Associations between Diabetes-Specific Medication Regimen Complexity and Cardiometabolic Outcomes among Underserved Non-Hispanic Black Adults Living with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Methods
2.1. Design and Setting
2.2. Study Population
2.3. Data Collection and Quality Control
2.4. Outcome Measures
2.5. Medication Regimen Complexity
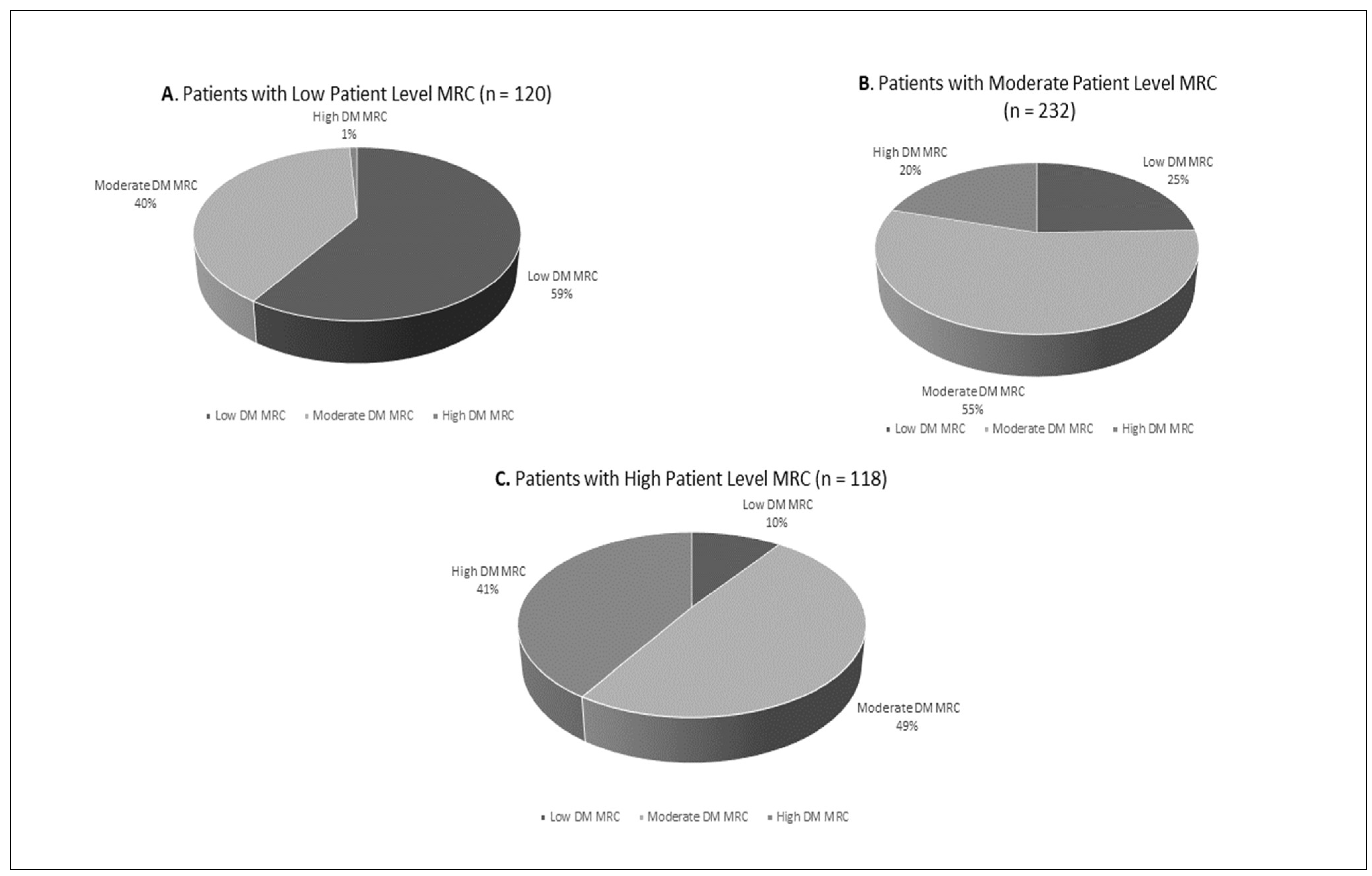
2.6. Medication Regimen Complexity Index Stratification Categories
2.7. Charlson Comorbidity Index
2.8. Sample Size Calculation and Hypotheses
2.9. Statistical Analyses
2.10. Institutional Review Board
3. Results
3.1. Population Characteristics
3.2. Associations between Medication Regimen Complexity and Cardiometabolic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | n (%) |
|---|---|
| Glycemic Control 1,2 | |
| Controlled | 143 (30.4) |
| Uncontrolled | 258 (54.9) |
| Atherogenic Cholesterol Control 1,3 | |
| Controlled | 172 (36.6) |
| Uncontrolled | 197 (41.9) |
| Systolic Blood Pressure Control 1,4 | |
| Controlled | 291 (61.9) |
| Uncontrolled | 169 (36.0) |
| Diastolic Blood Pressure Control 1,5 | |
| Controlled | 370 (78.7) |
| Uncontrolled | 90 (19.1) |
| Diabetes Mellitus Severity 1 | ||||||
|---|---|---|---|---|---|---|
| Diabetes-Specific MRC | Diet-Controlled n (%) | Uncomplicated n (%) | Complicated n (%) | |||
| p | p | p | ||||
| Low | 21 (100.0) | <0.001 | 89 (30.6) | <0.001 | 29 (18.4) | <0.001 |
| Moderate | 0 | 148 (50.9) | 87 (55.1) | |||
| High | 0 | 54 (18.6) | 42 (26.6) | |||
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/?CDC_AAref_Val=https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 6 April 2024).
- United States Census Bureau. Quick Facts. Available online: https://www.census.gov/quickfacts/fact/table/US/PST045218 (accessed on 6 April 2024).
- National Center for Health Statistics. Percentage of Diagnosed Hypertension for Adults Aged 18 and Over, United States, 2019–2021. National Health Interview Survey. Generated Interactively. Available online: https://wwwn.cdc.gov/NHISDataQueryTool/SHS_ADULT3YR/index.html (accessed on 6 April 2024).
- National Center for Health Statistics. Percentage of Obesity for Adults Aged 18 and Over, United States. 2019–2021. National Health Interview Survey. Generated Interactively. Available online: https://wwwn.cdc.gov/NHISDataQueryTool/SHS_ADULT3YR/index.html (accessed on 6 April 2024).
- National Center for Health Statistics. Percentage of High Cholesterol for Adults Aged 18 and Over, United States, 2021. National Health Interview Survey. Generated Interactively. Available online: https://wwwn.cdc.gov/NHISDataQueryTool/SHS_adult/index.html (accessed on 7 April 2024).
- Centers for Disease Control and Prevention. Heart Disease Facts. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/heartdisease/facts.htm (accessed on 7 April 2024).
