A Systematic Review of Naldemedine and Naloxegol for the Treatment of Opioid-Induced Constipation in Cancer Patients
Abstract
1. Introduction
1.1. Mechanism of Action
1.2. Structure
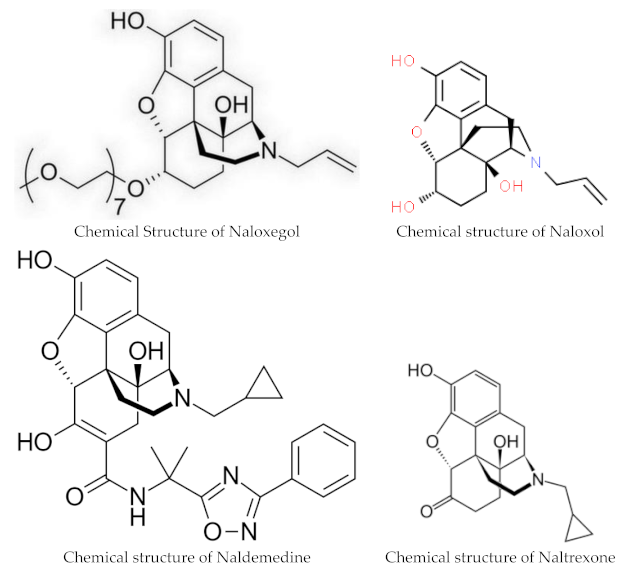
1.3. Pharmacokinetics
1.4. Interactions
1.5. Contraindications
1.6. Side Effects
1.7. Clinical Trials
2. Materials and Methods
2.1. Focal Question
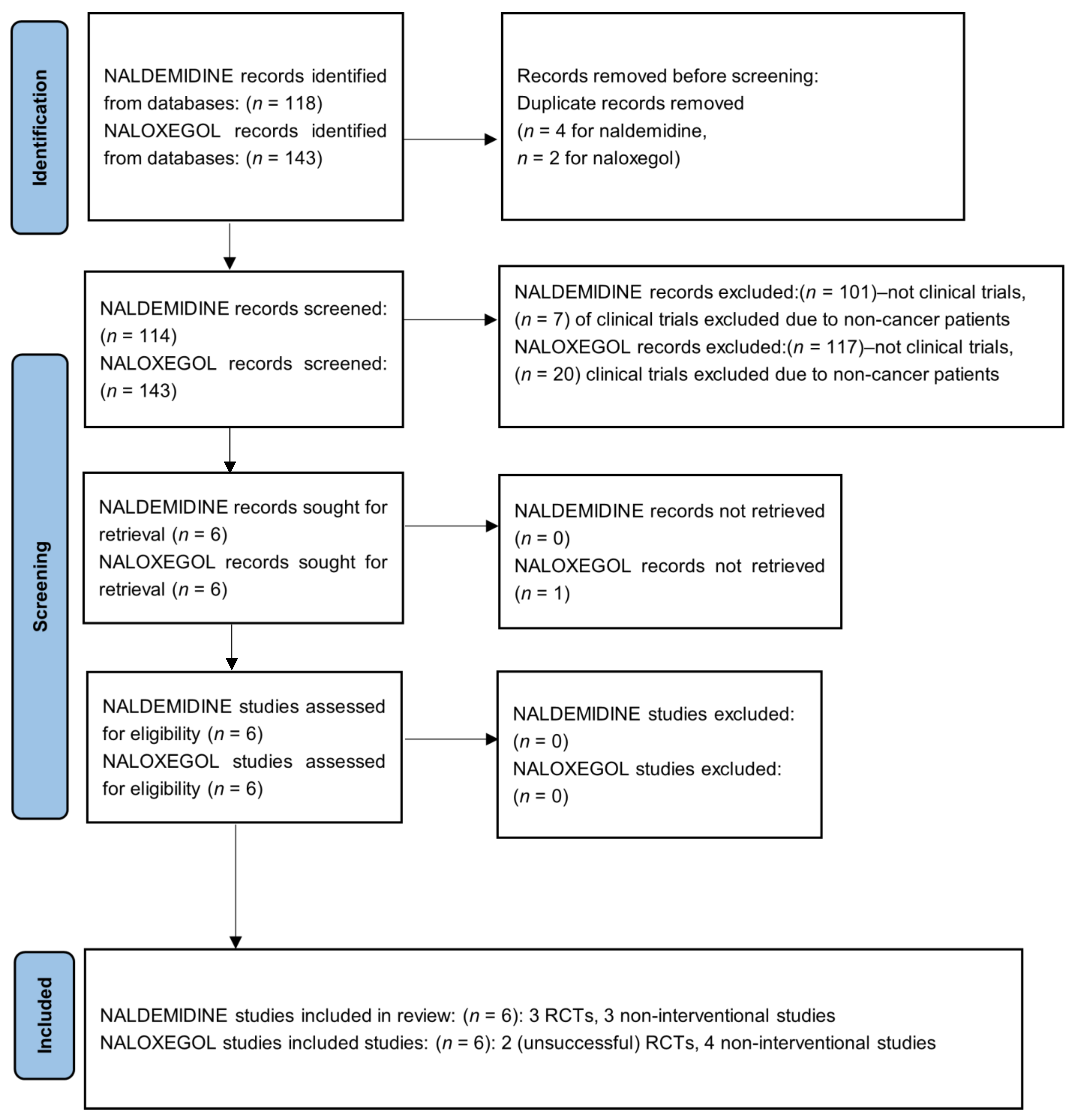
2.2. Search and Information Sources
2.3. Eligibility Criteria and Study Selection
2.4. Outcomes Assessed
2.5. Quality Assessment
3. Results
Retrospective Studies Excluded
4. Discussion
4.1. Limitations and Study Quality
4.2. Implications for Pharmacy Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rang, H.P.; Dale, M.M.; Ritter, J.M. Analgesic drugs. Pharmacology 1999, 13, 579–603. [Google Scholar]
- Kurz, A.; Sessler, D.I. Opioid-induced bowel dysfunction: Pathophysiology and potential new therapies. Drugs 2003, 63, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Sykes, N.P. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat. Med. 1998, 12, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.J.; Panchal, S.J.; Miaskowski, C.; Bolge, S.C.; Milanova, T.; Williamson, R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med. 2009, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI disorders: Disorders of gut-brain interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Schmulson, M.J.; Drossman, D.A. What