuropathic pain. Jaapa 2017, 30, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, P.; Krysinski, J.; Religioni, U.; Merks, P. Access to Medicines via Non-Pharmacy Outlets in European Countries—A Review of Regulations and the Influence on the Self-Medication Phenomenon. Healthcare 2021, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Wastesson, J.W.; Martikainen, J.E.; Zoëga, H.; Schmidt, M.; Karlstad, Ø.; Pottegård, A. Trends in Use of Paracetamol in the Nordic Countries. Basic. Clin. Pharmacol. Toxicol. 2018, 123, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Alchin, J.; Dhar, A.; Siddiqui, K.; Christo, P.J. Why paracetamol (acetaminophen) is a suitable first choice for treating mild to moderate acute pain in adults with liver, kidney or cardiovascular disease, gastrointestinal disorders, asthma, or who are older. Curr. Med. Res. Opin. 2022, 38, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Hoel, R.W.; Giddings Connolly, R.M.; Takahashi, P.Y. Polypharmacy Management in Older Patients. Mayo Clin. Proc. 2021, 96, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; Cox, N.J.; Stevenson, J.M.; Lim, S.; Fraser, S.D.S.; Roberts, H.C. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr. 2021, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Granås, A.G.; Bakken, M.S.; Ruths, S.; Finckenhagen, M. Deprescribing. In Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, ny Raekke; Cammermeyers Forlag: Christiania og Kjobenhavn, Norway, 2018; Volume 138. [Google Scholar]
- Spinewine, A.; Reeve, E.; Thompson, W. Revisiting systematic reviews on deprescribing trials to better inform future practice and research. Br. J. Clin. Pharmacol. 2023, 89, 3758–3764. [Google Scholar] [CrossRef] [PubMed]
- Junius-Walker, U.; Viniol, A.; Michiels-Corsten, M.; Gerlach, N.; Donner-Banzhoff, N.; Schleef, T. MediQuit, an Electronic Deprescribing Tool for Patients on Polypharmacy: Results of a Feasibility Study in German General Practice. Drugs Aging 2021, 38, 725–733. [Google Scholar] [CrossRef]
- Delara, M.; Murray, L.; Jafari, B.; Bahji, A.; Goodarzi, Z.; Kirkham, J.; Chowdhury, M.; Seitz, D.P. Prevalence and factors associated with polypharmacy: A systematic review and Meta-analysis. BMC Geriatr. 2022, 22, 601. [Google Scholar] [CrossRef]
- Lunsky, Y.; Modi, M. Predictors of Psychotropic Polypharmacy Among Outpatients With Psychiatric Disorders and Intellectual Disability. Psychiatr. Serv. 2018, 69, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.I.; Ansari, Z.; Vaughan, L.; Matters, H.; Emerson, E. Prevalence and factors associated with polypharmacy in Victorian adults with intellectual disability. Res. Dev. Disabil. 2014, 35, 3071–3080. [Google Scholar] [CrossRef] [PubMed]
- Halli-Tierney, A.D.; Scarbrough, C.; Carroll, D. Polypharmacy: Evaluating Risks and Deprescribing. Am. Fam. Physician 2019, 100, 32–38. [Google Scholar] [PubMed]
- Dalin, D.A.; Frandsen, S.; Madsen, G.K.; Vermehren, C. Exploration of Symptom Scale as an Outcome for Deprescribing: A Medication Review Study in Nursing Homes. Pharmaceuticals 2022, 15, 505. [Google Scholar] [CrossRef] [PubMed]
- Carollo, M.; Boccardi, V.; Crisafulli, S.; Conti, V.; Gnerre, P.; Miozzo, S.; Omodeo Salè, E.; Pieraccini, F.; Zamboni, M.; Marengoni, A.; et al. Medication review and deprescribing in different healthcare settings: A position statement from an Italian scientific consortium. Aging Clin. Exp. Res. 2024, 36, 63. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.; Raae Hansen, C.; Byrne, S.; O’Mahony, D.; Kearney, P.; Sahm, L. Challenges of deprescribing in the multimorbid patient. Eur. J. Hosp. Pharm. 2017, 24, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, J.; Kouch, L.; Christian, D.; Peterson, P.L.; Gruss, I. Barriers and Facilitators to the Deprescribing of Nonbenzodiazepine Sedative Medications among Older Adults. Perm. J. 2018, 22, 17–157. [Google Scholar] [CrossRef] [PubMed]