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621, Erratum in Circulation 2023, 147, e622; Erratum in Circulation 2023, 148, e4. [Google Scholar] [CrossRef]
- Chehal, P.K.; Uppal, T.S.; Turbow, S.; Fernandes, G.; Haw, J.S.; Shah, M.K.; Rajpathak, S.; Narayan, K.M.V.; Ali, M.K. Continuity of Medication Use by US Adults with Diabetes, 2005–2019. JAMA Netw. Open 2023, 6, e2253562. [Google Scholar] [CrossRef]
- Hofer, R.; Choi, H.; Mase, R.; Fagerlin, A.; Spencer, M.; Heisler, M. Mediators and Moderators of Improvements in Medication Adherence. Health Educ. Behav. 2017, 44, 285–296. [Google Scholar] [CrossRef]
- Tichy, E.M.; Hoffman, J.M.; Suda, K.J.; Rim, W.H.; Tadrous, M.; Cuellar, S.; Clark, J.S.; Ward, J.; Schumock, G.T. National trends in prescription drug expenditures and projections for 2022. Am. J. Health Syst. Pharm. 2022, 79, 1158–1172. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635, Erratum in Circulation 2024, 149, e1023. [Google Scholar] [CrossRef]
- Hirsch, J.D.; Metz, K.R.; Hosokawa, P.W.; Libby, A.M. Validation of a patient-level medication regimen complexity index as a possible tool to identify patients for medication therapy management intervention. Pharmacotherapy 2014, 34, 826–835. [Google Scholar] [CrossRef]
- Bazargan, M.; Smith, J.; Yazdanshenas, H.; Movassaghi, M.; Martins, D.; Orum, G. Non-adherence to medication regimens among older African-American adults. BMC Geriatr. 2017, 17, 163. [Google Scholar] [CrossRef]
- Ayele, A.A.; Tegegn, H.G.; Ayele, T.A.; Ayalew, M.B. Medication regimen complexity and its impact on medication adherence and glycemic control among patients with type 2 diabetes mellitus in an Ethiopian general hospital. BMJ Open Diabetes Res. Care 2019, 7, e000685. [Google Scholar] [CrossRef]
- Ab Rahman, N.; Lim, M.T.; Thevendran, S.; Ahmad Hamdi, N.; Sivasampu, S. Medication Regimen Complexity and Medication Burden Among Patients with Type 2 Diabetes Mellitus: A Retrospective Analysis. Front. Pharmacol. 2022, 13, 808190. [Google Scholar] [CrossRef]
- Wakai, E.; Ikemura, K.; Kato, C.; Okuda, M. Effect of number of medications and complexity of regimens on medication adherence and blood pressure management in hospitalized patients with hypertension. PLoS ONE 2021, 16, e0252944. [Google Scholar] [CrossRef]
- Yeh, A.; Shah-Manek, B.; Lor, K.B. Medication Regimen Complexity and A1C Goal Attainment in Underserved Adults with Type 2 Diabetes. Ann. Pharmacother. 2017, 51, 111–117. [Google Scholar] [CrossRef]
- Russell, A.M.; Opsasnick, L.; Yoon, E.; Bailey, S.C.; O’Brien, M.; Wolf, M.S. Association between medication regimen complexity and glycemic control among patients with type 2 diabetes. J. Am. Pharm. Assoc. 2023, 63, 769–777. [Google Scholar] [CrossRef]
