Medication Reviews and Clinical Outcomes in Persons with Dementia: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Step 1: Identifying the Research Question
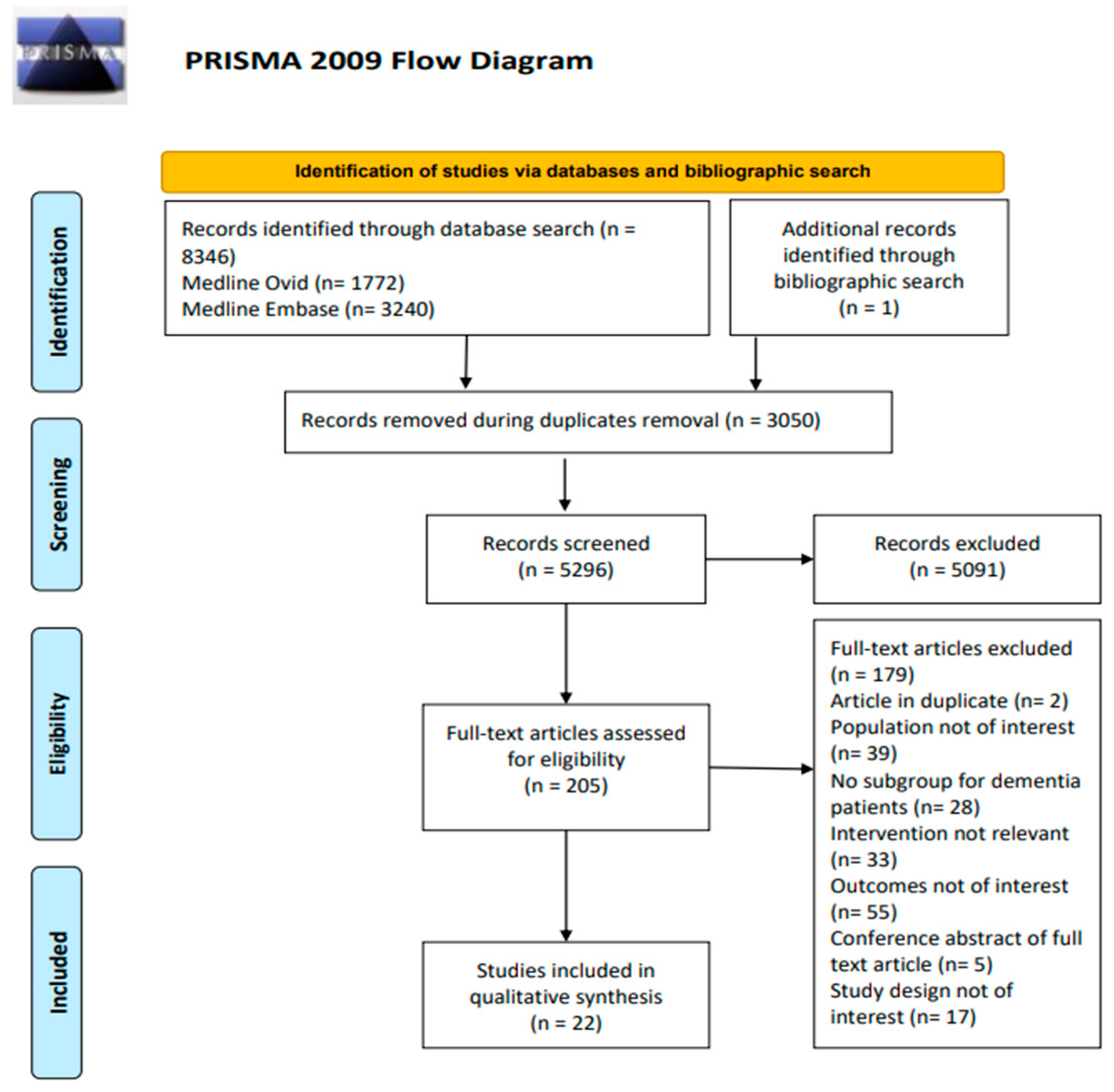
2.2. Step 2: Identifying the Relevant Studies
2.3. Step 3: Study Selection
2.4. Step 4: Data Charting
2.5. Step 5: Collating, Summarizing, and Reporting the Results
3. Results
3.1. Study Characteristics
3.2. Information about Interventions
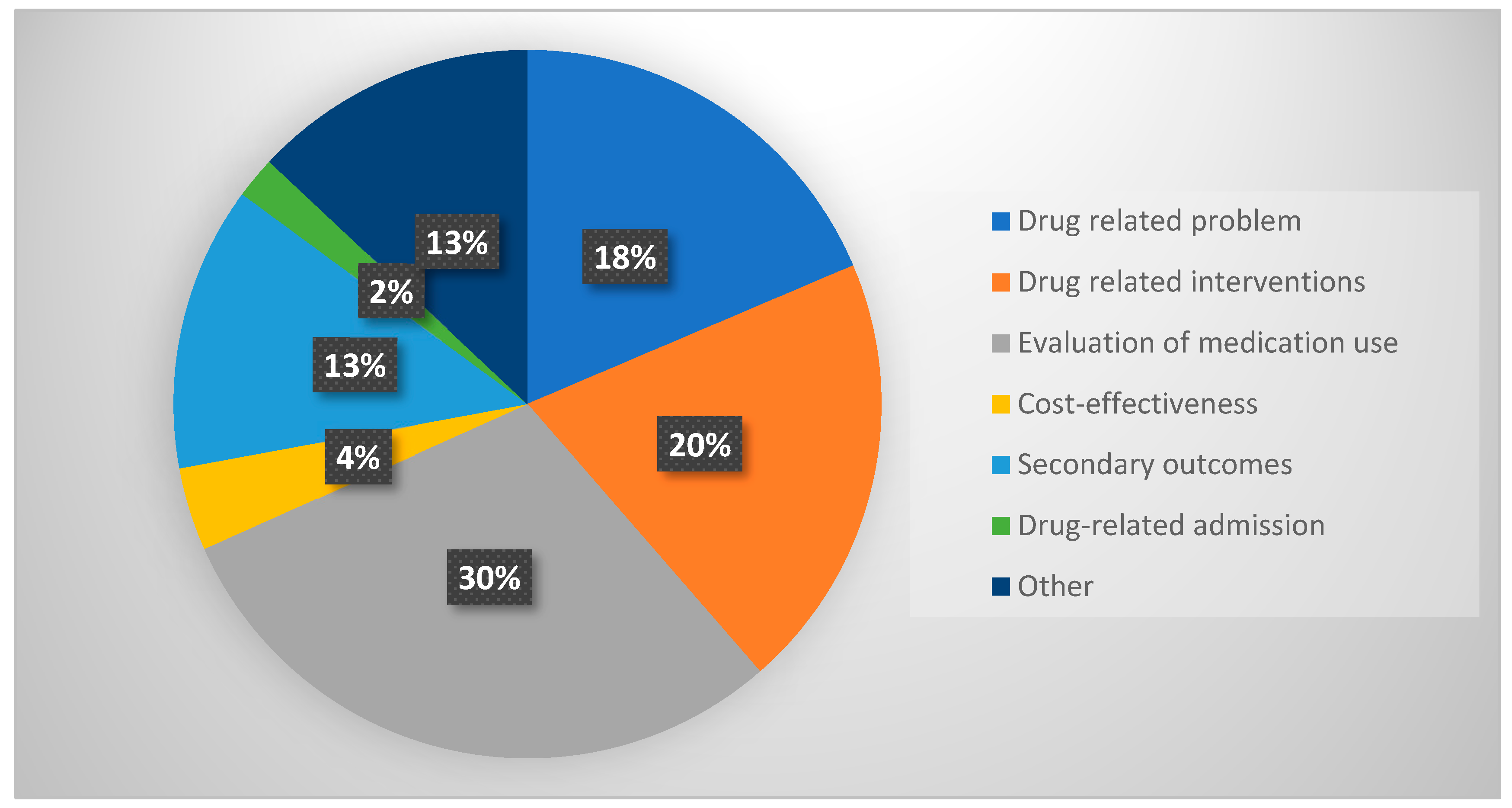
3.3. Type of Outcomes Reported
Effect of Medication Review
- (A)
- Evaluation of medication use
- (B)
- Drug-related problems
- (C)
- Drug-related interventions
4. Discussion
- The results obtained from RCTs are the most reliable evidence to assess an intervention’s effectiveness because the randomization process can minimize the risk of bias influencing the results [57]. No RCT was conducted in the community setting for patients with dementia.