- Olesen, A.E.; Vaever, T.J.; Simonsen, M.; Simonsen, P.G.; Høj, K. Deprescribing in primary care without deterioration of health-related outcomes: A real-life, quality improvement project. Basic. Clin. Pharmacol. Toxicol. 2024, 134, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, A.; Xing, G.; Tancredi, D.J.; Magnan, E.; Jerant, A.; Fenton, J.J. Association of Dose Tapering With Overdose or Mental Health Crisis Among Patients Prescribed Long-term Opioids. JAMA 2021, 326, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.M.; Bowe, T.; Manhapra, A.; Kertesz, S.; Hah, J.M.; Henderson, P.; Robinson, A.; Paik, M.; Sandbrink, F.; Gordon, A.J.; et al. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: Observational evaluation. BMJ 2020, 368, m283. [Google Scholar] [CrossRef]
- Riordan, D.O.; Byrne, S.; Fleming, A.; Kearney, P.M.; Galvin, R.; Sinnott, C. GPs’ perspectives on prescribing for older people in primary care: A qualitative study. Br. J. Clin. Pharmacol. 2017, 83, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Pizetta, B.; Raggi, L.G.; Rocha, K.S.S.; Cerqueira-Santos, S.; de Lyra, D.P., Jr.; Dos Santos Júnior, G.A. Does drug dispensing improve the health outcomes of patients attending community pharmacies? A systematic review. BMC Health Serv. Res. 2021, 21, 764. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.; Byrne, S. Role of the pharmacist in reducing healthcare costs: Current insights. Integr. Pharm. Res. Pr. 2017, 6, 37–46. [Google Scholar] [CrossRef]
- Elbeddini, A.; Zhang, C.X.Y. The pharmacist’s role in successful deprescribing through hospital medication reconciliation. Can Pharm. J. 2019, 152, 177–179. [Google Scholar] [CrossRef]
- Niznik, J.D.; Collins, B.J.; Armistead, L.T.; Larson, C.K.; Kelley, C.J.; Hughes, T.D.; Sanders, K.A.; Carlson, R.; Ferreri, S.P. Pharmacist interventions to deprescribe opioids and benzodiazepines in older adults: A rapid review. Res. Soc. Adm. Pharm. 2022, 18, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Gendre, P.; Mayol, S.; Mocquard, J.; Huon, J.F. Physicians’ views on pharmacists’ involvement in hospital deprescribing: A qualitative study on proton pump inhibitors. Basic. Clin. Pharmacol. Toxicol. 2023, 133, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.G.; Mok, V.; Wong, J.G.M.; Bhalla, A. Deprescribing initiative of NSAIDs (DIN): Pharmacist-led interventions for pain management in a federal correctional setting. Can Pharm. J. 2023, 156, 85–93. [Google Scholar] [CrossRef]
- Groot, P.C.; van Os, J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320932452. [Google Scholar] [CrossRef]
- Trenaman, S.C.; Kennie-Kaulbach, N.; d’Entremont-MacVicar, E.; Isenor, J.E.; Goodine, C.; Jarrett, P.; Andrew, M.K. Implementation of pharmacist-led deprescribing in collaborative primary care settings. Int. J. Clin. Pharm. 2022, 44, 1216–1221. [Google Scholar] [CrossRef]
- Schneider, J.L.; Firemark, A.J.; Papajorgji-Taylor, D.; Reese, K.R.; Thorsness, L.A.; Sullivan, M.D.; DeBar, L.L.; Smith, D.H.; Kuntz, J.L. “I really had somebody in my corner.” Patient experiences with a pharmacist-led opioid tapering program. J. Am. Pharm. Assoc. 2023, 63, 241–251.e241. [Google Scholar] [CrossRef]
- Alaa Eddine, N.; Schreiber, J.; El-Yazbi, A.F.; Shmaytilli, H.; Amin, M.E.K. A pharmacist-led medication review service with a deprescribing focus guided by implementation science. Front. Pharmacol. 2023, 14, 1097238. [Google Scholar] [CrossRef] [PubMed]
- Bužančić, I.; Kummer, I.; Držaić, M.; Ortner Hadžiabdić, M. Community-based pharmacists’ role in deprescribing: A systematic review. Br. J. Clin. Pharmacol. 2022, 88, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.P.; Sanyal, C.; Martin, P.; Tannenbaum, C. Economic evaluation of sedative deprescribing in older adults by community pharmacists. J. Gerontol. Ser. A 2021, 76, 1061–1067. [Google Scholar] [CrossRef]