- Wisseh, C.; Hildreth, K.; Marshall, J.; Tanner, A.; Bazargan, M.; Robinson, P. Social Determinants of Pharmacy Deserts in Los Angeles County. J. Racial Ethn. Health Disparit. 2021, 8, 1424–1434. [Google Scholar] [CrossRef]
- Canedo, J.R.; Miller, S.T.; Schlundt, D.; Fadden, M.K.; Sanderson, M. Racial/Ethnic Disparities in Diabetes Quality of Care: The Role of Healthcare Access and Socioeconomic Status. J. Racial Ethn. Health Disparit. 2018, 5, 7–14. [Google Scholar] [CrossRef]
- Charlson Comorbidity Index. Available online: https://www.mdcalc.com/calc/3917/charlson-comorbidity-index-cci (accessed on 17 April 2024).
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115, Erratum in Hypertension 2018, 71, e140–e144. [Google Scholar] [CrossRef]
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, 2183–2185, Erratum in Diabetes Care 2021, 44 (Suppl. S1), S125–S150. [Google Scholar] [CrossRef]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, 2186–2187, Erratum in Diabetes Care 2021, 44 (Suppl. S1), S151–S167. [Google Scholar] [CrossRef]
- Adinkrah, E.; Bazargan, M.; Wisseh, C.; Assari, S. Medication Complexity among Disadvantaged African American Seniors in Los Angeles. Pharmacy 2020, 8, 86. [Google Scholar] [CrossRef]
- A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 21 April 2024).
- American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S111–S125. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; Lefevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520, Erratum in JAMA 2014, 311, 1809. [Google Scholar] [CrossRef] [PubMed]
- Libby, A.M.; Fish, D.N.; Hosokawa, P.W.; Linnebur, S.A.; Metz, K.R.; Nair, K.V.; Saseen, J.J.; Griend, J.P.V.; Vu, S.P.; Hirsch, J.D. Patient-level medication regimen complexity across populations with chronic disease. Clin. Ther. 2013, 35, 385–398.e1. [Google Scholar] [CrossRef]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, M.; Leite, R.; Ahmed, H.; Saab, P.G.; Garg, R. Complexity of antidiabetic medication regimen is associated with increased diabetes-related distress in persons with type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2021, 9, e002348. [Google Scholar] [CrossRef] [PubMed]
- Hessler, D.; Fisher, L.; Glasgow, R.E.; Strycker, L.A.; Dickinson, L.M.; Arean, P.A.; Masharani, U. Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care 2014, 37, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.M.; Lutes, L.; Littlewood, K.; DiNatale, E.; Hambidge, B.; Schulman, K.; Morisky, D.E. Regimen-Related Distress, Medication Adherence, and Glycemic Control in Rural African American Women with Type 2 Diabetes Mellitus. Ann. Pharmacother. 2014, 48, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Label: Metformin [Package Insert]. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b857eccf-b9ff-45ba-8241-f47f5caada2a (accessed on 13 April 2024).