is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Streicher, J.M.; Bilsky, E.J. Peripherally Acting μ-Opioid Receptor Antagonists for the Treatment of Opioid-Related Side Effects: Mechanism of Action and Clinical Implications. J. Pharm. Pract. 2017, 31, 658–669. [Google Scholar] [CrossRef]
- Brock, C.; Olesen, S.S.; Olesen, A.E.; Frøkjaer, J.B.; Andresen, T.; Drewes, A.M. Opioid Induced Bowel Dysfunction. Drugs 2012, 72, 1847–1865. [Google Scholar] [CrossRef]
- Floettmann, E.; Bui, K.; Sostek, M.; Payza, K.; Eldon, M. Pharmacologic Profile of Naloxegol, a Peripherally Acting µ-Opioid Receptor Antagonist, for the Treatment of Opioid-Induced Constipation. J. Pharmacol. Exp. Ther. 2017, 361, 280–291. [Google Scholar] [CrossRef]
- Hu, K.; Bridgeman, M.B. Naldemedine (Symproic) for the Treatment of Opioid-Induced Constipation. Pharm. Ther. 2018, 43, 601–627. [Google Scholar]
- Garnock-Jones, K.P. Naloxegol: A review of its use in patients with opioid-induced constipation. Drugs 2015, 75, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Essmat, N.; Karádi, D.Á.; Zádor, F.; Király, K.; Fürst, S.; Al-Khrasani, M. Insights into the Current and Possible Future Use of Opioid Antagonists in Relation to Opioid-Induced Constipation and Dysbiosis. Molecules 2023, 28, 7766. [Google Scholar] [CrossRef] [PubMed]
- FDA. Symproic (Naldemedine) Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208854Orig1s000TOC.cfm (accessed on 28 November 2023).