- Only one study each was identified in this scoping review for Canada, Australia, the Netherlands, Slovenia, France, Taiwan, northern Sweden, Germany, Denmark, and Hong Kong. The studies conducted in these countries only included patients from one care setting. There is a need for more evidence for these counties in which patients are included from all types of care settings.
- Nine studies reported data from the LTC setting, and only one, by Hernandez et al., reported DRPs in persons with dementia from the LTC setting [45]. There is a scarcity of studies reporting DRPs in persons with dementia from the LTC setting.
- A lack of studies examining medication management and medication adherence as outcomes of medication reviews: A scoping review conducted by Hudani et al. in 2016 reported the nonadherence prevalence in older adults with CI or dementia, which ranged from 2 to 59%, which is not surprising considering the polypharmacy use, cognitive impairment, and complex medication regimens in this population [58]. Furthermore, the situation is much more difficult for individuals with CI or dementia due to various cognitive deficiencies, leading to increased nonadherence rates [58]. In this scoping review, we identified only one study that reported on medication nonadherence as an outcome of medication reviews in persons with CI and dementia [20]. Clinical practice in memory clinics includes evaluating the medication management capacity in this group. Still, it is not apparent why most of the studies did not report the effects of medication reviews on the medication management and adherence in this population. Any medication review conducted in this population should examine the medication adherence.
- A lack of research examining the cost-effectiveness of conducting a medication review: No study in this scoping review examined the impact of medication reviews on the overall cost, such as reductions in the medication cost, hospitalization cost, medical expenses, etc. Maidment et al. have reported data on costs, such as trainer and care home staff costs [50]. The authors conducted a mixed-method feasibility study that included a comprehensive clinical medication review conducted by a specialized dementia care pharmacist. Their findings revealed that the mean cost associated with the staff time for the medication review alone was GBP 104.41 per participant. In contrast, when accounting for both the medication review and the intervention (which included training), the mean cost rose to GBP 372.80 per participant. These cost assessments provide valuable insights into the financial aspects of implementing medication review interventions in dementia care. Only one other study has reported data on the clinical, economical, and organizational dimensions of DRI in the cognitive behavioral unit. Novais et al. conducted a study from retrospective data on medication reviews in a cognitive behavioral unit (CBU) [41]. These units are designed for people with responsive behavioural abnormalities linked to Alzheimer’s disease and related dementias (ADRD). Pharmacists discovered pertinent DRPs during medication reviews and made recommendations to the patients’ physicians. A total of 543 DRPs and DRIs were recorded for patients hospitalized in the CBU. According to pharmacists, 55.2% of pharmaceutical interventions decrease the costs of care, and 16.6% increase the costs [41]. No study was found in this scoping review that reported on the cost aspect in detail or whether the medication review conducted by a pharmacist decreases the overall cost, such as reduction in the medication cost, hospitalization cost, medical expenses, etc.
- A lack of patient and caregiver satisfaction as an outcome of medication reviews: The success of any intervention greatly relies on the patient receiving the care, the caregivers, and other healthcare professionals. The studies included in this scoping review reported no data on the satisfaction levels of patients, caregivers, or healthcare teams related to the medication review. The level of satisfaction will help the researcher to evaluate the patient, caregiver, and healthcare satisfaction and the potential acceptability of medication reviews by older adults with dementia.
- A lack of studies reporting on quality of life: There is a scarcity of studies examining the impact of medication reviews on the quality of life in people with dementia. In this scoping review, an RCT was conducted by Ballard et al. to measure whether a review of antipsychotic medications, either alone or in conjunction with evidence-based, non-pharmacological methods, has a substantial positive impact on health-related quality of life [31]. Two DEMQOL-Proxy domains (negative emotion and appearance) significantly worsened in individuals receiving antipsychotic reviews. The DEMQOL is a 28-item self-reported tool used to assess the health-related quality of life (HRQL) of people with dementia. The caregiver fills out a 31-item examination called the DEMQOL-Proxy, which examines the patient’s cognition, adverse emotions, positive emotions, daily activities, and appearance. More studies need to be conducted to see whether the medication review increases the quality of life among older adults with dementia or not.