- Hu, D.; Guo, Y.; Wu, R.; Shao, T.; Long, J.; Yu, B.; Wang, H.; Luo, Y.; Lu, H.; Zhang, J.; et al. New Insight Into Metformin-Induced Cholesterol-Lowering Effect Crosstalk between Glucose and Cholesterol Homeostasis via ChREBP (Carbohydrate-Responsive Element-Binding Protein)-Mediated PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Regulation. Arter. Thromb. Vasc. Biol. 2021, 41, e208–e223. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, F.; Mastroberardino, S.; Nusca, A.; Frau, L.; Guarino, L.; Napoli, N.; Ussia, G.P.; Grigioni, F. Novel Antidiabetic Agents and Their Effects on Lipid Profile: A Single Shot for Several Cardiovascular Targets. Int. J. Mol. Sci. 2023, 24, 10164. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Vellanki, P.; Blonde, L.; Christofides, E.A.; Galindo, R.J.; Hirsch, I.B.; Isaacs, S.D.; Izuora, K.E.; Wang, C.C.L.; Twining, C.L.; et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm—2023 Update. Endocr. Pract. 2023, 29, 305–340, Erratum in Endocr. Pract. 2023, 29, 746; Erratum in Endocr. Pract. 2023, 29, 1025. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S158–S178. [Google Scholar] [CrossRef]
- Feingold, K.R. Obesity and Dyslipidemia. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., Dhatariya, K., Dungan, K., Feingold, K.R., Hoflands, H., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK305895/ (accessed on 17 April 2024).
- Apovian, C.M.; Okemah, J.; O’Neil, P.M. Body Weight Considerations in the Management of Type 2 Diabetes. Adv. Ther. 2019, 36, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Belachew, E.A.; Netere, A.K.; Sendekie, A.K. Medication regimen complexity and its impact on medication adherence and asthma control among patients with asthma in Ethiopian referral hospitals. Asthma Res. Pract. 2022, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Negewo, N.A.; Gibson, P.G.; Wark, P.A.; Simpson, J.L.; McDonald, V.M. Treatment burden, clinical outcomes, and comorbidities in COPD: An examination of the utility of medication regimen complexity index in COPD. Int. J. Chronic Obstr. Pulmon. Dis. 2017, 12, 2929–2942. [Google Scholar] [CrossRef] [PubMed]
- Prosser, T.R.; Bollmeier, S.G. Assessment of Medication Regimen Complexity of COPD Regimens in Individuals Visiting Community Pharmacies. Int. J. Chronic Obstr. Pulmon. Dis. 2023, 18, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Cobretti, M.R.; Page, R.L., 2nd; Linnebur, S.A.; Deininger, K.M.; Ambardekar, A.V.; Lindenfeld, J.; Aquilante, C.L. Medication regimen complexity in ambulatory older adults with heart failure. Clin. Interv. Aging. 2017, 12, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.H.; Peterson, G.M.; Castelino, R.L.; McKercher, C.; Jose, M.D.; Wimmer, B.C.; Zaidi, S.T.R. Medication Regimen Complexity and Hospital Readmission in Older Adults with Chronic Kidney Disease. Ann. Pharmacother. 2019, 53, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Marienne, J.; Laville, S.M.; Caillard, P.; Batteux, B.; Gras-Champel, V.; Masmoudi, K.; Choukroun, G.; Liabeuf, S. Evaluation of Changes Over Time in the Drug Burden and Medication Regimen Complexity in ESRD Patients before and after Renal Transplantation. Kidney Int. Rep. 2020, 6, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Thrasher, J. Pharmacologic Management of Type 2 Diabetes Mellitus: Available Therapies. Am. J. Med. 2017, 130 (Suppl. S6), S4–S17. [Google Scholar] [CrossRef] [PubMed]