- FDA. Movantik (Naloxegol) Prescribing Information Highlights (PDF). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204760s000lbl.pdf (accessed on 28 November 2023).
- European Medicines Agency (EMA). “Rizmoic: EPAR—Product Information” (PDF). 2019. Available online: https://www.ema.europa.eu/en/documents/product-information/rizmoic-epar-product-information_en.pdf (accessed on 7 February 2024).
- European Medicines Agency (EMA). “Moventig”: EPAR—Product Information (PDF). 2014. Available online: https://www.ema.europa.eu/en/documents/product-information/moventig-epar-product-information_en.pdf (accessed on 31 January 2024).
- Crockett, S.D.; Greer, K.B.; Heidelbaugh, J.J.; Falck-Ytter, Y.; Hanson, B.J.; Sultan, S.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the Medical Management of Opioid-Induced Constipation. Gastroenterology 2019, 156, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.J.; Cherny, N.I.; La Carpia, D.; Guglielmo, M.; Ostgathe, C.; Scotté, F.; Ripamonti, C.I. ESMO Guidelines Committee. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv111–iv125. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Drewes, A.M.; Chiarioni, G.; De Giorgio, R.; O’Brien, T.; Morlion, B.; Tack, J. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United Eur. Gastroenterol. J. 2019, 7, 7–20, Erratum in: United Eur. Gastroenterol. J. 2019, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Schedules of Controlled Substances: Removal Of Naldemedine from Control (PDF). Federal Register. Available online: https://www.govinfo.gov/content/pkg/FR-2017-07-12/pdf/2017-14482.pdf (accessed on 28 November 2023).
- Bui, K.; Zhou, D.; Xu, H.; Floettmann, E.; Al-Huniti, N. Clinical Pharmacokinetics and Pharmacodynamics of Naloxegol, a Peripherally Acting µ-Opioid Receptor Antagonist. Clin. Pharmacokinet. 2017, 56, 573–582. [Google Scholar] [CrossRef]
- Federal Register. Available online: https://www.federalregister.gov/documents/2015/01/23/2015-01172/schedules-of-controlled-substances-removal-of-naloxegol-from-control (accessed on 28 November 2023).
- Kanemasa, T.; Koike, K.; Arai, T.; Ono, H.; Horita, N.; Chiba, H.; Nakamura, A.; Morioka, Y.; Kihara, T.; Hasegawa, M. Pharmacologic effects of naldemedine, a peripherally acting μ-opioid receptor antagonist, in in vitro and in vivo models of opioid-induced constipation. Neurogastroenterol. Motil 2019, 31, e13563. [Google Scholar] [CrossRef]
- Fukumura, K.; Yamada, T.; Yokota, T.; Kawasaki, A. The Influence of Renal or Hepatic Impairment on the Pharmacokinetics, Safety, and Tolerability of Naldemedine. Clin. Pharmacol. Drug Dev. 2020, 9, 162–174. [Google Scholar] [CrossRef]
- Bui, K.; She, F.; Sostek, M. The effects of mild or moderate hepatic impairment on the pharmacokinetics, safety, and tolerability of naloxegol. J. Clin. Pharmacol. 2014, 54, 1368–1374. [Google Scholar] [CrossRef]
- Webster, L.R.; Hale, M.E.; Yamada, T.; Wild, J.E. A Renal Impairment Subgroup Analysis of the Safety and Efficacy of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain Receiving Opioid Therapy. J. Pain Res. 2020, 13, 605–612. [Google Scholar] [CrossRef]