- A lack of application of a dementia-specific core outcome set: The studies included in this scoping review showed variations in the measuring techniques and reported results. For instance, some studies have reported drug-related interventions without identifying DRPs, and not all studies followed up with the patients to measure the effects of the medication review. An international core outcome set for clinical trials of medication reviews in polypharmacy and multimorbid older people has been published [59]. The creation of core outcome sets for clinical trials has produced a variety of advantages, reduced the possibility of reporting bias, increased the chance of clinically meaningful results, and decreased the trial-to-trial variation in results [60]. Establishing a core outcome set for medication management interventions in primary care for individuals with dementia simplifies the research process by providing a standardized set of outcomes to evaluate the intervention’s effectiveness in this population. This approach enhances the consistency and comparability across studies, making it easier for researchers to gauge the impacts of these interventions on individuals with dementia.
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hanjani, L.S.; Long, D.; Peel, N.M.; Peeters, G.; Freeman, C.R.; Hubbard, R.E. Interventions to optimise prescribing in older people with dementia: A systematic review. Drugs Aging 2019, 36, 247–267. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Dementia. Available online: http://www.who.int/mediacentre/factsheets/fs362/en/ (accessed on 20 March 2023).
- World Health Organization. Available online: https://www.who.int/news/item/07-12-2017-dementia-number-of-people-affected-to-triple-in-next-30-years (accessed on 20 March 2023).
- Dementia in Canada, Including Alzheimer’s Disease. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/dementia-highlights-canadian-chronic-disease-surveillance.html (accessed on 20 March 2023).
- Chung, S.D.; Liu, S.P.; Sheu, J.J.; Lin, C.C.; Lin, H.C.; Chen, C.H. Increased healthcare service utilizations for patients with dementia: A population-based study. PLoS ONE 2014, 9, e105789. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer Society Canada. Dementia Numbers in Canada. Available online: https://alzheimer.ca/en/about-dementia/what-dementia/dementia-numbers-canada#ref5 (accessed on 20 March 2023).
- Schubert, C.C.; Boustani, M.; Callahan, C.M.; Perkins, A.J.; Carney, C.P.; Fox, C.; Unverzagt, F.; Hui, S.; Hendrie, H.C. Comorbidity profile of dementia patients in primary care: Are they sicker? J. Am. Geriatr. Soc. 2006, 54, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Clague, F.; Mercer, S.W.; McLean, G.; Reynish, E.; Guthrie, B. Comorbidity and polypharmacy in people with dementia: Insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing 2017, 46, 33–39. [Google Scholar] [CrossRef]
- Growdon, M.E.; Gan, S.; Yaffe, K.; Steinman, M.A. Polypharmacy among older adults with dementia compared with those without dementia in the United States. J. Am. Geriatr. Soc. 2021, 69, 2464–2475. [Google Scholar] [CrossRef]
- Lau, D.T.; Mercaldo, N.D.; Harris, A.T.; Trittschuh, E.; Shega, J.; Weintraub, S. Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 56. [Google Scholar] [CrossRef]
- Johnell, K. Inappropriate drug use in people with cognitive impairment and dementia: A systematic review. Curr. Clin. Pharmacol. 2015, 10, 178–184. [Google Scholar] [CrossRef]