- Stack, R.J.; Elliott, R.A.; Noyce, P.R.; Bundy, C. A qualitative exploration of multiple medicines beliefs in co-morbid diabetes and cardiovascular disease. Diabet. Med. 2008, 25, 1204–1210. [Google Scholar] [CrossRef]
- Iskedjian, M.; Einarson, T.R.; MacKeigan, L.D.; Shear, N.; Addis, A.; Mittmann, N.; Ilersich, A.L. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: Evidence from a meta-analysis. Clin. Ther. 2002, 24, 302–316. [Google Scholar] [CrossRef]
- Stewart, K.D.; Johnston, J.A.; Matza, L.S.; Curtis, S.; Havel, H.; Sweetana, S.; Gelhorn, H.L. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer. Adher. 2016, 10, 1385–1399. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.T.; Keers, J.C.; Visser, R.; de Zeeuw, D.; Haaijer-Ruskamp, F.W.; Voorham, J.; Denig, P. Medication beliefs, treatment complexity, and non-adherence to different drug classes in patients with type 2 diabetes. J. Psychosom. Res. 2014, 76, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.M.; Ringel, J.B.; Rajan, M.; Colantonio, L.D.; Casalino, L.P.; Reshetnyak, E.; Pinheiro, L.C.; Safford, M.M. Ambulatory Care Fragmentation and Incident Stroke. J. Am. Heart Assoc. 2021, 10, e019036. [Google Scholar] [CrossRef] [PubMed]
- Snowden, L.R.; Michaels, E. Racial Bias Correlates with States Having Fewer Health Professional Shortage Areas and Fewer Federally Qualified Community Health Center Sites. J. Racial Ethn. Health Disparit. 2023, 10, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.V.; Levasseur, J.L.; Maxim, A.; Benavidez, G.A.; Pollack Porter, K.M. Structural Racism, Place, and COVID-19: A Narrative Review Describing How We Prepare for an Endemic COVID-19 Future. Health Equity 2022, 6, 356–366. [Google Scholar] [CrossRef]
- The Roots of Structural Racism Project. Available online: https://belonging.berkeley.edu/roots-structural-racism (accessed on 18 April 2024).
- Preventing Chronic Disease. Pharmacy Contributions to Improved Population Health: Expanding the Public Health Roundtable. Available online: https://www.cdc.gov/pcd/issues/2020/20_0350.htm (accessed on 15 May 2024).
- Morello, C.M.; Rotunno, T.; Khoan, J.; Hirsch, J.D. Improved Glycemic Control with Minimal Change in Medication Regimen Complexity in a Pharmacist-Endocrinologist Diabetes Intense Medical Management (DIMM) “Tune Up” Clinic. Ann. Pharmacother. 2018, 52, 1091–1097. [Google Scholar] [CrossRef]
| Variable | n (%) |
|---|---|
| Gender | |
| Female | 288 (61.3) |
| Male | 182 (38.7) |
| Age | |
| Mean ± SD | 60.3 ± 12.0 |
| 18–54 | 121 (25.7) |
| 55–64 | 172 (36.6) |
| 65–74 | 142 (30.2) |
| ≥75 | 35 (7.4) |
| Federal Poverty Level 1 | |
| 0–99% | 375 (79.8) |