- Bui, K.; She, F.; Sostek, M. The effects of renal impairment on the pharmacokinetics, safety, and tolerability of naloxegol. J. Clin. Pharmacol. 2014, 54, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.; Webster, L.; Yamada, T.; Hale, M. Safety and Efficacy of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain Receiving Opioid Therapy: A Subgroup Analysis of Patients ≥ 65 Years of Age. Drugs Aging 2020, 37, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Imai, H.; Fujita, Y.; Hiruta, E.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Sandoh, M.; Takei, S.; Arai, K.; et al. A Retrospective Study of the Efficacy and Safety of Naldemedine for Treatment of Opioid-Induced Constipation in Patients with Hepatobiliary Pancreatic Cancer. Medicina 2023, 59, 492. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.; Wild, J.; Reddy, J.; Yamada, T.; Arjona Ferreira, J.C. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): Two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol. Hepatol. 2017, 2, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Hale, M.; Morlion, B.; Tack, J.; Webster, L.; Wild, J. Naldemedine Improves Patient-Reported Outcomes of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain in the COMPOSE Phase 3 Studies. J. Pain Res. 2021, 14, 2179–2189. [Google Scholar] [CrossRef]
- von Roenn, J.H.; Tack, J.; Barker, P.N.; Lowe, E.S.; Fleischmann, C.; Sostek, M. Challenges in patient recruitment during KODIAC-06, a randomized, placebo-controlled, double-blind, multicenter, phase 3 trial of naloxegol in patients with neoplasia and opioid-induced constipation (OIC). In Multinational Association of Supportive Care in Cancer, Proceedings of the MASCC/ISOO International Symposium on Supportive Care in Cancer, Berlin, Germany, 27–29 June 2013; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Bull, J.; Bonsignore, L.; Massie, L.; Riggs, A.; Knotkova, H.; Wellman, C.; Portenoy, R. Challenges in Recruiting Patients to a Controlled Feasibility Study of a Drug for Opioid-Induced Constipation: Lessons from the Population With Advanced Cancer. J. Pain Symptom Manag. 2019, 57, e5–e8. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP Randomised Controlled Trial Checklist. 2020. Available online: https://casp-uk.net/checklists/casp-rct-randomised-controlled-trial-checklist-fillable.pdf (accessed on 7 February 2024).
- Imai, H.; Fujita, Y.; Hiruta, E.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Sandoh, M.; Takei, S.; Arai, K.; et al. A retrospective study of the efficacy and safety of naldemedine for opioid-induced constipation in thoracic cancer patients. Thorac. Cancer 2022, 13, 2301–2308. [Google Scholar] [CrossRef]
- Nishiba, H.; Imai, H.; Fujita, Y.; Hiruta, E.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Ito, M.; Takei, S.; et al. Efficacy and safety of naldemedine treatment for opioid-induced constipation in gastrointestinal cancer: A retrospective analysis. Ann. Palliat. Med. 2023, 12, 697–707. [Google Scholar] [CrossRef]
- Fujita, Y.; Imai, H.; Hiruta, E.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Sandoh, M.; Takei, S.; Arai, K.; et al. Efficacy and Safety of Naldemedine Administration for Opioid-Induced Constipation in Cancer Patients with Poor Performance Status. J. Palliat. Med. 2023, 26, 548–553. [Google Scholar] [CrossRef]