- Ramsey, C.M.; Gnjidic, D.; Agogo, G.O.; Allore, H.; Moga, D. Longitudinal patterns of potentially inappropriate medication use following incident dementia diagnosis. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Renom-Guiteras, A.; Thürmann, P.A.; Miralles, R.; Klaaßen-Mielke, R.; Thiem, U.; Stephan, A.; Bleijlevens, M.H.; Jolley, D.; Leino-Kilpi, H.; Rahm Hallberg, I.; et al. Potentially inappropriate medication among people with dementia in eight European countries. Age Ageing 2018, 47, 68–74. [Google Scholar] [CrossRef]
- Brunet, N.M.; Sevilla-Sánchez, D.; Novellas, J.A.; Jané, C.C.; Gómez-Batiste, X.; McIntosh, J.; Panicot, J.E. Optimizing drug therapy in patients with advanced dementia: A patient-centered approach. Eur. Geriatr. Med. 2014, 5, 66–71. [Google Scholar] [CrossRef]
- Smith, D.; Lovell, J.; Weller, C.; Kennedy, B.; Winbolt, M.; Young, C.; Ibrahim, J. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS ONE 2017, 12, e0170651. [Google Scholar] [CrossRef] [PubMed]
- Eshetie, T.C.; Nguyen, T.A.; Gillam, M.H.; Ellett, L.M.K. A narrative review of problems with medicines use in people with dementia. Expert Opin. Drug. Saf. 2018, 17, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Pfister, B.; Jonsson, J.; Gustafsson, M. Drug-related problems and medication reviews among old people with dementia. BMC Pharmacol. Toxicol. 2017, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Van Spall, H.G.; Toren, A.; Kiss, A.; Fowler, R.A. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: A systematic sampling review. JAMA 2007, 297, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Reeve, E.; Trenaman, S.C.; Rockwood, K.; Hilmer, S.N. Pharmacokinetic and pharmacodynamic alterations in older people with dementia. Expert Opin. Drug. Metab. Toxicol. 2017, 13, 651–668. [Google Scholar] [CrossRef]
- Dong, X.; Tsang, C.C.S.; Zhao, S.; Browning, J.A.; Wan, J.Y.; Chisholm-Burns, M.A.; Finch, C.K.; Tsao, J.W.; Hines, L.E.; Wang, J. Effects of the Medicare Part D comprehensive medication review on medication adherence among patients with Alzheimer’s disease. Curr. Med. Res. Opin. 2021, 37, 1581–1588. [Google Scholar] [CrossRef]
- PCNE Working Group on Medication Review. Available online: https://www.pcne.org/working-groups/1/medication-review (accessed on 20 March 2023).
- Krska, J.; Cromarty, J.A.; Arris, F.; Jamieson, D.; Hansford, D.; Duffus, P.R.; Downie, G.; Seymour, D.G. Pharmacist-led medication review in patients over 65: A randomized, controlled trial in primary care. Age Ageing 2001, 30, 205–211. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- van Mil, J.F.; Westerlund, L.T.; Hersberger, K.E.; Schaefer, M.A. Drug-related problem classification systems. Ann. Pharmacother. 2004, 38, 859–867. [Google Scholar] [CrossRef]
- Gustafsson, M.; Sjölander, M.; Pfister, B.; Jonsson, J.; Schneede, J.; Lövheim, H. Pharmacist participation in hospital ward teams and hospital readmission rates among people with dementia: A randomized controlled trial. Eur. J. Clin. Pharmacol. 2017, 73, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.; Sjölander, M.; Pfister, B.; Schneede, J.; Lövheim, H. Effects of Pharmacists’ Interventions on Inappropriate Drug Use and Drug-Related Readmissions in People with Dementia—A Secondary Analysis of a Randomized Controlled Trial. Pharmacy 2018, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Abramsson, L.; Gustafsson, M. Prevalence of drug-related problems using STOPP/START and medication reviews in elderly patients with dementia. Res. Soc. Adm. Pharm. 2020, 16, 308–314. [Google Scholar]