| 100–199% | 68 (14.5) |
| ≥200% | 13 (2.8) |
| Insurance Status | |
| Uninsured | 37 (7.9) |
| Insured | 433 (92.1) |
| Insurance Type 1,2 | |
| Private | 11 (2.3) |
| Medicaid | 308 (65.5) |
| Medicare | 110 (23.4) |
| My Health LA | 8 (1.7) |
| Other 3 | 18 (3.8) |
| Housing Status | |
| Housing Secure | 467 (99.4) |
| Housing Insecure | 3 (0.6) |
| Employment Status 1 | |
| Unemployed | 310 (66.0) |
| Employed | 72 (15.3) |
| Retired | 33 (7.0) |
| Smoking Status 1 | |
| Never | 292 (62.1) |
| Past | 45 (9.6) |
| Current | 111 (23.6) |
| Alcohol Use 1 | |
| No | 291 (61.9) |
| Yes | 150 (31.9) |
| Variable | n (%) |
|---|---|
| Condition | |
| Diabetes | |
| Diet-Controlled | 21 (4.5) |
| Uncomplicated | 291 (61.9) |
| Complicated 1 | 158 (33.6) |
| Hypertension | 392 (83.4) |
| Dyslipidemia | 329 (70) |
| Obesity (kg/m2) 2,3 | |
| 18.5–24.9 | 61 (13.0) |
| 25–29.9 (Pre-Obesity) | 108 (23.0) |
| 30–34.9 (Class 1 Obesity) | 98 (20.9) |
| 35–39.9 (Class 2 Obesity) | 76 (16.2) |
| ≥40 (Class 3 Obesity) | 85 (18.1) |
| Coronary Heart Disease | 21 (4.5) |
| Chronic Kidney Disease 4 | 48 (10.2) |
| Myocardial Infarction | 9 (1.9) |
| Congestive Heart Failure | 26 (5.5) |
| Peripheral Vascular Disease | 11 (2.3) |
| Cerebrovascular Accident or Transient Ischemic Attack | 32 (6.8) |
| Asthma | 73 (15.5) |
| Chronic Obstructive Pulmonary Disorder | 24 (5.1) |
| Charlson Comorbidity Index 5 | 3.6 ± 1.9 |
| Cardiometabolic Measures Overall (units) 3,5 | |
| HbA1c (%) | 8.4 ± 2.4 |
| SBP (mm Hg) | 135.9 ± 19.0 |
| DBP (mm Hg) | 81.2 ± 10.7 |
| LDL-C (mg/dL) | 109 ± 43.4 |
| HDL-C (mg/dL) | 53.3 ± 16.7 |
| TG (mg/dL) | 132.3 ± 76.2 |
| TC (mg/dL) | 187.1 ± 48.6 |
| BMI (kg/m2) | 33.4 ± 8.8 |
| Variable | n (%) |
|---|---|
| Patient Level (Overall) | |
| Number of medications, mean (SD) | 8.5 ± 4.7 |
| Patient-level MRCI, mean (SD) | 22.4 ± 14.5 |
| Number of medications taken | |
| ≤4 | 90 (20.2) |
| 5–9 | 205 (43.6) |
| 10–19 | 162 (34.5) |
| ≥20 | 8 (1.7) |
| Polypharmacy 1 | |
| No | 91 (19.4) |
| Yes | 379 (80.6) |
| Patient-level MRCI Categories: tertile ranges | |
| Low: ≤11.5 | 115 (25.5) |
| Moderate: 11.6–31.4 | 245 (52.1) |
| High: ≥31.5 | 110 (23.4) |
| Number of diabetes medications, mean (SD) | 1.8 ± 1.2 |
| Diabetes-specific MRCI, mean (SD) | 6.0 ± 4.7 |
| Number of diabetes medications taken | |
| ≤2 | 368 (78.3) |
| 3–6 | 102 (21.7) |
| Diabetes-specific MRCI Categories: tertile ranges | |
| Low: ≤3 | 149 (29.6) |
| Moderate: 4–8 | 235 (50.0) |
| High: ≥9 | 96 (20.4) |
| Antihyperglycemic Pharmacological Classes | |
| Sulfonylureas (2nd generation) | 160 (33.8) |
| Meglitinides | 1(0.21) |
| Biguanides | 310 (66.4) |
| Thiazolidinediones | 14 (2.8) |
| Dipeptidyl-peptidase- IV Inhibitors | 44 (9.4) |
| Sodium Glucose co-Transporter 2 Inhibitors | 19 (3.8) |
| Glucagon-Like peptide 1 Receptor Agonists (oral) | 1 (0.21) |
| Glucagon-Like peptide 1 Receptor Agonists (injectable) | 4 (0.85) |
| Combination Medications (oral) | 9 (2.1) |
| Rapid-Acting Insulin | 42 (9.0) |
| Short-acting insulin | 61 (13.2) |