- Katakami, N.; Oda, K.; Tauchi, K.; Nakata, K.; Shinozaki, K.; Yokota, T.; Suzuki, Y.; Narabayashi, M.; Boku, N. Phase IIb, Randomized, Double-Blind, Placebo-Controlled Study of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Cancer. J. Clin. Oncol. 2017, 35, 1921–1928. [Google Scholar] [CrossRef]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized Phase III and Extension Studies of Naldemedine in Patients with Opioid-Induced Constipation and Cancer. J. Clin. Oncol. 2017, 35, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized phase III and extension studies: Efficacy and impacts on quality of life of naldemedine in subjects with opioid-induced constipation and cancer. Ann. Oncol. 2018, 29, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Ishiki, H.; Yokota, T.; Tada, Y.; Sato, H.; Okamoto, M.; Satomi, E. Safety and efficacy of naldemedine in cancer patients with opioid-induced constipation: A pooled, subgroup analysis of two randomised controlled studies. ESMO Open 2019, 4, e000527. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Nakazawa, M.; Honda, K.; Hashimoto, S. Post-marketing surveillance of the safety and effectiveness of naldemedine in the management of opioid-induced constipation in patients with cancer pain in Japan. Support. Care Cancer 2022, 30, 3943–3954. [Google Scholar] [CrossRef] [PubMed]
- Naya, N.; Oka, H.; Hashimoto, S.; Morioka, Y.; Kizawa, Y. Real-World Evidence for the Safety and Effectiveness of Naldemedine in the Management of Opioid-Induced Constipation in Patients with Cancer Pain: Post-hoc Subgroup Analysis of Post-marketing Surveillance in Japan. Cureus 2023, 15, e46090. [Google Scholar] [CrossRef] [PubMed]
- Cobo Dols, M.; Beato Zambrano, C.; Cabezón Gutiérrez, L.; Chicas Sett, R.; Blancas López-Barajas, M.I.; García Navalón, F.; Fírvida Pérez, J.L.; Serrano Bermúdez, G.; Togores Torres, P.; Delgado Mingorance, I.; et al. Efficacy of naloxegol on symptoms and quality of life related to opioid-induced constipation in patients with cancer: A 3-month follow-up analysis. BMJ Support. Palliat. Care 2021, 11, 25–31. [Google Scholar] [CrossRef]
- Lemaire, A.; Pointreau, Y.; Narciso, B.; Piloquet, F.X.; Braniste, V.; Sabaté, J.M. Effectiveness of naloxegol in patients with cancer pain suffering from opioid-induced constipation. Support. Care Cancer 2021, 29, 7577–7586, Erratum in: Support. Care Cancer 2021, 29, 7587–7589. [Google Scholar] [CrossRef]
- Davies, A.; Cinieri, S.; Dupoiron, D.; España Fernandez, S.; Leclerc, J.; Montesarchio, V.; Mystakidou, K.; Serna, J.; Tack, J.; on Behalf of the Nacasy Study Group. A Prospective, Real-World, Multinational Study of Naloxegol for Patients with Cancer Pain Diagnosed with Opioid-Induced Constipation-The NACASY Study. Cancers 2022, 14, 1128. [Google Scholar] [CrossRef]
- Cobo Dols, M.; Beato Zambrano, C.; Cabezón-Gutiérrez, L.; Chicas-Sett, R.; Blancas López-Barajas, M.I.; García Navalón, F.J.; Fírvida Pérez, J.L.; Serrano Bermúdez, G.; Togores Torres, P.; Delgado Mingorance, I.; et al. One-year efficacy and safety of naloxegol on symptoms and quality of life related to opioid-induced constipation in patients with cancer: KYONAL study. BMJ Support Palliat. Care 2023, 13, e318–e326. [Google Scholar] [CrossRef]
- Tobben, D.; Carpenter, S.; Kolar, R.; Merritt, T.; Young, T.; Hauser, P.; Collier, T. Naloxegol versus Methylnaltrexone for Opioid-Induced Constipation in Critically Ill Patients. Ann. Pharmacother. 2023, 10600280231205023. [Google Scholar] [CrossRef]
- Naldemedine (SYMPROIC) Criteria for Use, February 2019 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Available online: https://www.va.gov/formularyadvisor/DOC_PDF/Naldemedine_SYMPROIC.pdf (accessed on 1 December 2023).
- Naloxegol (MOVANTIK) Criteria for Use March 2018 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Available online: https://www.pbm.va.gov/ (accessed on 1 December 2023).