- Sanford, A.M.; Orrell, M.; Tolson, D.; Abbatecola, A.M.; Arai, H.; Bauer, J.M.; Cruz-Jentoft, A.J.; Dong, B.; Ga, H.; Goel, A.; et al. An international definition for “nursing home”. J. Am. Med. Dir. Assoc. 2015, 16, 181–184. [Google Scholar] [CrossRef]
- Lee, C.; Ivo, J.; Carter, C.; Faisal, S.; Shao, Y.W.; Patel, T. Pharmacist interventions for persons with intellectual disabilities: A scoping review. Res. Soc. Adm. Pharm. 2021, 17, 257–272. [Google Scholar]
- Ballard, C.; Orrell, M.; Yongzhong, S.; Moniz-Cook, E.; Stafford, J.; Whittaker, R.; Woods, B.; Corbett, A.; Garrod, L.; Khan, Z.; et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: A factorial cluster-randomized controlled trial by the well-being and health for people with dementia (WHELD) program. Am. J. Psychiatry 2016, 173, 252–262. [Google Scholar]
- Ballard, C.; Orrell, M.; Sun, Y.; Moniz-Cook, E.; Stafford, J.; Whitaker, R.; Woods, B.; Corbett, A.; Banerjee, S.; Testad, I.; et al. Impact of antipsychotic review and non-pharmacological intervention on health-related quality of life in people with dementia living in care homes: WHELD—A factorial cluster randomised controlled trial. Int. J. Geriatr. Psychiatry 2017, 32, 1094–1103. [Google Scholar] [CrossRef]
- Smeets, C.H.W.; Smalbrugge, M.; Koopmans, R.T.C.M.; Nelissen-Vrancken, M.H.J.M.G.; Van Der Spek, K.; Teerenstra, S.; Gerritsen, D.L.; Zuidema, S.U. Can the PROPER intervention reduce psychotropic drug prescription in nursing home residents with dementia? Results of a cluster-randomized controlled trial. Int. Psychogeriatr. 2021, 33, 577–586. [Google Scholar] [CrossRef]
- van der Spek, K.; Koopmans, R.T.; Smalbrugge, M.; Nelissen-Vrancken, M.H.; Wetzels, R.B.; Smeets, C.H.; De Vries, E.; Teerenstra, S.; Zuidema, S.U.; Gerritsen, D.L. The effect of biannual medication reviews on the appropriateness of psychotropic drug use for neuropsychiatric symptoms in patients with dementia: A randomised controlled trial. Age Ageing 2018, 47, 430–437. [Google Scholar] [CrossRef]
- Stuhec, M.; Lah, L. Clinical pharmacist interventions in elderly patients with mental disorders in primary care focused on psychotropics: A retrospective pre–post observational study. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211011007. [Google Scholar] [CrossRef]
- Wilchesky, M.; Mueller, G.; Morin, M.; Marcotte, M.; Voyer, P.; Aubin, M.; Carmichael, P.H.; Champoux, N.; Monette, J.; Giguère, A.; et al. The OptimaMed intervention to reduce inappropriate medications in nursing home residents with severe dementia: Results from a quasi-experimental feasibility pilot study. BMC Geriatr. 2018, 18, 204. [Google Scholar]
- Mesquida, M.M.; Casas, M.T.; Sisó, A.F.; Muñoz, I.G.; Vian, Ó.H.; Monserrat, P.T. Consensus and evidence-based medication review to optimize and potentially reduce psychotropic drug prescription in institutionalized dementia patients. BMC Geriatr. 2019, 19, 7. [Google Scholar]
- Pearson, S.M.; Osbaugh, N.A.; Linnebur, S.A.; Fixen, D.R.; Brungardt, A.; Marcus, A.M.; Lum, H.D. Implementation of Pharmacist Reviews to Screen for Potentially Inappropriate Medications in Patients with Cognitive Impairment. Sr. Care Pharm. 2021, 36, 508–522. [Google Scholar] [PubMed]
- Levine, A.M.; Emonds, E.E.; Smith, M.A.; Rickles, N.M.; Kuchel, G.A.; Steffens, D.C.; Ohlheiser, A.; Fortinsky, R.H. Pharmacist identification of medication therapy problems involving cognition among older adults followed by a home-based care team. Drugs Aging 2021, 38, 157–168. [Google Scholar] [PubMed]