- Suzuki, S.; Uchida, M.; Suga, Y.; Sugawara, H.; Kokubun, H.; Uesawa, Y.; Nakagawa, T.; Takase, H. A nationwide survey of community pharmacist contributions to polypharmacy in opioid-using and non-using cancer patients in Japan. Biol. Pharm. Bull. 2019, 42, 1164–1171. [Google Scholar] [CrossRef]
- Shirdel, A.; Pourreza, A.; Daemi, A.; Ahmadi, B. Health-promoting services provided in pharmacies: A systematic review. J. Educ. Health Promot. 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Meaadi, J.; Obara, I.; Nazar, H. A qualitative study to investigate community pharmacists’ perceptions about identifying and addressing inappropriately prescribed analgesia. Int. J. Pharm. Pract. 2023, 31, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Owusu, Y.B.; Elkhalifa, W.H.; Awaisu, A.; Kheir, N. Assessment of Qatar community pharmacists’ competence and practices related to renal and gastrointestinal adverse effects of nonprescription NSAIDs. Saudi Pharm. J. 2022, 30, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, C.H.; Donovan, M.D. Assessing community pharmacists’ attitudes towards identifying opportunities for deprescribing in clinical practice in Ireland. Int. J. Pharm. Pract. 2022, 30, 28–35. [Google Scholar] [CrossRef]
- Gemmeke, M.; Koster, E.S.; Rodijk, E.A.; Taxis, K.; Bouvy, M.L. Community pharmacists’ perceptions on providing fall prevention services: A mixed-methods study. Int. J. Clin. Pharm. 2021, 43, 1533–1545. [Google Scholar] [CrossRef]
- Huffmyer, M.J.; Keck, J.W.; Harrington, N.G.; Freeman, P.R.; Westling, M.; Lukacena, K.M.; Moga, D.C. Primary care clinician and community pharmacist perceptions of deprescribing. J. Am. Geriatr. Soc. 2021, 69, 1686–1689. [Google Scholar] [CrossRef]
- Korenvain, C.; MacKeigan, L.D.; Dainty, K.N.; Guilcher, S.J.T.; McCarthy, L.M. Exploring deprescribing opportunities for community pharmacists using the Behaviour Change Wheel. Res. Soc. Adm. Pharm. 2020, 16, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Pap, E. Pseudo-Analysis as a Tool of Information Processing. In Proceedings of the Conference on Theoretical and Foundational Problems in Information Studies, IS4SI Summit 2021, online, 12–19 September 2021; p. 116. [Google Scholar]
- Wu, M.-J.; Zhao, K.; Fils-Aime, F. Response rates of online surveys in published research: A meta-analysis. Comput. Hum. Behav. Rep. 2022, 7, 100206. [Google Scholar] [CrossRef]
- Kishore, K.; Jaswal, V.; Kulkarni, V.; De, D. Practical Guidelines to Develop and Evaluate a Questionnaire. Indian Dermatol. Online J. 2021, 12, 266–275. [Google Scholar] [CrossRef] [PubMed]
| Null Hypothesis | Independent Variable | Variable, Measured Value | Dependent Variable | Variable, Measured Value | Statistical Analysis |
|---|---|---|---|---|---|
| There is no significant association between work experience and whether respondents report that deprescription of NSAIDs should be included in the medication review. | Work experience | Continuous | Deprescription should take place during a medication review | Categorical, ordinal | Ordinary regression |
| There is no significant difference between women and men in their perception that there may be reluctance on the part of the patient or their relatives to implement deprescription. | Gender | Categorical, nominal | The perception that there may be reluctance from the patient or relatives to implement deprescription | Categorical, ordinal | Chi-square test |
| There is no correlation between the level of education and pharmacists’ ability to identify cases where deprescription should be considered. | Level of education | Categorical, ordinal | The ability to identify cases for deprescription | Categorical, ordinal | Chi-square test |