| Strong Inhibitors of CYP3A4: Increase Naldemedine Concentration | Inducers of CYP3A4: Decrease Naldemedine Concentration |
|---|---|
| Itraconazole | Rifampine |
| Ketoconaxole | St. John’s wort |
| Clarithromycin | |
| Grapefruit juice | |
| Moderate Inhibitors of CYP3A4: Diltiazem Erythromycin Verapamil |
| Authors | Study Design | Medication Regimen | Inclusion Criteria | Outcomes Assessed | Results |
|---|---|---|---|---|---|
| Katakami N et al., J Clin Onc, June 2017, Japan [38] | Phase II b randomized double-blind placebo-controlled study | Naldemedine 1:1:1:1:1 assigned to either 0.1 mg, 0.2 mg, 0.4 mg, or placebo oral daily for 14 days | Adults 18 years or older with OIC and cancer, ECOG ≤ 2, on stable opioid regimen for ≥2 weeks | Primary: Change in spontaneous bowel movement frequency/week from baseline Secondary:
|
|
| |||||
| Katakami N et al., J Clin Onc, December 2017, Japan [39] | COMPOSE-4: randomized Phase III placebo-controlled double-blind study COMPOSE-5: open-label extension study | COMPOSE-4: 1:1 random assignment to Naldemedine 0.2 mg vs. placebo daily for 14 days COMPOSE-5: open-label 12-week extension | Adults 20 years or older with OIC and cancer, ECOG ≤ 2, on stable opioid regimen for ≥2 weeks | COMPOSE-4 Primary endpoint: Proportion of SBM responders (≥3 SBMs/week and increase ≥1 SBM/week from baseline) COMPOSE-5 primary end point: safety. |
COMPOSE-4: N = 193 (97 naldemedine, 96 placebo); COMPOSE-5: N = 131
|
| |||||
| Katakami N et al., Ann Onc, 2018, Japan [40] | COMPOSE-4: randomized Phase III placebo-controlled double-blind study COMPOSE-5: open-label extension study | COMPOSE-4: 1:1 random assignment to Naldemedine 0.2 mg vs. placebo daily for 14 days COMPOSE-5: open-label 12-week extension study of Naldemedine 0.2 mg | Adults 20 years or older with OIC and cancer, ECOG ≤ 2, on stable opioid regimen for ≥2 weeks | Secondary endpoints:
|
|
| |||||
| Osaka I et al., Esmo Open 2019, Japan [41] | Subgroup analysis of pooled data from both Katakami 2017 studies | Naldemedine 0.2 mg vs. placebo | Adults 18 years or older with OIC and cancer, ECOG ≤ 2, on stable opioid regimen for ≥2 weeks | Proportions of SBM responders and patients with diarrhea. For patient subgroups with or without possible blood–brain barrier (BBB) disruptions, changes in Numerical Rating Scale (NRS) and Clinical Opioid Withdrawal Scale (COWS) scores. |
|
| |||||
| Takata K et al., Support Care Cancer 2022, Japan [42] | Non-interventional multi-center prospective post-marketing surveillance | Naldemedine 0.2 mg, for up to 12 weeks | Adult patients with opioid-induced constipation (OIC) and cancer pain | Safety & effectiveness | Effectiveness analysis set (N = 953): Improved frequency (75.0% and 83.2%) and condition of bowel movement (80.0% and 88.0%) at 2 and 12 weeks, respectively |
| Safety analysis set (N = 1177), 145 ADRs occurred in 133 (11.3%) patients, diarrhea was the most frequent event (n = 107, 9.09%) but most cases of diarrhea were non-serious (98.1%). Most ADRs were non-serious (93.8%) and resolved within 2 weeks (75.9%). | |||||
| Naya N, 2023, Cureus, Japan [43] | Non-interventional exploratory post hoc subgroup analysis of post-marketing surveillance, same dataset as [42] | naldemedine 0.2 mg, for up to 12 weeks | Adult patients with opioid-induced constipation (OIC) and cancer pain | Safety & effectiveness with subgroup analysis by:
|
|
| Incidence of AE, including diarrhea, among subgroups ranged from 7.74% to 16.08% (diarrhea: 5.95% to 13.19%), compared to 11.30% (diarrhoea: 9.09%) in the total population. | |||||