- Melville, B.L.; Bailey, J.; Moss, J.; Bryan, W.; Davagnino, J.; Twersky, J.; Pepin, M. Description of pharmacist recommendations in the Caring for Older Adults and Caregivers at Home (COACH) Program. Sr. Care Pharm. 2020, 35, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Novais, T.; Maldonado, F.; Grail, M.; Krolak-Salmon, P.; Mouchoux, C. Clinical, economic, and organizational impact of pharmacists’ interventions in a cognitive-behavioral unit in France. Int. J. Clin. Pharm. 2021, 43, 613–620. [Google Scholar]
- Weeks, W.B.; Mishra, M.K.; Curto, D.; Petersen, C.L.; Cano, P.; Hswen, Y.; Serra, S.V.; Elwyn, G.; Godfrey, M.M.; Soro, P.S.; et al. Comparing three methods for reducing psychotropic use in older demented Spanish care home residents. J. Am. Geriatr. Soc. 2019, 67, 1444–1453. [Google Scholar] [CrossRef]
- Wucherer, D.; Thyrian, J.R.; Eichler, T.; Hertel, J.; Kilimann, I.; Richter, S.; Michalowsky, B.; Zwingmann, I.; Dreier-Wolfgramm, A.; Ritter, C.A.; et al. Drug-related problems in community-dwelling primary care patients screened positive for dementia. Int. Psychogeriatr. 2017, 29, 1857–1868. [Google Scholar]
- Bach, L.L.; Lazzaretto, D.L.; Young, C.F.; Lofholm, P.W. Improving nursing home compliance via revised antipsychotic use survey tool. Consult. Pharm. 2017, 32, 228–238. [Google Scholar] [CrossRef]
- Hernandez, M.; Mestres, C.; Junyent, J.; Costa-Tutusaus, L.; Modamio, P.; Lastra, C.F.; Mariño, E.L. Effects of a multifaceted intervention in psychogeriatric patients: One-year prospective study. Eur. J. Hosp. Pharm. 2020, 27, 226–231. [Google Scholar]
- Liang, C.K.; Chou, M.Y.; Chen, L.Y.; Wang, K.Y.; Lin, S.Y.; Chen, L.K.; Lin, Y.T.; Liu, T.Y.; Loh, C.H. Delaying cognitive and physical decline through multidomain interventions for residents with mild-to-moderate dementia in dementia care units in Taiwan: A prospective cohort study. Geriatr. Gerontol. Int. 2017, 17, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.M.; Wollsen, M.G.; Aagaard, L. Pain monitoring and medication assessment in elderly nursing home residents with dementia. J. Res. Pharm. Pract. 2016, 5, 126. [Google Scholar] [PubMed]
- Wong, Y.L.; Cheung, K.L.; Chan, C.C.; Yung, C.Y. P3-329: Pharmacist-Managed Medication Review in a Novel Multidisciplinary Care Model for Elderly with Dementia. Alzheimer’s Dement. 2016, 12, P972–P973. [Google Scholar] [CrossRef]
- Aziz, V.M.; Hill, N.; Kumar, S. Completed audit cycle to explore the use of the STOPP/START toolkit to optimise medication in psychiatric in-patients with dementia. BJPsych. Bull. 2018, 42, 37–41. [Google Scholar] [CrossRef]
- Maidment, I.D.; Barton, G.; Campbell, N.; Shaw, R.; Seare, N.; Fox, C.; Iliffe, S.; Randle, E.; Hilton, A.; Brown, G.; et al. MEDREV (pharmacy-health psychology intervention in people living with dementia with behaviour that challenges): The feasibility of measuring clinical outcomes and costs of the intervention. BMC Health Serv. 2020, 20, 157. [Google Scholar]
- Cross, A.J.; George, J.; Woodward, M.C.; Le, V.J.; Elliott, R.A. Deprescribing potentially inappropriate medications in memory clinic patients (DePIMM): A feasibility study. Res. Soc. Adm. Pharm. 2020, 16, 1392–1397. [Google Scholar]
- American Society of Health-System Pharmacists. ASHP guidelines on a standardized method for pharmaceutical care. Am. J. Health-Syst. Pharm. 1996, 53, 1713–1716. [Google Scholar] [CrossRef]
- Cipolle, R.; Strand, L.; Morely, P. Pharmaceutical Care Practice; The McGraw-Hill Companies Inc.: New York, NY, USA, 1998. [Google Scholar]
- Pharmaceutical Care Network Europe Foundation. The PCNE Classification V 6.2—Classification for Drug Related Problems. 2010. Available online: http://www.pcne.org/upload/files/11_PCNE_classification_V6-2.pdf (accessed on 20 March 2023).