| Intermediate-acting insulin | 66 (14.5) |
| Long-acting insulin | 75 (15.5) |
| Combination Insulin | 42 (9.2) |
| Cardiovascular Medication Use | |
| Statin Use | 258 (54.9) |
| Aspirin Use | 228 (48.5) |
| Blood Pressure Medication Use 2 | 345 (88.0) |
| Glycemic Control 1,2 | Atherogenic Cholesterol Control 1,3 | |||||
|---|---|---|---|---|---|---|
| Patient Level MRC | Yes, n (%) | No, n (%) | p | Yes, n (%) | No, n (%) | p |
| Low | 34 (43.6) | 44 (56.4) | 0.041 | 18 (26.9) | 49 (73.1) | 0.001 |
| Moderate | 82 (37.3) | 138 (62.7) | 99 (48.3) | 106 (51.7) | ||
| High | 27 (26.2) | 76 (73.8) | 55 (56.7) | 42 (43.3) | ||
| Diabetes-specific MRC | ||||||
| Low | 67 (67.7) | 32 (32.3) | 0.000 | 34 (35.4) | 62 (64.6) | 0.031 |
| Moderate | 65 (31.0) | 145 (69.0) | 100 (51.8) | 93 (48.2) | ||
| High | 11 (12.0) | 81 (88.0) | 38 (47.5) | 42 (52.5) | ||
| Glycemic Control 1 | Atherogenic Cholesterol Control 2 | |||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | p | AOR | 95% CI | p | |
| Diabetes-specific MRC | ||||||
| Low * | 1 | <0.001 | 1 | 0.104 | ||
| Moderate | 5.329 | 2.816–10.083 | <0.001 | 0.505 | 0.266–0.958 | 0.036 |
| High | 23.814 | 9.023–62.852 | <0.001 | 0.546 | 0.256–1.165 | 0.118 |
| Age | 0.979 | 0.952–1.005 | 0.116 | 0.979 | 0.954–1.005 | 0.117 |
| Gender | ||||||
| Male * | 1 | 1 | ||||
| Female | 0.983 | 0.547–1.767 | 0.955 | 0.880 | 0.514–1.504 | 0.639 |
| Insurance Status | ||||||
| Uninsured * | 1 | 1 | ||||
| Insured | 0.962 | 0.232–3.998 | 0.958 | 2.090 | 0.396–11.033 | 0.385 |
| Employment Status | ||||||
| Unemployed * | 1 | 0.271 | 1 | |||
| Employed | 1.861 | 0.863–4.014 | 0.113 | 1.060 | 0.541–2.078 | 0.865 |
| Retired | 1.323 | 0.466–3.754 | 0.599 | 0.973 | 0.360–2.629 | 0.957 |
| Alcohol Use | ||||||
| No * | 1 | 1 | ||||
| Yes | 1.236 | 0.670–2.281 | 0.497 | 1.886 | 1.074–3.311 | 0.027 |
| Smoking Status | ||||||
| Never * | 1 | 0.953 | 1 | |||
| Past | 0.967 | 0.377–2.482 | 0.944 | 0.817 | 0.350–1.905 | 0.639 |
| Current | 0.891 | 0.429–1.849 | 0.756 | 0.865 | 0.447–1.677 | 0.668 |
| Federal Poverty Level | ||||||
| 0–99% * | 1 | 0.629 | 1 | |||
| 100–199% | 0.819 | 0.382–1.753 | 0.607 | 1.387 | 0.683–2.814 | 0.365 |
| ≥ 200% | 2.582 | 0.235–28.350 | 0.438 | 1.425 | 0.327–6.205 | 0.637 |
| WHO BMI Groups | ||||||
| 18.5–24.9 * | 1 | 0.448 | 1 | |||
| 25–29.9 Pre-Obesity | 2.279 | 0.911–5.700 | 0.078 | 3.209 | 1.298–7.934 | 0.012 |
| 30–34.9 Class 1 Obesity | 1.489 | 0.603–3.680 | 0.388 | 2.330 | 0.936–5.800 | 0.069 |
| 35–39.9 Class 2 Obesity | 1.212 | 0.460–3.194 | 0.697 | 3.350 | 1.278–8.780 | 0.014 |
| ≥ 40 Class 3 Obesity | 1.461 | 0.544–3.919 | 0.452 | 3.310 | 1.255–8.729 | 0.016 |
| Charlson Comorbidity Index | 1.054 | 0.913–1.216 | 0.472 | 0.976 | 0.856–1.112 | 0.711 |
| Glycemic Control 1 | Atherogenic Cholesterol Control 2 | |||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | p | AOR | 95% CI | p | |
| Patient-Level Specific MRC | ||||||
| Low * | 1 | 0.010 | 1 | 0.009 | ||
| Moderate | 1.959 | 0.977–3.930 | 0.058 | 0.396 | 0.172–0.916 | 0.030 |