| Von Roenn JH et al., 2013, USA, published as poster only [32] | KODIAC-06, planned as a randomized, placebo-controlled, double-blind, multicenter, phase 3 trial | Naloxegol 12.5 or 25 mg | Adult cancer patients with OIC |
| Study was closed early due to inability to enroll sufficient patients. No further details available, no response from author received by date of submission. |
| Bull J et al., J Pain Sym Man, 2019, USA [33] | Feasibility study, planned as 3-center randomized, placebo-controlled trial | Naloxegol 25 mg, with or without concomitant use of laxatives, (14 days of double-blind naloxegol vs. placebo followed by 14-day open-label naloxegol daily) | Adult advanced cancer patients aged ≥ 18 years, with life expectancy > 8 weeks, PPS ≥ 30, on at least 20 Morphine equivalents/d for >1 week, with OIC on laxatives |
| Study closed early after 24 months due to inability to enroll sufficient patients:
|
| Cobo Dols M et al., BMJ Support Palliat Care, 2020, Spain [44] | Non-interventional, 3-month follow-up observational cohortstudy | Naloxegol 12.5 or 25 mg, with or without concomitant use of laxatives | Adult cancer patients ≥ 18 years, on opioids for pain with OIC on laxatives, Karnofsky ≥ 50 |
|
|
| Lemaire A. et al., Supp Care Cancer, 2021, France [45] | Non-interventional “real life” outpatient multi-center, 4-week follow-up observational study | Naloxegol 12.5 or 25 mg, with or without concomitant use of laxatives | Adult cancer patients aged 18–70 years old with OIC on laxatives, any ECOG, on any opioid regimen |
| N = 124 cancer patients of which 79% had ECOG ≤ 2, metastatic stage, 80%. At inclusion, the median opioid dosage was 60 mg of oral morphine or equivalent.
|
| Davies A et. al., Cancers, 2022, 26 European countries [46] | Non-interventional, prospective “real world” singles arm open label multi-national 4-wek study | Naloxegol 12.5 or 25 mg (−50 mg), with or without concomitant use of laxatives | Adult cancer patients ≥ 18 years old who had been on opioids for at least 4 weeks and had OIC, any ECOG, on any opioid regimen. Colorectal cancer pts were excluded |
|
|
| Cobo Dols M et al., BMJ Support Palliat Care, 2023, Spain [47] | Non-interventional, 1-year prospective observational “real-world” study (continuation of Cobo 2020 study) | Naloxegol 12.5 or 25 mg, with or without concomitant laxative use | Adult cancer patients ≥ 18 years, on opioids for pain with OIC on laxatives, Karnofsky ≥ 50 | Long-term efficacy, quality of life (QOL) and safety of naloxegol. Assessed by the patient assessment of constipation QOL questionnaire (PAC-QOL), the PAC symptoms (PAC-SYM), the response rate at day 15, and months 1-3-6-12, and global QOL (EuroQoL-5D-5L) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, U.K.; Jackson, L.K.; Garcia, M.A.; Imam, S.N. A Systematic Review of Naldemedine and Naloxegol for the Treatment of Opioid-Induced Constipation in Cancer Patients. Pharmacy 2024, 12, 48. https://doi.org/10.3390/pharmacy12020048
Braun UK, Jackson LK, Garcia MA, Imam SN. A Systematic Review of Naldemedine and Naloxegol for the Treatment of Opioid-Induced Constipation in Cancer Patients. Pharmacy. 2024; 12(2):48. https://doi.org/10.3390/pharmacy12020048
Chicago/Turabian StyleBraun, Ursula K., Leanne K. Jackson, Mary A. Garcia, and Syed N. Imam. 2024. "A Systematic Review of Naldemedine and Naloxegol for the Treatment of Opioid-Induced Constipation in Cancer Patients" Pharmacy 12, no. 2: 48. https://doi.org/10.3390/pharmacy12020048
APA StyleBraun, U. K., Jackson, L. K., Garcia, M. A., & Imam, S. N. (2024). A Systematic Review of Naldemedine and Naloxegol for the Treatment of Opioid-Induced Constipation in Cancer Patients. Pharmacy, 12(2), 48. https://doi.org/10.3390/pharmacy12020048