- McGrattan, M.; Ryan, C.; Barry, H.E.; Hughes, C.M. Interventions to improve medicines management for people with dementia: A systematic review. Drugs Aging 2017, 34, 907–916. [Google Scholar]
- Liu, A.K.; Possin, K.L.; Cook, K.M.; Lynch, S.; Dulaney, S.; Merrilees, J.J.; Braley, T.; Kiekhofer, R.E.; Bonasera, S.J.; Allen, I.E.; et al. Effect of collaborative dementia care on potentially inappropriate medication use: Outcomes from the Care Ecosystem randomized clinical trial. Alzheimer’s Dement. 2022, 19, 1865–1875. [Google Scholar]
- Akobeng, A.K. Understanding randomised controlled trials. Arch. Dis. Childh. 2005, 90, 840–844. [Google Scholar] [CrossRef]
- Hudani, Z.K.; Rojas-Fernandez, C.H. A scoping review on medication adherence in older patients with cognitive impairment or dementia. Res. Soc. Adm. Pharm. 2016, 12, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Beuscart, J.B.; Knol, W.; Cullinan, S.; Schneider, C.; Dalleur, O.; Boland, B.; Thevelin, S.; Jansen, P.A.; O’Mahony, D.; Rodondi, N.; et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018, 16, 21. [Google Scholar]
- McGrattan, M.; Barry, H.E.; Ryan, C.; Cooper, J.A.; Passmore, A.P.; Robinson, A.L.; Molloy, G.J.; Darcy, C.M.; Buchanan, H.; Hughes, C.M. The development of a core outcome set for medicines management interventions for people with dementia in primary care. Age Ageing 2019, 48, 260–266. [Google Scholar] [PubMed]
| Study | Types of Drug-Related Problems Reported |
|---|---|
| Pearson et al., 2021 [38] | 2019 Beers Criteria
|
| Levine et al., 2021 [39] |
|
| Aziz et al., 2018 [49] | 2015 STOPP Criteria
|
| Melville et al., 2020 [40] | 2012 Beers Criteria
|
| Novais et al., 2021 [41] | Westerlund System [25]
|
| Hernandez et al., 2020 [45] |
|
| Cross et al., 2020 [51] | Beer’s 2015 Criteria or 2015 STOPP Criteria
|
| Gustafsson et al., 2017 [17,26,27,28] | 2015 STOPP/START Criteria
|
| Wucherer et al., 2017 [43] | Inappropriate drugs according to the PRISCUS list reported in 105 (22.9%) patients. PCNE Classification V 6.2 [54]
|
| Wong et al., 2016 [48] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.; Mahajan, N.; Fadaleh, S.A.; Patel, H.; Ivo, J.; Faisal, S.; Chang, F.; Lee, L.; Patel, T. Medication Reviews and Clinical Outcomes in Persons with Dementia: A Scoping Review. Pharmacy 2023, 11, 168. https://doi.org/10.3390/pharmacy11050168
Sharma R, Mahajan N, Fadaleh SA, Patel H, Ivo J, Faisal S, Chang F, Lee L, Patel T. Medication Reviews and Clinical Outcomes in Persons with Dementia: A Scoping Review. Pharmacy. 2023; 11(5):168. https://doi.org/10.3390/pharmacy11050168
Chicago/Turabian StyleSharma, Rishabh, Neil Mahajan, Sarah Abu Fadaleh, Hawa Patel, Jessica Ivo, Sadaf Faisal, Feng Chang, Linda Lee, and Tejal Patel. 2023. "Medication Reviews and Clinical Outcomes in Persons with Dementia: A Scoping Review" Pharmacy 11, no. 5: 168. https://doi.org/10.3390/pharmacy11050168
APA StyleSharma, R., Mahajan, N., Fadaleh, S. A., Patel, H., Ivo, J., Faisal, S., Chang, F., Lee, L., & Patel, T. (2023). Medication Reviews and Clinical Outcomes in Persons with Dementia: A Scoping Review. Pharmacy, 11(5), 168. https://doi.org/10.3390/pharmacy11050168