| High | 3.625 | 1.572–8.358 | 0.003 | 0.230 | 0.090–0.592 | 0.002 |
| Age | 0.976 | 0.951–1.001 | 0.058 | 0.981 | 0.955–1.007 | 0.146 |
| Gender | ||||||
| Male* | 1 | 1 | ||||
| Female | 1.098 | 0.644–1.870 | 0.732 | 0.912 | 0.530–1.567 | 0.738 |
| Insurance Status | ||||||
| Uninsured * | 1 | 1 | ||||
| Insured | 0.741 | 0.200–2.750 | 0.655 | 1.789 | 0.331–9.650 | 0.499 |
| Employment Status | ||||||
| Unemployed * | 1 | 0.150 | 1 | 0.773 | ||
| Employed | 2.025 | 0.978–4.193 | 0.057 | 0.855 | 0.430–1.701 | 0.655 |
| Retired | 1.388 | 0.530–3.635 | 0.505 | 0.715 | 0.250–2.052 | 0.533 |
| Alcohol Use | ||||||
| No * | 1 | 1 | ||||
| Yes | 1.401 | 0.799–2.454 | 0.239 | 1.761 | 0.995–3.117 | 0.052 |
| Smoking Status | ||||||
| Never * | 1 | 0.708 | 1 | 0.970 | ||
| Past | 0.733 | 0.318–1.691 | 0.467 | 0.954 | 0.407–2.235 | 0.914 |
| Current | 1.090 | 0.565–2.103 | 0.796 | 0.922 | 0.473–1.799 | 0.812 |
| Federal Poverty Level | ||||||
| 0–99% * | 1 | 0.579 | 1 | 0.733 | ||
| 100–199% | 0.978 | 0.483–1.979 | 0.950 | 1.387 | 0.683–2.814 | 0.556 |
| ≥200% | 3.097 | 0.366–26.223 | 0.300 | 1.425 | 0.327–6.205 | 0.559 |
| WHO BMI Groups | ||||||
| 18.5–24.9 * | 1 | 0.330 | 1 | 0.051 | ||
| 25–29.9 Pre-Obesity | 2.014 | 0.868–4.676 | 0.103 | 3.226 | 1.290–8.065 | 0.012 |
| 30–34.9 Class 1 Obesity | 1.409 | 0.625–3.178 | 0.408 | 2.270 | 0.903–5.704 | 0.081 |
| 35–39.9 Class 2 Obesity | 0.929 | 0.388–2.227 | 0.869 | 3.885 | 1.454–10.380 | 0.007 |
| ≥40 Class 3 Obesity | 1.281 | 0.521–3.146 | 0.589 | 3.816 | 1.420–10.258 | 0.008 |
| Charlson Comorbidity Index | 1.058 | 0.926–1.209 | 0.407 | 1.008 | 0.800–1.154 | 0.908 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisseh, C.; Adinkrah, E.; Opara, L.; Melone, S.; Udott, E.; Bazargan, M.; Shaheen, M. Associations between Diabetes-Specific Medication Regimen Complexity and Cardiometabolic Outcomes among Underserved Non-Hispanic Black Adults Living with Type 2 Diabetes Mellitus. Pharmacy 2024, 12, 83. https://doi.org/10.3390/pharmacy12030083
Wisseh C, Adinkrah E, Opara L, Melone S, Udott E, Bazargan M, Shaheen M. Associations between Diabetes-Specific Medication Regimen Complexity and Cardiometabolic Outcomes among Underserved Non-Hispanic Black Adults Living with Type 2 Diabetes Mellitus. Pharmacy. 2024; 12(3):83. https://doi.org/10.3390/pharmacy12030083
Chicago/Turabian StyleWisseh, Cheryl, Edward Adinkrah, Linda Opara, Sheila Melone, Emem Udott, Mohsen Bazargan, and Magda Shaheen. 2024. "Associations between Diabetes-Specific Medication Regimen Complexity and Cardiometabolic Outcomes among Underserved Non-Hispanic Black Adults Living with Type 2 Diabetes Mellitus" Pharmacy 12, no. 3: 83. https://doi.org/10.3390/pharmacy12030083
APA StyleWisseh, C., Adinkrah, E., Opara, L., Melone, S., Udott, E., Bazargan, M., & Shaheen, M. (2024). Associations between Diabetes-Specific Medication Regimen Complexity and Cardiometabolic Outcomes among Underserved Non-Hispanic Black Adults Living with Type 2 Diabetes Mellitus. Pharmacy, 12(3), 83. https://doi.org/10.3390/pharmacy12030083