| There is no correlation between age and perception of communication and the availability of prescribers as a barrier. | Age | Categorical, ordinal | Perception of communication and accessibility to prescribers a barrier | Categorical, ordinal | Chi-square test |
| There is no correlation between the place of work and the perception of lack of time as a barrier. | Location | Categorical, nominal | Lack of time | Categorical, ordinal | Chi-square test |
| There is no correlation between gender and those who believe that prescribers are not very receptive to recommendations. | Gender | Categorical, nominal | The perception that prescribers are not very receptive to recommendations | Categorical, ordinal | Chi-square test |
| There is no correlation between having a pharmacy degree from Norway and reported confidence in discussing deprescription with patients. | Pharmacy education in Norway | Categorical, dichotomous | Confidence to discuss deprescription with patients | Categorical, nominal | Chi-square test |
| Question | Description | Number | Percent |
|---|---|---|---|
| Gender | |||
| Man | 8 | 11.0% | |
| Woman | 64 | 87.7% | |
| Other | 1 | 1.4% | |
| Does not want to answer | 0 | 0.0% | |
| Age (years) | |||
| 21–26 | 22 | 30.1% | |
| 27–32 | 17 | 23.3% | |
| >32 | 34 | 46.6% | |
| Work experience (years) | |||
| Newly qualified pharmacist (0–1) | 17 | 24.7% | |
| <5 | 19 | 26.0% | |
| 5–10 | 12 | 16.4% | |
| >10 | 24 | 32.9% | |
| Level of education | |||
| Bachelor in Pharmacy | 27 | 37.0% | |
| Master in Pharmacy | 42 | 57.5% | |
| Other | 4 | 5.5% | |
| Place of work in Norway | |||
| Northern Norway | 8 | 11.0% | |
| Central Norway | 7 | 9.5% | |
| Western Norway | 7 | 9.6% | |
| Eastern Norway | 48 | 65.8% | |
| Southern Norway | 3 | 4.1% | |
| Pharmacy education obtained in Norway | |||
| Yes | 65 | 89.0% | |
| No | 8 | 11.0% |
| # | Challenge | Number of Responses | Mean | Median | Minimal (1) | Small (2) | Moderate (3) | Large (4) | Major (5) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lack of time—not enough time to assess prescriptions for deprescription options | 73 | 3.92 | 4 | 0 | 8 | 16 | 23 | 26 |
| 2 | Lack of financial compensation for review of drug use | 73 | 3.68 | 4 | 2 | 11 | 20 | 15 | 25 |
| 3 | Lack of knowledge about tools and methods for deprescription | 73 | 3.42 | 4 | 6 | 9 | 20 | 24 | 14 |
| 4 | Concerns related to negative consequences after performing deprescription | 73 | 3.19 | 3 | 5 | 13 | 27 | 19 | 9 |
| 5 | Communication and the availability of prescribers are problematic | 73 | 4.15 | 4 | 1 | 1 | 11 | 33 | 27 |
| 6 | Prescribers are not very receptive to recommendations | 73 | 3.58 | 4 | 0 | 5 | 30 | 21 | 17 |
| 7 | Reluctance from the patient or relatives | 73 | 3.33 | 3 | 2 | 10 | 28 | 28 | 5 |
| Null Hypothesis | Statistical Analysis | p-value | Null Hypothesis Discarded/Retain | Result |
|---|---|---|---|---|
| There is no significant association between work experience and opinions that deprescription of NSAIDs should be included in the medication review | Ordinary regression | 0.988 | Retained | The analysis revealed no significant correlation between work experience and attitudes toward the inclusion of deprescription of NSAIDs in medication reviews, which is reflected in a p-value of 0.988 from logistic regression analysis. |
| There is no significant difference between women and men in their perception that there may be reluctance from the patient or relatives to implement deprescription | Chi-square test | 0.240 | Retained | The Chi-square test showed no significant gender differences in perception of resistance to deprescription among patients or relatives, with a p-value of 0.240. Confirmatory analyses, including the Likelihood Ratio test (p = 0.228) and the Fisher’s Exact Test (two-sided p = 0.287; unilateral p = 0.215), also underlined the absence of significant differences. |
| There is no correlation between the level of education and community pharmacists’ ability to identify cases where deprescription should be considered | Chi-square test | 0.954 | Retained | The results showed that there is no statistically significant association (Pearson Chi-Square = 1.582, df = 6, p = 0.954). This is confirmed by the Likelihood Ratio (p = 0.909) and the Fisher–Freeman–Halton Exact Test (p = 0.969), both of which support the null hypothesis. |
| There is no correlation between age and perception of communication and availability of prescribers as a barrier | Chi-square test | 0.498 | Retained | The Chi-Square test indicated no statistically significant difference in the perception of communication and accessibility to prescribers as a barrier based on age, with a p-value of 0.498. The Likelihood Ratio test confirmed this finding with a p-value of 0.437. Due to a low number of expected observations in several cells, a Fisher–Freeman–Halton Exact Test was also used, which further supported the null hypothesis with a p-value of 0.459. |
| There is no correlation between the place of work and the perception of lack of time as a barrier | Chi-square test | 0.935 | Retained | In this study, the Chi-square test revealed no statistically significant correlation between the place of work and the perception of lack of time as a barrier in deprescription, with a p-value of 0.935. The Likelihood Ratio test and the Fisher–Freeman–Halton Exact Test, with p-values of 0.855 and 0.925, respectively, confirmed the absence of a significant difference. |
| There is no correlation between gender and those who believe that prescribers are not very receptive to recommendations | Chi-square test | 0.522 | Retained | The analysis revealed no statistically significant association between gender and the perception that prescribers are not very receptive to recommendations, with a p-value of 0.522. Confirmatory results from the Likelihood Ratio test (p = 0.431) and the Fisher–Freeman–Halton Exact Test (p = 0.756) also supported the null hypothesis. |
| There is no correlation between having a pharmacy degree from Norway and reported confidence in discussing deprescription with patients | Chi-square test | 0.890 | Retained | Analysis of the relationship between pharmacy education in Norway and self-confidence in discussing deprescription with patients revealed no statistically significant correlation, as shown by the Chi-square test and the Fisher–Freeman–Halton exact test, with p-values of 0.890 and 0.731, respectively. The use of the Fisher–Freeman–Halton exact test was particularly justified given that over 20% of the cells in the cross-table had expected frequencies below five, which could compromise the reliability of the Chi-square test, especially with a small sample size. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amedi, D.; Gazerani, P. Deprescribing NSAIDs: The Potential Role of Community Pharmacists. Pharmacy 2024, 12, 116. https://doi.org/10.3390/pharmacy12040116
Amedi D, Gazerani P. Deprescribing NSAIDs: The Potential Role of Community Pharmacists. Pharmacy. 2024; 12(4):116. https://doi.org/10.3390/pharmacy12040116
Chicago/Turabian StyleAmedi, Delsher, and Parisa Gazerani. 2024. "Deprescribing NSAIDs: The Potential Role of Community Pharmacists" Pharmacy 12, no. 4: 116. https://doi.org/10.3390/pharmacy12040116
APA StyleAmedi, D., & Gazerani, P. (2024). Deprescribing NSAIDs: The Potential Role of Community Pharmacists. Pharmacy, 12(4), 116. https://doi.org/10.3390/pharmacy12040116







